Abstract
New animal models are greatly needed in interstitial cystitis/painful bladder syndrome (IC/PBS) research. We recently developed a novel transgenic cystitis model (URO-OVA mice) that mimics certain key aspects of IC/PBS pathophysiology. This paper aimed to determine whether URO-OVA cystitis model was responsive to intravesical dimethyl sulfoxide (DMSO) and if so identify the mechanisms of DMSO action. URO-OVA mice developed acute cystitis upon adoptive transfer of OVA-specific OT-I splenocytes. Compared to PBS-treated bladders, the bladders treated with 50% DMSO exhibited markedly reduced bladder histopathology and expression of various inflammatory factor mRNAs. Intravesical DMSO treatment also effectively inhibited bladder inflammation in a spontaneous chronic cystitis model (URO-OVA/OT-I mice). Studies further revealed that DMSO could impair effector T cells in a dose-dependent manner in vitro. Taken together, our results suggest that intravesical DMSO improves the bladder histopathology of IC/PBS patients because of its ability to interfere with multiple inflammatory and bladder cell types.
1. Introduction
IC/PBS is a chronic inflammatory condition of the urinary bladder characterized by pelvic pain, irritative voiding symptoms (frequency, urgency, and nocturia), and sterile and cytologically normal urine [1, 2]. The symptoms of IC/PBS are often associated with significant fatigue, depression, anxiety, and suicidal tendency [3, 4], and thus affect every aspect of an individual's life. Although the etiology of IC/PBS remains unknown, many theories have been proposed including mast cell activation, sensory neuron irritation, inflammation, and autoimmunity [2, 5–7]. Accordingly, IC/PBS models reflecting various pathophysiological pathways have been developed [8]. Among IC/PBS models, the rodent model of experimental autoimmune cystitis (EAC) [9] in which animals develop cystitis after immunization with bladder homogenate, represents one of the most actively used models in IC/PBS research [9–13]. Although this conventional EAC model can reproduce many clinical correlates seen in IC/PBS, this model does not facilitate the studies of detailed mechanisms because of its lack of defined self-antigen (Ag) and its corresponding T cell receptor (TCR) specificity. To improve this model, we recently developed a novel transgenic EAC model, designated as URO-OVA mice [14]. URO-OVA mice express a membrane form of the model Ag ovalbumin (OVA) as a self-Ag on the bladder urothelium driven by the uroplakin II gene promoter and develop bladder inflammation upon introduction of OT-I CD8+ T cells that express the transgenic TCR specific for H2-Kb/OVA257–264 epitope [15]. The inflamed bladder resembles the acute phase of IC/PBS histopathology as manifested by prominent cellular infiltration, interstitial edema, mucosal hyperemia, and high mast cell counts [14]. The inflamed bladder also resembles neurogenic inflammation as elevated mast cell- and sensory neuron-derived inflammatory factors such as tumor necrosis factor (TNF)-α, nerve growth factor (NGF), and substance P are detectable in the bladder prior to the detection of histological changes [14]. In addition, URO-OVA/OT-I mice, a derived line of URO-OVA mice after crossed with OT-I mice, can spontaneously develop bladder inflammation with predominant cellular infiltration, epithelial hyperplasia, and high mast cell counts [14], which are the characteristics of the chronic phase of IC/PBS. To date, the URO-OVA cystitis models have proven to be unique and reproducible, permitting controlled studies on bladder inflammation including antibladder inflammatory studies [16, 17].
Due to its diverse pharmacologic properties, DMSO has been used for the treatment of various diseases including IC/PBS [18, 19]. Since approved by the U.S. Food and Drug Administration (FDA) in 1978, intravesical DMSO has served as one of the mainstays in the pharmacologic treatment of IC/PBS. Although its mechanisms of action have not yet been fully elucidated, intravesical DMSO has shown its favorable effects on treating both classic and nonulcer IC/PBS patients [20–23]. Intravesical DMSO relieves pain and voiding symptoms likely via its properties of anti-inflammation and mast cell stabilization [24, 25]. In a protamine sulfate-induced rat cystitis model, intravesical DMSO has also been demonstrated to be effective on treating non-bacterial bladder inflammation [26, 27]. Studies in vitro have further demonstrated that DMSO could inhibit stretch-activated ATP release by bladder urothelial cells from IC/PBS patients [28], relax rabbit bladder detrusor muscle contractility [29], improve rat bladder muscle compliance [30], and increase rat bladder sensory afferent neuron release of nitric oxide [31]. In this study, we used transgenic EAC models to evaluate the effect of intravesical DMSO on treating autoimmune cystitis. We observed that DMSO could inhibit both acute and chronic autoimmune cystitis in vivo and effector T cell activity in vitro. Our results support the use of intravesical DMSO for the treatment of IC/PBS patients and provide a potential mechanism underlying the DMSO action.
2. Materials and Methods
2.1. Mice
URO-OVA mice (B6 and Thy1.2 background) were developed in our laboratory and used to provide an acute EAC model [14]. URO-OVA/OT-I mice were generated through crossbreeding of URO-OVA mice with OT-I mice (B6 background), a transgenic line originally generated by Kurts and associates that expresses the CD8+ TCR specific for Kb/OVA257–264 epitope [15], and used to provide a chronic EAC model [14]. Female mice were used due to their feasibility for intravesical procedures. In addition, OT-I mice with both B6 and Thy1.1 backgrounds were used to provide effector T cells for cystitis induction. Mice were used at 8–10 weeks. All mice were housed in a pathogen-free facility at the University of Iowa Animal Care Facility and used according to the procedures approved by University of Iowa Animal Care and Use Committee.
2.2. Cystitis Induction and DMSO Treatment
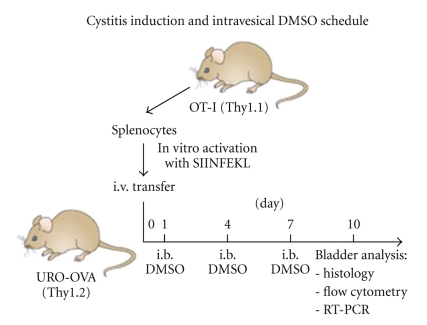
As described previously [14, 17], OT-I splenocytes (Thy1.1) were prepared, activated with OVA257–264 peptide in vitro, and transferred i.v. into URO-OVA mice (Thy1.2) for acute cystitis induction. Each mouse received 1 × 106 preactivated OT-I splenocytes at day 0. DMSO (Fisher Scientific, Fair Lawn, New Jersey) was dissolved in PBS at a 50% concentration and used as an intravesical therapeutic agent. Mice were anesthetized i.p. with 100 μL of a mixture solution of ketamine (87.5 mg/kg) and xylazine (12.5 mg/kg). The bladder was then catheterized via the urethra with a 24-gauge plastic intravenous cannula and instilled with 50 μL of 50% DMSO solution through the cannula for 1 hour. Control bladders were instilled with 50 μL of PBS. In our previous studies, URO-OVA mice developed acute cystitis with peak inflammation at 7–14 days after cystitis induction whereas URO-OVA/OT-I mice spontaneously developed chronic cystitis at 10 weeks of age that sustained for at least 4 months [14]. Based on these observations, URO-OVA mice were treated at 1, 4, and 7 days after cystitis induction and sacrificed at day 10 for analysis (Figure 1). Accordingly, URO-OVA/OT-I mice were treated once weekly for a total of 3 treatments staring at week 10 and sacrificed 3 days after last treatment for analysis.
Figure 1.
Cystitis induction and intravesical DMSO treatment schedule in URO-OVA mice. URO-OVA mice (Thy1.2) were transferred i.v. with in vitro preactivated OT-I splenocytes (Thy1.1) for cystitis induction at day 0, treated intravesically (i.b.) with 50% DMSO at days 1, 4, and 7 and sacrificed for analysis at day 10. SIINFEKL: OVA257–264 peptide.
2.3. Bladder Histology
The standard histology was performed as described previously [14, 17]. Briefly, bladder sections were paraffin-embedded, deparaffined, stained with hematoxylin and eosin (H&E) solution, and photographed using an Olympus BX-51 microscope. Bladder inflammation was scored according to the criteria established in our previous studies: 1+ (mild infiltration with no or mild edema), 2+ (moderate infiltration with moderate edema), and 3+ (moderate to severe infiltration with severe edema) [14, 17].
2.4. Bladder Cell Flow Cytometry
Bladder single-cell suspensions were prepared through mechanical dispersion as described previously [14, 17]. Cells were washed with staining buffer (1% FBS, 0.09% (w/v) NaN3 in Mg2+- and Ca-2+ free PBS), double stained with a FITC-CD8 antibody (eBioscience, San Diego, California) and a PE-Thy1.1 antibody (eBioscience), fixed in 2% formalin, and analyzed using a FACScan equipped with CellQuest software (BD Biosciences, Franklin Lakes, New Jersey). Post acquisition analyses were carried out using FlowJo software (Tree Star Inc, Ashland, Oregan).
2.5. Bladder RT-PCR
As described previously [14, 17], bladder total RNAs were extracted using Qiagen RNAeasy Kit (Qiagen, Valencia, California) and used for cDNA synthesis by Invitrogen Superscript III RNase H Reverse Transcriptase (Carlsbad, California) and Oligo dT. The cDNA products were then processed for PCR amplification using sequence-specific primer pairs and Invitrogen Taq DNA polymerase. The following primer pairs were used: 5′-TGAACGCTACACACTGCATCT and 5′-GACTCCTTTTCCGCTTCCTGA for IFN-γ (459 bp), 5′-CAAGAAGGAATGGGTCCAGA and 5′-TGAGGTGGTTGTGGAAAAGG for MCP-1 (175 bp), 5′-CTGTGGACCCCAGACTGTTT and 5′-CACTGAGAACTCCCCCATGT for NGF (194 bp), 5′-CGTCAGCCGATTTGCTATCT and 5′-CGGACTCCGCAAAGTCTAAG for TNF-α (206 bp), 5′-GTTCTCTGGGAAATCGTGGA and 5′-GGAAATTGGGGTAGGAAGGA for IL-6 (339 bp), and 5′-GTTCCAGTATGACTCCACT and 5′-GTGCAGGATGCATTGCTG for GAPDH (321 bp). The PCR kinetics for each of these molecules was initially established to achieve a desirable discrepancy between the control PBS-treated bladders and the DMSO-treated bladders. Based on the established kinetics, 30 cycles were used for GAPDH, 36 cycles were used for IFN-γ, MCP-1, NGF, and TNF-α, and 40 cycles were used for IL-6. The DNA fragments were run on a 1% agarose gel and imaged using EpiChemi digital image analysis system (UVP Inc., Upland, California).
2.6. Effector T Cell Growth Inhibition and Colorimetric MTT Assay
OT-I splenocytes were prepared and incubated with various concentrations of DMSO (ranging 1.563%–75%) in a 96-well flat-bottom plate at 4 × 105 cells/well in 200 μL of RPMI 1640 medium containing 10% fetal bovine serum, 100 units/mL of penicillin and 100 μL/mL of streptomycin. Cells treated with PBS were used as a control. After incubation for 24 hours, 20 μL of MTT (5 mg/mL; Sigma, St. Louis, Missouri) was added to each well and the incubation continued for 4 hours. The medium overlying cells was then aspirated and cells were solubilized with 200 μL of DMSO. The optical density was read at 570 nm. Percent of cell growth inhibition was calculated and presented as mean ± standard deviation of 5 duplicate wells referring to PBS-treated wells (100% growth).
2.7. Effector T Cell Apoptosis and Flow Cytometry
OT-I splenocytes were prepared and incubated with various concentrations of DMSO (ranging 1.563%–25%) in the above-mentioned culture medium for 2 hours. Cells treated with PBS were used as a control. After incubation, cells were double stained with FITC-annexin V (BD Biosciences) and propidium iodide, followed by flow cytometry as described previously [17].
2.8. Statistical Analysis
Student's t-Test (StatView 4.5 software, SAS Institute Inc., Cary, North Carolina) was used to determine statistical significance for bladder T cell infiltration and growth inhibition. A P-value of <.05 was considered statistically significant.
3. Results
3.1. Intravesical DMSO Treatment Reduces Bladder Histopathology in Acute Autoimmune Cystitis
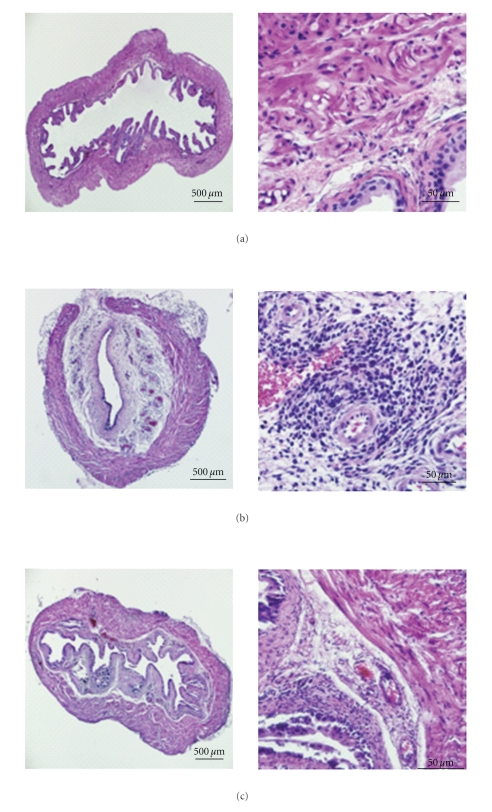
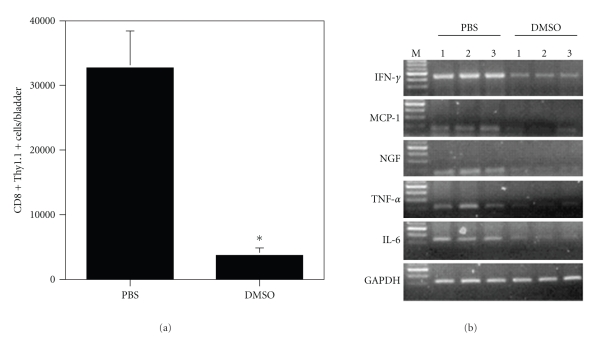
To evaluate the effect of DMSO on treating acute autoimmune cystitis, URO-OVA mice (Thy1.2) were transferred i.v. with preactivated OT-I splenocytes (Thy1.1) for cystitis induction at day 0, treated intravesically with 50% DMSO at days 1, 4, and 7 and sacrificed for analysis at day 10 (Figure 1). Compared to the normal bladders (Figure 2(a)), adoptive transfer of preactivated OT-I splenocytes induced clear bladder histopathology seen in the control PBS-treated bladders (Figure 2(b); score: 3+). The inflamed bladders showed prominent cellular infiltration, edema, and hyperemia in the lamina propria. Compared to the PBS-treated bladders, the DMSO-treated bladders exhibited markedly reduced histopathology with minimum defined morphologic changes (Figure 2(c); score: <1+). The normal bladders treated with 50% DMSO showed no clear histological changes (data not shown). In addition to the bladder histopathology, the DMSO-treated bladders also exhibited a significantly reduced number of infiltrating effector CD8+ T cells compared to the PBS-treated bladders (Figure 3(a); P < .001).
Figure 2.
Intravesical DMSO treatment reduces bladder histopathology in acute autoimmune cystitis. Acute cystitis was induced and treated in URO-OVA mice as shown in Figure 1. At day 10, the bladders were collected, prepared for histological cross-sections, and stained with H&E solution. (a) The normal bladder showing unremarkable mucosa and muscularis. (b) The PBS-treated bladder showing remarkable cellular infiltration, interstitial edema, and mucosal hyperemia in the lamina propria. (c) The DMSO-treated bladder showing scattered cellular infiltration and minimum edema and hyperemia. The slides are representative of 5 bladders in each group. Magnification: ×40 for the left pane and ×400 for the right panel.
Figure 3.
Intravesical DMSO treatment reduces infiltrating effector CD8+ T cells and bladder expression of inflammatory factor mRNAs in acute autoimmune cystitis. (a) The DMSO-treated bladders show reduced infiltrating effector CD8+ T cells. Bladder single-cell suspensions were prepared, stained with anti-Thy1.1 and anti-CD8 antibodies, and analyzed by flow cytometry. Gate was set on lymphocytes according to scatter criteria. The number of double positive T cells per bladder is presented as mean ± standard deviation of 5 bladders. *P < .001 (compared to the PBS-treated bladders). (b) The DMSO-treated bladders show reduced production of IFN-γ, MCP-1, NGF, TNF-α, and IL-6 mRNAs. Bladder total RNAs were extracted and analyzed by RT-PCR for the indicated inflammatory factors. GAPDH was used as an internal control. Three bladders for each of PBS- and DMSO-treated groups are presented. The results are representative of two separate experiments consisting of 4-5 animals per group. M: 100 bp ladder.
3.2. Intravesical DMSO Treatment Reduces Bladder Production of Inflammatory Factor mRNAs in Acute Autoimmune Cystitis
URO-OVA mice are known to produce a number of inflammatory factors in the bladder upon cystitis induction such as IFN-γ, MCP-1, NGF, TNF-α, and IL-6 [14, 17]. Aberrant expression of these inflammatory factors could reflect the abnormal activities of multiple cell types in site including T cells, mast cells, urothelial cells, detrusor muscle cells, and sensory neurons. To investigate whether the improved bladder histopathology after intravesical DMSO treatment was correlated with reduced bladder production of inflammatory factors, total RNAs were extracted from the bladders and analyzed by RT-PCR (Figure 3(b)). The normal bladders expressed a basal level of MCP-1 and IL-6 mRNAs but not IFN-γ, NGF, and TNF-α mRNAs at the experimental setting (data not shown). Induction of cystitis resulted in increased mRNA expression for all factors tested as manifested in the control PBS-treated bladders. Compared to the PBS-treated bladders the DMSO-treated bladders showed reduced production of these mRNAs, although the magnitude of the reduction varied among the mRNAs.
3.3. Intravesical DMSO Treatment Reduces Bladder Histopathology in Chronic Autoimmune Cystitis
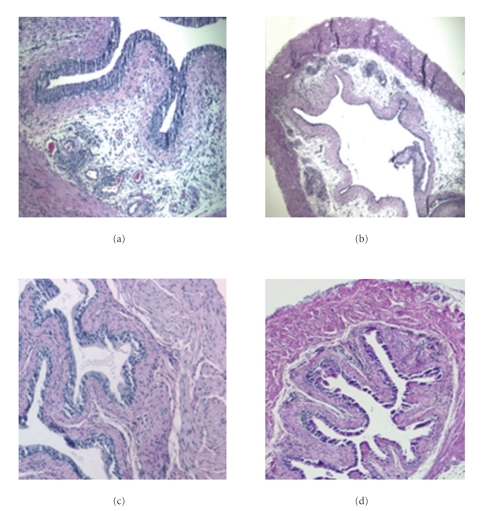
Due to the presence of deletion-escaped autoreactive OT-I CD8+ T cells URO-OVA/OT-I mice can spontaneously develop bladder inflammation at 10 weeks of age that sustains for at least 4 months [14]. To assess whether DMSO is effective on treating chronic autoimmune cystitis, URO-OVA/OT-I mice were treated intravesically with 50% DMSO once weekly for a total of 3 treatments starting at week 10 and sacrificed for analysis 3 days after last treatment. Compared to the PBS-treated bladders that exhibited predominant cellular infiltration with mild edema (Figure 4(a); score: 2+), the DMSO-treated bladders showed minimum histopathological changes that retained over next 4 weeks tested (Figure 4(c); score: <1+). To investigate whether intravesical DMSO might affect the endogenous effector T cells in site and thus led to improved bladder histopathology, we transferred a parallel set of mice with 5 × 106 naive OT-I splenocytes 7 days after last treatment. Mice were sacrificed for analysis 3 days after cell transfer. In our previous studies, we observed that adoptive transfer of naïve OT-I splenocytes could trigger an acute inflammatory response, resulting in severe bladder inflammation in URO-OVA/OT-I mice (data not shown). Similarly, in the present study URO-OVA/OT-I mice treated with PBS, upon transfer of naive OT-I splenocytes, developed severe acute bladder inflammation (Figure 4(b); score: 3+). In contrast, URO-OVA/OT-I mice treated with DMSO developed only mild bladder inflammation (Figure 4(d); score: 1+), presumably due to the DMSO elimination of endogenous autoreactive OT-I CD8+ T cells.
Figure 4.
Intravesical DMSO treatment reduces bladder histopathology in chronic autoimmune cystitis. URO-OVA/OT-I mice were treated intravesically with PBS (a) or 50% DMSO (c) once weekly for a total of 3 treatments starting at week 10 and sacrificed for analysis 3 days after last treatment. The bladders were collected, prepared for histological cross-sections, and stained with H&E solution. A parallel set of mice treated with PBS (b) or DMSO (d) were further transferred with 5 × 106 naive OT-I splenocytes 7 days after last treatment. Mice were sacrificed 3 days after cell transfer and the bladders analyzed by histological H&E staining. The slides are representative of 5 bladders in each group. Magnification: ×200 for the left pane and ×100 for the right panel.
3.4. DMSO Impairs Effector T Cell Viability In Vitro
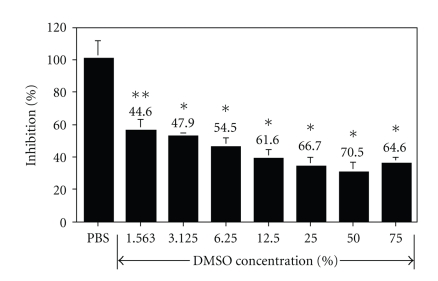
To test whether DMSO could directly affect effector T cells, we incubated OT-I splenocytes with various concentrations of DMSO (ranging 1.563%–75%) in vitro for 24 hours, followed by analysis of cell viability using MTT assay. Compared to control PBS-treated cells, the DMSO-treated cells exhibited significantly reduced cell growth in a dose-dependent manner (Figure 5; P < .05 for 1.563% DMSO and P < .001 for all other DMSO concentrations). The highest cell growth inhibition (70.5%) was observed at 50% DMSO. The DMSO effect was so potent as a 44.6% growth inhibition was observed even at a very low DMSO concentration (1.563%).
Figure 5.
DMSO inhibits effector T cell growth in vitro. OT-I splenocytes were prepared and incubated with indicated concentrations of DMSO (ranging 1.563%–75%) for 24 hours. MTT assay was then used to assess cell viability. Percent of cell growth inhibition was calculated and presented as mean ± standard deviation of 5 duplicate wells referring to the PBS-treated wells (100% growth). *= P < .001 and **= P < .05 (compared to the PBS-treated cells). The results are representative of two separate experiments.
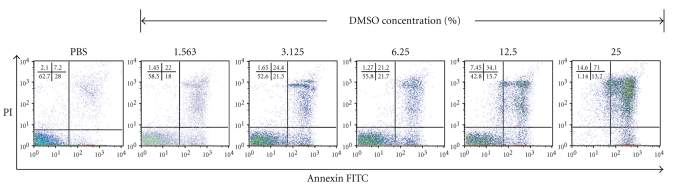
We further assessed the effect of DMSO on the induction of effector T cell apoptosis. OT-I splenocytes were incubated with various concentrations of DMSO (ranging 1.563%–25%) for 2 hours, double stained with annexin V and propidium iodide, and analyzed by flow cytometry (Figure 6). Compared to the control PBS-treated cells (7.2% for double-stained cells), DMSO-treated cells showed a marked increase in the double-stained cell population in a dose-dependent manner (from 21.2% at 6.25% DMSO to 71% at 25% DMSO).
Figure 6.
DMSO induces effector T cell apoptotic death in vitro. OT-I splenocytes were prepared and incubated with indicated concentrations of DMSO (ranging 1.563%–25%) for 2 hours. Cells were then double stained with FITC-annexin V and propidium iodide (PI) and analyzed by flow cytometry. Gate was set on lymphocytes according to scatter criteria. The percent of single- and double-stained cells is indicated. The results are representative of two separate experiments.
4. Discussion
We evaluated the effect of intravesical DMSO on treating acute and chronic autoimmune cystitis developed in URO-OVA mice and URO-OVA/OT-I mice, respectively, and observed that DMSO was effective in treating both types of cystitis in these novel EAC models. Compared to the control PBS-treated bladders, the DMSO-treated bladders showed markedly reduced histopathology and expression of inflammatory factor mRNAs. In addition, DMSO also showed its direct impairment on effector T cells as cells treated with DMSO exhibited reduced growth and apoptotic death in vitro.
The present EAC models mimic both acute and chronic phases of IC/PBS in bladder histopathology. Unlike the conventional EAC models that require immunization of syngeneic bladder homogenates that contain a mixture of bladder tissue antigens, both URO-OVA mice and URO-OVA/OT-I mice express a defined self-Ag (i.e., OVA) on the urothelium and develop bladder inflammation upon introduction of OVA-specific OT-I CD8+ T cells [14, 17]. Thus, these transgenic EAC models are unique, allowing quality-controlled studies generating predictable, reliable and reproducible information on bladder inflammation. In addition, the URO-OVA/OT-I cystitis model is particularly relevant to the natural history of IC/PBS patients as this model can spontaneously develop bladder inflammation over time during the animal life span. Further studies will focus on characterizing bladder functional changes such as voiding alternations and pain in these transgenic EAC models.
Prior studies involving intravesical DMSO in animal models are limited. It was observed that intravesical DMSO could increase the pressure threshold in a protamine sulfate-induced rat bladder hyperactivity model, suggesting its effect on desensitizing nociceptive bladder afferent [26]. A separate study also demonstrated that intravesical DMSO could facilitate the desensitization of nociceptive bladder afferent via its stimulation of bladder reflex pathways in rats [31]. In addition, Intravesical DMSO also showed its effect on reducing urinary level of hyaluronic acid in a similar protamine sulfate-induced rat model, suggesting that it could replenish the damaged glycosaminoglycan (GAG) layer [27]. In this study, we used transgenic mouse EAC models and observed that intravesical DMSO reduced bladder histopathology and expression of inflammatory factor mRNAs. All animal studies are consistent with the clinical observations that intravesical DMSO is beneficial for the treatment of IC/PBS patients [20–23]. Since little is known with regard to bladder histological changes in response to intravesical DMSO, our results provide such histological evidence for the effect of DMSO on treating the bladder disorders.
Excessive expression of inflammatory factors such as NGF and IL-6 in the bladder is considered to be responsible for the development and propagation of IC/PBS symptoms [32, 33]. As a model for IC/PBS studies, the inflamed bladders of URO-OVA mice expressed elevated NGF and IL-6 mRNAs as well as other inflammatory factor mRNAs including IFN-γ, MCP-1, and TNF-α [14, 17]. These increased mRNA expressions could reflect abnormality of multiple cell types in site including T cells, mast cells, urothelial cells, detrusor muscle cells, and sensory neurons. Although the functional outcomes in response to intravesical DMSO require further investigation in this model, intravesical DMSO could reduce bladder production of the inflammatory factors, suggesting the beneficial effects of DMSO in antibladder inflammation and symptomatic relief.
In both URO-OVA and URO-OVA/OT-I cystitis models, OT-I CD8+ T cells that express the OVA-specific TCR serve as effector T cells in cystitis induction [14, 17]. Studies have shown that DMSO is inhibitory for various cell types including T cells [34, 35]. In consistent with these studies, we observed that DMSO directly impaired T cell viability and caused T cell apoptotic death in vitro. Our observations on intravesical DMSO treatment in the URO-OVA/OT-I cystitis model also suggest the inhibitory effect of DMSO in vivo. Mice treated with intravesical DMSO remained minimum bladder histopathology for 4 weeks tested without further treatment, presumably due to the DMSO elimination of endogenous autoreactive OT-I CD8+ T cells. To support this assumption, DMSO-treated URO-OVA/OT-I mice, after adoptive transfer of naïve OT-I splenocytes, failed to develop acute cystitis with severity comparable to that seen in PBS-treated URO-OVA/OT-I mice. In addition to T cells, DMSO may also affect other inflammatory cell types in site. In fact, the observed reduction of NGF, MCP-1, and IL-6 mRNAs in the DMSO-treated bladders could suggest the inhibitory effect of DMSO on abnormal activation of sensory neurons, urothelial cells, mast cells, and detrusor muscle cells. Thus, the overall anti-inflammatory effects of DMSO may involve in its inhibition on multiple inflammatory cell types in the inflamed bladder.
Currently, intravesical instillation of DMSO is one of the primary treatments for IC/PBS patients and one of the only two FDA-approved treatments for IC/PBS. This therapeutic method has proven to be feasible and effective in the treatment of this painful urinary condition. Our present study in transgenic EAC models provides histological evidence for the effect of intravesical DMSO and a potential mechanism of DMSO action.
Acknowledgments
The authors thank Dr. Timothy Ratliff for providing OT-I/Thy1.1 mice and Ms. Kris Greiner for editorial review of this paper. This work was supported in part by NIH U01DK082344-020005 and DOD W81XWH-04-1-0070.
Abbreviations
- Ag:
Antigen
- DMSO:
Dimethyl sulfoxide
- EAC:
Experimental autoimmune cystitis
- GAPDH:
Glyceraldehyde-3-phosphate dehydrogenase
- H&E:
Hematoxylin and eosin
- i.b.:
Intravesical
- IC/PBS:
Interstitial cystitis/painful bladder syndrome
- IFN-γ:
Interferon-γ
- IL-6:
Interleukin-6
- i.p.:
Intraperitoneal
- i.v.:
Intravenous
- MCP-1:
Monocyte chemoattractant protein-1
- MHC:
Major histocompatibility complex
- MTT:
Thiazolyl blue tetrazolium bromide assay
- NGF:
Nerve growth factor
- OVA:
Chicken ovalbumin
- PBS:
Phosphate buffered saline
- RT-PCR:
Reverse transcription-polymerase chain reaction
- TCR:
T cell receptor
- TNF-α:
Tumor necrosis factor-α.
References
- 1.Warren JW, Brown J, Tracy JK, Langenberg P, Wesselmann U, Greenberg P. Evidence-based criteria for pain of interstitial cystitis/painful bladder syndrome in women. Urology. 2008;71(3):444–448. doi: 10.1016/j.urology.2007.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selo-Ojeme DO, Onwude JL. Interstitial cystitis. Journal of Obstetrics and Gynaecology. 2004;24(3):216–225. doi: 10.1080/01443610410001660652. [DOI] [PubMed] [Google Scholar]
- 3.Sairanen J, Leppilahti M, Tammela TLJ, et al. Evaluation of health-related quality of life in patients with painful bladder syndrome/interstitial cystitis and the impact of four treatments on it. Scandinavian Journal of Urology and Nephrology. 2009;43(3):212–219. doi: 10.1080/00365590802671031. [DOI] [PubMed] [Google Scholar]
- 4.Rothrock NE, Lutgendorf SK, Hoffman A, Kreder KJ. Depressive symptoms and quality of life in patients with interstitial cystitis. Journal of Urology. 2002;167(4):1763–1767. [PubMed] [Google Scholar]
- 5.Ratliff TL, Klutke CG, Hofmeister M, He F, Russell JH, Becich MJ. Role of the immune response in interstitial cystitis. Clinical Immunology and Immunopathology. 1995;74(3):209–216. doi: 10.1006/clin.1995.1031. [DOI] [PubMed] [Google Scholar]
- 6.van de Merwe JP. Interstitial cystitis and systemic autoimmune diseases. Nature Clinical Practice Urology. 2007;4(9):484–491. doi: 10.1038/ncpuro0874. [DOI] [PubMed] [Google Scholar]
- 7.Peeker R, Atanasiu L, Logadottir Y. Intercurrent autoimmune conditions in classic and non-ulcer interstitial cystitis. Scandinavian Journal of Urology and Nephrology. 2003;37(1):60–63. doi: 10.1080/00365590310008721. [DOI] [PubMed] [Google Scholar]
- 8.Westropp JL, Buffington CAT. In vivo models of interstitial cystitis. Journal of Urology. 2002;167(2):694–702. doi: 10.1016/S0022-5347(01)69129-8. [DOI] [PubMed] [Google Scholar]
- 9.Bullock AD, Becich MJ, Klutke CG, Ratliff TL. Experimental autoimmune cystitis: a potential murine model for ulcerative interstitial cystitis. Journal of Urology. 1992;148(6):1951–1956. doi: 10.1016/s0022-5347(17)37091-x. [DOI] [PubMed] [Google Scholar]
- 10.Lin Y-H, Liu G, Kavran M, et al. Lower urinary tract phenotype of experimental autoimmune cystitis in mouse: a potential animal model for interstitial cystitis. British Journal of Urology International. 2008;102(11):1724–1730. doi: 10.1111/j.1464-410X.2008.07891.x. [DOI] [PubMed] [Google Scholar]
- 11.Phull H, Salkini M, Purves T, Funk J, Copeland D, Comiter CV. Angiotensin II plays a role in acute murine experimental autoimmune cystitis. British Journal of Urology International. 2007;100(3):664–667. doi: 10.1111/j.1464-410X.2007.07035.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitra S, Dagher A, Kage R, Dagher RK, Luber-Narod J. Experimental autoimmune cystitis: further characterization and serum autoantibodies. Urological Research. 1999;27(5):351–356. doi: 10.1007/s002400050162. [DOI] [PubMed] [Google Scholar]
- 13.Luber-Narod J, Austin-Ritchie T, Banner B, et al. Experimental autoimmune cystitis in the Lewis rat: a potential animal model for interstitial cystitis. Urological Research. 1996;24(6):367–373. doi: 10.1007/BF00389795. [DOI] [PubMed] [Google Scholar]
- 14.Liu W, Evanoff DP, Chen X, Luo Y. Urinary bladder epithelium antigen induces CD8+ T cell tolerance, activation, and autoimmune response. Journal of Immunology. 2007;178(1):539–546. doi: 10.4049/jimmunol.178.1.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurts C, Heath WR, Carbone FR, et al. Constitutive class I-restricted exogenous presentation of self antigens in vivo. Journal of Experimental Medicine. 1996;184(3):923–930. doi: 10.1084/jem.184.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu W, Luo Y. Interruption of mast cell function effectively inhibits bladder inflammation in an autoimmune cystitis model. Journal of Urology. 2008;179:62–63. [Google Scholar]
- 17.Liu W, DeYoung BR, Chen X, Evanoff DP, Luo Y. RDP58 inhibits T cell-mediated bladder inflammation in an autoimmune cystitis model. Journal of Autoimmunity. 2008;30(4):257–265. doi: 10.1016/j.jaut.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: pharmacological, cellular, and molecular aspects. Biochemical Pharmacology. 2003;65(7):1035–1041. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 19.Sant GR. Intravesical 50% dimethyl sulfoxide (Rimso-50) in treatment of interstitial cystitis. Urology. 1987;29(4, supplement):17–21. [PubMed] [Google Scholar]
- 20.Parkin J, Shea C, Sant GR. Intravesical dimethyl sulfoxide (DMSO) for interstitial cystitis—a practical approach. Urology. 1997;49(5):105–107. doi: 10.1016/s0090-4295(97)00181-7. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Marrero R, Emerson LE, Feltis JT. A controlled study of dimethyl sulfoxide in interstitial cystitis. Journal of Urology. 1988;140(1):36–39. doi: 10.1016/s0022-5347(17)41478-9. [DOI] [PubMed] [Google Scholar]
- 22.Rössberger J, Fall M, Peeker R. Critical appraisal of dimethyl sulfoxide treatment for interstitial cystitis: discomfort, side-effects and treatment outcome. Scandinavian Journal of Urology and Nephrology. 2005;39(1):73–77. doi: 10.1080/00365590410018738. [DOI] [PubMed] [Google Scholar]
- 23.Peeker R, Haghsheno M-A, Holmäng S, Fall M. Intravesical bacillus Calmette-Guerin and dimethyl sulfoxide for treatment of classic and nonulcer interstitial cystitis: a prospective, randomized double-blind study. Journal of Urology. 2000;164(6):1912–1916. doi: 10.1016/s0022-5347(05)66916-9. [DOI] [PubMed] [Google Scholar]
- 24.Ghoniem GM, McBride D, Sood OP, Lewis V. Clinical experience with multiagent intravesical therapy in interstitial cystitis patients unresponsive to single-agent therapy. World Journal of Urology. 1993;11(3):178–182. doi: 10.1007/BF00211416. [DOI] [PubMed] [Google Scholar]
- 25.Stout L, Gerspach JM, Levy SM, et al. Dimethyl sulfoxide does not trigger urine histamine release in interstitial cystitis. Urology. 1995;46(5):653–656. doi: 10.1016/S0090-4295(99)80295-7. [DOI] [PubMed] [Google Scholar]
- 26.Tyagi P, Hsieh VC, Yoshimura N, Kaufman J, Chancellor MB. Instillation of liposomes vs dimethyl sulphoxide or pentosan polysulphate for reducing bladder hyperactivity. British Journal of Urology International. 2009;104(11):1689–1692. doi: 10.1111/j.1464-410X.2009.08673.x. [DOI] [PubMed] [Google Scholar]
- 27.Soler R, Bruschini H, Truzzi JC, et al. Urinary glycosaminoglycans excretion and the effect of dimethyl sulfoxide in an experimental model of non-bacterial cystitis. International Brazilian Journal of Urology. 2008;34(4):503–511. doi: 10.1590/s1677-55382008000400013. [DOI] [PubMed] [Google Scholar]
- 28.Sun Y, Chai TC. Effects of dimethyl sulphoxide and heparin on stretch-activated ATP release by bladder urothelial cells from patients with interstitial cystitis. British Journal of Urology International. 2002;90(4):381–385. doi: 10.1046/j.1464-410x.2002.02912.x. [DOI] [PubMed] [Google Scholar]
- 29.Shiga K-I, Hirano K, Nishimura J, Niiro N, Naito S, Kanaide H. Dimethyl sulphoxide relaxes rabbit detrusor muscle by decreasing the Ca2+ sensitivity of the contractile apparatus. British Journal of Pharmacology. 2007;151(7):1014–1024. doi: 10.1038/sj.bjp.0707317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Melchior D, Packer CS, Johnson TC, Kaefer M. Dimethyl sulfoxide: does it change the functional properties of the bladder wall? Journal of Urology. 2003;170(1):253–258. doi: 10.1097/01.ju.0000071520.73686.3d. [DOI] [PubMed] [Google Scholar]
- 31.Birder LA, Kanai AJ, De Groat WC. DMSO: effect on bladder afferent neurons and nitric oxide release. Journal of Urology. 1997;158(5):1989–1995. doi: 10.1016/s0022-5347(01)64199-5. [DOI] [PubMed] [Google Scholar]
- 32.Liu H-T, Tyagi P, Chancellor MB, Kuo H-C. Urinary nerve growth factor level is increased in patients with interstitial cystitis-bladder pain syndrome and decreased in responders to treatment. British Journal of Urology International. 2009;104(10):1476–1481. doi: 10.1111/j.1464-410X.2009.08675.x. [DOI] [PubMed] [Google Scholar]
- 33.Lotz M, Villiger P, Hugli T, Koziol J, Zuraw BL. Interleukin-6 and interstitial cystitis. Journal of Urology. 1994;152(3):869–873. doi: 10.1016/s0022-5347(17)32594-6. [DOI] [PubMed] [Google Scholar]
- 34.Trubiani O, Ciancarelli M, Rapino M, Di Primio R. Dimethyl sulfoxide induces programmed cell death and reversible G1 arrest in the cell cycle of human lymphoid pre-T cell line. Immunology Letters. 1996;50(1-2):51–57. doi: 10.1016/0165-2478(96)02518-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Yoshikawa H, Nakajima Y, Tasaka K. Involvement of mitochondrial permeability transition and caspase-9 activation in dimethyl sulfoxide-induced apoptosis of EL-4 lymphoma cells. International Immunopharmacology. 2001;1(1):63–74. doi: 10.1016/s1567-5769(00)00016-3. [DOI] [PubMed] [Google Scholar]