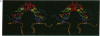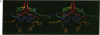Abstract
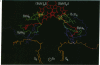
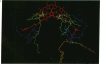
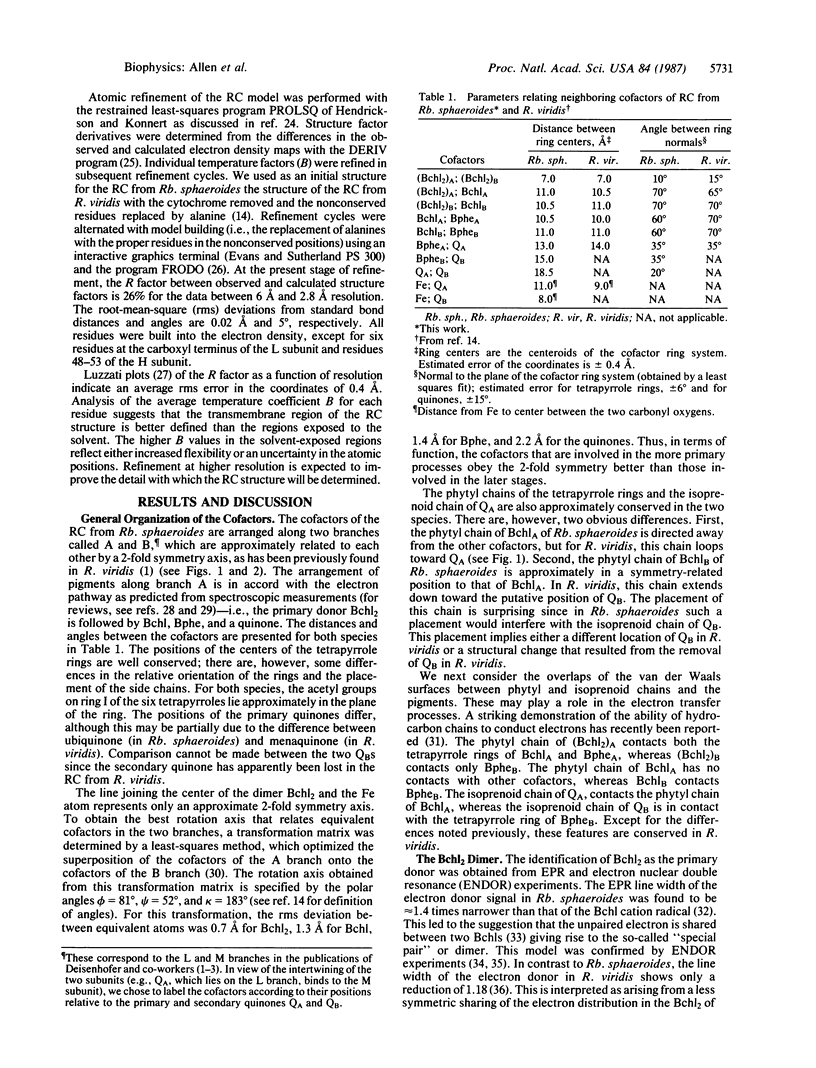
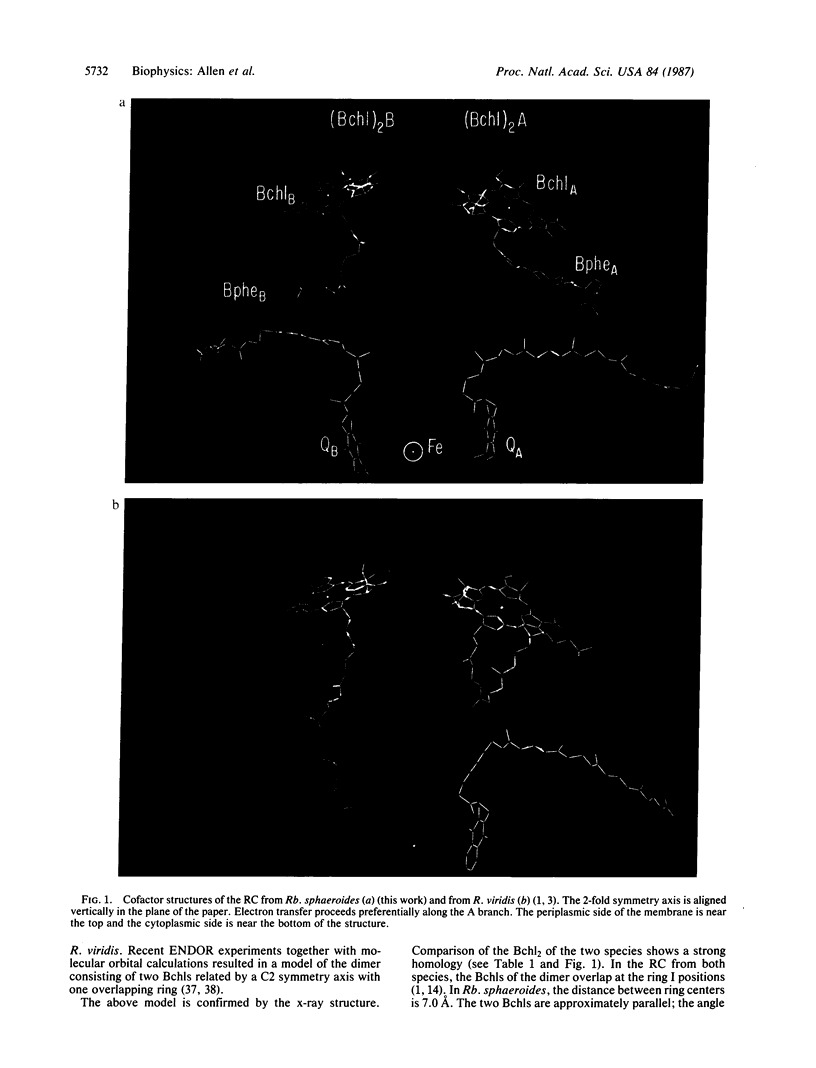
The three-dimensional structure of the cofactors of the reaction center of Rhodobacter sphaeroides R-26 has been determined by x-ray diffraction and refined at a resolution of 2.8 A with an R value of 26%. The main features of the structure are similar to the ones determined for Rhodopseudomonas viridis [Michel, H., Epp, O. & Deisenhofer, J. (1986) EMBO J. 5, 2445-2451]. The cofactors are arranged along two branches, which are approximately related to each other by a 2-fold symmetry axis. The structure is well suited to produce light-induced charge separation across the membrane. Most of the structural features predicted from physical and biochemical measurements are confirmed by the x-ray structure.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. P., Feher G. Crystallization of reaction center from Rhodopseudomonas sphaeroides: preliminary characterization. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4795–4799. doi: 10.1073/pnas.81.15.4795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J. P., Feher G., Yeates T. O., Rees D. C., Deisenhofer J., Michel H., Huber R. Structural homology of reaction centers from Rhodopseudomonas sphaeroides and Rhodopseudomonas viridis as determined by x-ray diffraction. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8589–8593. doi: 10.1073/pnas.83.22.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship R. E., Parson W. W. The involvement of iron and ubiquinone in electron transfer reactions mediated by reaction centers from photosynthetic bacteria. Biochim Biophys Acta. 1979 Mar 15;545(3):429–444. doi: 10.1016/0005-2728(79)90152-x. [DOI] [PubMed] [Google Scholar]
- Bunker G., Stern E. A., Blankenship R. E., Parson W. W. An x-ray absorption study of the iron site in bacterial photosynthetic reaction centers. Biophys J. 1982 Feb;37(2):539–551. doi: 10.1016/S0006-3495(82)84699-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. F., Calvo R., Fredkin D. R., Isaacson R. A., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. III. EPR measurements of the reduced acceptor complex. Biophys J. 1984 May;45(5):947–973. doi: 10.1016/S0006-3495(84)84241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. F., Johnston D. C., Shore H. B., Fredkin D. R., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. I. Static magnetization measurements. Biophys J. 1980 Dec;32(3):967–992. doi: 10.1016/S0006-3495(80)85030-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. H., Tiede D., Tang J., Smith U., Norris J., Schiffer M. Structure of Rhodopseudomonas sphaeroides R-26 reaction center. FEBS Lett. 1986 Sep 1;205(1):82–86. doi: 10.1016/0014-5793(86)80870-5. [DOI] [PubMed] [Google Scholar]
- Debus R. J., Feher G., Okamura M. Y. Iron-depleted reaction centers from Rhodopseudomonas sphaeroides R-26.1: characterization and reconstitution with Fe2+, Mn2+, Co2+, Ni2+, Cu2+, and Zn2+. Biochemistry. 1986 Apr 22;25(8):2276–2287. doi: 10.1021/bi00356a064. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Epp O., Miki K., Huber R., Michel H. X-ray structure analysis of a membrane protein complex. Electron density map at 3 A resolution and a model of the chromophores of the photosynthetic reaction center from Rhodopseudomonas viridis. J Mol Biol. 1984 Dec 5;180(2):385–398. doi: 10.1016/s0022-2836(84)80011-x. [DOI] [PubMed] [Google Scholar]
- Eisenberger P., Okamura M. Y., Feher G. The electronic structure of Fe2+ in reaction centers from Rhodopseudomonas sphaeroides. II. Extended x-ray fine structure studies. Biophys J. 1982 Feb;37(2):523–538. doi: 10.1016/S0006-3495(82)84698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher G., Hoff A. J., Isaacson R. A., Ackerson L. C. ENDOR experiments on chlorophyll and bacteriochlorophyll in vitro and in the photosynthetic unit. Ann N Y Acad Sci. 1975 Apr 15;244:239–259. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 1985;115:252–270. doi: 10.1016/0076-6879(85)15021-4. [DOI] [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Kleinfeld D., Okamura M. Y., Feher G. Electron transfer in reaction centers of Rhodopseudomonas sphaeroides. I. Determination of the charge recombination pathway of D+QAQ(-)B and free energy and kinetic relations between Q(-)AQB and QAQ(-)B. Biochim Biophys Acta. 1984 Jul 27;766(1):126–140. doi: 10.1016/0005-2728(84)90224-x. [DOI] [PubMed] [Google Scholar]
- Lubitz W., Lendzian F., Scheer H., Gottstein J., Plato M., Möbius K. Structural studies of the primary donor cation radical P(870) in reaction centers of Rhodospirillum rubrum by electron-nuclear double resonance in solution. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1401–1405. doi: 10.1073/pnas.81.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinetti T. D., Okamura M. Y., Feher G. Localization of the primary quinone binding site in reaction centers from Rhodopseudomonas sphaeroides R-26 by photoaffinity labeling. Biochemistry. 1979 Jul 10;18(14):3126–3133. doi: 10.1021/bi00581a033. [DOI] [PubMed] [Google Scholar]
- McElroy J. D., Feher G., Mauzerall D. C. On the nature of the free radical formed during the primary process of bacterial photosynthesis. Biochim Biophys Acta. 1969 Jan 14;172(1):180–183. doi: 10.1016/0005-2728(69)90105-4. [DOI] [PubMed] [Google Scholar]
- Michel H., Epp O., Deisenhofer J. Pigment-protein interactions in the photosynthetic reaction centre from Rhodopseudomonas viridis. EMBO J. 1986 Oct;5(10):2445–2451. doi: 10.1002/j.1460-2075.1986.tb04520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Katz J. J. Models for antenna and reaction center chlorophylls. Ann N Y Acad Sci. 1975 Apr 15;244:260–280. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Uphaus R. A., Crespi H. L., Katz J. J. Electron spin resonance of chlorophyll and the origin of signal I in photosynthesis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):625–628. doi: 10.1073/pnas.68.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura M. Y., Isaacson R. A., Feher G. Primary acceptor in bacterial photosynthesis: obligatory role of ubiquinone in photoactive reaction centers of Rhodopseudomonas spheroides. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3491–3495. doi: 10.1073/pnas.72.9.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. M. Calculation of molecular volumes and areas for structures of known geometry. Methods Enzymol. 1985;115:440–464. doi: 10.1016/0076-6879(85)15032-9. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Bryant A., Quate C. F., Rabe J. P., Gerber C., Swalen J. D. Images of a lipid bilayer at molecular resolution by scanning tunneling microscopy. Proc Natl Acad Sci U S A. 1987 Feb;84(4):969–972. doi: 10.1073/pnas.84.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeglio A. Secondary electron transfer in reaction centers of Rhodopseudomonas sphaeroides. Out-of-phase periodicity of two for the formation of ubisemiquinone and fully reduced ubiquinone. Biochim Biophys Acta. 1977 Mar 11;459(3):516–524. doi: 10.1016/0005-2728(77)90050-0. [DOI] [PubMed] [Google Scholar]
- Wraight C. A. Electron acceptors of photosynthetic bacterial reaction centers. Direct observation of oscillatory behaviour suggesting two closely equivalent ubiquinones. Biochim Biophys Acta. 1977 Mar 11;459(3):525–531. doi: 10.1016/0005-2728(77)90051-2. [DOI] [PubMed] [Google Scholar]