Abstract
After menarche, women have an increased prevalence of migraine compared to men. There is significant variability in the frequency and severity of migraine throughout the menstrual cycle. Women report migraines occur more frequently during menses, and that those are more severe than other migraines. This creates a unique challenge of effectively treating menstrually related and pure menstrual migraines. As with treatment of other migraines, both abortive and prophylactic treatment regimens are used. Triptans demonstrate efficacy in the abortive management of menstrually related and pure menstrual migraines. For migraines that occur primarily during menses or that are particularly resistant to other therapies, intermittent prophylactic therapies can be used. Naproxen and estrogens have been studied for this use. More recently, triptans have been examined and have shown efficacy for intermittent prophylaxis of menstrual migraine.
Keywords: Menstrual migraine, Headache, Triptan, Abortive therapy, Prophylactic therapy, Intermittent prophylaxis
Introduction
The increased prevalence of migraine in women has been well established [1, 2]. The cause of this increased prevalence has been the subject of much research, study, and debate. Epidemiologic studies have shown that, before the onset of puberty, the incidence of migraine is more common in boys than in girls. However, after menarche, the incidence and prevalence of migraine increases in girls and continues to increase until the mid-40 s in women [1]. In addition, migraine onset in the second and third decades is much more common in women and relatively rare in men [2]. Not only is the prevalence of migraine increased in women, but also women typically report more frequent and more painful headaches than their male counterparts [3].
This increased frequency in women has been widely attributed to the change in hormonal milieu that occurs after menarche [4]. This is further supported by the widely reported variability of frequency and severity of migraines throughout the menstrual cycle. Stewart et al. [4] evaluated 81 women of menstruating age with a daily assessment of headaches, headache features, and days of menstruation and noted an increased incidence of migraine without aura during menses. Participants also noted some increase in pain of migraines during menses but did not suggest these headaches were overall worse than other migraines in duration, intensity, or disability [4]. Other studies have suggested that migraines occurring during menses are more severe than others [5–7]. In addition, migraine is reported less frequently at and immediately after ovulation [4, 8].
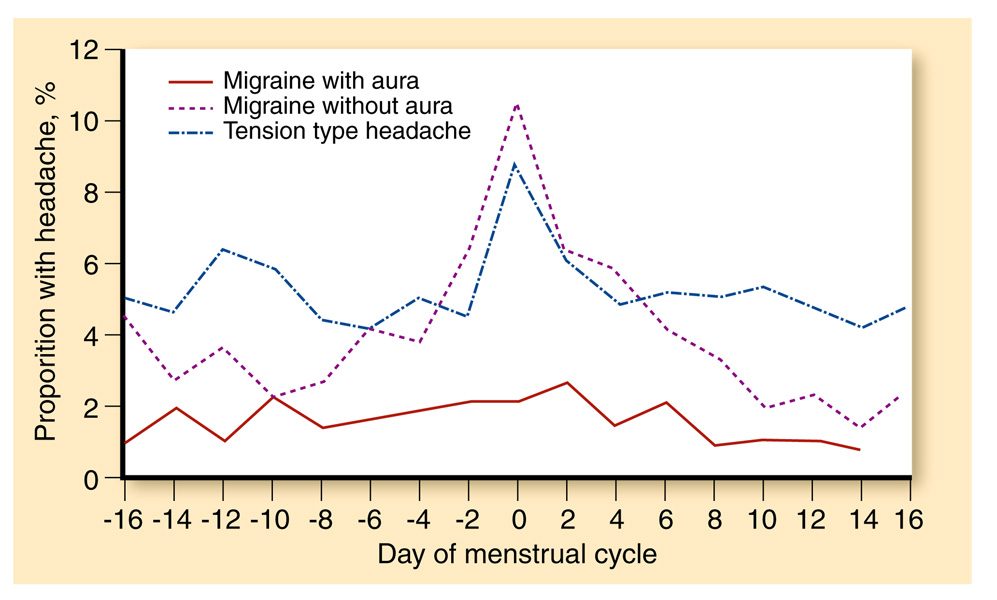
The cause of this cyclic variability has been the subject of much research (see Fig. 1). In 1972, Somerville [9] determined that administering 10 mg of intramuscular estradiol to female migraineurs in the perimenstrual phase would delay onset of their migraine attacks. When a similar study group was administered progesterone, the result was a delay in bleeding without a change in migraine pattern [10]. This led to the hypothesis that migraine could be triggered by estrogen withdrawal, a supposition that has been supported by multiple studies [6, 9, 11–14].
Fig. 1.
Incidence of headache by type of headache and the menstrual cycle. (Adapted from Stewart et al. [4])
In women who suffer migraines more frequently around menses than other migraineurs, the question becomes what is the appropriate management of these patients. This review will examine the current literature and research on abortive and prophylactic treatments for menstrual migraines.
Evidence Acquisition
A MEDLINE search (1950 through December 2009) was performed searching for English-language articles using the keywords menstrual migraine, pure menstrual migraine, menstrually related migraine, estrogen and migraine, treatment and menstrual migraine, and progesterone and migraine. This search yielded 744 citations that were reviewed for relevance. Selected articles were limited to those involving menstrually related (MRM) or pure menstrual migraine (PMM). The bibliography of each relevant citation then was reviewed and additional pertinent resources were examined. We emphasized inclusion of randomized, blinded, placebo-controlled studies or open-label studies. Articles published after 2004 primarily were included because most of the studies examining treatment via randomized controlled trials were performed after this date.
Diagnostic Criteria
This association between migraine attacks and menses has led to the terms pure menstrual migraine and menstrually related migraine. For the purposes of this review, diagnostic criteria from the International Headache Society’s (IHS) International Classification of Headache Disorders, 2nd Edition will be used. Per these criteria, PMM is defined as migraine without aura occurring exclusively on days −2 through +3 (with the first day of menses labeled +1) of at least two of three menstrual cycles without migraine at other times in the cycle [15]. MRM is defined as above, with the caveat that migraine can occur at other times during the cycle [15].
Abortive Therapies
For the most part, abortive therapies are not specifically tailored to menstrual migraine. Treatments used in traditional migraine therapy are used for PMM and MRM as well. These therapies include NSAIDs, triptans, ergotamine, dihydroergotamine, and opiates. However, in recent years, randomized controlled trials or subsets of larger trials have focused on the use of triptans for abortive therapy for menstrual migraine (see Table 1). As randomized controlled trials have not been conducted for these other therapies, this review will focus on triptans for abortive therapy.
Table 1.
Studies of triptans for abortive therapy of menstrual migraines
| Medication | Study | Study type | Dosage, mg | Results |
|---|---|---|---|---|
| Sumatriptan | Martin et al. [16•] | Prospective subgroup analysis | 10 | 63.5% 2-hour pain relief vs 29.0% in placebo (P=0.002) |
| Mannix et al. [17] | Prospective, double-blind, placebo-controlled analysis | 10 | 70% (study 1) and 73% (study 2) 2-hour pain relief vs 53% (study 1) and 50% (study 2) in placebo (study 1: P=0.001; study 2: P<0.001) |
|
| Nett et al. [18] | Prospective, double-blind, placebo-controlled analysis | 10 | 73% 2-hour pain relief in PMM vs 50% in placebo (P=0.006); 71% 2-hour pain relief in MRM vs 52% in placebo (P<0.001) |
|
| Frovatriptan | Newman et al. [19] | Open-label, postmarketing surveillance study | 2.5 | Effectiveness and tolerability improved compared to previous migraine treatments in 84.2% (P<0.001) |
| Sumatriptan | Landy et al. [20] | Prospective, double-blind, placebo-controlled analysis | 50, 100 | 35% (study 1, 100 mg), 31% (study 2, 100 mg) and 30% (studies 1 and 2, 50 mg) 2-hour pain relief vs 8% (study 1) and 14% (study 2) in placebo (study 1:P<0.001; study 2: P<0.05) |
| Nett et al. [21] | Prospective, double-blind, placebo-controlled analysis | 50, 100 | 61% (100 mg) and 51% (50 mg) 2-hour pain relief vs 29% in placebo (P<0.001) |
|
| Sumatriptan-naproxen | Mannix et al. [22•] | Prospective, double-blind, placebo-controlled analysis | 85–500 | 42% (study 1) and 52% (study 2) 2-hour pain relief vs 23% (study 1) and 22% (study 2) in placebo (P<0.001) |
| Zolmitriptan | Tuchman et al. [23] | Prospective, double-blind, placebo-controlled analysis | 2.5 | 65.7% 2-hour pain relief vs 32.8% in placebo (P<0.0001) |
MRM menstrually related migraine, PMM pure menstrual migraine
Rizatriptan
In 2004 and 2005, two multicenter, randomized, parallel, placebo-controlled, double-blind studies were conducted to evaluate the efficacy of rizatriptan versus placebo in migraine sufferers [16•]. These combined to form the TAME (Treat A Migraine Early) studies. Martin et al. [16•] conducted a prospective subgroup analysis of efficacy in PMM and MRM within the TAME studies. IHS criteria were used, and 94 patients were randomized in a 2:1 ratio. Comparisons of 2-hour pain-free reporting and 24-hour sustained pain freedom were made. They found that rizatriptan, 10 mg, was more effective than placebo for treating PMM and MRM, with significantly higher pain freedom at 2 h in the rizatriptan group (63.5%; 95% CI: 51.6%, 75.4%) versus the placebo group (29%; 95% CI: 13%, 45%). Similarly, Mannix et al. [17] examined 707 patients who treated an MRM or PMM over the course of three menstrual cycles. Again, women in the rizatriptan group experienced a significant improvement in 2-hour pain relief compared with the placebo group. Nett et al. [18] reanalyzed the Mannix data, comparing 2-hour migraine relief in the PMM group to the MRM group. They found a similar response to rizatriptan in both groups.
Frovatriptan
Although frovatriptan has been well established for the acute treatment of migraine headaches, current studies on its use for abortive therapy are limited. This largely is due to recent investigation of frovatriptan as a short-term prophylactic therapy (discussed in the next section). Newman et al. [19] conducted a postmarketing surveillance study in a multicenter study in Germany evaluating the use of frovatriptan as abortive therapy for migraine. A subanalysis of this study examined efficacy in patients with menstrual migraine. Patients with menstrual migraine were asked to compare efficacy of frovatriptan with previous therapies. Both the menstrual migraine and nonmenstrual migraine groups reported significant improvement with frovatriptan as an abortive compared with previous therapies. Both groups also reported frovatriptan was better tolerated than previous triptan and nontriptan therapies.
Sumatriptan
Landy et al. [20] and Nett et al. [21] examined the efficacy of sumatriptan for abortive therapy in menstrual migraine. Both of these studies were randomized, double-blind, placebo-controlled trials and examined 50 mg and 100 mg sumatriptan doses versus placebo. Nett et al. [21] examined only a single menstrual migraine attack and reported 61% and 51% of patients taking 100 mg and 50 mg, respectively, were pain-free at the 2-hour mark, compared to 29% of patients receiving placebo. Landy et al. [20] examined two separate studies. The first group reported sustained pain freedom from 2 to 24 h after their dose in 30% of the 50 mg group and 35% of the 100 mg group; this was compared to 8% of patients receiving placebo. The second study group reported 30% and 31% pain freedom in the 50 mg and 100 mg groups, respectively, compared to 14% in the placebo group [20]. Neither of these studies was powered to examine the efficacy at individual doses. Both reported sumatriptan was well tolerated, with only about 2% of patients reporting mild nausea, fatigue, dizziness, or paresthesia in both studies [20, 21].
Mannix et al. [22•] examined the use of combination sumatriptan–naproxen for treatment of menstrual migraine and for efficacy in managing dysmenorrhea. Two randomized, double-blind, placebo-controlled, multicenter trials with patients diagnosed with menstrual migraines and dysmenorrhea were included. Doses used in these studies were sumatriptan, 85 mg, and naproxen, 500 mg. Study one reported 2-hour freedom from pain in 42% of treated patients compared to 23% of control patients. Study two demonstrated pain freedom at 2 h in 52% of treated patients compared to 22% of control patients. In study one, 29% of treated patients and 18% of control patients experienced 2- to 24-hour pain freedom; in study two, 38% of treated patients and 10% of control patients experienced 2- to 24-hour pain freedom. Again, no significant adverse events were noted, with only a few patients reporting dizziness or nausea [22•].
Zolmitriptan
As with other triptans, zolmitriptan also demonstrates good efficacy in the acute treatment of menstrual migraine. Tuchman et al. [23] conducted a 27-site randomized, double-blind, placebo-controlled, outpatient study examining acute treatment on menstrual migraine with zolmitriptan, 2.5 mg, versus placebo. Again, the primary end point was 2-hour headache response. Of patients taking zolmitriptan, 65.7% reported pain-free response at 2 h, compared to 32.8% of control patients. Similar results were noted at all times evaluated, including 1 h, 4 h, and 24 h. This medication also was well tolerated, with no significant adverse effects reported [23].
Prophylactic Therapy
While triptans have shown efficacy in aborting PMM and MRM, the ultimate goal is to prevent migraines from occurring. As such, recent research has focused on this topic. Multiple therapies have been examined for intermittent prophylactic treatment, from ergots to hormonal therapies to scheduled usage of NSAIDs and triptans (see Table 2).
Table 2.
Studies of triptans for intermittent prophylaxis of menstrual migraines
| Medication | Study | Study type | Dosage | Results |
|---|---|---|---|---|
| Sumatriptan | Newman et al. [28] | Open-label study | 25 mg three times daily, starting 2–3 days before expected menstrual onset through total of 5 days |
In 126 treated menstrual cycles, headache was absent in 66 patients (52.4%) or reduced in severity by 50% or more in 53 patients (42%) |
| Zolmitriptan | Tuchman et al. [29•] | Prospective, double-blind, placebo-controlled analysis |
2.5 mg three times daily vs 2.5 mg twice daily, starting 2 days before expected menstrual onset through total of 7 days |
58.6% reduction in the frequency of migraine attack with zolmitriptan three times daily (P=0.0007) vs 54.7% with zolmitriptan twice daily (P=0.002) vs 37.8% in placebo |
| Naratriptan | Newman et al. [30] | Prospective, double-blind, placebo-controlled analysis |
1 mg twice daily vs 2.5 mg twice daily, starting 2 days before expected menstrual onset through total of 5 days |
50% of patients treated with naratriptan, 1 mg twice daily, reported headache freedom vs 25% in placebo (P=0.003); no statistically significant difference seen in naratriptan, 2.5 mg daily, vs placebo |
| Moschiano et al. [31] | Open-label study | 1 mg twice daily, starting 2 days before expected menstrual onset through total of 6 days |
61.4% of patients reported a greater than 50% reduction in migraine attacks |
|
| Mannix et al. [32] | Prospective, double-blind, placebo-controlled analysis |
1 mg twice daily, starting 2 days before expected onset of headache through total of 5 days |
Mean percentage of perimenstrual periods without MRM is 38% (study 1) and 34% (study 2) in the naratriptan, 1 mg twice daily, groups compared to 29% (study 1) and 24% (study 2) in the placebo groups |
|
| Frovatriptan | Silberstein et al. [33] | Prospective, double-blind, placebo-controlled analysis |
2.5 mg daily vs 2.5 mg twice daily, starting 2 days before expected menstrual onset through total of 6 days |
52% incidence of migraine in frovatriptan, 2.5 mg daily, vs 41% in frovatriptan, 2.5 mg twice daily, vs 67% in placebo (P<0.0001) |
| Brandes et al. [35] | Prospective, double-blind, placebo-controlled analysis |
2.5 mg daily vs 2.5 mg twice daily, starting 2 days before expected menstrual onset through total of 6 days |
0.92 mean headache-free cycles with frovatriptan, 2.5 mg twice daily (P<0.001), vs 0.69 with frovatriptan, 2.5 mg daily (P<0.02), vs 0.42 with placebo |
|
| Guidotti et al. [36] | Open-label, nonrandomized, parallel group study |
2.5 mg daily vs 25 µg transdermal estrogen vs 500 mg daily naproxen, starting 2 days before expected menstrual onset through total of 6 days |
2.5 median incidence of migraine in frovatriptan vs3.0 in transdermal oestrogens vs 3.9 in naproxen sodium (P=0.049) |
|
| Wade et al. [37•] | Prospective, double-blind, placebo-controlled analysis |
2.5 mg daily vs 2.5 mg twice daily, starting 2 days before expected menstrual onset through total of 6 days |
Maximum-to-minimum blood concentration ratios were more stable with frovatriptan, 2.5 mg twice-daily, than frovatriptan, 2.5 mg daily |
MRM menstrually related migraine
Naproxen
Although few studies have examined naproxen for intermittent prophylactic treatment of menstrual migraine, some evidence supports its use for intermittent prophylactic therapy. A small study in 2007 examined use of naproxen in 25 patients with PMM [24]. These patients took 550 mg of naproxen daily for 7 days before and 7 days after the start of menses and kept record of their headaches during this time. This timeframe later was narrowed to 5 days before and 5 days after the start of menses. In addition, blood samples were obtained and prostaglandin levels evaluated throughout the study. Results after 3 and 6 months were compared to the start of the study, with lower prostaglandin levels after treatment. Subjects also reported a small decrease in the number of migraine attacks after treatment (mean number of migraines: 1.7±0.11 pretreatment to 1.2±0.10 at the third month [P<0.001], to 1.1±0.06 at the sixth month [P<0.0001]). More significant was the report of shorter duration (25.6 h±4.42 h pretreatment to 15.5 h±4.43 h in the third month [P<0.02], to 13.35 h±4.26 h in the sixth month [P<0.001]). In addition, this study showed decreased intensity of migraine after treatment using a 4-point scale, with 4 being most severe pain (2.4±0.11 pretreatment to 1.2±0.10 in the third month of treatment [P<0.0001], and 1.1±0.07 in the sixth month [P<0.0001]). However, this study was limited by its small size and lack of a placebo group for comparison [24].
Hormone Therapies
If PMM and MRM have been related to estrogen withdrawal, it stands to reason that estrogen replacement or supplementation around the time of menses may improve headache control. Multiple older studies have examined the use of estrogen patches in MRM treatment [25]. At lower doses (25 and 50 µg), these interventions did not demonstrate a significant change in recurrence of MRM. At higher doses (100 µg), some benefit was reported. More recently, estrogen has been examined for use in intermittent prophylaxis in patch, gel, and oral form. A multicenter, parallel-group, randomized clinical trial examined the effect of cycle versus extended contraceptive progestin/ethinylestradiol patch use on headache (though not specifically migraine) frequency [26]. Patients were randomized to receive either 12 consecutive weeks of weekly patch use followed by 1 patch-free week versus 3 consecutive weeks of weekly patch use followed by 1 patch-free week. Researchers examined headache frequency and mean headache days, total bleeding days, and patient satisfaction. Both regimens demonstrated an increase in mean headache days during the patch-free weeks, with the extended-use group demonstrating a higher mean compared to the cyclic group. However, both groups had a downward trend of mean headache days over the course of the study [26]. A double-blind, placebo-controlled, crossover study by MacGregor et al. [27•] examined the use of estradiol gel. Estradiol or placebo gel was applied beginning 10 days after ovulation (determined using urine studies and a fertility monitor) and was continued through the second day of menses. While receiving estradiol gels, participants had a 22% reduction in migraine days per patient. Patients also reported less severe headaches and less nausea associated with their headache. As in the previous study, participants had an increase in migraine occurrence 5 days after cessation of estradiol gel when compared to placebo [27•].
Overall, the use of estrogen supplementation appears to be effective in lowering the number of headache days in MRM; however, many studies have reported an increase in headache days when supplementation is stopped. The overall downward trend of headache days, even during the patch-free intervals, in the study noted above would suggest that extended contraceptive progestin/ethinylestradiol delivery may decrease headache days and would not necessarily result in a more severe or prolonged headache compared to placebo [26].
Triptans
Much of the more recent research has focused on the use of scheduled triptans for intermittent prophylactic treatment of menstrual migraine. Research has been promising, particularly in triptans with longer half-lives.
Sumatriptan
The first study examining the use of triptans for intermittent prophylaxis in menstrual migraine was conducted using sumatriptan. This came on the heels of studies demonstrating the efficacy of sumatriptan in abortive therapy of menstrual migraine, although high recurrence rates were seen. In 1998, Newman et al. [28] conducted an open-label study examining efficacy of sumatriptan for short-term MRM or PMM prophylaxis. Patients in the study were menstruating women with MRM or PMM, with or without aura, who had been resistant to previous prophylactic regimens. Patients documented their headaches for 2 months on their usual treatment before beginning sumatriptan. They also rated pain intensity, degree of disability, and percentage of improvement of migraine pain. Patients were then given sumatriptan, 25 mg three times daily, starting 2 to 3 days before expected onset of menstrual migraine for a total of 5 days per cycle. They could continue their usual prophylactic treatment and could use extra sumatriptan for abortive therapy up to a 300 mg per day limit. With 20 patients in the final analysis, a total of 126 menstrual cycles were treated. Of these, 52.4% of cycles had no headaches charted, and 42% of cycles demonstrated a 50% or greater reduction of headache severity. Based on these findings, examiners felt that 94.8% of cycles had responded to sumatriptan to some degree [28]. This study was not placebo-controlled or double-blinded. It also did not examine side effects and adverse reactions in subjects. It did open the door to more extensive study of triptans for use in intermittent prophylaxis.
Zolmitriptan
The study discussed above for acute menstrual migraine treatment with zolmitriptan represents the first phase of a two-phase, multicenter, randomized, double-blind, placebo-controlled study [23]. Phase two examined use of zolmitriptan for intermittent prophylaxis. Patients who successfully completed phase one and had migraines during, on average, three out of four menstrual cycles were enrolled in the phase two study [29•]. They then were randomized to receive zolmitriptan, 2.5 mg twice daily; zolmitriptan, 2.5 mg three times daily; or placebo three times daily. Patients were to begin medication 2 days before the anticipated onset of menses and continue for a total of 7 days per cycle for three cycles. Zolmitriptan was effective in reducing both frequency of headaches (54.7% in twice-daily dosing, 58.6% in three times–daily dosing, and 37.8% in placebo) and mean number of headaches (0.75 in twice-daily dosing, 0.56 in three times–daily dosing, and 0.95 in placebo) compared to placebo [29•]. Zolmitriptan was well tolerated and no adverse events were reported.
Naratriptan
The first study conducted for short-term prophylaxis of MRM with naratriptan was a double-blind, placebo-controlled, three-group parallel study [30]. Participants were randomized into three groups: placebo; naratriptan, 1 mg twice daily; or naratriptan, 2.5 mg twice daily. Treatment was started 2 days before expected onset of menses and continued for 5 days each cycle over four cycles. More patients taking naratriptan, 1 mg, reported increased headache freedom than placebo patients (50% vs 25%, P=0.003). Patients taking naratriptan, 2.5 mg, also reported increased headache freedom compared to control patients, but this was not statistically significant. Also, patients taking naratriptan, 2.5 mg, reported increased adverse events, although none of these were severe or life threatening [30]. A similar study conducted at Italian centers was not placebo controlled, but evaluated patients both before treatment and after 3 months of short-term naratriptan, 1 mg twice-daily dosing [31]. This study also reported increase in headache-free days and decreased headache severity [31]. More recently, Mannix et al. [32] in 2007 conducted another double-blind, placebo-controlled study evaluating two separate study groups, each with naratriptan, 1 mg twice daily, versus placebo. Treatment was initiated 2 days before expected onset of menstrual migraine and was continued for a 5-day course. Patients taking naratriptan, 1 mg, in this study reported more perimenstrual periods without migraine versus the placebo group (38% and 34% vs 29% and 24%; P<0.05 in each study). Although no significant adverse effects were reported with this study, an increased number of posttreatment headaches were noted in the naratriptan group [32].
Frovatriptan
Given the benefit demonstrated with other triptans in the treatment of MRM and PMM, it stands to reason that frovatriptan also would be beneficial for short-term prophylaxis, especially given its longer half-life. The seminal study of frovatriptan for intermittent prophylaxis in MRM and PMM was conducted by Silberstein et al. [33] in 2004. This multicenter, double-blind, randomized, placebo-controlled, three-way crossover study evaluated women with history of MRM or PMM. Participants were randomized into one of three groups: frovatriptan, 2.5 mg daily; frovatriptan, 2.5 mg twice daily; or placebo. Treatment was started 2 days before the anticipated start of their menstrual migraine and continued for a total of 6 days per cycle. Consistent with the crossover design, each participant was treated with each medication regimen by the end of the study and logged their headaches and headache severity in diaries. The study was divided into two arms so that, at any one time, two groups were taking the same medication regimen. Efficacy was determined by reduction of both headache frequency and severity. Of the placebo groups, 67% of one arm and 69% of another reported MRM. This was reduced to 52% in both arms of the frovatriptan, 2.5 mg daily, groups and 41% and 43% in the arms of the frovatriptan, 2.5 mg twice daily, groups. In addition, headache severity improved, with 51% of the placebo group reporting headaches of moderate to severe intensity, compared with 37% of the frovatriptan, 2.5 mg daily, group and 28% of the frovatriptan, 2.5 mg twice daily, group. Mean headache duration with placebo was 31.1 h, compared to 20.3 h with frovatriptan, 2.5 mg daily, and 16.6 h with frovatriptan, 2.5 mg twice daily. Both medications regimens were well tolerated, with the most common side effects of nausea and dizziness occurring in a small percentage of patients [33]. In 2009, Silberstein et al. [34] reexamined their data from this study and focused on patients with PMM. Efficacy was demonstrated in this group as well, with 51% and 37% of patients reporting menstrual migraine in the frovatriptan, 2.5 mg daily, and frovatriptan, 2.5 mg twice daily, groups, respectively, compared to 67% of the placebo group. Severity also was improved, with 32% and 25% of patients reporting moderate to severe headaches in the 2.5 mg daily and 2.5 mg twice-daily groups, respectively, compared to 46% of the placebo group [34].
A similar study with the same dosing regimens and durations of treatment was conducted by Brandes et al. [35] and was a placebo-controlled parallel-group study. This study examined headache-free perimenstrual periods over three cycles and reported the mean number of headache-free perimenstrual periods per patient as 0.69 in the 2.5 mg daily group, 0.92 in the 2.5 mg twice-daily group, and 0.42 in the placebo group. The severity of migraines and migraine-associated symptoms were significantly improved with frovatriptan. Again, these medications were well tolerated [35].
An open-label, nonrandomized, parallel-group study evaluated patients receiving frovatriptan, 2.5 mg daily; transdermal estrogen, 25 µg; or naproxen sodium, 500 mg per day, for menstrual migraine. All treatments were started 2 days before expected onset of menstrual migraine and continued for 6 days per cycle. Patients reported decreased incidence of headache on frovatriptan compared to the estrogen or naproxen groups. However, this was limited by its relatively small sample size (10–14 patients per group) [36].
A randomized, double-blind, placebo-controlled, two-period, crossover pharmacokinetic study performed in 2009 evaluated concentrations of frovatriptan and its metabolites in serum samples of patients [37•]. Daily and twice-daily dosing regimens were evaluated. Both regimens reached steady state by day 2. Twice-daily dosing demonstrated less fluctuation in blood concentrations of frovatriptan than daily dosing. Both regimens were well tolerated [37•].
Conclusions
MRM and PMM represent unique challenges in headache treatment and management. Many women report that migraines associated with their menses are more severe and more debilitating than migraines occurring at other times in the menstrual cycle. As with other migraines, triptans have demonstrated efficacy in use as abortive therapy of menstrual migraine. For women who experience an increased frequency of migraines during menses, these headaches can be treated with intermittent prophylaxis. Intermittent prophylactic therapies can be used alone, especially in women with PMM, or in conjunction with compatible, long-term, prophylactic medications. Hormonal therapies can result in increased headaches when therapy is withdrawn, but demonstrate an overall decrease in headache frequency. Loder et al. [38•] examined the use of hormonal therapy in migraines and concluded that the risk-to-benefit ratio has not been sufficiently studied to make hormones a first-line treatment. Long-term risk of estrogen use is difficult to assess and, although some women enjoy the side effect of decreased menstrual bleeding, others are frustrated by frequent spotting or even suffer from estrogen-induced headaches [38•]. Intermittent prophylaxis with naproxen also has shown benefit and may be a safe and cost-effective treatment. More recently, studies have examined the use of triptans in intermittent prophylaxis of menstrual migraine. Most studies examined treatment 2 days before the anticipated start of menses or menstrual migraine through a 6- to 7-day course per month. Triptans demonstrated good efficacy for decreasing headache days and debility associated with migraines. They also seem to be well tolerated. Triptans do carry a risk of medication rebound headache, although this risk seems increased with greater than 10 days per month use rather than the 5 to 7 days per month noted in these studies [39]. Of note, only the Mannix et al. [32] study of naratriptan reported the occurrence of rebound headaches. The high cost of triptans also is of concern. In a paper by Perfetto et al. [40], the lowest cost for abortive treatment of 100 migraines using triptans was calculated at $1560. Although there are no studies evaluating the cost of triptans for intermittent prophylaxis, the economic impact on patients and insurance companies likely would be considerable. Recent studies have focused on the triptan with the longest half-life, frovatriptan. Frovatriptan has demonstrated efficacy and tolerability in multiple placebo-controlled randomized trials. Although the cost of triptans may be prohibitive, the evidence from these studies suggests that intermittent MRM prophylaxis with scheduled dosing of triptans is a viable treatment option for appropriate patients.
Footnotes
Disclosures No potential conflicts of interest relevant to this article were reported.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Scher AI, Stewart WF, Lipton RB. Migraine and headache: a meta-analytic approach. In: Crombie IK, Croft PR, Linton SJ, et al., editors. In Epidemiology of Pain. Seattle: IASP Press; 1999. pp. 159–170. [Google Scholar]
- 2.Stewart WF, Linet MS, Celentano DD, et al. Age- and sex-specific incidence rates of migraine with and without visual aura. Am J Epidemiol. 1991;134:1111–1120. doi: 10.1093/oxfordjournals.aje.a116014. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Schechter A, Lipton RB. Migraine heterogeneity. Disability, pain intensity, and attack frequency and duration. Neurology. 1994;44(6 Suppl 4):S24–S39. [PubMed] [Google Scholar]
- 4.Stewart WF, Lipton RB, Chee E, et al. Menstrual cycle and headache in a population sample of migraineurs. Neurology. 2000;55:1517–1523. doi: 10.1212/wnl.55.10.1517. [DOI] [PubMed] [Google Scholar]
- 5.Dowson AJ, Kilminster SG, Salt R, et al. Disability associated with headaches occurring inside and outside the menstrual period in those with migraine: a general practice study. Headache. 2005;45:274–282. doi: 10.1111/j.1526-4610.2005.05064.x. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor EA, Hackshaw A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology. 2004;63:351–353. doi: 10.1212/01.wnl.0000133134.68143.2e. [DOI] [PubMed] [Google Scholar]
- 7.Granella F, Sances G, Allais G, et al. Characteristics of menstrual and nonmenstrual attacks in women with menstrually related migraine referred to headache centres. Cephalalgia. 2004;24:707–716. doi: 10.1111/j.1468-2982.2004.00741.x. [DOI] [PubMed] [Google Scholar]
- 8.Martin VT, Wernke S, Mandell K, et al. Defining the relationship between ovarian hormones and migraine headache. Headache. 2005;45:1190–1201. doi: 10.1111/j.1526-4610.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 9.Somerville BW. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology. 1972;22:355–365. doi: 10.1212/wnl.22.4.355. [DOI] [PubMed] [Google Scholar]
- 10.Somerville BW. The role of progesterone in menstrual migraine. Neurology. 1971;21:853–859. doi: 10.1212/wnl.21.8.853. [DOI] [PubMed] [Google Scholar]
- 11.Martin VT, Lipton RB. Epidemiology and biology of menstrual migraine. Headache. 2008;48 Suppl 3:S124–S130. doi: 10.1111/j.1526-4610.2008.01310.x. [DOI] [PubMed] [Google Scholar]
- 12.Lokken C, Holm JE, Myers TC. The menstrual cycle and migraine: a time-series analysis of 20 women migraineurs. Headache. 1997;37:235–239. doi: 10.1046/j.1526-4610.1997.3704235.x. [DOI] [PubMed] [Google Scholar]
- 13.Brandes JL. The influence of estrogen on migraine: a systematic review. JAMA. 2006;295:1824–1830. doi: 10.1001/jama.295.15.1824. [DOI] [PubMed] [Google Scholar]
- 14.Martin VT. New theories in the pathogenesis of menstrual migraine. Curr Pain Headache Rep. 2008;12:453–462. doi: 10.1007/s11916-008-0077-3. [DOI] [PubMed] [Google Scholar]
- 15.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders. 2nd edition. Vol. 24. Cephalalgia: 2004. pp. 9–160. [DOI] [PubMed] [Google Scholar]
- 16. Martin V, Cady R, Mauskop A, et al. Efficacy of rizatriptan for menstrual migraine in an early intervention model: a prospective subgroup analysis of the rizatriptan TAME (Treat A Migraine Early) studies. Headache. 2008;48:226–235. doi: 10.1111/j.1526-4610.2007.00947.x.This well-modeled study evaluated the efficacy of rizatriptan in a subgroup of menstrual migraineurs from the larger TAME study.
- 17.Mannix LK, Loder E, Nett R, et al. Rizatriptan for the acute treatment of ICHD-II proposed menstrual migraine: two prospective, randomized, placebo-controlled, double-blind studies. Cephalalgia. 2007;27:414–421. doi: 10.1111/j.1468-2982.2007.01313.x. [DOI] [PubMed] [Google Scholar]
- 18.Nett R, Mannix LK, Mueller L, et al. Rizatriptan efficacy in ICHD-II pure menstrual migraine and menstrually related migraine. Headache. 2008;48:1194–1201. doi: 10.1111/j.1526-4610.2008.01093.x. [DOI] [PubMed] [Google Scholar]
- 19.Newman LC, Harper S, Jones BA, Campbell J. Frovatriptan for acute treatment of migraine associated with menstruation: Results from an open-label postmarketing surveillance study. J Women’s Health (Larchmt) 2009;18:1265–1273. doi: 10.1089/jwh.2008.1031. [DOI] [PubMed] [Google Scholar]
- 20.Landy S, Savani N, Shackelford S, et al. Efficacy and tolerability of sumatriptan tablets administered during the mild-pain phase of menstrually associated migraine. Int J Clin Pract. 2004;58:913–919. doi: 10.1111/j.1368-5031.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- 21.Nett R, Landy S, Shackelford S, et al. Pain-free efficacy after treatment with sumatriptan in the mild pain phase of menstrually associated migraine. Obstet Gynecol. 2003;102:835–842. doi: 10.1016/s0029-7844(03)00659-8. [DOI] [PubMed] [Google Scholar]
- 22. Mannix LK, Martin VT, Cady RK, et al. Combination treatment for menstrual migraine and dysmenorrhea using sumatriptan-naproxen: two randomized controlled trials. Obstet Gynecol. 2009;114:106–113. doi: 10.1097/AOG.0b013e3181a98e4d.This randomized, double-blind, multicenter, placebo-controlled trial examines the efficacy of sumatriptan- naproxen in menstrual migraine and connects this with efficacy in dysmenorrhea.
- 23.Tuchman M, Hee A, Emeribe U, Silberstein S. Efficacy and tolerability of zolmitriptan oral tablet in the acute treatment of menstrual migraine. CNS Drugs. 2006;20:1019–1026. doi: 10.2165/00023210-200620120-00005. [DOI] [PubMed] [Google Scholar]
- 24.Allais G, Bussone G, De Lorenzo C, et al. Naproxen sodium in short-term prophylaxis of pure menstrual migraine: pathophysiological and clinical considerations. Neurol Sci. 2007;28 Suppl 2:S225–S228. doi: 10.1007/s10072-007-0783-3. [DOI] [PubMed] [Google Scholar]
- 25.Pfaffenrath V. Efficacy and safety of percutaneous estradiol vs placebo in menstrual migraine. Cephalalgia. 1993;13 Suppl 13:244. [Google Scholar]
- 26.LaGuardia KD, Fisher AC, Bainbridge JD, et al. Suppression of estrogen-withdrawal headache with extended transdermal contraception. Fertil Steril. 2005;83:1875–1877. doi: 10.1016/j.fertnstert.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 27. MacGregor EA, Frith A, Ellis J, et al. Prevention of menstrual attacks of migraine: a double-blind placebo-controlled crossover study. Neurology. 2006;67:2159–2163. doi: 10.1212/01.wnl.0000249114.52802.55.This double-blind, placebo-controlled, crossover study evaluates the efficacy of estradiol gel in reduction of menstrual migraine.
- 28.Newman LC, Lipton RB, Lay CL, Solomon S. A pilot study of oral sumatriptan as intermittent prophylaxis of menstruation-related migraine. Neurology. 1998;51:307–309. doi: 10.1212/wnl.51.1.307. [DOI] [PubMed] [Google Scholar]
- 29. Tuchman MM, Hee A, Emeribe U, Silberstein S. Oral zolmitriptan in the short-term prevention of menstrual migraine: a randomized, placebo-controlled study. CNS Drugs. 2008;22:877–886. doi: 10.2165/00023210-200822100-00007.This well-designed study evaluated the efficacy of two dosing regimens of zolmitriptan for intermittent prophylaxis of menstrual migraine.
- 30.Newman L, Mannix LK, Landy S, et al. Naratriptan as short-term prophylaxis of menstrually associated migraine: a randomized, double-blind, placebo-controlled study. Headache. 2001;41:248–256. doi: 10.1046/j.1526-4610.2001.111006248.x. [DOI] [PubMed] [Google Scholar]
- 31.Moschiano F, Allais G, Grazzi L, et al. Naratriptan in the short-term prophylaxis of pure menstrual migraine. Neurol Sci. 2005;26 Suppl 2:S162–S166. doi: 10.1007/s10072-005-0435-4. [DOI] [PubMed] [Google Scholar]
- 32.Mannix LK, Savani N, Landy S, et al. Efficacy and tolerability of naratriptan for short-term prevention of menstrually related migraine: data from two randomized, double-blind, placebo-controlled studies. Headache. 2007;47:1037–1049. doi: 10.1111/j.1526-4610.2007.00855.x. [DOI] [PubMed] [Google Scholar]
- 33.Silberstein SD, Elkind AH, Schreiber C, Keywood C. A randomized trial of frovatriptan for the intermittent prevention of menstrual migraine. Neurology. 2004;63:261–269. doi: 10.1212/01.wnl.0000134620.30129.d6. [DOI] [PubMed] [Google Scholar]
- 34.Silberstein SD, Berner T, Tobin J, et al. Scheduled short-term prevention with frovatriptan for mi-graine occurring exclusively in association with menstruation. Headache. 2009;49:1283–1297. doi: 10.1111/j.1526-4610.2009.01509.x. [DOI] [PubMed] [Google Scholar]
- 35.Brandes JL, Poole A, Kallela M, et al. Short-term frovatriptan for the prevention of difficult-to-treat menstrual migraine attacks. Cephalalgia. 2009;29:1133–1148. doi: 10.1111/j.1468-2982.2009.01840.x. [DOI] [PubMed] [Google Scholar]
- 36.Guidotti M, Mauri M, Barrilà C, et al. Frovatriptan vs. transdermal estrogens or naproxen sodium for the prophylaxis of menstrual migraine. J Headache Pain. 2007;8:283–288. doi: 10.1007/s10194-007-0417-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wade A, Pawsey S, Whale H, et al. Pharmacokinetics of two 6-day frovatriptan dosing regimens used for the short-term prevention of menstrual migraine: A phase I, randomized, double-blind, placebo-controlled, two-period crossover, single-centre study in healthy female volunteers. Clin Drug Investig. 2009;29:325–337. doi: 10.2165/00044011-200929050-00005.This very useful study evaluates the pharmacodynamics of frovatriptan and evaluates serum levels with different dosing regimens.
- 38. Loder E, Rizzoli P, Golub J. Hormonal management of migraine associated with menses and the menopause: A clinical review. Headache. 2007;47:329–340. doi: 10.1111/j.1526-4610.2006.00710.x.This is an excellent review of current data regarding estrogen and migraine treatment.
- 39.Smith TR, Stoneman J. Medication overuse headache from antimigraine therapy: clinical features, pathogenesis and management. Drugs. 2004;64:2503–2514. doi: 10.2165/00003495-200464220-00002. [DOI] [PubMed] [Google Scholar]
- 40.Perfetto EM, Weis KA, Mullins CD, et al. An economic evaluation of triptan products for migraine. Value Health. 2005;8:647–655. doi: 10.1111/j.1524-4733.2005.00056.x. [DOI] [PubMed] [Google Scholar]