SUMMARY
The cellular and molecular mechanisms mediating histamine-independent itch in primary sensory neurons are largely unknown. Itch induced by chloroquine (CQ) is a common side-effect of this widely used anti-malarial drug. Here we show that Mrgprs, a family of G protein-coupled receptors expressed exclusively in peripheral sensory neurons, function as itch receptors. Mice lacking a cluster of Mrgpr genes display significant deficits in itch induced by CQ but not histamine. CQ directly excites sensory neurons in an Mrgpr-dependent manner. CQ specifically activates mouse MrgprA3 and human MrgprX1. Loss- and gain-of-function studies demonstrate that MrgprA3 is required for CQ responsiveness in mice. Furthermore, MrgprA3-expressing neurons respond to histamine and co-express Gastrin-Releasing Peptide, a peptide involved in itch sensation, and MrgprC11. Activation of these neurons with MrgprC11-specific agonist BAM8-22 induces itch in wild-type but not mutant mice. Therefore, Mrgprs may provide molecular access to itch-selective neurons and constitute novel targets for itch therapeutics.
INTRODUCTION
Itch, formally known as pruritus, has been defined as an “unpleasant skin sensation that elicits the desire or reflex to scratch” (Ikoma et al., 2006). Primary sensory neurons in dorsal root ganglia (DRG) play an essential role in generating itch by detecting pruritogenic stimuli through their peripheral axons in the skin and mucosal surfaces and sending the signals to the spinal cord via their central axons (Paus et al., 2006). The best characterized itch mediator is histamine, which is mainly secreted by skin mast cells and excites nearby sensory fibers by acting on histamine receptors (Alving et al., 1991). Histamine-induced itch in human can be almost completely blocked by histamine receptor H1 antagonists. However, the blockers are ineffective in many other itch conditions, such as those arising from atopic dermatitis, renal and liver diseases, the side effects of drugs, plant toxins and mechanical stimuli (Paus et al., 2006). These observations, together with other electrophysiological and molecular studies, strongly imply the existence of histamine-independent types of itch (Davidson et al., 2007; Johanek et al., 2007; Johanek et al., 2008). A major hurdle to investigating histamine-independent itch is the lack of information about the receptors directly activated by non-histaminergic pruritogens as well as molecular markers for itch-sensing neurons in the DRG.
Chloroquine (CQ) is a drug which has long been used in the treatment and prevention of malaria. One major side effect of this drug is itch, which is very common among black Africans (up to 70%), but less common in other races. Pruritus is a major cause of non-compliance in the treatment of malaria as ~30% of African patients refused further CQ treatment because of unbearable itch (Mnyika and Kihamia, 1991; Sowunmi et al., 2000). This non-compliance may lead to the development and spread of CQ-resistant Plasmodium falciparum. CQ-induced itch is not considered an allergic reaction since pruritus is seen after first exposure (Ademowo, 1998; Olatunde, 1977). More importantly, it cannot be treated effectively by anti-histamine drugs suggesting a histamine-independent pathway is involved (Abila et al., 1994; Ezeamuzie et al., 1990). CQ-induced itch is also well documented in mouse. Subcutaneous CQ injection in wild- type (WT) mice acutely evokes a pronounced scratching behavior (Green et al., 2006). Interestingly, mice lacking gastrin-releasing peptide receptor (GRPR), which is specifically expressed in dorsal horn neurons of the spinal cord, exhibit severely reductions in itch responses evoked by various pruritogens including CQ (Sun and Chen, 2007). Furthermore, mice with GRPR-expressing dorsal horn neurons selectively ablated showed profound scratching deficits whereas pain behaviors were unaffected in these animals (Sun et al., 2009). These findings suggest that both GRPR and the second-order neurons in the spinal cord marked by GRPR are important for transmitting itch signals from primary sensory afferents. However, it is unknown whether CQ directly activates primary sensory fibers in the skin and if cell surface receptors are involved in the process.
Several G protein-coupled receptors (GPCRs) have been shown to be essential in generating itch including histamine receptors and protease activated receptors (PARs) (Shim and Oh, 2008; Steinhoff et al., 2003). Mrgprs (also named Mrg/SNSR) are a family of orphan GPCRs consisting of more than 50 members in the mouse genome that can be grouped into several subfamilies: MrgprA1-22, MrgprB1-13, MrgprC1-14, and MrgprD-G (Dong et al., 2001; Zylka et al., 2003). Strikingly, the expression of Mrgprs, including MrgprAs, MrgprB4, MrgprB5, MrgprC11 and MrgprD, is restricted to subsets of small-diameter sensory neurons in DRG and trigeminal ganglia, and has not been detected in the central nervous system or in the rest of the body (Dong et al., 2001; Zylka et al., 2003). Similarly, human MrgprXs are also selectively expressed in DRG neurons (Lembo et al., 2002).
Mrgprs can be activated by peptides terminating in RF/Y-G or RF/Y-amide such as molluscan FMRFamide and mammalian neuropeptide FF (NPFF), neuropeptide AF (NPAF), γ2-melanocyte-stimulating hormone (γ2-MSH) and bovine adrenal medulla peptide (BAM). These peptides can activate heterologously expressed mouse MrgprA1, MrgprA4, MrgprC11, and human MrgprX1 with different sensitivities (Dong et al., 2001; Han et al., 2002; Lembo et al., 2002). The highly restricted expression of these receptors suggests that Mrgprs are likely involved in somatosensation including pain or itch, but direct evidence for this is lacking. Here we demonstrate that certain Mrgprs function as receptors for CQ and mediate its direct activation of a small subset of DRG neurons and CQ-induced itch. More importantly, CQ-sensitive neurons, comprising only 4–5% of total DRG neurons, may define a subpopulation of DRG neurons that mediate itch.
RESULTS
Targeted deletion of a cluster of Mrgpr genes
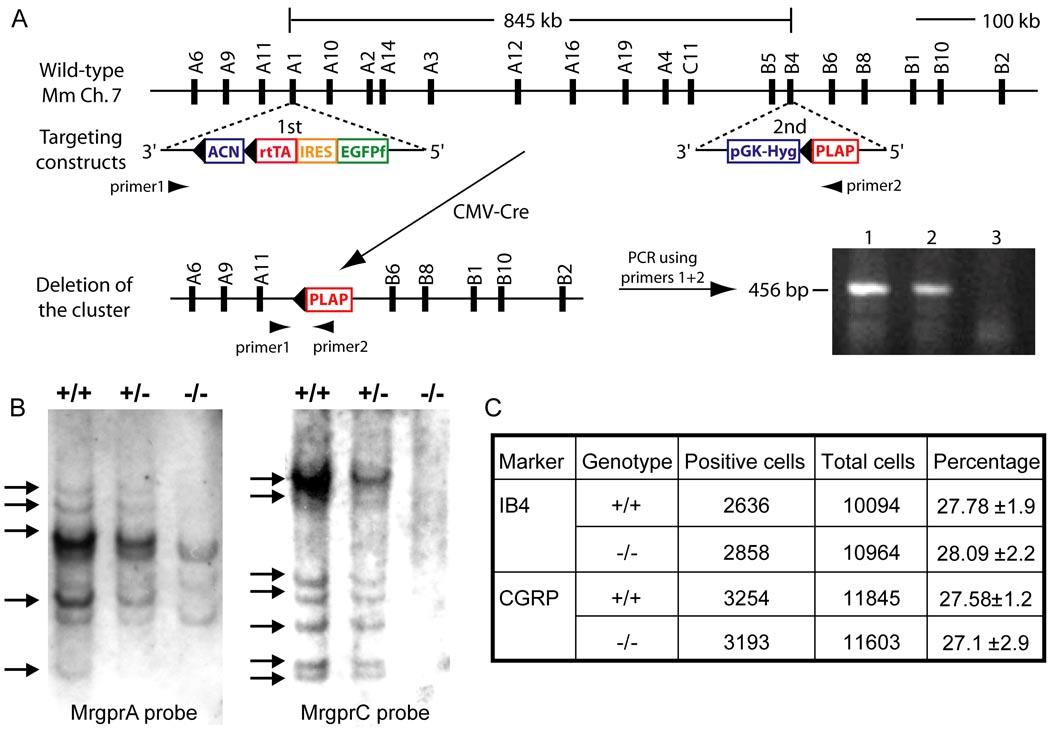
Many Mrgpr genes are clustered together on mouse chromosome 7 (Dong et al., 2001; Zylka et al., 2003). To determine the function of Mrgprs in vivo while overcoming the potential problem of gene redundancy, we generated a mouse line in which a cluster of Mrgpr genes was deleted (Figure 1A and 1B). The deleted 845-kilobase region comprises ~30 Mrgpr genes, twelve of which (MrgprA1-4, A10, A12, A14, A16, A19, B4, B5 and C11) have intact open reading frames (ORFs, Figure 1A). No other ORF is present in this region according to the Mouse Genome Project. Although the mouse Mrgpr superfamily consists of over 50 members, more than half are pseudogenes and only ~24 genes have intact ORFs (Dong et al., 2001; Han et al., 2002). Therefore, the deleted cluster represents ~50% of the potentially functional Mrgpr repertoire and contains most MrgprA and MrgprC genes as well as some members of the MrgprB subfamily. The deleted Mrgpr genes are specifically expressed in DRG (Dong et al., 2001; Han et al., 2002; Zylka et al., 2003). MrgprA6, A9, A11, B1, B2, B6, B8, B10, and D-G are not included in this deletion based on the Mouse Genome Project and RT-PCR experiments (data not shown). Notably, MrgprB1 and MrgprB2, which were not deleted, are expressed in the skin but not in DRG (Zylka et al., 2003).
Figure 1.
Targeted deletion of a cluster of Mrgpr genes. (A) Top horizontal line represents Mrgpr gene cluster on WT mouse chromosome 7. The distance between MrgprA1 and MrgprB4 is 845 kilobases, which contains 12 intact Mrgprs (each represented by a black bar with its name on top). Targeting constructs containing loxP sites (black triangles) and the selection marker genes were introduced to the MrgprA1 and MrgprB4 loci in ES cells by two rounds (1st and 2nd) of electroporation and homologous recombination. Positive ES clones with correct targeting in the two loci underwent a third round of electroporation with CMV-Cre construct. Cre-mediated recombination resulted in deletion of an Mrgpr cluster between loxP sites. The deletion event in ES cells (lane 1 and 2; lane 3 as negative control using WT ES cells) was detected by PCR amplification using primers 1 and 2 flanking the cluster (shown as arrowheads). The PCR product (456 bp) was further confirmed by sequencing. (B) Southern blot of genomic DNA with an MrgprA or MrgprC probe. The genomic DNA was digested with BglII. Due to cross-hybridization, a single MrgprC or MrgprA probe can label multiple members of the MrgprC or MrgprA subfamily in WT (+/+) and cluster heterozygous mice (+/−) DNA. In homozygous mice (−/−), most of the positive bands are absent (arrows). (C) The deletion of Mrgpr genes does not affect cell fate determination of small-diameter sensory neurons. The proportion of nonpeptidergic (IB4+) and peptidergic (CGRP+) small-diameter sensory neurons does not differ between WT and Mrgpr-clusterΔ−/− mice (n = 3).
Mating between mice heterozygous for the cluster deletion (Mrgpr-clusterΔ−/+) produced offspring with the expected Mendelian distribution of gender and genotype. Homozygous Mrgpr-clusterΔ−/− mice are viable, fertile and generally indistinguishable from WT littermates in appearance, body weight, overt behavior and gross anatomy. The motor function of Mrgpr-clusterΔ−/− mice is also normal as determined by the rotarod test (data not shown). Furthermore, Mrgprs are not required for neuronal survival, fate determination or differentiation of small-diameter sensory neurons (Figure 1C; Supplemental Data).
Mrgpr-clusterΔ−/− mice exhibit severe reduction in CQ-induced scratching
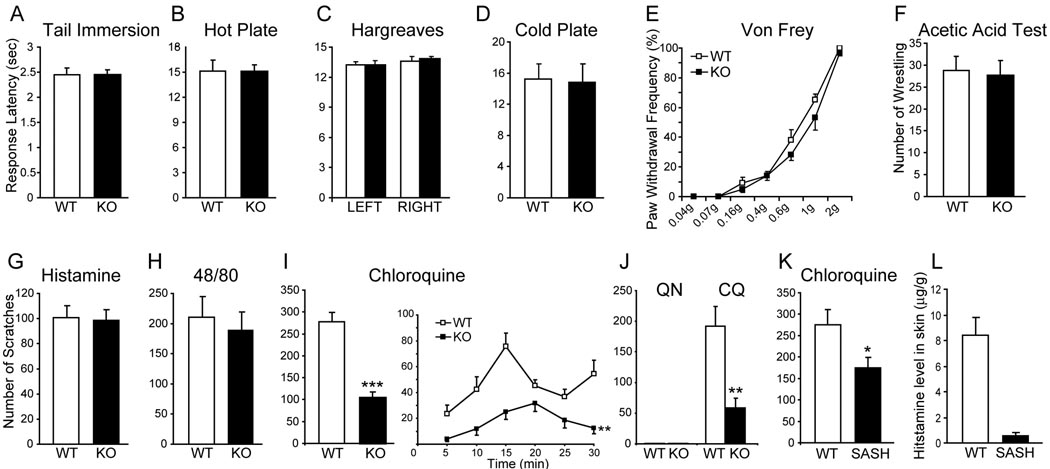
Activation of small-diameter sensory neurons in DRG can generate different types of somatosensation including pain and itch with specific and distinct behavioral responses. For instance, pain and itch cause withdrawal and scratching responses, respectively (Ikoma et al., 2006). We next investigated whether the deletion of Mrgpr genes affects behavioral responses to pain- and itch-inducing stimuli. Mrgpr-clusterΔ−/− mice responded normally to acute noxious heat, cold, mechanical and chemical stimulation as compared with WT littermates (Figure 2A–2F). Thus, acute pain sensation appears unaffected in Mrgpr-clusterΔ−/− mice. In addition, Mrgpr mutant mice exhibited modest but statistically significant increases only at certain testing time points in inflammatory hyperalgesia induced by complete Freund’s adjuvant (CFA) or carrageenen injection. No significant difference was found in neuropathic pain caused by L5 spinal nerve ligation between mutant and wild-type mice (Figure S1).
Figure 2.
Mrgpr-clusterΔ−/− mice show severe deficiency in CQ-induced itch. (A–D) Mrgpr-clusterΔ−/− mice respond normally to noxious acute thermal stimuli. Response latencies in tail immersion (50°C, n = 12 per genotype, A), hot plate (50°C, n = 11 per genotype, B), Hargreaves (n = 24 per genotype, C) and cold plate (0 °C, WT n = 13, KO n = 9, D) tests did not differ between WT and Mrgpr-clusterΔ−/− mice. (E) The paw withdrawal threshold of Mrgpr-clusterΔ−/− mice to punctate mechanical stimuli (Von Frey) was comparable to that of WT mice (n = 12 per genotype). (F) Mrgpr-clusterΔ−/− mice responded normally to noxious acute chemical stimuli. The writhing responses to intraperitoneal injection of acetic acid (0.6%, 15 ml/kg) were indistinguishable between WT and Mrgpr-clusterΔ−/− mice (n = 12 per genotype). (G and H) Mrgpr-clusterΔ−/− mice displayed normal histamine-dependent itch. The total scratching bouts were not significantly different between WT and Mrgpr-clusterΔ−/− mice during the first 30 min after subcutaneous injection of histamine (10 µmol; WT n = 7, KO n = 10, G) or compound 48/80 (100 µg/50 µl; WT n = 8, KO n = 7, H). (I) Mrgpr-clusterΔ−/− mice showed deficiency in CQ-induced itch. The total scratching bouts during the first 30 min after CQ injection (200 µg/50 µl, 8 mM) were significantly decreased in Mrgpr-clusterΔ−/− mice (n = 9) than in WT littermates (n = 8). The time course shows bouts of scratching at 5 min intervals. (J) Quinoline (QN) failed to induce itch in both WT and Mrgpr-clusterΔ−/− mice. However, subsequent injection of CQ induced a strong scratch response in WT and a much weaker response in Mrgpr-clusterΔ−/− mice (n = 5 per genotype). (K) SASH mice showed a mild but significant reduction in CQ-induced itch compared with WT mice (SASH n = 8, WT n = 7). (L) SASH mice released significantly less histamine than WT mice after IgE stimulation of the skin (n = 5 per genotype), which provides strong evidence for the mast cell deficiency in SASH mice. The data are presented as mean ± SEM. *, P<0.05; **, P<0.01; ***, P<0.005; two-tailed unpaired t-test or two-way ANOVA.
In addition to pain, we evaluated chemically induced itch responses in Mrgpr-clusterΔ−/− mice. No significant difference was found between Mrgpr-clusterΔ−/− and WT mice in the total number of scratching bouts induced by histamine over a period of 30 min (Figure 2G). Consistent with this result, WT and mutant mice also showed similar responses to compound 48/80, a drug that elicits mast cell degranulation and induces histamine-dependent itch (Kuraishi et al., 1995; Nakayama et al., 2002) (Figure 2H). These results suggest that Mrgprs are not involved in histamine-dependent itch.
Strikingly, itch induced by CQ was strongly reduced in Mrgpr-clusterΔ−/− mice. Figure 2I shows the time course of scratching bouts at 5 min intervals after CQ injection. Typically, the first bout was observed within 1 to 2 min after injection, and scratching peaked within 15 min in WT mice. In contrast, Mrgpr-clusterΔ−/− mice showed a delayed occurrence of the first scratching behavior (WT 57.8 ± 25.4 sec versus KO 280.5 ± 35.0 sec; P=0.0004). The total number of scratching bouts induced by CQ were 278 ± 21 in WT mice and 104 ± 13 in Mrgpr-clusterΔ−/− mice (Figure 2I). Interestingly, injection of a CQ precursor, quinoline, did not evoke any scratching behavior (see below for quinoline’s structure and inability of activating Mrgprs) in both WT and mutant mice (Figure 2J). However, immediately after quinoline treatment, CQ injection at the same location induced robust scratching behavior in WT mice; and the number of scratches induced by this treatment was again severely reduced in Mrgpr-clusterΔ−/− mice (Figure 2J). These results suggest that CQ-induced itch, but not histaminergic itch, is affected in the cluster deletion mice. Similarly, intradermal injection of CQ in rats evoked profound scratching responses whereas quinoline did not (Figure S2A). These data provide further evidence that CQ-evoked itch is well-conserved.
Previous studies have indicated that CQ can cause mast cell degranulation (Green and Lim, 1989; Nosal et al., 1991). To determine if this effect on mast cells contributes to CQ-evoked scratching behavior, we repeated the experiment on SASH mice, which lack mast cells due to a chromosomal inversion in the regulatory element of the Kit gene (Yamazaki et al., 1994). As compared to WT controls, SASH mice exhibited a modest but significant reduction in CQ-induced scratching behavior (Figure 2K). The mast cell deficiency in these mice was confirmed by a dramatic decrease in the level of histamine released upon skin mast cell degranulation (Figure 2L). These results confirm that degranulation of mast cells induced by CQ contributes to scratching behavior, which may account for the residual response to CQ in Mrgpr-clusterΔ−/− mice.
CQ directly excites DRG neurons in an Mrgpr-dependent manner
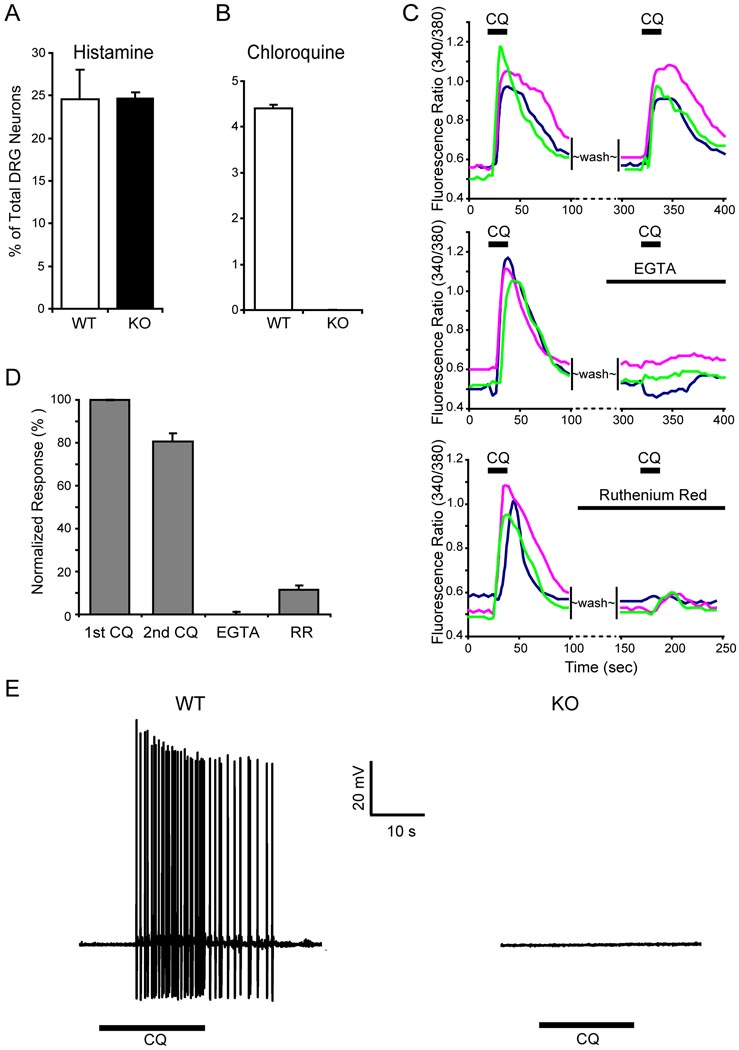
Since CQ-induced itch is not considered an allergic reaction, we hypothesized given our results that this type of itch results from a direct activation of DRG neurons by the drug. If so, then the behavioral deficit seen in mutant mice would be attributed to a loss of CQ responsiveness in primary sensory neurons. Indeed, 1 mM CQ treatment of cultured DRG neurons evoked a robust intracellular calcium ([Ca2+]i) increase in ~4–5% of the cells from WT mice. In contrast, none of the neurons in cultures derived from Mrgpr-deficient mice exhibited any significant response to CQ (Figure 3B). These data indicate that the CQ-evoked [Ca2+]i increases seen in WT DRG cultures reflect specific activation of a subset of cells, and that this activation is Mrgpr-dependent. In contrast, the percentage of DRG neurons responding to histamine was identical between WT and mutant cultures, consistent with the behavioral data and providing further evidence that histamine-induced itch is unaffected in Mrgpr-clusterΔ−/− mice (Figure 3A).
Figure 3.
The response of DRG neurons to CQ is Mrgpr-dependent. (A) The response to histamine was not impaired in Mrgpr-deficient DRG neurons. Calcium imaging showed that the percentage of Mrgpr-clusterΔ−/− DRG neurons responding to histamine (50 µM) was similar to that of WT neurons (n = 3 per genotype). (B) ~4.4% of WT DRG neurons responded to CQ (1 mM) with increased [Ca2+]i whereas Mrgpr-clusterΔ−/− DRG neurons failed to respond to the drug (n = 3 per genotype). (C–D) Extracellular calcium was required for the CQ-induced [Ca2+]i increase in DRG neurons. (C) shows representative traces from 3 different DRG neurons in calcium imaging assays. (D) The CQ-induced increase in [Ca2+]i was almost completely blocked with EGTA treatment. Ruthenium red (RR) also significantly attenuated [Ca2+]i increase evoked by CQ. As a control, sequential treatment of CQ only caused a ~20% reduction in [Ca2+]i increase. (E) CQ (1 mM) induced APs in DRG neurons. In WT DRG neurons, all CQ-sensitive neurons (as determined by calcium imaging, n=5) elicited a train of APs evoked by subsequent CQ treatment. In contrast, none of the neurons tested (n=11) from Mrgpr-clusterΔ−/− mice showed any response to the drug.
We performed additional experiments to further characterize [Ca2+]i increases in WT neurons. Sequential application of CQ caused a ~20% reduction of calcium responses. In addition, extracellular Ca2+ was necessary for the CQ-induced increase in [Ca2+]i since the CQ effect was almost completely blocked in Ca2+-free bath solution. Ruthenium red, an inhibitor of several TRP channels (Fujita et al., 2007), also severely attenuated the effect, indicating that TRPs are likely involved in the CQ signaling pathway (Figure 3C and D). Signaling via TRP channels has been observed for many GPCRs in DRG neurons including the histamine and bradykinin receptors (Chuang et al., 2001; Shim et al., 2007).
We also examined whether CQ can directly induce action potentials (APs) in dissociated DRG neurons, using whole-cell patch clamp recording. In WT DRG, all CQ-sensitive neurons (identified by calcium imaging) displayed a train of APs upon subsequent CQ treatment (Figure 3E). In contrast, all neurons that fail to show a calcium response to CQ also failed to generate APs with a similar treatment (n=5). As in the calcium imaging experiments, none of 11 size-matched neurons (diameter: ~20 µm) tested from Mrgpr-clusterΔ−/− mice showed any response to the drug. These studies provide strong evidence that CQ can directly excite a small subpopulation of sensory neurons in DRG and that Mrgprs are required for this effect. Similar to the mouse results, CQ induces robust activation in a subset of rat DRG neurons as determined by both an increase in [Ca2+]i and generation of APs (Figure S2).
CQ specifically activates mouse MrgprA3 and human MrgprX1
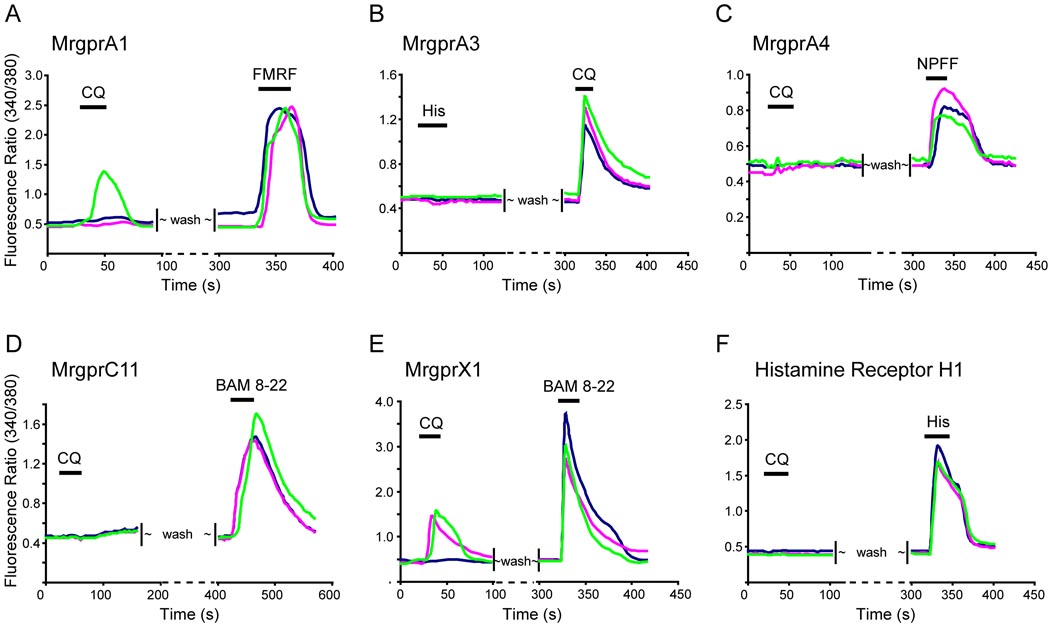
The Mrgpr-clusterΔ−/− behavioral and cellular loss-of-function phenotypes strongly suggest that Mrgprs function as cell surface receptors for CQ. To test this possibility directly, we examined whether Mrgprs have the ability to confer sensitivity to CQ on heterologous cells. We cloned each of the twelve Mrgprs that were deleted in Mrgpr-clusterΔ−/− mice (Figure 1A) into a mammalian expression vector, and transfected them individually into human embryonic kidney (HEK) 293 cells. By fusing green fluorescent protein (GFP) to the C-termini of the Mrgpr coding sequences, we were able to visualize both transfected cells and the proper membrane localization of the receptors. Our previous studies demonstrated that GFP does not disturb the normal function of Mrgprs (Dong et al., 2001; Han et al., 2002). Increased [Ca2+]i resulting from activation of the receptors was monitored by calcium imaging. Among the twelve mouse Mrgprs, only MrgprA3 conferred a strong response to CQ on HEK cells, whereas the other receptors conferred either weak or no responses to the drug (Figure 4A–D, Figure 5B, data not shown). MrgprA1, MrgprA4, and MrgprC11 were activated by their peptide agonists FMRF, NPFF, and BAM8-22, respectively, confirming they are functional receptors but insensitive to CQ (Figure 4A, C and D). Furthermore, MrgprA3-expressing HEK cells did not respond to BAM8-22 or histamine, indicating that MrgprA3 is a specific receptor for CQ (Figure 4B). Conversely, histamine receptor H1-expressing HEK293 cells failed to show any response to CQ (Figure 4F).
Figure 4.
Mouse MrgprA3 and human MrgprX1 are the predominant receptors for CQ. HEK293 cells were transfected with expression constructs for Mrgprs and histamine H1 receptor. The effects of different agonists on these transfected cells were tested via calcium imaging. Each figure shows a typical response from three different cells. (A) Fewer than half of MrgprA1-tranfected cells responded to CQ (1 mM) with increased [Ca2+]i whereas all transfected cells responded to FMRF (2 µM). (B) All MrgprA3-expressing cells responded to CQ but not histamine. (C) MrgprA4-expressing cells respond to NPFF (2 µM) but not CQ. (D) MrgprC11-expressing cells failed to respond to CQ whereas they responded to BAM8-22 (2 µM). (E) Human MrgprX1 responded to both CQ and BAM8-22 (2 µM). (F) Cells expressing the histamine H1 receptor exhibited a strong response to histamine (50 µM) but failed to respond to CQ.
Figure 5.
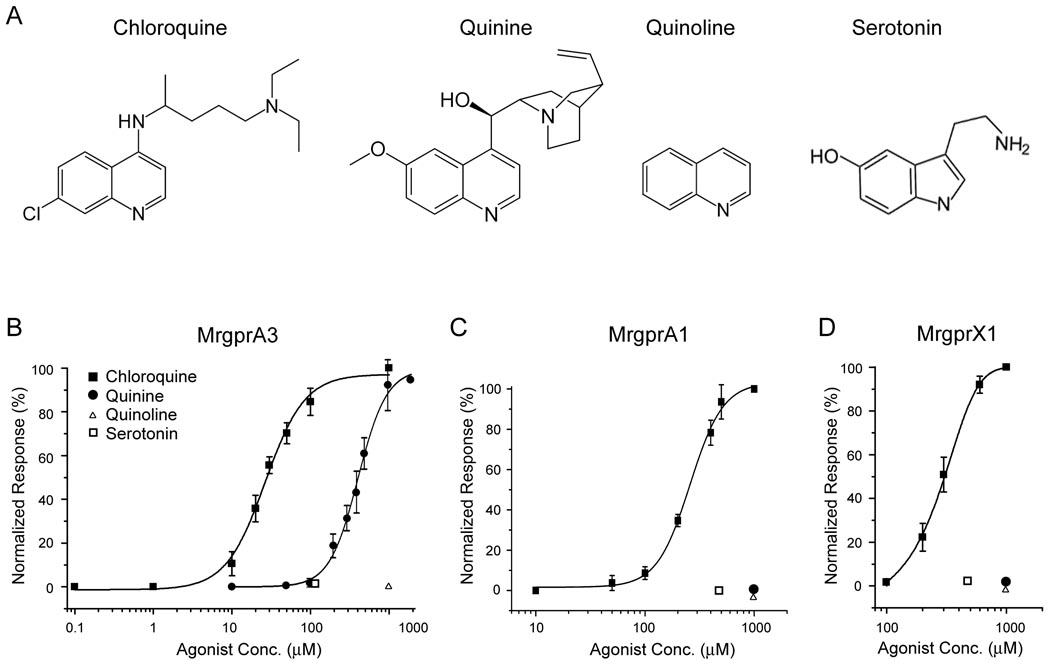
Mrgprs are selectively activated by CQ. (A) Molecules with structures related to CQ. Dose-response curves for MrgprA3 (B), MrgprA1 (C), and MrgprX1 (D) expressed in HEK 293 cells to the molecules in (A). Each data point represents the mean ± SEM of at least three independent experiments and at least 50 GFP+ cells were analyzed each time. Calcium responses at each ligand concentration were normalized to the maximal response subsequently elicited.
The human Mrgpr family (i.e. MrgprXs) is much smaller than the murine family. Although the human and mouse genes share strong sequence homology, they do not form clear orthologous pairs. We found that MrgprX1-expressing HEK293 cells responded to CQ whereas MrgprX2- and X3-expressing cells were completely insensitive to the drug (Figure 4E, data not shown). Together these data suggest that CQ directly activates mouse MrgprA3 and human MrgprX1 in heterologous cells with high specificity.
In order to determine the lowest concentrations of CQ capable of activating MrgprA3 and X1, we performed dose-response experiments in HEK293 cells. These experiments indicated that the receptors could be activated by the drug at micromolar concentrations with the mouse receptor showing 10-fold higher sensitivity than the human receptor (Figure 5B and D). EC50s for MrgprA3 and MrgprX1 are 27.55 ± 2.03 and 297.68 ±2.10 µM, respectively (see Discussion regarding the low potency of CQ on MrgprX1). Besides CQ, we also determined the sensitivities of these receptors to other structurally related compounds (i.e. quinoline, quinine and serotonin) (Figure 5A). Quinoline is used as an intermediate in the production of various compounds including CQ. Despite the presence of a bicyclic structure, quinoline completely failed to activate MrgprA3 and MrgprX1 suggesting that the side chain in CQ is also necessary for activation (Figure 5B and D). Consistently, quinoline does not induce any scratching behavior in mice (Figure 2J). Serotonin also has a bicyclic structure. But unlike CQ or quinoline, its bicyclic structure consists of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring (Figure 5A). Serotonin failed to activate MrgprA3 at a concentration of 100 µM (Figure 5B). Quinine is another drug used to treat malaria and its side effects also include itch. However, it is unclear if quinine-induced itch is an allergic response (Gonzalez et al., 2002; Kanny et al., 2003). Unlike CQ, quinine weakly activates MrgprA3 (Figure 5B).
MrgprA3 is the major receptor mediating CQ responsiveness in DRG neurons
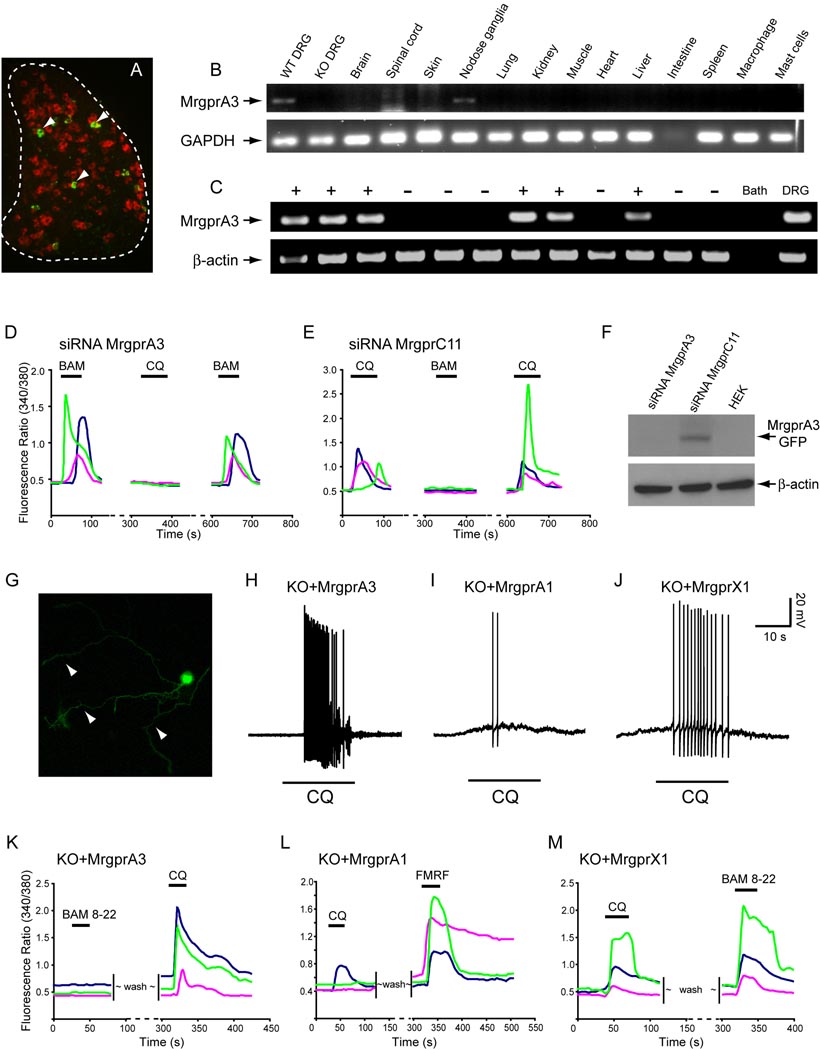
We and other groups have shown that MrgprA3 is expressed in a small subset (i.e. 4–5%) of WT DRG neurons (Dong et al., 2001; Liu et al., 2008; Zylka et al., 2003). The population of MrgprA3+ neurons is small in comparison to that expressing another Mrgpr member, MrgprD (Figure 6A). According to our previous studies, MrgprA3 has the highest expression level among all MrgprAs in adult mouse DRG whereas MrgprA1 is dramatically down-regulated to expression in few neurons, all of which are also MrgprA3+ (Zylka et al., 2003). To confirm the expression profile of MrgprA3, we performed RT-PCR on various adult mouse tissues. Among the tissues tested, MrgprA3 is found exclusively in DRG and nodose ganglia (Figure 6B). The low intensity of the MrgprA3 band as compared to that of GAPDH is consistent with the fact that only a small percentage of neurons express MrgprA3 in these ganglia. Human MrgprX1 exhibits a similar expression pattern (Lembo et al., 2002). This result also suggests that mast cells are unlikely to express MrgprA3. We did not see MrgprA3 in the skin, which contains many mast cells, nor did we see MrgprA3 expression in primary mast cells enriched from skin (Figure 6B) or bone marrow- derived mast cells (data not shown). Therefore, the transmission of CQ-induced itch signal by MrgprA3 likely occurs in primary sensory neurons in DRG and not other cell types in the skin.
Figure 6.
MrgprA3 is required for CQ responsiveness in mouse DRG neurons. (A) Fluorescent in situ hybridization of DRG sections with MrgprA3 (green, arrowheads) and MrgprD (red). The white dashed line outlines the DRG. (B) RT-PCR analysis of 14 mouse tissues or cell types for expression of MrgprA3. The only tissues containing MrgprA3 are WT DRG and nodose ganglia. Notably no band was found in Mrgpr-clusterΔ−/− DRG, confirming MrgprA3 was deleted in Mrgpr-clusterΔ−/− mice. (C) Single cell RT-PCR was performed on individual DRG neurons with the responsiveness to CQ (1 mM) established by calcium imaging (shown here are 12 representative neurons). MrgprA3 mRNA was detected in 8/9 CQ-responsive neurons (+), but was not detected in any of 11 CQ-unresponsive neurons (−). For a negative control, sample of bath solution was used (Bath); Diluted total DRG cDNA was used as positive control (DRG). Arrows indicate predicted product size for MrgprA3 (150 bp) and β-actin (302 bp). No product was detected in RT- controls from MrgprA3-expressing cells (n=8). (D) and (E) show representative traces from 3 different WT DRG neurons electroporated with siRNAs in calcium imaging assays. (D) CQ-induced increase in [Ca2+]i was completely lost in WT neurons electroporated with MrgprA3 siRNA. However, these neurons (normally express both MrgprA3 and MrgprC11) are still sensitive to BAM8-22 (BAM). 24 BAM8-22 sensitive neurons were analyzed. (E) As a control, CQ responsiveness in WT neurons electroporated with MrgprC11 siRNA remained intact (10 CQ sensitive neurons analyzed). But MrgprC11 siRNA completely abolished BAM8-22 sensitivity. (F) The efficiency and specificity of MrgprA3 siRNA were tested by co-transfecting HEK293 cells with MrgprA3 siRNA and expression constructs of MrgprA3 or MrgprC11. Western blot shows that MrgprA3 siRNA specifically knocked-down the expression of MrgprA3, but not MrgprC11. (G–M) Mrgpr A3 and MrgprX1 selectively rescued CQ responsiveness in Mrgpr-clusterΔ−/− DRG neurons. (G) Visualization of Mrgpr-clusterΔ−/− DRG neurons that express MrgprA3-GFP protein. Note the membrane and axon localization (arrowheads) of MrgprA3-GFP in DRG neurons. (H) All Mrgpr-clusterΔ−/− neurons electroporated with MrgprA3 fired a train of APs upon CQ treatment (n=6). (I) Fewer than half of MrgprA1-electroporated neurons (3 out of 7) elicited a few APs upon CQ treatment. (J) Most Mrgpr-clusterΔ−/− neurons electroporated with MrgprX1 (5 out of 7 GFP-positive neurons recorded) also generated a train of APs in response to CQ. (K–M) Each figure shows typical calcium traces from three different neurons. (K) All MrgprA3-expressing Mrgpr-clusterΔ−/− neurons showed increased [Ca2+]i in response to CQ (1 mM) but not BAM8-22 (2 µM). (L) All MrgprA1-electroporated mutant neurons showed a strong response to FMRF (2 µM) whereas only a small portion responded to CQ. (M) Electroporation of MrgprX1 rendered Mrgpr-clusterΔ−/− DRG neurons sensitivity to both CQ and BAM8-22.
To determine whether the expression of MrgprA3 in DRG neurons correlates with CQ sensitivity (also 4–5%), we performed single cell RT-PCR for the gene on individual DRG neurons responsive to CQ as determined by calcium imaging. 8 of 9 CQ-responding neurons expressed MrgprA3 mRNA whereas none of 11 CQ-insensitive neurons showed detectable levels of the receptor transcript (Figure 6C). Since MrgprA3 expression in mouse DRG neurons correlates very well with CQ sensitivity, we examined whether specific knock-down of MrgprA3 would abolish CQ responsiveness. Strikingly, WT DRG neurons failed to respond CQ after electroporation with siRNA specifically targeted against MrgprA3, whereas a control siRNA had no effect on CQ sensitivity (Figure 6D–F). These data strongly suggest that MrgprA3 is the main receptor mediating CQ-evoked responses in mice. Unlike mouse MrgprA subfamily which consists of 22 members, rats have only one MrgprA. Consistently, using single neuron RT-PCR technique, we found that all of CQ-sensitive rat DRG neurons express rat MrgprA (n=10).
MrgprA3 and MrgprX1 rescue the phenotypes of Mrgpr-deficient neurons
We next asked whether MrgprA3 or MrgprX1 can rescue the phenotypes of DRG neurons from Mrgpr-clusterΔ−/− mice. To answer this question, we electroporated the Mrgpr expression constructs used in the heterologous studies into dissociated adult DRG neurons from these mice. After 24 hour in culture, expression and membrane localization of the transfected Mrgprs in the mutant neurons could be readily visualized by GFP (Figure 6G). Strikingly, all MrgprA3-expressing mutant neurons generated numerous APs in response to CQ treatment (Figure 6H) whereas neighboring GFP-negative neurons remained silent (n=6, not shown). The number of APs generated in the GFP-positive neurons was comparable to that produced by CQ treatment of WT DRG neurons, indicating a nearly complete rescue by MrgprA3. Similar results were obtained for Mrgpr-deficient neurons electroporated with MrgprX1 (Figure 6J). In contrast, fewer than half of the MrgprA1-electroporated neurons elicited a few APs in response to CQ (Figure 6I). Rescue by MrgprA3 and MrgprX1 was also seen using calcium imaging, with an increase in [Ca2+]i induced by CQ (Figure 6K–M). Together these results strongly suggest that mouse MrgprA3 and human MrgprX1 are the major CQ receptors in DRG neurons. Expression of rat MrgprA in Mrgpr-clusterΔ−/− DRG neurons conferred CQ sensitivity upon them whereas rat MrgprC did not (Figure S2D, E).
CQ-sensitive neurons also respond to histamine and capsaicin
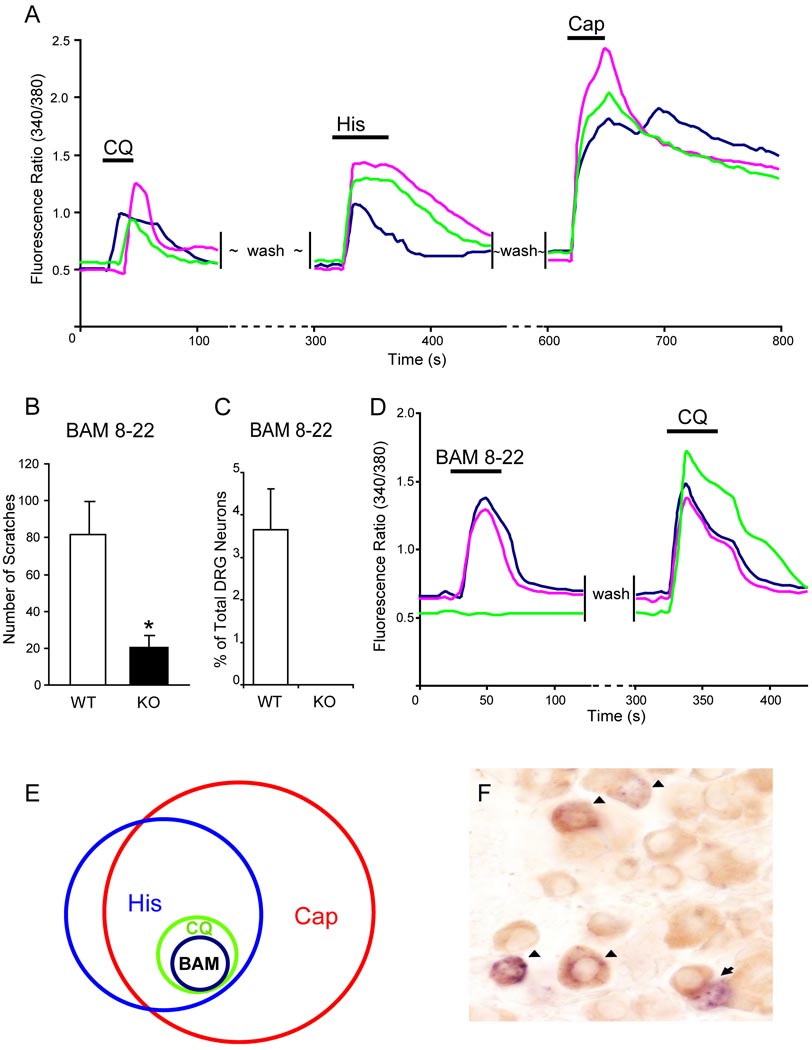
To further define the population of CQ-sensitive neurons in DRG, we examined the responses of these cells to other well-characterized chemicals. Many studies utilizing multiple approaches have shown that histamine- and capsaicin-responding cells largely overlap (Schmelz et al., 2003; Shim et al., 2007). Consistent with previous reports, we found that 87% of histamine-sensitive DRG neurons also responded to capsaicin as monitored by an increase in [Ca2+]i using calcium imaging. Interestingly, all CQ-responding neurons in DRG cultures were also activated by both histamine and capsaicin (Figure 7A). Furthermore, we noticed that CQ-sensitive cells have a narrow range of cell diameters whereas histamine-sensitive neurons have a wide range (Figure S3). Therefore, the small population of CQ-sensitive neurons in WT DRG defines a unique and specific subset of histamine- and capsaicin-sensitive neurons.
Figure 7.
CQ-responsiveness defines a specific subpopulation of DRG neurons. (A) CQ-responsive neurons represented a small population of DRG neurons that also responded to histamine (50 µM) and capsaicin (1 µM) with increased [Ca2+]i monitored by calcium imaging. (B) The total scratching bouts during the first 30 min after BAM8-22 intradermal injection (50 µI of 1 mM). WT mice exhibited significantly stronger scratching responses after injection than Mrgpr-clusterΔ−/− littermates did (n=8 per genotype; * p<0.05). (C) As determined by calcium imaging, 3.6% of WT DRG neurons responded to BAM8-22 (2 µM) with increased [Ca2+]i and all of them are also CQ-sensitive (D), whereas Mrgpr-clusterΔ−/− DRG neurons failed to respond to the drug (n=3 per genotype) (C). (E) The Venn diagram illustrates the relationships of histamine- (His), capsaicin- (Cap), chloroquine- (CQ), and BAM8-22- (BAM) responsive neurons in adult DRG. The sizes of the circles are proportional to the sizes of the cell populations. (F) WT adult DRG sections were doubly stained by in situ hybridization for MrgprA3 (blue) and immunostaining using anti-GRP antibody (brown). Most MrgprA3+ cells (51 out of 55) express GRP. Arrowheads indicate MrgprA3/GRP co-expressing neurons. Arrows indicate MrgprA3+/GRP− cells.
MrgprA3-expressing neurons are likely itch-selective neurons
The finding that MrgprA3-positive neurons are sensitive to both histamine and CQ raises the interesting possibility that these neurons are itch-selective neurons. Gastrin-releasing peptide (GRP), a ligand for GRPR, is expressed in a subset of DRG neurons (Sun and Chen, 2007). To look for overlap between GRP and MrgprA3 expression in DRG neurons, we carried out double staining experiments for these two genes. Strikingly, 93% of MrgprA3-positive neurons also expressed GRP, providing strong evidence that MrgprA3-expressing neurons may play important roles in itch sensation (Figure 7F and Figure S4).
Our previous data have shown that expression of MrgprC11 largely overlaps with that of MrgprA3 (Zylka et al., 2003). Consistently, all BAM8-22-responsive neurons (i.e. 3.6% of total WT DRG neurons) also responded to CQ (Figure 7C–E). Importantly, no DRG neurons from Mrgpr-clusterΔ−/− mice responded to BAM8-22 (Figure 7C). As we expected, intradermal injection of BAM8-22 induced strong scratching behavior in WT mice whereas mutant mice exhibited a dramatic reduction in the response evoked by the peptide (Figure 7B). Together these data suggest that activation of MrgprA3- or MrgprC11-expressing neurons by its specific agonist (i.e. CQ and BAM8-22, respectively) can evoke scratching behavior and further support that these neurons are involved in itch sensation.
DISCUSSION
It has been known for a long time that both pain and itch are initiated and modulated by small-diameter sensory neurons in DRG (Caterina and Julius, 1999; Ikoma et al., 2006; Shim and Oh, 2008). Compared to pain, our knowledge of itch especially histamine-independent itch at cellular and molecular levels is poor. Here we present evidence showing that sensory neuron-specific Mrgprs are receptors mediating CQ-induced itch.
Mrgpr-clusterΔ−/− mice exhibit a severe reduction in CQ-induced scratching behavior whereas histamine-mediated itch and acute pain are completely normal. The residual CQ-induced scratching behavior seen in mutant animals is likely due to an indirect effect on skin sensory nerves. Both our behavioral data from SASH mice and complete elimination of CQ-sensitive neurons in Mrgpr-clusterΔ−/− DRG support this notion and suggest that the residual CQ-induced response in Mrgpr-clusterΔ−/− mice results from degranulation of skin mast cells caused by the drug, a phenomenon observed in previous studies (Green and Lim, 1989; Nosal et al., 1991).
Since Mrgpr members are highly homologous to each other, especially the MrgprA subfamily (70%-80% identity), it is surprising to find that only MrgprA3 shows strong activation by CQ. The most divergent regions of MrgprAs are localized to the extracellular loops consistent with the differences in their ligand preferences (Dong et al., 2001; Yang et al., 2005). Bioinformatic analysis of Mrgpr sequences suggest that positive selection likely accounts for the amino acid substitutions in the extracellular domains (Choi and Lahn, 2003; Yang et al., 2005). Interestingly, human MrgprX1 can respond to both CQ and BAM8-22 while mouse MrgprA3 and MrgprC11 are specific receptors for these two agonists, respectively. The agonist selectivity of the mouse receptors supports the conclusion made from statistical analysis that adaptive evolution of Mrgpr family contributes to its expansion in the mouse genome.
According to our dose-response curves, EC50s of CQ for MrgprA3 and human MrgprX1 are 27.55 ± 2.03 and 297.68 ±2.10 µM, respectively. Although the concentration of CQ in patient plasma is in the micromolar range, excretion of the drug is quite slow and it is deposited in tissues in considerable amounts (Adam, 2004; Evans et al., 2005; Onyeji and Ogunbona, 2001). Since CQ binds strongly to melanin that is synthesized by melanocytes, it accumulates at very high levels in the skin and other pigmented tissues to reach high micromolar to millimolar concentrations (Dencker et al., 1975; Olatunde, 1971; Sams and Epstein, 1965; Tanaka et al., 2004). The high level of CQ (i.e. high micromolar to millimolar concentrations) is also required to induce scratching behavior in mice based on our and other group’s dose-response studies (data not shown; Green et al., 2006). In addition, patients prone to CQ-induced itch accumulate higher concentrations of CQ in their skin than those not prone to the side effect (Olatunde, 1971). The different levels of CQ in the skin of the two groups are likely due to different rates of metabolism of the drug (Onyeji and Ogunbona, 2001). Besides the level of CQ in the skin, mouse strain comparison and human familial clustering of itch studies suggest that genetic variability also contributes to phenotypic differences in CQ-induced itch (Ajayi et al., 1989; Green et al., 2006). The high polymorphism seen in both mouse and human Mrgpr genes may provide a molecular explanation for the variability in itch levels among different individuals (Dong et al., 2001; Yang et al., 2005).
Our heterologous studies indicate that MrgprA3 is the major receptor for CQ among the twelve deleted Mrgprs. Other Mrgprs excluded in the cluster deletion are unlikely involved in CQ signaling in DRG neurons because of the total loss of CQ response in mutant DRG. Consistently, the percentage of CQ-sensitive DRG neurons (i.e. 4–5% of total DRG neurons) matches that of MrgprA3-expressing cells determined by in situ hybridization on adult DRG sections (Liu et al., 2008). More importantly, our single neuron RT-PCR results indicate that MrgprA3 expression correlates almost perfectly to CQ responsiveness. Furthermore, both gain-and loss-of-function studies firmly establish that MrgprA3 is required for CQ responsiveness in mice.
This small population of CQ-sensitive neurons marks a subset of histamine- and capsaicin-responsive cells in DRG and it has a uniform cell size as compared to the total histamine-sensitive population. According to different reports, the percentage of histamine-sensitive cells in the DRG ranges from 15% to 40% (Han et al., 2006; Kim et al., 2004; Nicolson et al., 2002). It is unlikely that all of these cells are pruriceptive neurons. In fact, human microneurography studies suggest the sensory fibers that respond to histamine with sustained discharges are responsible for itch whereas those weakly activated by histamine are involved in pain processing (Schmelz et al., 1997). The strong histamine-responsive fibers comprise only a small portion of all unmyelinated sensory fibers and the majority of them are heat-responsive. Recent studies have shown that TRPV1, a molecular sensor for capsaicin and heat, functions downstream of histamine receptors and is required for histamine-induced DRG neuron activation and itch behavior (Shim et al., 2007). These studies also raise the interesting possibility that a subset of capsaicin- and heat-sensitive neurons mediates itch. Therefore, it would be important to know whether the 4–5% of total DRG neurons activated by CQ is selective for itch. Activation of CQ-sensitive neurons by BAM8-22 through MrgprC11 also induces scratching response, providing further evidence that CQ-sensitive neurons may be itch-selective neurons. Finally, overlap between MrgprA3- and GRP-expressing neurons in DRG leads us to propose a model for CQ signal transduction: CQ directly activates a subset of primary sensory fibers in the skin through MrgprA3. This leads to the release of GRP into the dorsal horn of the spinal cord where it activates a subset of dorsal horn neurons through GRPR. Therefore, the identification of Mrgprs as receptors for CQ may open new avenues for the exploration of itch-selective neuron development and function as well as developing novel anti-itch drugs.
EXPERIMENTAL PROCEDURES
Generation of Mrgpr-clusterΔ−/− mice
To delete a cluster of Mrgpr genes in the mouse germline, we constructed two replacement vectors for MrgprA1 and MrgprB4, which reside on either end of the Mrgpr cluster. The entire open reading frames (ORFs) of both MrgprA1 and MrgprB4 are encoded by a single exon. The arms of the MrgprA1 and MrgprB4 targeting constructs were obtained by PCR amplification from 129/SvJ genomic DNA using Expand High Fidelity PCR System (Roche). Details of the generation of Mrgpr-clusterΔ−/− mice are available in the Supplemental Data.
Behavioral studies
All behavioral tests were performed with an experimenter blind to genotype. The mice were 2 to 3 month-old males (20–30 g) that had been backcrossed to C57Bl/6 mice for at least ten generations. Rat studies were done using four week old CD® animals (Charles River Laboratories). All experiments were performed under the protocol approved by the Animal Care and Use Committee of Johns Hopkins University School of Medicine. Pruritic compounds (i.e. histamine, compound 48/80, and CQ) were subcutaneously injected into the nape of the neck after acclimatization and scratching behavior was observed for 30 min. A bout of scratching was defined as continuous scratch movements with hind paws directed at the area around the injection site. Scratching behavior was quantified by recording the number of scratching bouts at 5 min intervals over the 30 min observation period. Details for other behavior assays are available in the Supplemental Data.
Cultures of dissociated DRG neurons
DRG from all spinal levels of 4-week old mice or rats were collected in cold DH10 medium and treated with enzyme solution at 37°C. Following trituration and centrifugation, cells were resuspended in DH10, plated on glass cover slips coated with poly-D-lysine and laminin, cultured in an incubator at 37°C and used within 24 hours. Details are available (see Supplemental Data).
Electroporation of dissociated adult DRG neurons with Mrgpr-expression constructs was carried out using Mouse Neuron Nucleofector Kit (Amaxa Biosystems) following the manufacturer’s instructions. Electroporated neurons were plated and cultured as described above.
Calcium imaging
Neurons or HEK293 cells were loaded with fura 2-acetomethoxy ester (Molecular Probes) for 30 min in the dark at room temperature or 45 min at 37°C, respectively. After washing, cells were imaged at 340 and 380 nm excitation to detect intracellular free calcium. Calcium imaging assays were performed with an experimenter blind to genotype. Each experiment was done at least three times and at least 100 cells (neurons or HEK293 cells) were analyzed each time.
RNA interference
MrgprA3 and MrgprC11 on-target siRNAs were purchased from Thermo Scientific. 0.175 nmol MrgprA3 or MrgprC11 siRNA was electroporated into WT DRG neurons, respectively. After three days culture, neurons were re-plated on glass coverslips for calcium imaging. HEK293 cells in 12-well plate were co-transfected with 2 µg Mrgpr-expression constructs and 2 µg siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. After one day culture, cells were lysated for western blot.
Whole-cell current-clamp recordings of cultured DRG neurons
Neurons plated on cover slips were transferred into a chamber with the extracellular solution. Patch pipettes had resistances of 2–4 MΩ. In current clamp recordings, action potential measurements were performed with an Axon 700B amplifier and the pCLAMP 9.2 software package (Axon Instruments). Neurons were perfused with 1 mM CQ for 20 sec. All experiments were performed at room temperature (~25°C). Details are available in the Supplemental Data.
In situ hybridization and immunostaining
In situ hybridization was performed as previously described (Dong et al., 2001). For MrgprA3 and GRP double staining, after in situ hybridization for MrgprA3, DRG sections were incubated overnight at 4°C with Rabbit anti-GRP antibody (Immunostar, 1:1000) in PBS/1% donkey serum/0.3% Triton X-100. After washing twice in PBS, sections were incubated with biotinylated secondary antibody (Jackson, 1:200) for 2 hours at RT and then washed twice in PBS and incubated with ABC mix (Elite ABC kit, Vectastain) in PBS at RT for 1 hour. Sections were washed twice in PBS and incubated with 3,3'-Diaminobenzidine/H2O2 in PBS for color development.
Single cell RT-PCR
Plated neurons with CQ responsiveness established by calcium imaging were individually harvested into a glass pipette and transferred into PCR tube. Reverse transcription was done by Super-Script III CellsDirect (Invitrogen) as previously described (Kwong et al., 2008). Details for PCR conditions are available in the Supplemental Data.
Data analysis
Data are presented as mean ± standard error of mean (S.E.M). Statistical comparisons were made using unpaired Student’s t-test and differences were considered significant at p < 0.05.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Shirley Pease and the staff of the Caltech GEMS facility for assistance with ES cell manipulation. We also thank M. Ringkamp and R. Meyer for helpful comments on the manuscript. The work was supported by Alfred P. Sloan Neuroscience grant, Whitehall Foundation grant, Blaustein Pain Research Fund award, and grants from the NIH to X.D. (NS054791), B.J.U. (HL038095), M.K. (DK074480), Z.F.C. (AR056318), and D.J.A. (NS048499). L.S. is supported by VEGA 1/0018/08. D.J.A and X.D. are an investigator and an Early Career Scientist of the Howard Hughes Medical Institute, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abila B, Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, Asomugha L. Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. Afr J Med Med Sci. 1994;23:139–142. [PubMed] [Google Scholar]
- Adam ME, Karim EF, Elkadaru AY, Ibrahim KE, Berger BJ, Wiese M, Babiker HA. Imipramine induced complete reversal of chloroquine resistance in plasmodium falciparum infections in Sudan. Saudi Pharmaceutical Journal. 2004;12:130–135. [Google Scholar]
- Ademowo OG, Walker O, Sodeinde O. Prevalence of chloroquine-induced pruritus: evidence for heredofamilial factors. Malaria Infect Dis Afr. 1998;8:14–18. [Google Scholar]
- Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol. 1989;37:539–540. doi: 10.1007/BF00558141. [DOI] [PubMed] [Google Scholar]
- Alving K, Sundstrom C, Matran R, Panula P, Hokfelt T, Lundberg JM. Association between histamine-containing mast cells and sensory nerves in the skin and airways of control and capsaicin-treated pigs. Cell Tissue Res. 1991;264:529–538. doi: 10.1007/BF00319042. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- Choi SS, Lahn BT. Adaptive evolution of MRG, a neuron-specific gene family implicated in nociception. Genome Res. 2003;13:2252–2259. doi: 10.1101/gr.1431603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, Giesler GJ., Jr The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker L, Lindquist NG, Ullberg S. Distribution of an 125I-labelled chloroquine analogue in a pregnant macaca monkey. Toxicology. 1975;5:255–265. doi: 10.1016/0300-483x(75)90122-5. [DOI] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Evans JA, May J, Tominski D, Eggelte T, Marks F, Abruquah HH, Meyer CG, Timmann C, Agbenyega T, Horstmann RD. Pre-treatment with chloroquine and parasite chloroquine resistance in Ghanaian children with severe malaria. Qjm. 2005;98:789–796. doi: 10.1093/qjmed/hci121. [DOI] [PubMed] [Google Scholar]
- Ezeamuzie CI, Igbigbi PS, Asomugha L, Ambakederemo AW, Abila B, Assem ES. Urine methylhistamine concentrations before and after chloroquine in healthy black subjects. J Trop Med Hyg. 1990;93:423–425. [PubMed] [Google Scholar]
- Fujita F, Moriyama T, Higashi T, Shima A, Tominaga M. Methyl p-hydroxybenzoate causes pain sensation through activation of TRPA1 channels. Br J Pharmacol. 2007;151:153–160. doi: 10.1038/sj.bjp.0707219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Merchan R, Crespo JF, Rodriguez J. Allergic urticaria from tonic water. Allergy. 2002;57:52. doi: 10.1046/j.0105-4538.2001.00001.x-i10. [DOI] [PubMed] [Google Scholar]
- Green AD, Young KK, Lehto SG, Smith SB, Mogil JS. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain. 2006;124:50–58. doi: 10.1016/j.pain.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Green KB, Lim HW. Effects of chloroquine on release of mediators from mast cells. Skin Pharmacol. 1989;2:77–85. doi: 10.1159/000210804. [DOI] [PubMed] [Google Scholar]
- Han SK, Dong X, Hwang JI, Zylka MJ, Anderson DJ, Simon MI. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci U S A. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Friedman RM, Greenquist KW, Shim B, Borzan J, Hartke T, LaMotte RH, Ringkamp M. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Meyer RA, Hartke T, Hobelmann JG, Maine DN, LaMotte RH, Ringkamp M. Psychophysical and physiological evidence for parallel afferent pathways mediating the sensation of itch. J Neurosci. 2007;27:7490–7497. doi: 10.1523/JNEUROSCI.1249-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanny G, Flabbee J, Morisset M, Moneret Vautrin DA. Allergy to quinine and tonic water. Eur J Intern Med. 2003;14:395–396. doi: 10.1016/s0953-6205(03)90011-2. [DOI] [PubMed] [Google Scholar]
- Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Kuraishi Y, Nagasawa T, Hayashi K, Satoh M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur J Pharmacol. 1995;275:229–233. doi: 10.1016/0014-2999(94)00780-b. [DOI] [PubMed] [Google Scholar]
- Kwong K, Kollarik M, Nassenstein C, Ru F, Undem BJ. P2X2 receptors differentiate placodal vs. neural crest C-fiber phenotypes innervating guinea pig lungs and esophagus. Am J Physiol Lung Cell Mol Physiol. 2008;295:L858–L865. doi: 10.1152/ajplung.90360.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O'Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, et al. Proenkephalin A gene products activate a new family of sensory neuron--specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yang FC, Okuda T, Dong X, Zylka MJ, Chen CL, Anderson DJ, Kuner R, Ma Q. Mechanisms of compartmentalized expression of Mrg class G-protein-coupled sensory receptors. J Neurosci. 2008;28:125–132. doi: 10.1523/JNEUROSCI.4472-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyika KS, Kihamia CM. Chloroquine-induced pruritus: its impact on chloroquine utilization in malaria control in Dar es Salaam. J Trop Med Hyg. 1991;94:27–31. [PubMed] [Google Scholar]
- Nakayama Y, Mio M, Sugimoto Y, Fujii Y, Kamei C. Changes in membrane potential induced by compound 48/80 in the peritoneal mast cells of rats. Methods Find Exp Clin Pharmacol. 2002;24:267–273. doi: 10.1358/mf.2002.24.5.802303. [DOI] [PubMed] [Google Scholar]
- Nicolson TA, Bevan S, Richards CD. Characterisation of the calcium responses to histamine in capsaicin-sensitive and capsaicin-insensitive sensory neurones. Neuroscience. 2002;110:329–338. doi: 10.1016/s0306-4522(01)00561-9. [DOI] [PubMed] [Google Scholar]
- Nosal R, Drabikova K, Pecivova J. Effect of chloroquine on isolated mast cells. Agents Actions. 1991;33:37–40. doi: 10.1007/BF01993121. [DOI] [PubMed] [Google Scholar]
- Olatunde A. The practical and therapeutic implications of chloroquine-induced itching in tropical Africa. Afr J Med Med Sci. 1977;6:27–32. [PubMed] [Google Scholar]
- Olatunde IA. Chloroquine concentrations in the skin of rabbits and man. Br J Pharmacol. 1971;43:335–340. [PMC free article] [PubMed] [Google Scholar]
- Onyeji CO, Ogunbona FA. Pharmacokinetic aspects of chloroquine-induced pruritus: influence of dose and evidence for varied extent of metabolism of the drug. Eur J Pharm Sci. 2001;13:195–201. doi: 10.1016/s0928-0987(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Paus R, Schmelz M, Biro Tamas, Steinhoff M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J. Clin. Invest. 2006;116:1174–1185. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sams WM, Jr, Epstein JH. The affinity of melanin for chloroquine. J Invest Dermatol. 1965;45:482–487. doi: 10.1038/jid.1965.162. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmidt R, Bickel A, Handwerker HO, Torebjork HE. Specific C-receptors for itch in human skin. J Neurosci. 1997;17:8003–8008. doi: 10.1523/JNEUROSCI.17-20-08003.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M, Schmidt RC, Weidner C, Hilliges M, Torebjork HE, Handwerker HO. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J. Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- Shim WS, Oh U. Histamine-induced itch and its relationship with pain. Mol Pain. 2008;4:29. doi: 10.1186/1744-8069-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, Tak MH, Lee MH, Kim M, Kim M, Koo JY, Lee CH, Kim M, Oh U. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowunmi A, Fehintola FA, Adedeji AA, Falade AG, Falade CO, Akinyinka OO, Oduola AM. Comparative efficacy of chloroquine plus chlorpheniramine alone and in a sequential combination with sulfadoxine-pyrimethamine, for the treatment of acute, uncomplicated, falciparum malaria in children. Ann Trop Med Parasitol. 2000;94:209–217. doi: 10.1080/00034980050006375. [DOI] [PubMed] [Google Scholar]
- Steinhoff M, Neisius U, Ikoma A, Fartasch M, Heyer G, Skov PS, Luger TA, Schmelz M. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takashina H, Tsutsumi S. Comparative assessment of ocular tissue distribution of drug-related radioactivity after chronic oral administration of 14C-levofloxacin and 14C-chloroquine in pigmented rats. J Pharm Pharmacol. 2004;56:977–983. doi: 10.1211/0022357043932. [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Tsujimura T, Morii E, Isozaki K, Onoue H, Nomura S, Kitamura Y. C-kit gene is expressed by skin mast cells in embryos but not in puppies of Wsh/Wsh mice: age-dependent abolishment of c-kit gene expression. Blood. 1994;83:3509–3516. [PubMed] [Google Scholar]
- Yang S, Liu Y, Lin AA, Cavalli-Sforza LL, Zhao Z, Su B. Adaptive evolution of MRGX2, a human sensory neuron specific gene involved in nociception. Gene. 2005;352:30–35. doi: 10.1016/j.gene.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.