Summary
The Fas cell surface receptor induces apoptosis upon receptor oligomerization. We have identified a novel signaling protein, termed Daxx, that binds specifically to the Fas death domain. Overexpression of Daxx enhances Fas-mediated apoptosis and activates the Jun N-terminal kinase (JNK) pathway. A C-terminal portion of Daxx interacts with the Fas death domain, while a different region activates both JNK and apoptosis. The Fas-binding domain of Daxx is a dominant-negative inhibitor of both Fas-induced apoptosis and JNK activation, while the FADD death domain partially inhibits death but not JNK activation. The Daxx apoptotic pathway is sensitive to both Bcl-2 and dominant-negative JNK pathway components and acts cooperatively with the FADD pathway. Thus, Daxx and FADD define two distinct apoptotic pathways downstream of Fas.
Introduction
Fas (also known as CD95 or APO-1) is a widely expressed cell death receptor that has a critical role in the regulation of the immune system and tissue homeostasis. Fas is activated by Fas ligand (FasL), a trimeric transmembrane protein (reviewed by Nagata, 1997). Fas is thought to have an essential role in deleting autoreactive lymphocytes and maintaining peripheral tolerance. Inherited Fas mutations in humans and mice cause a syndrome of massive lymphoproliferation and autoantibody production (reviewed by Nagata, 1997). Fas-induced apoptosis is also a major mechanism in cytotoxic T lymphocyte–mediated cytolysis and in the maintenance of immune privilege sites (reviewed by Abbas, 1996). Moreover, depending on the signal from the B cell antigen receptor, Fas may induce either apoptosis or proliferation of B cells in vivo (Rathmell et al., 1996).
Fas belongs to the tumor necrosis factor (TNF) receptor superfamily, which includes TNF receptor 1 (TNFR1), TNFR2, CD40, and the p75 low affinity NGF receptor; these receptors share characteristic cysteine-rich repeats in their extracellular domains (reviewed by Smith et al., 1994). The intracellular tails of Fas and TNFR1 share homologous death domains, an approximately 80 amino acid protein motif that is critical for signaling apoptosis (Itoh and Nagata, 1993; Tartaglia et al., 1993). Over the last two years, elucidation of the mechanism for Fas-mediated apoptosis has begun (reviewed by Cleveland and Ihle, 1995; Fraser and Evan, 1996). FADD, also known as MORT1, is a cytoplasmic protein that has a C-terminal death domain that interacts with Fas and an N-terminal domain that can induce cell death (Boldin et al., 1995b; Chinnaiyan et al., 1995). The N terminus of FADD interacts with MACH/FLICE, an interleukin-1β-converting enzyme (ICE) family cysteine protease (caspase) that potently induces apoptosis (Boldin et al., 1996; Muzio et al., 1996). Although the details are not yet clear, other caspases, including ICE and CPP32, are sequentially activated to execute the apoptotic dissolution of the cell (Enari et al., 1996). TNFR1 also interacts with FADD via an adaptor protein termed TRADD (Hsu et al., 1996). The emerging model from these molecular studies is that Fas, via FADD, directly engages and activates apoptotic ICE family proteases. However, this model fails to explain how Bcl-2 and other physiologic signals may modulate Fas-mediated apoptosis (Fraser and Evan, 1996). It remains possible that other signaling molecules in addition to FADD are involved in Fas-mediated apoptosis.
Fas can also activate the Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) pathway (Latinis and Koretzky, 1996; Goillot et al., 1997; Lenczowski et al., 1997). Analogous to the MAP kinase cascade, the prototypical JNK/SAPK pathway involves the sequential activation of the proteins MEKK1, SEK1, JNK, and c-Jun. Other targets of the JNK pathway include the transcription factors Elk-1 and ATF-2 (reviewed by Kyriakis and Avruch, 1996). This pathway was initially characterized by the ability of UV irradiation and transforming Ha-Ras to activate the AP-1 transcription factor; subsequently, it was shown that TNF-α and other stress-activated signals may also activate this pathway. The significance of Fas-mediated JNK activation has been unclear. One hypothesis is that activation of the JNK pathway contributes to Fas-mediated apoptosis (Goillot et al., 1997). Dominant-negative constituents of the JNK pathway can block stress- and TNF-induced apoptosis in several cell lines, suggesting that activation of the JNK pathway is required for these apoptotic inducers (Verheij et al., 1996). Similarly, in PC12 cells that undergo apoptosis in response to nerve growth factor withdrawal, activation of the JNK pathway in concert with the suppression of the ERK pathway is critical to induction of programmed cell death (Xia et al., 1995). Alternatively, Fas-mediated JNK activation may drive cellular proliferation via activation of the proto-oncogene c-Jun and AP-1 transcriptional activity (Rathmell et al., 1996).
Recently, Liu et al. have demonstrated that overexpression of FADD, the established downstream signal transducer of Fas, cannot activate JNK but that two other proteins engaged by TNFR1—RIP and TRAF2—are responsible for JNK activation by TNF (Liu et al., 1996). This raises the question of whether Fas also engages other proteins to activate the JNK pathway.
In this report, we describe the molecular cloning and characterization of Daxx, a novel Fas-binding protein. Daxx binds to the Fas death domain, yet lacks a death domain of its own. Overexpression of Daxx leads to JNK activation and potentiates Fas-induced apoptosis. The Fas-binding domain of Daxx acts as a dominant-negative inhibitor of Fas-induced apoptosis and JNK activation. Furthermore, using dominant-negative and constitutively active forms of Daxx and FADD, we show that Fas engages two independent pathways to induce cell death: one pathway via Daxx that involves JNK activation and is blocked by Bcl-2, and a second pathway via FADD that is Bcl-2 insensitive.
Results
Two-Hybrid Screen for Novel Fas-Interacting Proteins
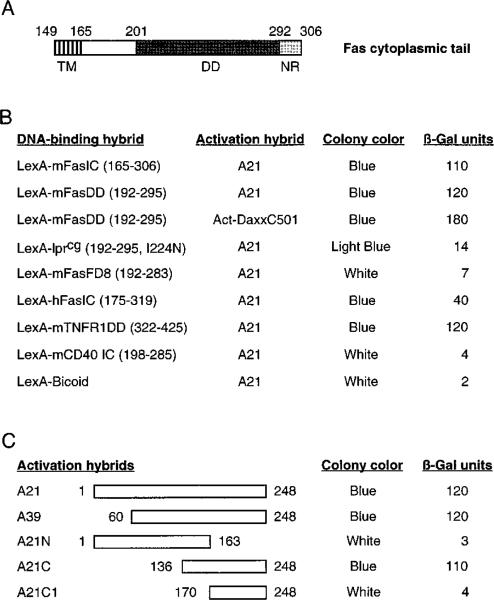
To identify novel Fas-interacting proteins, we performed a two-hybrid screen with the death domain of murine Fas fused to the DNA-binding protein LexA (LexA-mFasDD). A plasmid library of fusions between a transcription activation domain and cDNAs from human HeLa cells was screened for interaction with LexA-mFasDD in a yeast reporter strain. One group of positive interactors, typified by clone A21, interacted strongly with FasDD. However, it interacted poorly with either Fas-lprcg, an I224A mutation in the Fas death domain that abrogates Fas signaling and causes lymphoproliferation in mice (reviewed by Nagata, 1997), or with Fas-FD8, a functionally inactive deletion mutation of the Fas death domain (Itoh and Nagata, 1993) (Figure 1B). Sequence analysis of clone A21 revealed it to encode a portion of a novel protein.
Figure 1. Interaction of Clone A and Daxx with Fas Death Domain in Yeast.
(A) Schematic representation of the cytoplasmic domain of murine Fas (Watanabe-Fukunaga et al., 1992). Boundaries of the transmembrane domain (TM), death domain (DD), and the negative regulatory domain (NR) are labeled.
(B) Protein interactions in the two-hybrid system. LexA constructs contained the indicated sequences (amino acids in parentheses) of receptors that expressed similar level of fusion proteins in yeast. Colony color and β-galactosidase units were determined as described in Experimental Procedures. The activation hybrid Act-DaxxC501 contained amino acids 501–739 of Daxx.
(C) The C terminus of clone A interacts with Fas death domain. Amino acids contained in each activation hybrid are indicated.
In the two-hybrid system, clone A21 also interacted with the intracellular domain of human Fas and the death domain of TNFR1, but not with the intracellular region of CD40, a closely related receptor that lacks a death domain (Figure 1B). The sequence C-terminal to the Fas death domain has been shown to inhibit the cytotoxicity of Fas death domain (Itoh and Nagata, 1993), and it also inhibits the binding of FADD to Fas (Chinnaiyan et al., 1995). The presence of this inhibitory region had no effect on the binding of clone A21 to Fas because clone A21 interacted equally well with FasDD and FasIC (Figure 1B).
We mapped the Fas interaction domain on clone A21 to its C-terminal 112 amino acids by deletion analysis (Figure 1C). This region showed no evident sequence similarity to death domains, suggesting that the interaction between clone A21 and Fas is not through a homo-typic death domain association.
Cloning of Daxx cDNA and Northern Analysis
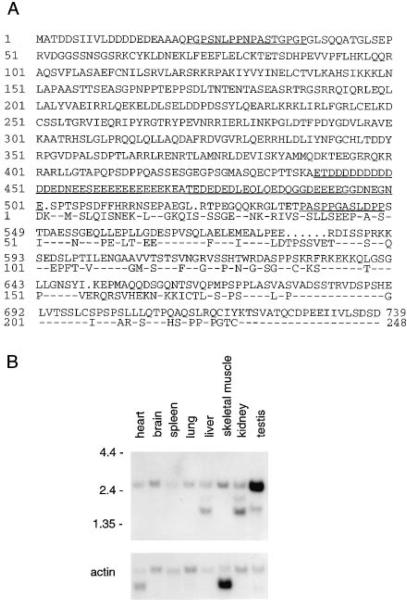
Using clone A21 as the probe, we cloned a cross-hybridizing full-length murine cDNA. Sequence analysis revealed an open reading frame able to encode a protein of 739 amino acids with a predicted molecular mass of 81.4 kDa (Figure 2A). The C terminus of this protein is homologous to clone A. We call this protein Daxx for Fas death domain–associated protein. A database search using BLAST revealed that Daxx is a novel protein with no significant sequence similarity to any other protein. Daxx contains a region of 62 amino acids with a high content (71%) of glutamic acid and aspartic acid and contains two small proline-rich regions (Figure 2A).
Figure 2. Daxx Sequence and mRNA Distribution.
(A) Conceptually translated amino acid sequence of Daxx protein. The open reading frame of murine Daxx follows an in-frame stop codon and begins with a Kozak consensus sequence. The regions enriched for acidic residues and proline are underlined. The partial human cDNA sequence from A21 is shown below the mouse sequence with identical amino acids indicated by dashes.
(B) Tissue distribution of Daxx. A mouse multiple tissue Northern blot was probed with a C-terminal 0.7 kb fragment of Daxx and a human β-actin cDNA.
To determine the tissue distribution of Daxx, we performed a Northern analysis with a Daxx C-terminal probe. A 2.6 kb transcript, consistent with the length of the open reading frame, was detected in various adult mouse tissues (Figure 2B). The expression of Daxx appeared uniform, with the exception of stronger expression in testis. Shorter hybridizing transcripts were also detected in liver, kidney, and testis. Cell lines from many tissues have been reported to support the ability of ectopically expressed Fas to induce apoptosis, suggesting that the downstream signaling mechanism is present in most tissues. Similarly, FADD is expressed ubiquitously in adult tissues (Boldin et al., 1995b; Chinnaiyan et al., 1995).
Daxx Interacts with Fas Both In Vitro and In Vivo
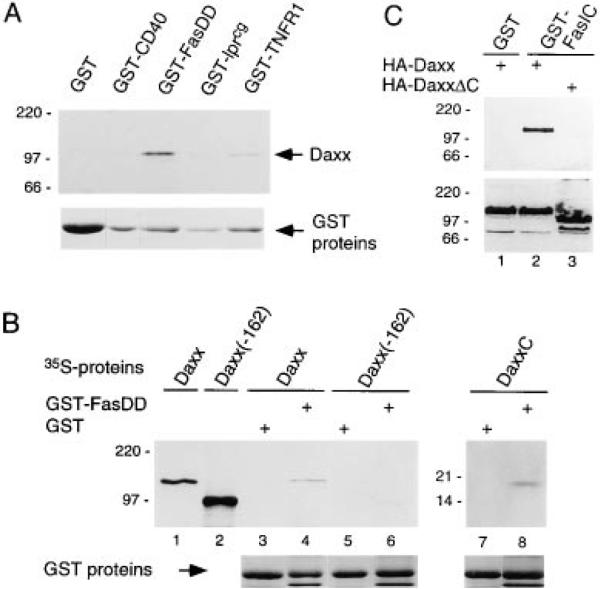
In the two-hybrid system, the Daxx C-terminal region interacted strongly with Fas, confirming that Daxx is the functional homolog of clone A21 (Figure 1B). We then tested the binding of full-length murine Daxx protein in vitro and in mammalian cells. In vitro translated, 35S-labeled Daxx bound to immobilized glutathione S–transferase (GST) fusion proteins of Fas death domain and TNFR1 intracellular tail but not to immobilized GST, GST-CD40 intracellular tail, or GST-Fas lprcg death domain (Figure 3A). Daxx migrated with an apparent molecular weight of approximately 120 kDa on SDS–PAGE; this slower than expected migration may reflect the high content of acidic residues in Daxx. In the GST pull-down assay, 35S-Daxx bound to GST-FasDD but only very weakly to GST-TNFR1. This discrepancy with the two-hybrid result (Figure 1) may be due to the nonlinear readout of the two-hybrid system. Deletion of 162 amino acids from the C terminus of Daxx abrogated binding to GST-FasDD, while the C-terminal 112 amino acids (DaxxC) were sufficient to bind GST-FasDD (Figure 3B), consistent with the two-hybrid results.
Figure 3. Interaction of Daxx with Fas In Vitro and in Mammalian Cells.
(A) Binding of in vitro translated 35S-Daxx to GST-fusion proteins. Positions of MW standards (in kDa) are shown at left. Coomassiestained GST fusion proteins from the same gel were aligned to show protein levels.
(B) Binding of full-length and truncated 35S-Daxx to GST (lanes 3, 5, and 7) and GST-FasDD (lanes 2, 4, and 6). Daxx(–162) lacked the C-terminal 162 aa of Daxx; DaxxC corresponded to aa 628–739. Input of 35S-Daxx and 35S-Daxx(–162) proteins in binding assays are shown in lanes 1 and 2, respectively. GST fusion proteins are shown on the bottom panel.
(C) Association of HA-Daxx and HA-DaxxΔC (lacking aa 626–739) with the GST fusion of Fas intracellular tail (GST-FasIC) in 293 cells (top panel). The presence of HA-Daxx and HA-DaxxΔC in extracts was verified by immunoblotting for HA (bottom panel).
To determine whether Daxx interacted with Fas in mammalian cells, human embryonic kidney 293 cells were cotransfected with constructs expressing hemagglutinin-tagged Daxx (HA-Daxx) and GST-Fas intracellular tail (GST-FasIC). HA-Daxx was coprecipitated with GST-FasIC but not with GST using glutathione beads. Again, this interaction was dependent on the C-terminus of Daxx (Figure 3C). GST-FasIC was also able to coprecipitate HA-tagged Fas death domain, confirming that death domains may multimerize (data not shown). Because Fas is overexpressed and thereby activated, we are uncertain whether Daxx binds to the inactive Fas. Collectively, these data show that the C terminus of Daxx mediates an interaction between the Fas death domain and Daxx, and that this interaction is likely to occur directly and in vivo.
Daxx Potentiates Fas-Mediated Apoptosis
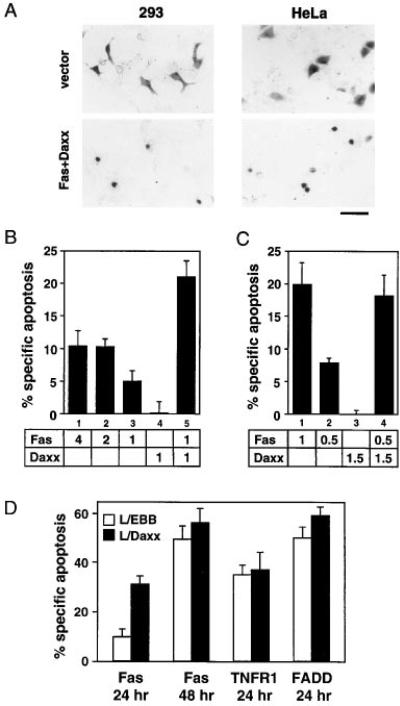
To study the role of Daxx in Fas signaling, we chose 293 cells and HeLa cells. Both are sensitive to Fas- and TNF-mediated apoptosis, and their normally flat morphology facilitates the scoring of apoptotic cells, characterized by membrane blebbing, pyknosis, and cell body condensation (Figure 4A). Cells scored to be apoptotic by morphology also exhibited nuclear condensation and fragmentation as judged by Hoechst staining (data not shown). HeLa and 293 cells were transfected with various expression constructs and an expression construct for β-galactosidase; at defined times after the transfection, cells were stained for β-galactosidase activity to mark the transfected cells and scored for apoptotic morphology. In 293 cells, transient overexpression of Fas induced apoptosis in a dose-dependent and saturable manner (Figure 4B). Fas activation in the absence of activating ligand is due to a documented propensity of death domains to multimerize (Boldin et al., 1995a). However, the addition of activating anti-Fas antibodies, Jo2, did not increase cell death in Fas-transfected 293 cells, implying that a function downstream of receptor activation may be limiting (data not shown). Overexpression of Daxx by itself did not induce apoptosis, but Daxx coexpression significantly enhanced Fas-mediated apoptosis (Figure 4B). Parallel experiments with TNFR1 did not show any enhancement of apoptosis by Daxx, consistent with the much lower affinity of Daxx for TNFR1 (Figure 3A).
Figure 4. Daxx Potentiates Fas-Induced Apoptosis.
(A) Normal and apoptotic 293 and HeLa cells. Cells were transiently transfected with the indicated plasmids, stained with X-Gal, and examined by light microscopy. Fields were chosen to illustrate morphologic differences but not relative percentages of apoptosis. Scale bar = 50 μm.
(B) Daxx potentiates Fas-induced apoptosis in 293 cells. Indicated amounts (in μg) of pEBB-Fas and pEBB-HA-Daxx plasmids were cotransfected with 0.5 μg of pCMV-lacZ. Amounts of transfected DNA were equalized by adding vector DNA. The cells were stained with X-Gal 20 hr after transfection and analyzed for apoptotic morphology as described in Experimental Procedures.
(C) Daxx potentiates Fas-induced apoptosis in HeLa cells. Transfection and specific apoptosis were done and measured as in 293 cells except that X-Gal staining was done 24 hr after transfection.
(D) L929 cells stably overexpressing Daxx have accelerated apoptosis in response to Fas. L/EBB and L/Daxx were transfected with 1 μg of pEBB-Fas, pEBB-TNFR1, or pRK-FADD plus 0.2 μg of pCMV-lacZ. Specific apoptosis was determined as in Figure 4B at indicated time after transfection. Similar results were obtained with multiple L/Daxx lines.
In HeLa cells, transient transfection of Fas led to robust, dose-dependent, and saturable cell death (Figures 4A and 4C), which was further enhanced by the addition of Jo2. As in 293 cells, overexpression of Daxx alone did not induce apoptosis in HeLa cells. In the range where apoptosis was proportional to input Fas DNA, coexpression of Daxx significantly increased Fas-mediated apoptosis (Figure 4C), suggesting that Daxx activity may be a rate-limiting step downstream of receptor engagement.
In an analogous approach to assess the function of Daxx, we established murine fibroblast L929 cell lines that stably overexpressed Daxx (L/Daxx). L/Daxx cells are substantially more susceptible to Fas killing compared to vector-transfected cells (L/EBB). This stimulation effect appeared to be a kinetic one: compared to L/EBB, the L/Daxx culture had greater than 3-fold more apoptotic cells 24 hours after Fas transfection, but L/EBB cells caught up by 48 hours after transfection (Figure 4D). TNF-α-, TNFR1-, or FADD-mediated apoptosis was not increased in L/Daxx cells (Figure 4D and data not shown). Therefore, L/Daxx cells are not generally more sensitive to apoptosis, but are specifically sensitized to the Fas signal, suggesting that Daxx is a mediator of Fas-induced killing.
Daxx Activates the JNK/SAPK Pathway
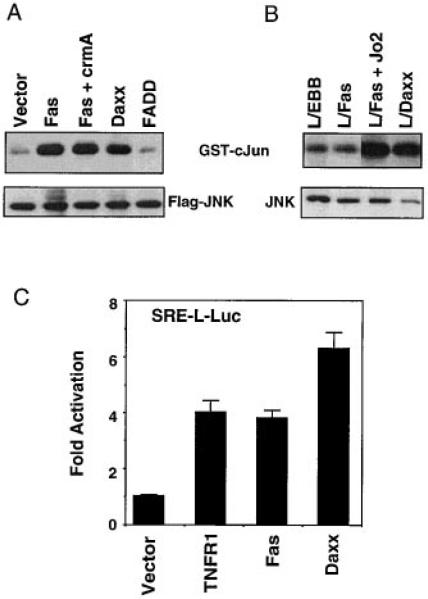
The lack of death domain homology or constitutive cell death activity suggests that Daxx may play a different role from previously identified death domain–binding proteins in Fas signaling. Fas has been reported to activate the JNK pathway (Latinis and Koretzky, 1996; Goillot et al., 1997; Lenczowski et al., 1997), which is required in certain cell lines for the analogous TNF-α-induced apoptosis (Verheij et al., 1996). We therefore analyzed the ability of Fas and Daxx to activate the kinase activity of JNK and JNK-dependent transcription. In transient transfection assays in 293 cells, Fas activated JNK-1, the major JNK activity in cells (Derijard et al., 1994). Fas-induced JNK activation was not blocked by the serpin ICE inhibitor crmA (Figure 5A), a peptide ICE inhibitor Z-VAD, or a peptide CPP32 inhibitor Z-DEVD (data not shown; Goillot et al., 1997). FADD overexpression did not induce JNK activation (Figure 5A). Therefore, Fas activation of JNK is not secondary to FADD activity or apoptosis. Interestingly, Daxx overexpression activated JNK-1 to a level similar to that of Fas (Figure 5A). To assay endogenous JNK activation by Fas and Daxx, we used L929 cells stably expressing murine Fas (L/Fas) and the L/Daxx and L/EBB cells. In L/Fas cells, Fas-induced JNK activation was observed approximately 15 minutes after Fas ligation and reached maximal activity in about one hour (Figure 5A and data not shown). L/Daxx cells had constitutive activation of JNK activity compared to L/EBB cells (Figure 5B), and the level of JNK activation correlated with the level of Daxx overexpression in various L/Daxx cell lines (data not shown).
Figure 5. Daxx Activates the JNK Pathway.
(A) Daxx activates JNK in transient transfection. Flag-tagged JNK1 (Flag-JNK) and the indicated plasmids (1 μg each) were cotransfected into 293 cells. Top: phosphorylation of GST-cJun. Bottom: expression of Flag-JNK. The data shown are representative of four independent assays.
(B) Stable expression of Daxx constitutively activates JNK. Top: phosphorylation of GST-cJun. Bottom: expression of endogenous JNK. The data shown are representative of three independent assays.
(C) Daxx activates a JNK-dependent reporter gene. The data shown are the average and SD of three independent experiments in duplicate.
As an independent measure of JNK activity, we tested the ability of Fas and Daxx to stimulate signaling to SRF (Serum Response Factor). JNK can phosphorylate and activate SRF independent of the MEK/MAPK pathway, and the level of JNK activation in vivo can be assayed using a reporter gene driven by SRE-L, a derivative of SRE that specifically binds SRF (Hill et al., 1995). In 293 cells, Fas induced SRF-dependent transcription about 4-fold, and Daxx induced it about 6-fold. TNF-α, a known inducer of the JNK pathway, stimulated the SRF-reporter gene to a level similar to that induced by Fas or Daxx (Figure 5C).
Collectively, these data show that Daxx is an activator of the JNK pathway.
Deletion Mutagenesis of Daxx
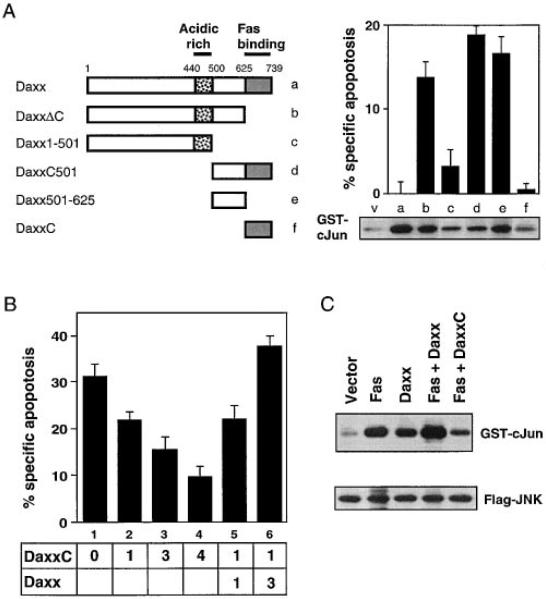
To further dissect Daxx signaling, we asked which regions of Daxx were required for its three activities: Fas binding, enhancement of apoptosis, and activation of JNK. We have already determined that the C-terminal 112 amino acids of Daxx (DaxxC) are necessary and sufficient for Fas binding (Figure 3B). Significantly, Daxx mutants missing either the C terminus (DaxxΔC) or N-terminal 500 amino acids (DaxxC501) acquired a modest constitutive cell death activity for 293 cells in the absence of Fas (Figure 6A). HeLa cells and L929 cells were not sensitive to this activity. The result in 293 cells suggested that deletion of either end of Daxx activated a normally latent cell death activity. Because DaxxΔC is unable to bind Fas death domain (Figures 3B and 3C), this cell death activity is likely to be independent of Fas or other death domain proteins. Further deletions revealed that a peptide containing amino acids 501–625, which lies immediately N-terminal to the Fas-binding domain, contained most of the cell death activity (Figure 6A).
Figure 6. Deletion Analysis of Daxx.
(A) Apoptosis and JNK activation by Daxx deletion mutants. The horizontal bars represent Daxx sequences present in deletion mutants. Apoptosis assay: 3 μg of each Daxx mutant construct was transfected into 293 cells as in Figure 4B. JNK assay: transient transfection of 1 μg of each Daxx mutant construct or pEBB vector (v) with 1 μg of Flag-JNK and in vitro JNK assay was doneas in Figure 5A. Equal Flag-JNK expression was verified by immunoblotting for Flag.
(B) DaxxC inhibits Fas-induced apoptosis. HeLa cells were transfected with 0.5 μg pEBB-Fas and pCMV-lacZ and the indicated amount (in μg) of HA-Daxx and HA-DaxxC. Total amount of transfected DNA was made constant by adding pEBB. Jo2 antibody (12.5 ng/ml) was added 16 hr later. X-Gal staining was done 24 hr after transfection.
(C) DaxxC inhibits Fas-induced JNK activation. Transient transfection of 1 μg of each indicated plasmid with 1 μg of Flag-JNK and in vitro JNK assay were done as in Figure 5A.
When these Daxx mutants were tested for their ability to activate JNK, we observed that each deletion mutant maintained some level of JNK activity with the exception of DaxxC; the majority of the activity came from just the region with amino acids 501–625 (Figure 6A). This result suggests that JNK activation may be involved in Daxx-stimulated apoptosis. However, full-length Daxx activates JNK but does not cause constitutive apoptosis, suggesting that full-length Daxx may activate other pathways that counterbalance the apoptotic JNK signal.
DaxxC Is a Dominant-Negative Inhibitor of Fas-Mediated Apoptosis and JNK Activation
Deletion mutagenesis showed that DaxxC, the C-terminal 112 amino acids of Daxx, was necessary and sufficient to bind Fas but more N-terminal domains were required to activate JNK and cell death. Thus, we tested whether DaxxC can act as a dominant-negative inhibitor of endogenous Daxx by competing with its binding to Fas. We chose to use HeLa cells in these experiments because the cells have a robust response to transfected Fas (Figure 4C) and are the source of clone A21 from the two-hybrid screen. In Figure 6B, we show that expression of DaxxC gave a dose-dependent suppression of Fas-mediated apoptosis. Fas-induced c-Jun phosphorylation was also inhibited by DaxxC in HeLa cells (data not shown) and in 293 cells (Figure 6C). To address the specificity of DaxxC, we then coexpressed full-length Daxx with DaxxC and asked if this combination now reversed the dominant-negative effect. If DaxxC were binding other death domain–containing proteins (e.g., FADD), coexpression of full-length Daxx would further titrate FADD away from Fas and inhibit apoptosis. Instead, coexpression of Daxx with Fas and DaxxC gave a dose-dependent rescue of Fas-induced apoptosis (Figure 6B). This result argues that the only functions made deficient by DaxxC are those of intact Daxx, implying that DaxxC specifically competes with endogenous Daxx but not other proteins for binding to Fas. These results suggest that endogenous Daxx is required for Fas-induced apoptosis and JNK activation.
Daxx and FADD Define Two Distinct Fas-Mediated Signaling Pathways
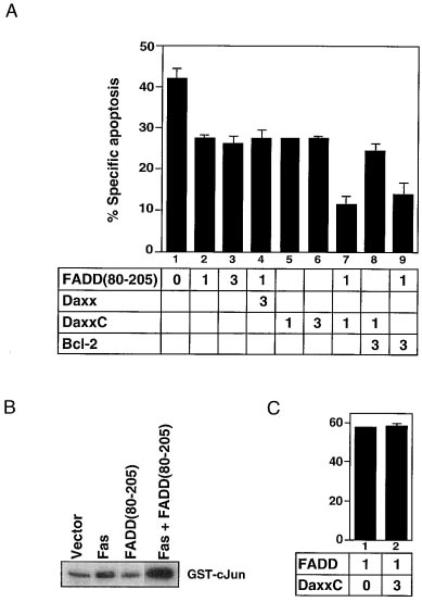
Because Daxx and FADD are both required for Fas-induced apoptosis, we assessed how these two effectors may be related to each other by a dominant-negative approach. The FADD death domain, FADD(80–205), has been shown to block Fas-induced death presumably by preventing the binding of endogenous FADD (Chinnaiyan et al., 1996). We found that FADD(80–205) partially inhibited Fas-induced death (Figure 7A, lanes 1–3) but did not inhibit JNK activation (Figure 7B). Moreover, the effect of FADD(80–205) on cell death was not reversed by coexpression of excess Daxx (Figure 7A, lane 4). These results contrast with the effects of DaxxC (Figure 6) and suggest that Daxx and FADD bind independently to Fas and activate distinct pathways. Consistent with this interpretation, FADD-induced cell death is not blocked by DaxxC (Figure 7C). In addition, DaxxC plus FADD(80–205) inhibited Fas-induced cell death substantially more than saturating amounts of either dominant-negative protein alone (Figure 7A, lanes 7). Thus, Daxx and FADD activate apoptosis downstream of Fas by distinct but cooperative pathways.
Figure 7. Daxx and FADD Activate Distinct Apoptotic Pathways.
(A) Inhibition of Fas-induced apoptosis by DaxxC and FADD(80–205). HeLa cells were transfected with pEBB-Fas (0.5 μg), pCMV-lacZ (0.5 μg), and plasmids expressing the indicated genes (in μg). Jo2 (12.5 ng/ml) was added 16 hr later; X-Gal staining was done 24 hr after transfection.
(B) FADD(80–205) fails to inhibit Fas-induced JNK activation. JNK kinase activity was assayed after transient transfection of the indicated plasmids (2 μg each) with Flag-JNK (2 μg).
(C) DaxxC does not inhibit FADD-mediated apoptosis. HeLa cells were transfected with the indicated amount (in μg) of FADD and DaxxC and assayed as Figure 4C.
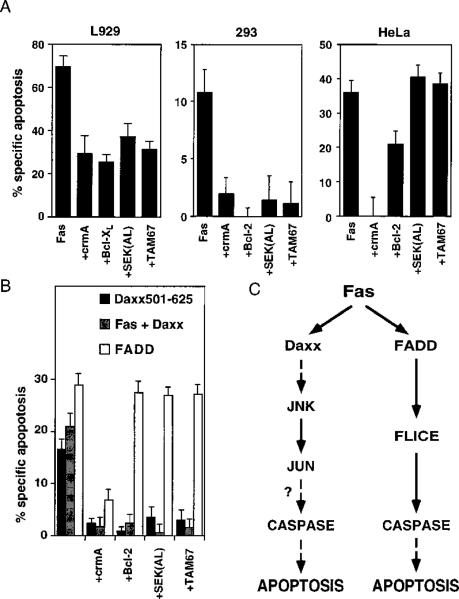
Fas-mediated apoptosis can be inhibited by crmA and, in some cell types, by Bcl-2, a negative regulator of cell death (Itoh et al., 1993; Enari et al., 1995; Los et al., 1995; Tewari and Dixit, 1995; Lacronique et. al., 1996). To dissect the apoptotic pathways initiated by overexpression of Fas, Daxx, and FADD, we tested the ability of crmA, Bcl-2, SEK(AL), and TAM67 to block each apoptotic inducer. SEK(AL) and TAM67 are dominant-negative inhibitors of the JNK pathway. SEK1 is the kinase that phosphorylates and activates JNK; SEK(AL) encodes a mutant that has a single mutation at the ATP-binding site, abrogating the kinase activity (Sanchez et al., 1994). TAM67 is a variant of c-Jun in which amino acids 3–122 have been deleted. This mutant can dimerize and bind DNA but lacks a transcriptional activation domain (Brown et al., 1994). First, we tested the ability of the panel of inhibitor genes to block Fas in several cell types commonly used in apoptosis studies. We found that Fas-induced apoptosis in L929, 293, and HeLa cells can be blocked by crmA and Bcl-2-type inhibitors, but only 293 cells and L929 cells required the JNK pathway for Fas-induced apoptosis (Figure 8A). This result is consistent with the work of Liu et al., who reported that the JNK pathway appeared dispensable for TNF-α-induced apoptosis in HeLa cells (Liu et al., 1996). Kolesnick and colleagues have reported that TNF-α-induced apoptosis is inhibited in U937 human monoblastic leukemia cells that stably express TAM67 (Verheij et al., 1996); these cells are also resistant to Fas-mediated apoptosis (data not shown). Taken together, these observations indicate that the requirement for the JNK pathway in Fas-mediated apoptosis is cell-type specific. We note that this type of dominant-negative experiment gives a positive result only if the protein in question is uniquely required for a particular process. In a case where a negative result (no inhibition) is obtained, it may be that the protein in question is involved but is functionally redundant or in great excess. The continued discovery of JNK relatives makes such scenarios plausible (Gupta et al., 1996).
Figure 8. Inhibition Profile of Daxx- and FADD-Induced Apoptosis.
(A) Inhibition profile of Fas-induced apoptosis in L929, 293, and HeLa cells. Transfection and apoptosis analysis in L/Fas cells were performed as described in Experimental Procedures. As in Figure 4B, 293 cells were cotransfected with pEBB-Fas (2 μg) plus vector or plasmids expressing indicated genes (2 μg each) and pCMV-lacZ (0.5 μg). HeLa cells were transfected with pEBB-Fas (1 μg) plus plasmids expressing the indicated genes (3 μg each) and pCMV-lacZ (0.5 μg); Jo2 (12.5 ng/ml) addition and X-Gal staining were done as in Figure 6B.
(B) Inhibition of profile of Fas+Daxx, Daxx 501–625, and FADD in 293 cells. As in Figure 4B, 293 cells were transiently transfected with Fas, Daxx, Daxx 501–625, or FADD (1 μg each) plus empty vector or plasmids expressing the indicated apoptotic inhibitor genes (3 μg each) and pCMV-lacZ (0.5 μg).
(C) Two pathways of Fas signaling that induce cell death.
Next, we tested the same panel of inhibitor genes on FADD- and Daxx-induced apoptosis. Since full-length Daxx does not induce apoptosis by itself, we used two alternative strategies: we examined the apoptotic response of Fas plus Daxx in 293 cells (where a large fraction of the apoptotic response is Daxx dependent, Figure 4B) and the apoptotic response of Daxx 501–625, the smallest domain that has constitutive cell death activity in 293 cells (Figure 6A). Daxx-dependent apoptosis was blocked by crmA and Bcl-2 and required the JNK pathway, which paralleled the inhibition profile of Fas in 293 cells (Figure 8B). In contrast, FADD, which can not activate JNK, was inhibited only by crmA, but not by Bcl-2, SEK(AL), or TAM67. Similarly, Hsu et al. have shown that TRADD-mediated apoptosis is not blocked by Bcl-2 (Hsu et al. 1995). Consistent with the two pathway model, the residual Fas-induced death remaining after either DaxxC or FADD(80–205) treatment are qualitatively different: the apoptosis remaining after FADD(80–205) treatment is Bcl-2 sensitive, but the apoptosis remaining after DaxxC treatment is not (Figure 7A, lanes 8 and 9). Our results suggest that Fas activates two distinct cell death pathways—one via FADD that is Bcl-2 insensitive and a second one via Daxx that activates JNK and is Bcl-2 sensitive.
Discussion
Daxx Is the Missing Link between Fas and the JNK Pathway
The Fas–FADD–FLICE connection is currently the best understood model for apoptotic signal transduction (Fraser and Evan, 1996; Nagata, 1997), but its inability to explain Fas-induced JNK activation suggests that the current model is at best incomplete. We and other investigators have demonstrated that Fas can robustly activate the JNK pathway (Figure 5; Latinis and Koretzky, 1996; Goillot et al., 1997; Lenczowski et al., 1997). JNK activation is unlikely to be secondary to apoptosis because it is not inhibited by blocking the apoptotic caspases (Figure 5A). The fact that FADD induces cell death but not JNK activation provides powerful evidence that Fas must engage additional signaling molecules to activate JNK. While RIP and TRAF2 are recruited by TNFR1 to activate JNK, currently no known Fas effector can account for its JNK activation. In this study, Daxx emerges as the missing link between Fas and the JNK pathway. Daxx directly binds to the death domain of Fas, and overexpression of Daxx or its effector domain is sufficient to activate the JNK pathway. In addition, a specific dominant-negative inhibitor of Daxx blocks Fas-induced JNK activation. The JNK pathway is activated by many stress stimuli (such as oxidative damage, irradiation, and ischemia-reperfusion) that culminate in apoptosis (Verheij et al., 1996), but the signaling events upstream of the kinase cascades are poorly understood. Daxx may have general importance in coupling other death signals to JNK, and its effector domain should be a useful probe for uncovering the molecular connections that lead to JNK activation.
It is becoming increasingly clear that activation of the JNK pathway can induce apoptosis. For example, ASK1, an activating kinase of the JNK pathway that functions in response to TNF-α is both sufficient to induce apoptosis and is required for TNF-induced cell death (Ichijo et al., 1997). Similarly, MEKK, the upstream kinase of the classical JNK kinase cascade, induced apoptosis upon ectopic expression (Johnson et al., 1996). Because the JNK pathway culminates in the activation of transcription factors, the JNK pathway may directly or indirectly counteract the expression of survival factors, such as NF-kB and Bcl-2. It is well known that in many cell types, Fas-mediated apoptosis is greatly enhanced by transcriptional and translational inhibitors, suggesting an important role for the modulation of gene expression during apoptosis.
However, the involvement of the JNK pathway in apoptosis is commonly analyzed by dominant-negative proteins and may be complicated by several factors. For example, Fas-mediated apoptosis has been reported to not involve JNK in Jurkat cells (Lenczowski et al., 1997) but to require JNK in SHEP cells (Goillot et al., 1997). These results may reflect a cell-type-dependent variation of the relative contribution of Daxx and FADD pathways. Alternatively, a negative result may reflect the functional redundancy of kinase isoforms in the MAP kinase cascades that make up the JNK and p38/Mpk2 pathways. Another possibility is that various tissue culture cell lines are originally derived from different tumors, and oncogenes, such as Ha-Ras, are known to activate the JNK pathway (Hibi et al., 1993; Derijard et al., 1994), giving different basal JNK activity in different cell lines. As the most receptor-proximal protein leading to JNK activation, Daxx may be an experimentally tractable target for dissecting the contribution of JNK signals to Fas-induced cell death. One prime example is in HeLa cells. Although dominant-negative inhibitors of the JNK pathway did not inhibit Fas-induced death in HeLa cells, HeLa cells clearly employ Daxx and the JNK pathway as shown by the ability of DaxxC to block Fas-induced JNK activation and cell death (Figures 6 and 7).
Daxx and FADD Define Two Distinct Fas-Induced Pathways to Apoptosis
In this study, we present several lines of evidence that Daxx and FADD bind to Fas independently and activate distinct cell death signals. Although both Daxx and FADD cannot bind to the lprcg point mutant of Fas, NMR structural analysis of the Fas death domain suggest that this mutant may be unfolded and is thus uninformative for mapping the exact site of binding (Huang et al., 1996). However, Daxx has no evident death domain and interacts with Fas death domain via a novel domain contained in the 112 amino acids of DaxxC, while FADD interacts with Fas via homotypic death domain–death domain interactions. In addition, the Daxx–Fas interaction is not affected by the C-terminal 15 amino acids of Fas (Figure 1), while the FADD–Fas interaction is partially inhibited by this C-terminal “salvation domain” of Fas (Chinnaiyan et al., 1995). These differences between FADD and Daxx are consistent with a model of FADD and Daxx binding to distinct surfaces of the Fas death domain. We have produced mutations in the intracellular tail of Fas that selectively bind either Daxx or FADD (X. Y., H. Y. C., and D. B., unpublished data). Moreover, dominant-negative forms of Daxx and FADD (each consisting of the minimal Fas-binding domains) did not inhibit the heterologous protein's biologic function even at saturating levels (Figure 7). This last result in particular suggests that Daxx and FADD bind to Fas independently in vivo.
Daxx and FADD not only appear to bind differently to Fas but transduce physiologically distinct death signals. As summarized by the model in Figure 8C, FADD directly binds FLICE and is thought to induce apoptosis by recruiting FLICE and activating a caspase cascade. However, this pathway cannot activate JNK. By contrast, Daxx transduces an apoptotic signal via JNK (Figure 8C). Because inhibition of caspases did not block JNK activation but blocked Daxx-induced cell death, the caspases are placed downstream of Daxx and JNK (Figure 8C). The Daxx pathway is also sensitive to Bcl-2 (Figures 7A and 8B). Although either the Daxx- or the FADD-induced pathway alone is sufficient to activate cell death, these two pathways probably work together in vivo. For example, in HeLa cells, both Daxx and FADD act downstream of Fas, and it is necessary to block both death signals to effectively protect the cell from Fas (Figure 7A). Why expand the number of potentially dangerous cell suicide signals if one signal is sufficient? As in the case of growth regulation, the collaboration of multiple signaling pathways allows fine-tuned regulation and creates multiple checkpoints for control. In this instance, incorporation of the Daxx pathway may allow the Fas death signal to be regulated by Bcl-2.
Experimental Procedures
Reagents and Cell Lines
Anti-murine Fas Jo2 antibody was the generous gift of S. Nagata (Ogasawara et al., 1993). Murine TNF-α (Genzyme), monoclonal antibody M2 against Flag epitope (Kodak/IBI), and anti-JNK-1 antibody C17 (Santa Cruz Biotech) were obtained from the indicated sources. HeLa, 293, and L929 were obtained originally from American Type Culture Collection (ATCC). To establish the L/Daxx cell line, HA-Daxx vector was cotransfected with pBabe-puro into L929 cells. Resistant cells were selected in media containing 2.5 μg/ml of puromycin, and HA-Daxx-expressing clones were identified by immunoblot analysis using anti-HA antibody 12CA9. The L929 cells expressing mFas (L/Fas) were established by transfecting pRc/CMV-mFas into L929 cells and subsequently selecting for resistant cells in 600 μg/ml G418. Resistant clones were then screened for Fas expression by FACScan using Jo2 antibody.
Plasmid Construction
DNA fragments for most plasmid constructs were obtained by PCR amplification using Pfu polymerase (Stratagene) and primers incorporated with appropriate restriction sites and epitope tags as needed. The fragments for LexA and transcription activator fusions were cloned into plasmid pEG202 and pJG4–5 (Gyuris et al., 1993), respectively. The I225N mutation in LexA-lprcg was made by PCR site-directed mutagenesis. GST constructs were made in pGEX vector (Pharmacia). In vitro translation constructs were made in pET3a (Novagen) or 6HisT-pRSET (A. Hoffmann, MIT) for DaxxC. Daxx(–162) was obtained by digestion of a full-length Daxx construct with HindIII. For expressing proteins in mammalian cells, full-length Daxx and FADD and their mutants were cloned into pEBB with a hemagglutinin (HA) epitope tag at the 5′ end. Full-length murine Fas was cloned into pEBB and pRc/CMV (Invitrogen). Fas intracellular region (amino acids 165–306) was fused to GST in pEBG, a derivative of pEBB expressing GST. Full-length murine TNFR1 was cloned into pEBB. Each construct was confirmed by partial DNA sequence and by immunoblot analysis.
The following plasmids were described in or obtained from the indicated sources: LexA-CD40IC (G. Cheng, UCLA), LexA-Bicoid (Gyuris et al., 1993), pHD1.2 (crmA) and pBabe-Bcl-2 (J. Yuan, Harvard), Bcl-XL (R. J. Lutz, Apoptosis Inc.), TAM67 and Flag-JNK (M. Karin, UCSD), pEBG-SEK(AL) (C. J. Der, UNC), SRE-L-Luc (R. Treisman, ICRF, UK).
Two-Hybrid Screen and β-Galactosidase Assay
The two-hybrid screen was performed essentially as described (Gyuris et al., 1993). Among 1 × 106 library plasmids screened, 33 interacting clones were obtained. The cDNA inserts of the library plasmids within those colonies were isolated and grouped. Representative plasmids from each group were retransformed back into yeast to test their interaction with different baits.
For assaying β-Galactosidase expression, each pair of DNA-binding (LexA) and activation hybrids were cotransfected together the lacZ reporter plasmid pSH18–34 (Gyuris et al., 1993) into EGY48 yeast cells. Filter lift assay for colony color and quantitative liquid assay were done as described (Yang et al., 1994) for 3–6 independent transformants.
cDNA Cloning and Northern Blot Analysis
The cDNA insert in clone A21 was used to screen a murine thymus cDNA library (Y. W. Choi, Rockefeller Univ.). Sequences of the longest cDNA clone (2.4kb) as well as the cDNA inserts of clone A21 were determined on both strands with an automated sequencer (Applied Biosystem). Sequence comparison was done by using GeneWorks program of IntelliGenetics, Inc. The Daxx amino acids 501–739 exhibit 57% sequence identity to clone A21.
For Northern analysis, the C-terminal 0.7 kb fragment of murine Daxx and a human β-actin cDNA (Clontech) were used to probe a mouse multiple tissue Northern blot (Clontech), according to the manufacturer's protocol.
In Vitro Binding and Coprecipitation Assays
GST fusions were purified as described (Smith and Johnson, 1988). 35S-proteins were made with TNT Reticulocyte Lysate System (Promega). 35S-proteins were incubated with 10 μg of each GST fusion protein in 0.1 ml of modified E1A buffer (Hsu et al., 1996) with 50 mM NaCl and 10% glycerol for 1–2 hr, washed three times, and analyzed by SDS–PAGE and autoradiography. A fraction of the reaction mixture was analyzed by Coomassie staining to visualize GST fusion proteins.
For testing association in mammalian cells, HA-Daxx and HA-DaxxΔC were cotransfected with GST-FasIC or GST into 293 cells by calcium phosphate method. Thirty-six hours after cotransfection, cells were solubilized in E1A buffer, precipitated with glutathione beads (Molecular Probes), washed three times, and immunoblotted for HA using ECL (Amersham). Comparable levels of GST fusions were verified by immunoblotting for GST.
Apoptosis Assays
Cells were plated onto 6-well dishes the day before transfection at 2 × 105 cells/well for Hela and 293 cells and 5 × 105 for L/Daxx and L/EBB cells. The wells were precoated with 0.2% gelatin for 293 cells. HeLa and 293 cells were transfected by the calcium phosphate precipitation method and L/Daxx and L/EBB with lipofectamine (GIBCO-BRL). X-Gal staining was done for 4 hr to overnight. The percentage of apoptotic cells was determined by the number of blue cells with apoptotic morphology divided by the total number of blue cells. Specific apoptosis was calculated as the percentage of blue cells with apoptotic morphology in each experimental condition minus the percentage of blue cells with apoptotic morphology in pEBB vector–transfected cells. pEBB vector control transfection was always done in parallel and had about 5% or less apoptotic cells. At least 400 cells from four random fields were counted in each experiment, and the data shown are the average and SD of at least three independent experiments.
For L929 apoptosis assay in Figure 8, L/Fas cells were seeded onto 6-well plates at 2.5 × 105 cells/well. On the next day, cells were cotransfected with 200 ng of pHook-1 plasmid (Invitrogen) and 400 ng of crmA, Bcl-xL, or SEK(AL) with lipofectamine. Twenty-four hours after transfection, cells were removed from the dish in 1 ml of PBS/3 mM EDTA. Magnetic beads (1.5 × 106) were added to the cells and incubated at 37°C for 30 min. The cells were then washed three times in media, counted, and plated in duplicate in 96-well plates (~5000 cells/well). After culture for 14–16 hours, Fas killing was induced using 1 μg/ml Jo2 antibody and 0.5 μg/ml actinomycin D and measured by counting the number of surviving cells in four random fields 24 hr later.
JNK Activity and Reporter Gene Assays
HeLa and 293 cells were transfected in 60 mm dishes with Flag-JNK plus the indicated expression plasmids by the calcium phosphate method. Approximately 24 hr after transfection, cells were serum starved for 14–16 hours. Cells transfected with Fas were treated with 0.5 μg/ml of Jo2 antibody for 30 min. To test for the effect of protease inhibitors, cells were treated with 0.5 μg/ml of Jo2 and 100 μM of ICE inhibitor Z-Val-Ala-Asp-CH2F or CPP32 inhibitor Z-Asp-Glu-Val-Asp-CH2F (Enzyme Systems Products, CA) for 30 min. Flag-JNK was immunoprecipitated with anti-Flag antibody, and in vitro kinase assay with 1 μg of GST-cJun (1–79) was performed as previously described (Khosravi-Far et al., 1996). JNK1 kinase activity in L929 cells stably expressing Fas or Daxx was measured by immunoprecipitation of the endogenous JNK-1 using anti-JNK-1 C-17 antibody. TNFR1- and Fas-transfected cells were treated with 20 ng/ml TNF-α for 10 min and 0.5 μg/ml Jo2 for 30 min, respectively.
SRE-L reporter gene assay: 293 cells were cotransfected with 1 μg of the indicated plasmids with 1 μg of SRE-L-Luc reporter construct (Hill et al., 1995). After an incubation of 24 hr, cells were switched to 0.5% FCS media for 14–16 hr. Cell lysates were prepared in 250 μl of reporter lysis buffer (Promega), and 20 μl of the lysate was assayed in Luminometer with 100 μl of ATP and Luciferin re-agents (Promega) as described (Khosravi-Far et al., 1996).
Acknowledgments
We are indebted to Dr. Genhong Cheng for his help in the initial phase of this project. We thank Drs. R. Brent, Y. W. Choi, C. J. Der, D. V. Goeddel, A. Hoffmann, M. Karin, R. N. Kolesnick, P. H. Krammer, R. J. Lutz, S. Nagata, R. Treisman, D. Wallach, and J. Yuan their generous gifts of reagents, and S. W. Fesik for examining the Daxx sequence. We thank P. Svec and Y. Li for excellent technical assistance, and Drs. A. J. Koleske, B. Chen, Z. Songyang, Y. Yamanashi, I. Stancovsky and other members of Baltimore lab for valuable advice. X. Y. and R. K.-F. are fellows of the Leukemia Society of America and Irvington Research Institute, respectively. H. Y. C. is supported by the Medical Scientist Training Program at Harvard Medical School. D. B. is an American Cancer Society Research Professor. This work was supported by the NIH grant CA51462.
Footnotes
References
- Abbas AK. Die and let live: eliminating dangerous lymphocytes. Cell. 1996;84:655–658. doi: 10.1016/s0092-8674(00)81042-9. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1-and TNF receptor–induced cell death. Cell. 1996;85:803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH, Wallach D. Self-association of the “death domains” of the p55 tumor necrosis factor (TNF) receptor and Fas/Apo1 prompts signalling for TNF and Fas/Apo1 effects. J. Biol. Chem. 1995a;270:387–391. doi: 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- Boldin MP, Varfolomev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J. Biol. Chem. 1995b;270:7795–7798. doi: 10.1074/jbc.270.14.7795. [DOI] [PubMed] [Google Scholar]
- Brown PH, Chen TK, Birrer MJ. Mechanism of action of a dominant-negative mutant of c-Jun. Oncogene. 1994;9:791–799. [PubMed] [Google Scholar]
- Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM. FADD, a novel death domain–containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- Chinnaiyan AM, Tepper CG, Seldin MF, O'Rourke K, Kischkel FC, Hellbardt S, Krammer PH, Peter ME, Dixit VM. FADD/MORT is a common mediator of CD95 (Fas/APO-1) and tumor necrosis factor receptor–induced apoptosis. J. Biol. Chem. 1996;271:4961–4965. doi: 10.1074/jbc.271.9.4961. [DOI] [PubMed] [Google Scholar]
- Cleveland JL, Ihle JN. Contenders in Fas/TNF death signaling. Cell. 1995;81:479–482. doi: 10.1016/0092-8674(95)90068-3. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu I-H, Barret T, Su B, Deng T, Karin M, Davis RJ. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- Enari M, Talanian RV, Wong WW, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- Fraser A, Evan G. A license to kill. Cell. 1996;85:781–784. doi: 10.1016/s0092-8674(00)81005-3. [DOI] [PubMed] [Google Scholar]
- Goillot E, Raingeaud J, Ranger A, Tepper RI, Davis RJ, Harlow E, Sanchez I. Mitogen-activated protein kinase– mediated Fas apoptotic signaling pathway. Proc. Natl. Acad. Sci. USA. 1997;94:3302–3307. doi: 10.1073/pnas.94.7.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B, Davis RJ. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15:2760–2770. [PMC free article] [PubMed] [Google Scholar]
- Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphotase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and phosphorylates the c-Jun activation domain. Genes Dev. 1993;7:2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hsu H, Xiong J, Goeddel DV. The TNF receptor 1–associated protein TRADD signals cell death and NF-kB activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- Hsu H, Shu H-B, Pan M-G, Goeddel DV. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature. 1996;384:638–641. doi: 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Itoh N, Nagata S. A novel protein domain required for apoptosis. J. Biol. Chem. 1993;268:10932–10937. [PubMed] [Google Scholar]
- Itoh N, Tsujimoto Y, Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J. Immunol. 1993;151:621–627. [PubMed] [Google Scholar]
- Johnson NL, Gardner AM, Diener KM, Lange-Carter CA, Gleavy J, Jarpe MB, Minden A, Karin M, Zon LI, Johnson GL. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J. Biol. Chem. 1996;271:3299–3237. doi: 10.1074/jbc.271.6.3229. [DOI] [PubMed] [Google Scholar]
- Khosravi-Far R, White MA, Westwick JK, Solski PA, Chrzanowska-Wodnicka M, van Aelst L, Wigler MH, Der CJ. Oncogenic Ras activation of Raf/mitogen-activated protein kinase–independent pathway is sufficient to cause tumorigenic transformation. Mol. Cell. Biol. 1996;16:3923–3933. doi: 10.1128/mcb.16.7.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis JM, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokine. BioEssay. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- Lacronique V, Mignon A, Fabre M, Viollet B, Rouquet N, Molina T, Porteu A, Henrion A, Bouscary D, Varlet P, et al. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat. Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- Latinis KM, Koretzky GA. Fas ligation induces apoptosis and Jun kinase activation independently of CD45 and Lck in human T cells. Blood. 1996;87:871–875. [PubMed] [Google Scholar]
- Lenczowski JM, Dominguez L, Eder AM, King LB, Zacharchuk CM, Ashwell JD. Lack of a role for Jun kinase and AP-1 in Fas-induced apoptosis. Mol. Cell. Biol. 1997;17:170–181. doi: 10.1128/mcb.17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.-g., Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NF-kB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- Los M, Van de Craen M, Penning LC, Shenk H, Westendorp M, Baeuerle PA, Droge W, Krammer PH, Fiers W, Schulze-Osthoff K. Requirement of an ICE/CED3 protease for Fas/APO1-mediated apoptosis. Nature. 1995;375:81–83. doi: 10.1038/375081a0. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/Apo1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Ogasawara J, Watanabe-Fukunaga R, Adachi M, Matsuzawa A, Kasugai T, Kitamura Y, Itoh N, Suda T, Nagata S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Townsend SE, Xu JC, Flavell RA, Goodnow CC. Expansion or elimination of B cells in vivo: dual roles for CD40- and Fas(CD95)-ligands modulated by the B cell antigen receptor. Cell. 1996;87:319–329. doi: 10.1016/s0092-8674(00)81349-5. [DOI] [PubMed] [Google Scholar]
- Sanchez I, Hughes RT, Mayer BJ, Yee K, Woodgett JR, Avruch J, Kyriakis JM, Zon LI. Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature. 1994;372:794–798. doi: 10.1038/372794a0. [DOI] [PubMed] [Google Scholar]
- Smith CA, Farrah T, Goodwin RG. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell. 1994;76:959–962. doi: 10.1016/0092-8674(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in E. coli as fusions with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, Ayres TM, Wong GHW, Goeddel DV. A novel domain within the 55 kD TNF receptor signals cell death. Cell. 1993;74:845–853. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Tewari M, Dixit VM. Fas- and tumor necrosis factor–induced apoptosis is inhibited by the poxvirus crmA gene product. J. Biol. Chem. 1995;270:3255–3260. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- Verheij M, Bose R, Lin XH, Yao B, Jarvis WD, Grant S, Birrer MJ, Haimovitz-Friedman A, Fuks Z, Kolesnick RN. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R, Brannan CI, Itoh N, Yonehara S, Copeland NG, Jenkins NA, Nataga S. The cDNA structure, expression, and chromosomal assignment of the mouse Fas antigen. J. Immunol. 1992;148:1274–1279. [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Yang X, Jiang R, Carlson M. A family of proteins containing a conserved domain that mediates interaction with the yeast SNF1 protein kinase complex. EMBO J. 1994;13:5878–5886. doi: 10.1002/j.1460-2075.1994.tb06933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]