Abstract
PURPOSE
To determine whether there are systematic changes in pupil location with changes in the state of pupil size and with other ocular variables.
METHODS
High-resolution images of the pupil of the eyes of 70 subjects were taken using an infrared-sensitive camera. Images were obtained under mesopic, photopic, and pharmacologically dilated conditions. From the images, the center and diameter of the corneal limbus and the pupil were computed. In addition, the location of the first Purkinje image was calculated.
RESULTS
The pupil center shifted consistently temporally as the pupil dilated. The total motion was relatively small, with a mean distance of 0.133 mm motion between the mesopic and photopic conditions, with the pupil diameter changing from 6.3 to 4.1 mm. Ninety percent of the subjects had a motion of less than 0.3 mm. One patient showed a motion of almost 0.6 mm. The change in location of the pupil center was not significantly related to refractive error, age, or the change of pupil diameter.
CONCLUSIONS
Changes in the location of the pupil center with changes in the dilation of the pupil are typically slight, but can be significant in a few subjects, especially in pharmacologically dilated pupils.
The pupil of the eye is a critical limiting factor in the optics of the visual system. The pupil, when it changes size, controls not only the amount of light available to the visual system, but also, and just as important, the optics of the eye. The eye has numerous optical aberrations1–6 that increase as the pupil dilates.7–9 The exact size and location of the pupil are important in corneal refractive surgery.9–11 For instance, in a typical LASIK procedure, the cornea is reshaped to provide an appropriate refractive correction in the optical zone, usually 5 to 6.5 mm in diameter. Beyond this optical zone is a transition zone to the peripheral cornea that has not been ablated. The relationship between the size and location of the optical zone and the pupil is critical for a successful surgical outcome. For instance, if the corneal ablation is well centered over the pupil of small diameter but becomes decentered with pupils of large diameter, then the patient may experience glare, halos, or ghost images in low illumination.
There have been several studies of the change in centration of the pupil with changing size. Walsh12 used a photographic technique to measure the changes between light-adapted, dark-adapted, and pharmacologically dilated conditions. In 39 subjects, he found an average movement of approximately 0.19 mm, with the center of the pupil moving slightly nasally and superiorly as the pupil constricted. Wilson et al.13 used a video apparatus to examine the horizontal changes in the pupil center with natural dilation in eight subjects. They found larger shifts in most subjects (up to 0.59 mm), with movement occurring in different directions in different subjects, although they used the achromatic axis of the eye as the reference position, rather than the limbus center used by Walsh,12 and therefore an exact comparison of direction is not possible. Wyatt14 used a modified slit lamp to examine the change in centration with natural dilation in 23 subjects and similar to Walsh12 found that the center of the pupil moved nasally and superiorly to the limbus, with an average movement of approximately 0.1 mm with constriction. These studies did not investigate the role of refractive status or age on the movements measured, and it is possible that these factors can explain some of the differences in results. Younger subjects with their larger absolute change in pupil size15 with dilation may show larger shifts.
If there are large changes in the pupil center with changes in size, there could be relatively large changes in the refractive state of the eye,16 because the curvature of the cornea changes with location. Similarly, with the advent of modern wave-front sensing technology for determining the aberrations of the eye,5,17–20 the location of the pupil becomes an important factor in determining effective optics.21,22 It is now generally accepted23 that the line from the fovea through the center of the pupil should be used in calculating the aberrations of the eye.23,24 However, if there are large changes in the location of the pupil center with changes in pupil size, this axis becomes less valuable, because its location depends on the measurement conditions. In the current study, we measured the location of the pupil, relative to the corneal limbus center of normal eyes under mesopic, photopic, and pharmacologically dilated conditions, by using an infrared pupillometer that is incorporated into the InterWave Scanner, a modification of the spatially resolved refractometer (SRR)18,25 wave-front sensing device. We also determined the location of the first Purkinje image (the reflection of anterior corneal surface) within the same coordinate system and using the same images as the limbus and pupil measurements.
METHODS
Apparatus
The InterWave sensor is an updated, solid-state version of the spatially resolved refractometer (SRR).18,25 It can measure pupil size by imaging the eye under infrared illumination using its pupillometer subsystem. This pupillometer has three major components: an infrared-sensitive video camera, a coaxial illuminator, and a series of six eccentric illuminators. The camera and the coaxial illuminator (an infrared LED) are aligned by a beam splitter and arranged so that the camera images the focal plane of the final lens of the SRR (which is the location of the eye’s pupil). The coaxial LED is located so that the final lens of the pupillometer collimates light from the LED, and this light illuminates the eye. Six additional LEDs are arranged in a hexagon around the final lens, so that they directly illuminate the eye from a distance of 100 mm. In addition, the SRR provides a coaxial fixation target, to help keep the eye aligned with the instrument.
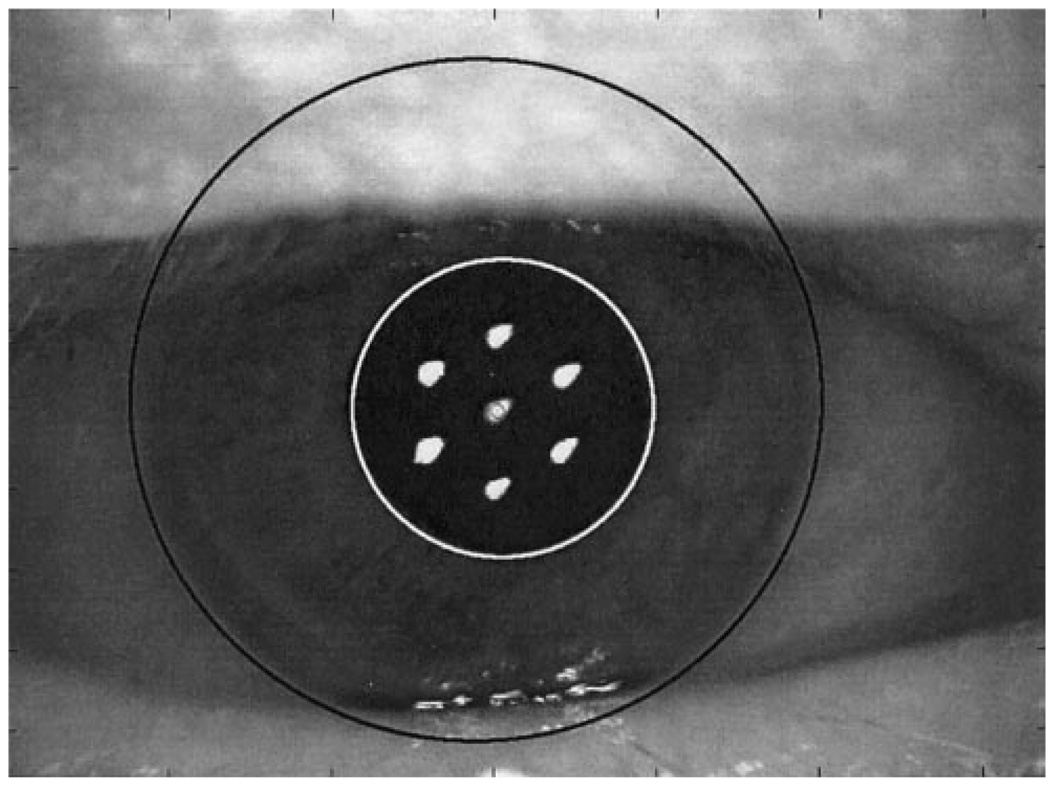
Figure 1 shows a typical pupil image, together with the superimposed estimates of the locations and radii for the corneal limbus, pupil margin, and the first Purkinje image. This system produces a high-magnification image of the eye, from which the corneal limbus, the pupil margins, and the first Purkinje image are readily identified.
FIGURE 1.
An external image of an eye, obtained with the pupillometer. Superimposed on the image are circles, fit using the method described to characterize the position and the size of the limbus and pupil. Also evident is the location of the first Purkinje image.
Subjects
The study sample consisted of 70 individuals (130 eyes) tested from April 25, 2001, through July 2, 2001, at Emory Vision Correction Center, including 31 females and 39 males, in whom 66 right eyes and 64 left eyes were tested. The mean age of the subjects was 44.6 ± 10.9 years (range, 12–70). The mean spherical equivalent refraction of the subjects was −2.61 ± 3.58 D (range, −11.00 to +6.63). The spherical error ranged from −11.00 to +5.25 D and the cylindrical error from 0 to +6.50 D. Most of subjects planned to have LASIK. None had a record of any other anterior ocular disease or anatomic abnormality.
All data were collected after informed consent was obtained from the subjects, and their participation was in accordance with the tenets of the Declaration of Helsinki.
Image Capture
At the start of each test session the technician aligned the subject to the apparatus by moving the instrument (using an X-Y-Z positioning stage) until the subject’s pupil was approximately centered in the video monitor and the image of the eye was in best focus. The pupil imaging was performed first under mesopic conditions with room lights off, then under photopic conditions with room lights on. After these two measurements were obtained, the eye was dilated (1 drop 1% cyclopentolate), and the same measurements were performed on the dilated pupil after approximately 30 minutes. Images of the eye were captured from the infrared camera for each condition, with a video frame-grabber under control computer. The three images were stored for later analysis.
Image Analysis
Images were analyzed using a custom computer program (Matlab; Mathworks, Inc., Natick, MA). Each image was displayed on a computer monitor at high magnification. The computer’s mouse was used to identify a series of locations along the edge of the limbus, marking at least six points and distributing them over as wide a region of the limbus as possible. A circle was then fit to these points, using a least-square error criterion, and the circle was superimposed on the image of the eye (Fig. 1). Similarly, the margins of the pupil and the location of the first Purkinje image were determined. All the sample images were analyzed by one of the authors, after a training session.
Repeatability of Scoring
To determine the repeatability of the scoring, data from 10 eyes (chosen randomly) were selected, and each eye was scored 10 times, as described.
Data Analysis
The results from the fittings of the corneal and pupillary diameters, the center locations (X, Y) of the limbus, the pupil, and the first Purkinje image were recorded by the computer. From these base data we calculated the center locations for the pupil and first Purkinje image relative to the geometric corneal center using standard geometric formulas. We corrected the frame-grabber coordinates to nasal–temporal coordinates. All statistical tests were performed on computer (StatView software, ver. 4.5; SAS Institute, Cary, NC).
RESULTS
Repeatability of Scoring
The scoring method used was highly reproducible. As an example, the mean SD of 10 measurements for each of the 10 eyes of the distance between the geometric corneal center and the pupil center (mesopic conditions) was 0.039 mm. Other measurement parameters were replicated with similar reproducibility.
Corneal Diameter
Corneal diameter varied between 11.18 and 14.36 mm in all eyes (mean 12.23 ± 0.46 mm). As expected, there were no significant differences in corneal diameter among the mesopic, photopic, and dilated conditions (paired t-test, n = 130, P > 0.05). The corneal diameter in the females was slightly smaller than in the males (unpaired t-test, mesopic conditions, t = −2.073, P = 0.040), but there was no correlation between corneal diameter and age (r = 0.09, P = 0.323) or corneal diameter and refractive error (r = 0.014, P = 0.87).
Pupil Diameter
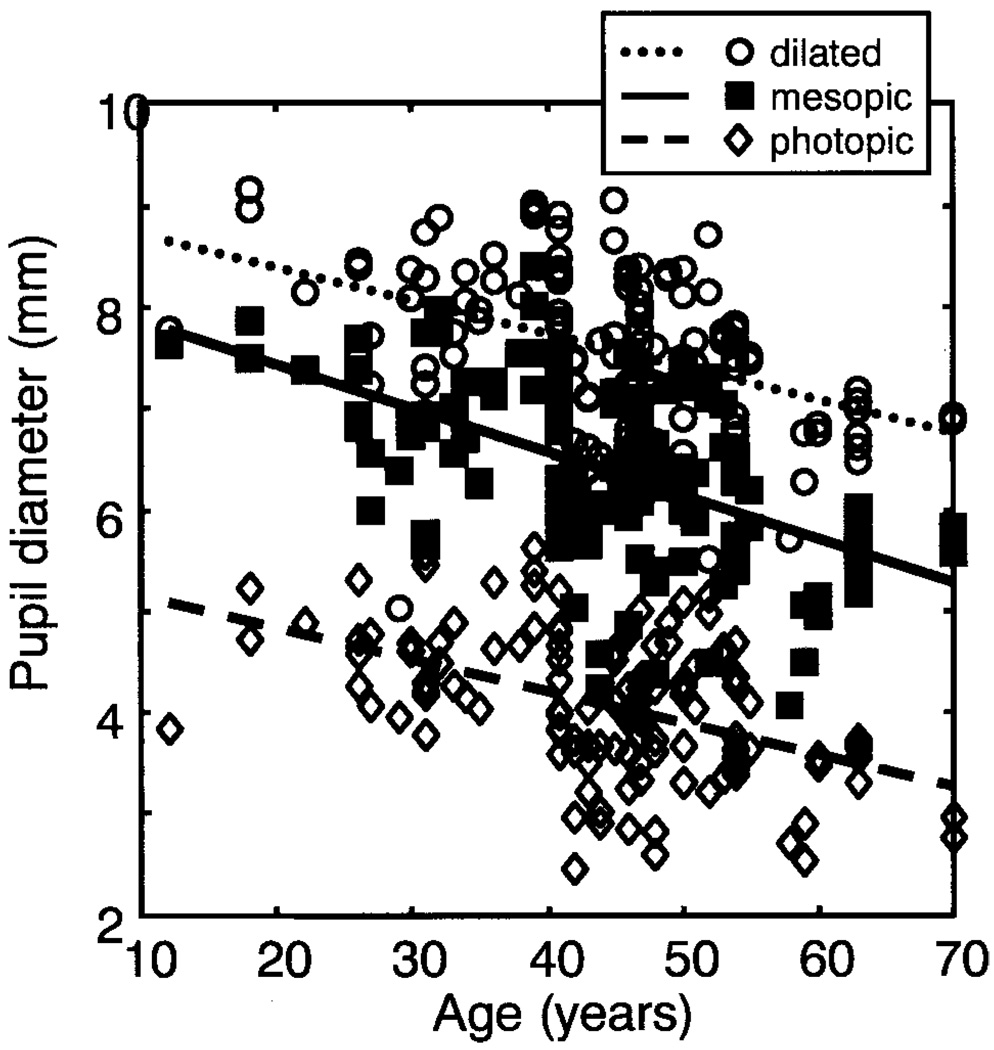
The mean mesopic, photopic, and dilated pupil diameter was 6.37 ± 0.89, 4.06 ± 0.70, and 7.58 ± 0.82 mm, respectively. As expected, there were significant differences between the pupil diameters under the three conditions (paired t-test, P < 0.0001), with negative correlations15,26 between age and pupil diameter under all three lighting conditions (P < 0.0001, Fig. 2). We did not find a significant correlation between pupil diameter and spherical equivalent refractive error when age was taken into account (ANOVA, P = 0.55). There was a significant association between the pupil diameter and the corneal diameter in all three luminance conditions (P < 0.01), and the mean ratio of pupil diameter to corneal diameter under mesopic conditions was 0.52 ± 0.07. The pupil diameter under mesopic conditions in females was not significantly different from that of the males (unpaired t-test, t = −0.71, P = 0.481).
FIGURE 2.
Pupil diameter as a function of age under mesopic (r = 0.523, P < 0.0001), photopic (r = 0.481, P < 0.0001), and dilated (r = 0.430, P < 0.0001) conditions.
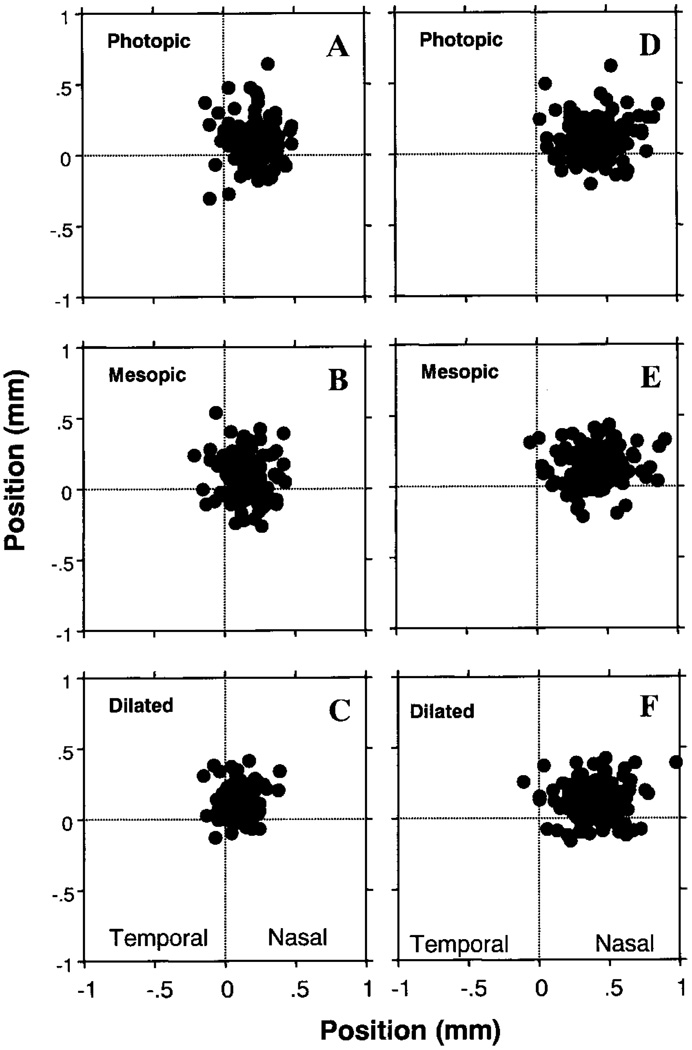
Pupil Center Location
The pupil center locations and their distances relative to the geometric corneal center under mesopic, photopic, and dilated conditions are shown in Table 1 and Figures 3A, 3B, and 3C. There was a small but significant shift of the pupil center location between illumination conditions (paired t-test, P < 0.0001). The pupil center moved nasally from mesopic to photopic conditions (mean distance, 0.13 ± 0.07 mm), and this motion was significant in the left and right eyes treated separately. From mesopic to pharmacologically dilated conditions, the pupil center moved superotemporally (mean distance, 0.162 ± 0.083 mm). This direction was approximately the same as for the movement between photopic and dilated conditions (mean distance moved, 0.183 ± 0.093 mm). No statistically significant correlations were found between the pupil center’s location and age or refraction in all three conditions (P > 0.05). The distance of the pupil center from the corneal center decreased slightly with increasing pupil diameter under mesopic conditions (r = 0.17, P = 0.047). The movements of the pupil center were not significantly correlated with age, refraction, or the change in pupil diameter between conditions (P > 0.05, data not shown).
TABLE 1.
Pupil Center Location and Distance Relative to Geometric Corneal Center
| Horizontal Pupil Location | Vertical Pupil Location | Pupil Distance | |
|---|---|---|---|
| Photopic | 0.202 ± 0.120 | 0.087 ± 0.151 | 0.272 ± 0.113 |
| Mesopic | 0.148 ± 0.123 | 0.083 ± 0.146 | 0.232 ± 0.107 |
| Dilated | 0.103 ± 0.092 | 0.124 ± 0.108 | 0.192 ± 0.095 |
Positive values of location represent superior and nasal directions relative to geometric corneal center. Data are expressed as the mean (millimeters) ± SD.
FIGURE 3.
Pupil center and Purkinje image locations relative to the geometric corneal center. Left: locations of the pupil center under photopic (A), mesopic (B), and pharmacologically dilated (C) conditions. Right: Purkinje image I locations in photopic (D), mesopic (E), and pharmacologically dilated (F) conditions. The crossing point of the dotted lines indicates geometric corneal center.
First Purkinje Image Location
Table 2 and Figures 3D, 3E, and 3F plot the locations of the first Purkinje image relative to the corneal center under mesopic, photopic, and dilated conditions, respectively. The mean location of the Purkinje image I centers in all three conditions was 0.41 mm nasal and 0.12 mm superior, and the average distances relative to the pupil center were 0.23, 0.19, and 0.24 mm under mesopic, photopic, and dilated conditions, respectively. Thus, the Purkinje image location was closest to the pupil center under photopic conditions. As expected,27,28 no significant movement of the Purkinje image center was found from mesopic to photopic conditions (t = −0.124, P = 0.90), however, there were significant movements from mesopic to pharmacologically dilated conditions (t = 3.651, P = 0.0004) and from photopic to pharmacologically dilated conditions (t = 3.914, P = 0.001). There were no significant correlations between the center locations of Purkinje image I and age, refraction, corneal diameter, or pupil diameter in all three conditions (P > 0.05, data not shown).
TABLE 2.
Purkinje Image I Center Location and Distance Relative to Geometric Corneal Center
| Horizontal Purkinje Image I Location | Vertical Purkinje Image I Location | Purkinje Image I Distance | |
|---|---|---|---|
| Photopic | 0.427 ± 0.164 | 0.110 ± 0.137 | 0.463 ± 0.159 |
| Mesopic | 0.417 ± 0.174 | 0.134 ± 0.130 | 0.462 ± 0.159 |
| Dilated | 0.391 ± 0.171 | 0.121 ± 0.127 | 0.434 ± 0.155 |
Positive values of location represent superior and nasal directions relative to geometric corneal center. Data are expressed as the mean (millimeters) ± SD.
DISCUSSION
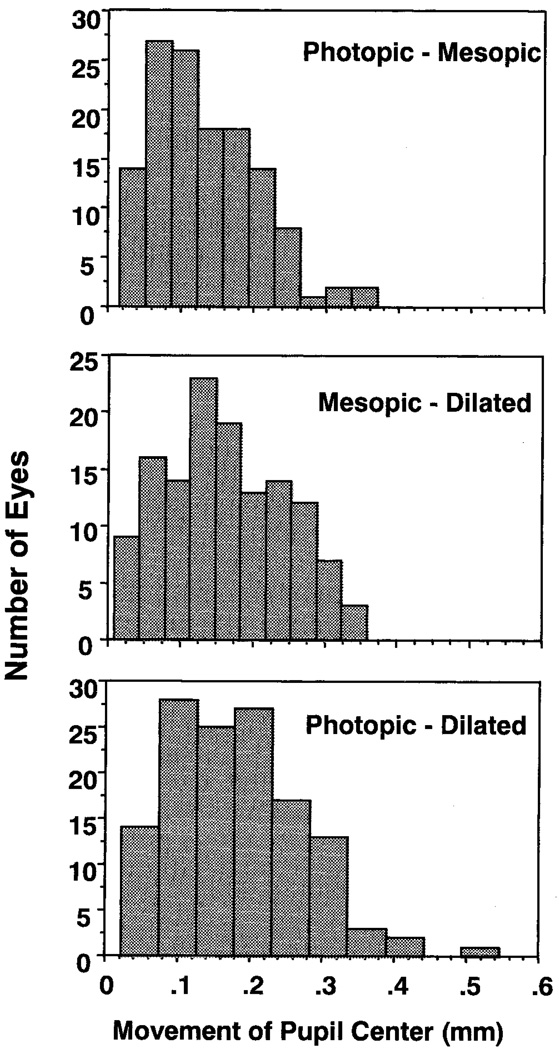
In general, the pupil center is located nasal and slightly superior to the geometric corneal center, and there is a significant shift in the pupil center’s location with increasing dilation, with its lateral position coming closest to the geometric center of the cornea at its maximal dilation. The vertical motion is less consistent, with natural dilation moving the average position only 0.004 mm toward the center of the pupil, but pharmacologic dilation then moving the pupil away from the center (0.04 mm superiorly). The average absolute change in position we measured was between the values reported by Wyatt14 and Walsh,12 but less than those reported by Wilson et al.,13 with an average movement of the pupil center from mesopic to photopic conditions of 0.13 ± 0.07 mm. One difference in the studies was the use of the achromatic axis in the study by Wilson et al. This axis is variable among observers,29,30 and this difference could explain the difference in inferred direction of the shifts. However, for a small Maxwellian entrance pupil, the location of the achromatic axis should be stable, and this cannot explain the difference in size of motions measured. Individual differences could explain this difference in the measured change in pupil centration, although we found few large motions. Histograms of the distribution of number of eyes versus the movement of the pupil center between conditions are shown in Figure 4. Whereas we found one eye where the motion was more than 0.5 mm, 85% of the eyes had motions of their pupil centers of less than 0.25 mm, and none had movements larger than 0.4 mm from mesopic to photopic conditions. The number of subjects showing a greater than 0.3-mm change in the location of the pupil center increased in the pharmacologically dilated conditions, with seven showing a change of center location of 0.3 mm or more. Although there was a trend for the pupils with the largest relative change in size to have the largest motion, as was suggested by Walsh,12 it was not significant (P > 0.25), and even restricting the analysis to younger ages (< 40 years) did not cause the trend to reach significance. Overall, we conclude that although occasional large shifts in pupil center occur, such movements are not the rule.
FIGURE 4.
The distribution of distances in pupil movements between three conditions in the 130 eyes in the study. Distance distribution of the movement of the pupil center from (top) photopic to mesopic, mesopic to pharmacologically dilated (middle), and photopic to pharmacologically dilated (bottom) conditions.
The Purkinje image has often been suggested as a landmark for aligning the eye in optical systems. The first Purkinje image is formed by reflection of light from the anterior corneal surface. When the cornea is illuminated with collimated light, the curvature causes the formation of an image at the focal point of the corneal curvature.27,28,31 Theoretically, there should be no change in the relative positions of the first Purkinje image and the geometric center of the cornea with dilation of the pupil, as long as there is no change in the direction of gaze relative to the optical axis of the pupillometer. Although the locations of the Purkinje images across conditions were very similar, with no significant change in center location, we found a small but significant difference between measurement conditions, in the distance from the corneal center to the Purkinje image. The first Purkinje image moved slightly to the temporal side under the pharmacologically dilated condition. These dilated measurements were made more than 20 minutes after measurements in the other two conditions. This suggests that some of the change in location could arise from slight changes in fixation strategy of the subjects.
In our data sample, it was necessary to eliminate data from two eyes, because after data collection was completed, it was realized that the subjects had not fixated correctly in at least one of the conditions. These conditions were recognizable in the images as a slight rotation of the eye and a large discrepancy between the positions of the Purkinje images across conditions. Eliminating these two outliers decreased the change in distance from the Purkinje image to the corneal center under dilated conditions, but did not eliminate it (mean distance moved 0.04 mm, P < 0.01, t-test). It is difficult to account for this movement, because, in general, small misfixations should be randomly distributed around the mean. It is not likely that these changes are attributable to changes in the point spread function of the optics with changes in pupil size, because any realistic change in the centroid of the point spread function would be quite small relative to the rotations required to move the Purkinje image. However, it is important to note that although the change in location is statistically significant, it was slight. The motion of the pupil center was larger and was in the same direction in most subjects. Thus, although the location of the Purkinje image is relatively stable in the cornea, it appears to move slightly, most likely because of small eye movements. We concluded that the limbus probably provides a more reliable coordinate system for the eye; however, we could not test this, because the present study assumed that the edge of the limbus provides a stable reference, a supposition supported by the small change in measured diameter (approximately 1% of the total diameter) across measurement condition. This conclusion is potentially important when considering the need for relating measurements of the optical quality of the eye to planning corneal surgery. Current recommendations are to base measurements of wave aberrations on the center of the pupil.10,23 However, these measurements must then be related to physical positions of the cornea. There is some tolerance for misalignment between surgery and the pupil, even for the correction of high-order aberrations,21,22 the possibility of inadvertent large changes in fixation could seriously influence references based on the location of the Purkinje image.
In summary, we confirmed that there are systematic changes in pupil diameter with age15,26 and that there are changes in the location of the pupil center between mesopic, photopic, and pharmacologically dilated conditions, but these motions are relatively small. Finally, it is of note that although most individuals had only slight changes of pupil location with dilation, occasional eyes showed large changes in centration, and these changes tended to be more common under pharmacologically dilated conditions.
Acknowledgments
Supported by Grant EYO4395 (SAB) from the National Eye Institute.
Footnotes
Commercial relationships policy: I (KT, SAB); N (YY).
References
- 1.Ivanoff A. About the spherical aberration of the eye. J Opt Soc Am. 1956;46:901–903. doi: 10.1364/josa.46.0901_1. [DOI] [PubMed] [Google Scholar]
- 2.Charman WN, Jennings JA. The optical quality of the monochromatic retinal image as a function of focus. Br J Physiol Opt. 1976;31:119–134. [PubMed] [Google Scholar]
- 3.Howland HC, Howland B. A subjective method for the measurement of monochromatic aberrations of the eye. J Opt Soc Am. 1977;67:1508–1518. doi: 10.1364/josa.67.001508. [DOI] [PubMed] [Google Scholar]
- 4.Charman WN. Wavefront aberration of the eye: a review. Optom Vis Sci. 1991;68:574–583. doi: 10.1097/00006324-199108000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Liang J, Grimm B, Goetz S, Bille JF. Objective measurements of wave aberrations of the human eye with the use of a Hartmann- Shack wave-front sensor. J Opt Soc Am A. 1994;11:1949–1957. doi: 10.1364/josaa.11.001949. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins TCA. Aberrations of the eye and their effects on vision. Br J Physiol Opt. 1963;20:59–91. [PubMed] [Google Scholar]
- 7.Walsh G, Charman WN. The effect of pupil centration diameter on ocular performance. Vision Res. 1988;28:659–665. doi: 10.1016/0042-6989(88)90114-9. [DOI] [PubMed] [Google Scholar]
- 8.Artal P, Navarro R. Monochromatic modulation transfer function of the human eye for different pupil diameters: an analytical expression. J Opt Soc Am A. 1994;11:246–249. doi: 10.1364/josaa.11.000246. [DOI] [PubMed] [Google Scholar]
- 9.Martinez CE, Applegate RA, Klyce SD, McDonald MB, Medina JP, Howland HC. Effect of pupillary dilation on corneal optical aberrations after photorefractive keratectomy. Arch Ophthalmol. 1998;116:1053–1062. doi: 10.1001/archopht.116.8.1053. [DOI] [PubMed] [Google Scholar]
- 10.Mrochen M, Kaemmerer M, Mierdel P, Seiler T. Increased higher-order optical aberrations after laser refractive surgery: a problem of subclinical decentration. J Cataract Refract Surg. 2001;27:362–369. doi: 10.1016/s0886-3350(00)00806-3. [DOI] [PubMed] [Google Scholar]
- 11.Pande M, Hillman JS. Optical zone centration in keratorefractive surgery. Entrance pupil center, visual axis, coaxially sighted corneal reflex, or geometric corneal center? Ophthalmology. 1993;100:1230–1237. [PubMed] [Google Scholar]
- 12.Walsh G. The effect of mydriasis on the pupillary centration of the human eye. Ophthalmic Physiol Opt. 1988;8:178–182. doi: 10.1111/j.1475-1313.1988.tb01034.x. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MA, Campbell MCW, Simonet P. Change of pupil centration with change of illumination and pupil size. Optom Vis Sci. 1992;69:129–136. doi: 10.1097/00006324-199202000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Wyatt HJ. The form of the human pupil. Vision Res. 1995;35:2021–2036. doi: 10.1016/0042-6989(94)00268-q. [DOI] [PubMed] [Google Scholar]
- 15.Winn B, Whitaker D, Elliott DB, Phillips NJ. Factors affecting light-adapted pupil size in normal human subjects. Invest Ophthalmol Vis Sci. 1994;35:1132–1137. [PubMed] [Google Scholar]
- 16.Charman WN, Walsh G. Variations in the local refractive correction of the eye across its entrance pupil. Optom Vis Sci. 1989;66:34–40. doi: 10.1097/00006324-198901000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Thibos LN, Hong X. Clinical applications of the Shack-Hartmann aberrometer. Optom Vis Sci. 1999;76:817–825. doi: 10.1097/00006324-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 18.He JC, Marcos S, Webb RH, Burns SA. Measurement of the wavefront aberration of the eye by a fast psychophysical procedure. J Opt Soc Am A. 1998;15:2449–2456. doi: 10.1364/josaa.15.002449. [DOI] [PubMed] [Google Scholar]
- 19.Navarro R, Losada MA. Aberrations and relative efficiency of light pencils in the living human eye. Optom Vis Sci. 1997;74:540–547. doi: 10.1097/00006324-199707000-00023. [DOI] [PubMed] [Google Scholar]
- 20.Howland HC. The history and methods of ophthalmic wavefront sensing. J Refract Surg. 2000;16:S552–S553. doi: 10.3928/1081-597X-20000901-11. [DOI] [PubMed] [Google Scholar]
- 21.Bara S, Mancebo T, Moreno-Barriuso E. Positioning tolerances for phase plates compensating aberrations of the human eye. Appl Opt. 2000;39:3413–3420. doi: 10.1364/ao.39.003413. [DOI] [PubMed] [Google Scholar]
- 22.Guirao A, Williams DR, Cox IG. Effect of rotation and translation on the expected benefit of an ideal method to correct the eye's higher-order aberrations. J Opt Soc Am A. 2001;18:1003–1015. doi: 10.1364/josaa.18.001003. [DOI] [PubMed] [Google Scholar]
- 23.Thibos LN, Applegate RA, Schwiegerling JT, Webb R. Report from the VSIA taskforce on standards for reporting optical aberrations of the eye. J Refract Surg. 2000;16:S654–S655. doi: 10.3928/1081-597X-20000901-34. [DOI] [PubMed] [Google Scholar]
- 24.Mrochen M, Eldine MS, Kaemmerer M, Seiler T, Hutz W. Improvement in photorefractive corneal laser surgery results using an active eye-tracking system. J Cataract Refract Surg. 2001;27:1000–1006. doi: 10.1016/s0886-3350(00)00884-1. [DOI] [PubMed] [Google Scholar]
- 25.Webb RH, Penney CM, Thompson KP. Measurement of ocular wavefront distortion with a spatially resolved refractometer. Appl Opt. 1992;31:3678–3686. doi: 10.1364/AO.31.003678. [DOI] [PubMed] [Google Scholar]
- 26.Calver RI, Cox MJ, Elliott DB. Effect of aging on the monochromatic aberrations of the human eye. J Opt Soc Am A. 1999;16:2069–2078. doi: 10.1364/josaa.16.002069. [DOI] [PubMed] [Google Scholar]
- 27.Barry JC, Branmann K, Dunne MC. Catoptric properties of eyes with misaligned surfaces studied by exact ray tracing. Invest Ophthalmol Vis Sci. 1997;38:1476–1484. [PubMed] [Google Scholar]
- 28.Barry JC, Pongs UM, Hillen W. Algorithm for Purkinje images I and IV and limbus centre localization. Comput Biol Med. 1997;27:515–531. doi: 10.1016/s0010-4825(97)00023-1. [DOI] [PubMed] [Google Scholar]
- 29.Rynders M, Lidkea B, Chisholm W, Thibos LN. Statistical distribution of foveal transverse chromatic aberration, pupil centration, and angle psi in a population of young adult eyes. J Opt Soc Am A. 1995;12:2348–2357. doi: 10.1364/josaa.12.002348. [DOI] [PubMed] [Google Scholar]
- 30.Marcos S, Burns SA, Prieto PM, Navarro R, Baraibar B. Investigating sources of variability of monochromatic and transverse chromatic aberrations across eyes. Vision Res. 2001;41:3861–3871. doi: 10.1016/s0042-6989(01)00133-x. [DOI] [PubMed] [Google Scholar]
- 31.Cornsweet TN, Crane HD. Accurate two-dimensional eye tracker using first and fourth Purkinje images. J Opt Soc Am A. 1973;63:921–928. doi: 10.1364/josa.63.000921. [DOI] [PubMed] [Google Scholar]