Abstract
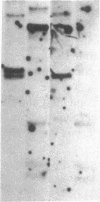
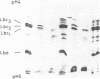
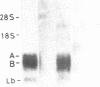
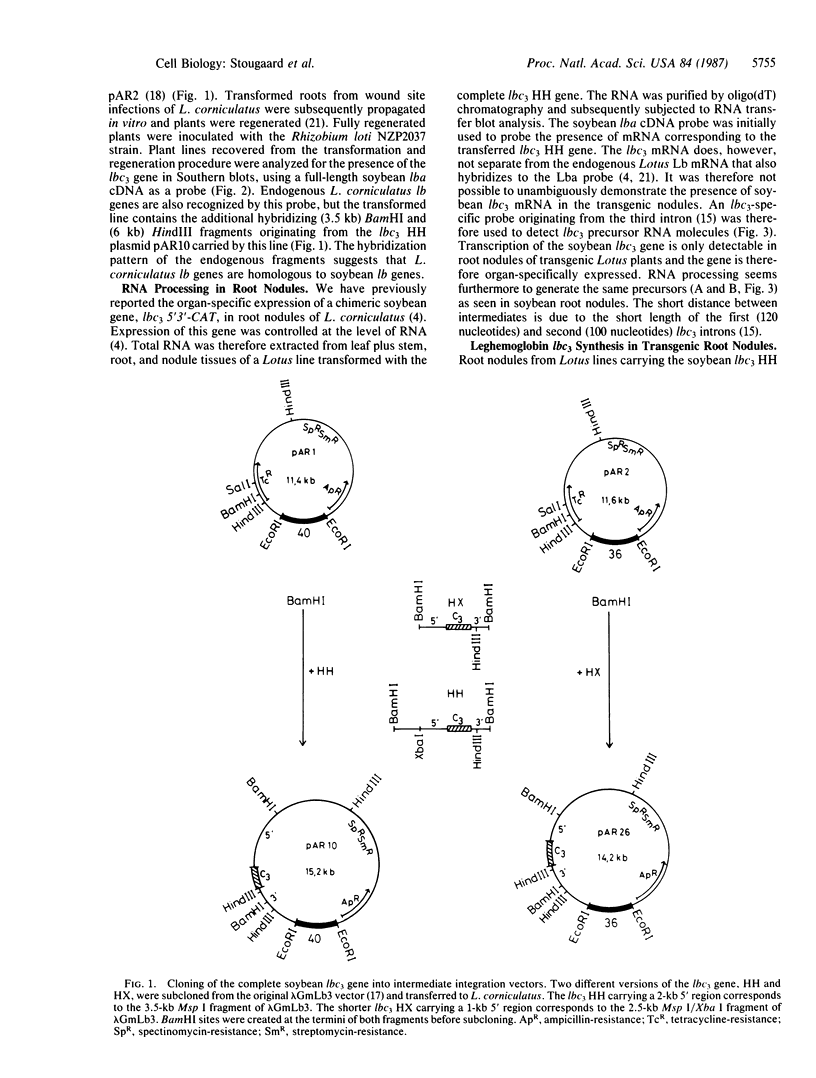
The complete soybean leghemoglobin lbc3 gene was transferred into the legume Lotus corniculatus using an Agrobacterium rhizogenes vector system. Organ-specific expression of the soybean gene was observed in root nodules formed on regenerated transgenic plants after infection with Rhizobium loti. The primary transcript was processed in the same way as in soybean nodules and the resulting mRNA was translated into Lbc3 protein. Quantitative determination of the Lbc3 protein in nodules of transgenic plants indicated that the steady-state level of the soybean protein is comparable to that of endogenous Lotus leghemoglobin.
Keywords: symbiotic nitrogen fixation, plant transformation, RNA processing, Lbc3 synthesis and stability
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojsen K., Abildsten D., Jensen E. Ø, Paludan K., Marcker K. A. The chromosomal arrangement of six soybean leghemoglobin genes. EMBO J. 1983;2(7):1165–1168. doi: 10.1002/j.1460-2075.1983.tb01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J. G., Llewellyn D. J., Dennis E. S., Peacock W. J. Maize Adh-1 promoter sequences control anaerobic regulation: addition of upstream promoter elements from constitutive genes is necessary for expression in tobacco. EMBO J. 1987 Jan;6(1):11–16. doi: 10.1002/j.1460-2075.1987.tb04711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchsman W. H., Appleby C. A. Separation and determination of the relative concentrations of the homogeneous components of soybean leghemoglobin by isoelectric focusing. Biochim Biophys Acta. 1979 Aug 28;579(2):314–324. doi: 10.1016/0005-2795(79)90059-x. [DOI] [PubMed] [Google Scholar]
- Govers F., Gloudemans T., Moerman M., van Kammen A., Bisseling T. Expression of plant genes during the development of pea root nodules. EMBO J. 1985 Apr;4(4):861–867. doi: 10.1002/j.1460-2075.1985.tb03711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Estrella L., Van den Broeck G., Maenhaut R., Van Montagu M., Schell J., Timko M., Cashmore A. Light-inducible and chloroplast-associated expression of a chimaeric gene introduced into Nicotiana tabacum using a Ti plasmid vector. Nature. 1984 Jul 12;310(5973):115–120. doi: 10.1038/310115a0. [DOI] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Wiborg O., Garrett R., Jørgensen P., Marcker K. A. The primary structures of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jan 22;10(2):689–701. doi: 10.1093/nar/10.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith B., Chua N. H. Monocot and dicot pre-mRNAs are processed with different efficiencies in transgenic tobacco. EMBO J. 1986 Oct;5(10):2419–2425. doi: 10.1002/j.1460-2075.1986.tb04516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G., Nagy F., Chua N. H. Light-regulated and organ-specific expression of a wheat Cab gene in transgenic tobacco. Nature. 1985 Aug 22;316(6030):750–752. doi: 10.1038/316750a0. [DOI] [PubMed] [Google Scholar]
- Legocki R. P., Verma D. P. Identification of "nodule-specific" host proteins (nodoulins) involved in the development of rhizobium-legume symbiosis. Cell. 1980 May;20(1):153–163. doi: 10.1016/0092-8674(80)90243-3. [DOI] [PubMed] [Google Scholar]
- Marcker A., Lund M., Jensen E. Ø, Marcker K. A. Transcription of the soybean leghemoglobin genes during nodule development. EMBO J. 1984 Aug;3(8):1691–1695. doi: 10.1002/j.1460-2075.1984.tb02033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzke M. A., Susani M., Binns A. N., Lewis E. D., Rubenstein I., Matzke A. J. Transcription of a zein gene introduced into sunflower using a Ti plasmid vector. EMBO J. 1984 Jul;3(7):1525–1531. doi: 10.1002/j.1460-2075.1984.tb02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy F., Morelli G., Fraley R. T., Rogers S. G., Chua N. H. Photoregulated expression of a pea rbcS gene in leaves of transgenic plants. EMBO J. 1985 Dec 1;4(12):3063–3068. doi: 10.1002/j.1460-2075.1985.tb04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Baumann G. Thermo-induced transcripts of a soybean heat shock gene after transfer into sunflower using a Ti plasmid vector. EMBO J. 1985 May;4(5):1119–1124. doi: 10.1002/j.1460-2075.1985.tb03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiborg O., Hyldig-Nielsen J. J., Jensen E. O., Paludan K., Marcker K. A. The nucleotide sequences of two leghemoglobin genes from soybean. Nucleic Acids Res. 1982 Jun 11;10(11):3487–3494. doi: 10.1093/nar/10.11.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]