Abstract
The standard method for the measurement of blood pressure (BP) in clinical practice has traditionally been to use readings taken with the auscultatory technique by a physician or nurse in a clinic or office setting. While such measurements are likely to remain the cornerstone for the diagnosis and management of hypertension for the foreseeable future, it is becoming increasingly clear that they often give inadequate or even misleading information about a patient’s true BP status. All clinical measurements of BP may be regarded as surrogate estimates of the “True” BP, which may regarded as the average level over prolonged periods of time. In the past 30 years there has been an increasing trend to supplement office or clinic readings with out-of-office measurements of BP, taken either by the patient or a relative at home (home or self-monitoring- HBPM) or by an automated recorder for 24 hours (ambulatory blood pressure monitoring- ABPM).
Of the two methods HBPM has the greatest potential for being incorporated into the routine care of hypertensive patients, in the same way that home blood glucose monitoring performed by the patient has become a routine part of the management of diabetes. The currently available monitors are relatively reliable, easy to use, inexpensive, and accurate, and are already being purchased in large numbers by patients. Despite this, their use has only been cursorily endorsed in current guidelines for the management of hypertension, and there have been no detailed recommendations as to how they should be incorporated into routine clinical practice. And despite the fact that there is strong evidence that HBPM can predict clinical outcomes and improve clinical care, the cost of the monitors is not generally reimbursed. It is the purpose of this Call to Action paper to address the issues of the incorporation of HBPM into the routine management of hypertensive patients and its reimbursement.
Health and economic consequences of hypertension and its inadequate control in the US
Hypertension affects over 65 million persons in the US, according to analyses of data from the National Health and Nutrition Examination Survey (NHANES), 1999 – 2000 (1). In this analysis, a person was classified as having high BP by having a systolic BP of 140 mm Hg or higher or a diastolic BP of 90 mm Hg or higher, taking BP lowering medications, or being told at least twice by a physician or other health professional that they had high BP(1). This estimate may be considered conservative because it does not include the additional persons with systolic BP of at least 130 mm Hg or diastolic BP of at least 80 mm Hg with either diabetes mellitus or chronic kidney disease who would be classified as having high BP according to the definition put forward by the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) (2). Worldwide estimates approach one billion people with high BP (3).
High BP increases the risk of total mortality, mortality due to heart disease, stroke, chronic kidney disease, and heart failure, as well as morbidity associated with non-fatal cardiovascular disease events (2). Based on estimates of population attributable fractions, high BP may account for 27% of total cardiovascular disease events in women and 37% in men (4), 14% of myocardial infarctions in men and 30% in women (5), 35% of ischemic strokes (6), 39% of chronic heart failure events in men and 59% in women (7), and 56% of chronic kidney disease (8). These results, based on North American populations, are supported by global estimates. In the Global Burden of Disease Project, a systolic BP threshold of 115 mm Hg was used to distinguish between optimal and non-optimal BP levels. Globally, 62% of stroke, 49% of coronary heart disease and 14% of other cardiovascular disease was attributable to non-optimal BP. Approximately 12.8% of all deaths (7.1 million) and 4.4% of all disability life years lost (64.3 million) in the year 2000 were due to cardiovascular disease attributable to non-optimal BP levels (9). Clearly, high BP is a major cause of mortality and morbidity in the US and worldwide.
Randomized, controlled trials have provided convincing evidence that BP-lowering treatment reduces the risk of total mortality, stroke, coronary heart disease, heart failure and chronic kidney disease (2). Consequently, clinical practice guidelines have been promulgated in the US and elsewhere to promote detection, treatment and control of high BP (2). Despite 30 years of attention to high BP control in the US, current levels of control are suboptimal. Based on data from NHANES 2003-4, 76% of persons with high BP had been told that their BP was high, 65% were on treatment with BP lowering medications, and only 37% were controlled to BP levels less than 140 mm Hg systolic and less than 90 mm Hg diastolic (10). These proportions mask ethnic disparities. The proportion aware of having high BP was 67% among non-Hispanic whites, 66% among non-Hispanic blacks, , and 63% among Mexican Americans. The proportion on treatment varied from 55% among non-Hispanic blacks, to 54% among non-Hispanic whites, and 48% among Mexican Americans. The proportion with controlled BP was highest in non-Hispanic whites (35%), intermediate in non-Hispanic blacks (29%) and lowest in Mexican Americans (26%) (10).
The direct and indirect cost of high BP and its complications was estimated to be $63.5 billion (B) in the US in 2006 (11). This figure is almost certainly an under-estimate of the true costs of the complications of high BP, because, in this analysis, the cost attributable to hypertensive disease was distinguished from the costs attributed to coronary heart disease ($142.5 B), stroke ($57.9 B) and chronic heart failure ($29.6 B) (11), and, as documented above, high BP is a major contributor to these forms of cardiovascular disease. Given the substantial mortality, morbidity and cost associated with poorly controlled BP in the US and other countries, identification of low cost strategies to improve control of high BP should be a high priority.
Recommendations of professional organizations on the use of HBPM
The use of HBPM is recommended by several national and international guidelines for the management of hypertension, including the European Society of Hypertension (12), the American Society of Hypertension (13), the American Heart Association (14), the British Hypertension Society (15), the European Society of Hypertension (16), the Japanese Hypertension Society (17), the World Health Organization –International Society of Hypertension (18) and JNC 7 (2), which is the generally accepted guideline for the US. For the most part, the recommendations from the various organizations are similar, as outlined below, although there are some minor differences.
The levels of HBPM considered normal by the majority of the guidelines is a BP of <135 mm Hg systolic and 85 mm Hg diastolic. The Japanese guidelines regard “definite normotension” as a pressure below 125/75 mmHg, and “definite hypertension” as above 135/85, and the British Hypertension Society stated that home BP levels of <130/85 can probably be regarded as normal (15). The World Health Organization –International Society of Hypertension Guidelines recommended an upper limit of 125/80 mmHg (18)
The use of accurate and properly validated automated digital BP monitors is strongly encouraged. Monitors must have passed at least one of three accepted validation protocols.
Adequate patient education on the use of BP monitors should precede any recommendation for self-monitoring of home BP.
The indications for HBPM include the assessment of white coat hypertension, and the monitoring of effective BP control in conjunction with office BP measurement.
There is a lack of data on the accuracy and use of HBPM in pregnant women and obese patients.
Current usage of HBPM
The use of home monitors has been increasing steadily over the past few years. A Gallup poll of hypertensive patients conducted in 2005 (19) obtained the following results:
The number of patients monitoring their BP at home has increased steadily over the past 5 years, being 38% in 2000 and 55% in 2005- an increase of 17%.
The proportion of patients owning a monitor has increased from 49% in 2000 to 64% in 2005.
In 2000, 35% of patients reported that a doctor recommended their using a home monitor, and in 2005 this was 47%.
86% of patients who had been advised to purchase a monitor had done so; only 46% of patients who had received no recommendation from their doctors had bought monitors.
The use of home monitors is more common in older and more affluent patients.
35% of hypertensive patients now check their BP at least once a week.
The most commonly used monitors are those that go on the upper arm and are self-inflating; the use of wrist monitors is growing rapidly, and they now are used by 22% of patients who own monitors.
Of patients who do not own monitors, 14% said that expense was the reason.
A recently published survey of 855 hypertensive patients attending specialized clinics in Italy found that 75% were regularly performing HBPM (20). Users tended to be younger and better educated than non-users; 58% used electronic devices that recorded from the upper arms, and 19% used wrist monitors. .
Physicians are also becoming enthusiastic about the use of HBPM. A survey of family practitioners in Hungary found that 90% recommended the use of HBPM (21). The physicians’ main concerns were the use of non-validated devices, the possibility that patients would become obsessional about their BP, and the lack of proper training in the use of the monitors. A survey of pediatric nephrologists in Germany found that 70% prescribed the use of HBPM for children with renal disease and hypertension (22).
Techniques for performing HBPM
When HBPM was first used, BP was measured using the auscultatory technique (23), but this has now been almost completely supplanted by the use of oscillometric devices specifically designed for use by patients in the home. These are mostly fully automatic, such that all the patient needs to do is to wrap the cuff round the upper arm, and press a button for the machine to take a reading, and display the values for systolic and diastolic pressure on a screen. Some require the patient to inflate the cuff manually.
Arm Monitors
Monitors which measure the BP in the brachial artery with a cuff placed on the upper arm continue to be the most reliable, and have the additional advantage that the brachial artery pressure is the measure that has been used in all the epidemiological studies of high BP and its consequences. For the majority of patients, these are the preferred type of monitor.
Wrist Monitors
Wrist monitors are the most convenient type to use, and are preferred by many patients. They have the potential advantage that they can be used in obese individuals in whom putting a cuff on the upper arm is difficult. A potential disadvantage is that the wrist must be held at the level of the heart when a reading is being taken, which increases the possibility of erroneous readings (24). A recently introduced model avoids this problem by only taking readings when the wrist is held over the heart. Experience with wrist monitors is relatively limited at present, and most of the monitors that have been tested have failed the validation studies (see (http://www.dableducational.org). They are therefore not generally recommended for routine clinical use.
Finger Monitors
These devices have been found to be very inaccurate and should not be used (25).
Testing and Validation of Monitors
Patients should be advised to use only monitors that have been validated for accuracy and reliability according to standard international testing protocols. The original two protocols that gained the widest acceptance were developed in the US by the Association for the Advancement of Medical Instrumentation (AAMI) in 1987 and the British Hypertension Society (BHS) in 1990, with revisions to both in 1993. These required testing of a device against two trained human observers in 85 subjects, which made validation studies difficult to perform. One consequence of this has been that there are still many devices on the market that have never been adequately validated. More recently, an international group of experts who are members of the European Society of Hypertension Working Group on Blood Pressure Monitoring have produced an International Protocol that is replacing the two earlier versions (26), and is easier to perform. Briefly, it requires comparison of the device readings (4 in all) alternating with 5 mercury readings taken by two trained observers in 33 patients. Devices are recommended for approval if both systolic and diastolic readings taken are at least within 5 mm Hg of each other for at least 2 of each subject’s 3 readings in 22 out of the 33 subjects.
Unfortunately, only a few of the devices that are currently on the market have been subjected to proper validation tests such as the AAMI and BHS protocols, and several devices have failed the tests. An up-to-date list of validated monitors is available on the Dabl Educational web site (http://www.dableducational.org) and the British Hypertension Society website (http://www.bhsoc.org/default.stm).
The fact that a device passed a validation test does not mean that it will provide accurate readings in all patients. There can be substantial numbers of individual subjects in whom the error is consistently greater than 5 mm Hg with a device that has achieved a passing grade (27). This may be more likely to occur in elderly (28) or diabetic patients (29). At least one home monitor has been found to be accurate in patients with end-stage renal disease (30). For this reason it is recommended that each oscillometric monitor should be validated on each patient before the readings are accepted. No formal protocol has yet been endorsed for doing this, but if sequential readings are taken with a mercury sphygmomanometer and the device as described below, major inaccuracies can be detected.
Checking Monitors for Accuracy
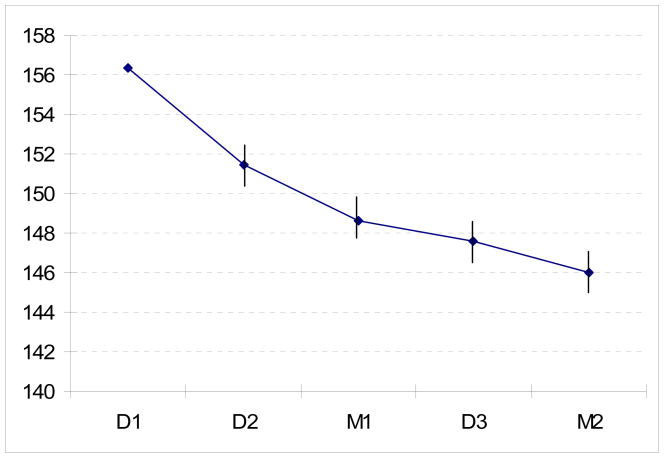
When patients get their own monitor it is very important to have them bring it in to the clinic to check their technique and also the accuracy of the monitor. A simple and practical version of the European Society of Hypertension Protocol has been developed for this purpose, and can be done in less than 10 minutes by the physician or other healthcare provider and the patient. The patient sits at the physician’s desk with the monitor set up and the arm resting on the desk. Five sequential same-arm BP readings are recorded with a gap of no more than about 30 seconds between readings. The first two (D1 and D2) are taken by the patient using their device; the third (M1) by the physician using a mercury sphygmomanometer; the fourth (D3) by the patient; and the fifth (M2) by the physician. There is a tendency for the BP to decline during this process (See Figure 1). The accuracy of the device can be assessed by comparing the device and mercury readings, although exact criteria for determining acceptability have not been established.
Figure 1.
Systolic pressure recorded during clinical validation of home monitors in 92 consecutive patients. D1-3 are readings taken with the patient’s device, and M1-2 are mercury readings taken by a physician. (Unpublished data).
Patient education
It is critical that patients should be educated in the proper use of home monitors. Automated oscillometric devices are much easier to use than auscultatory monitors, but still require some training. Patients should be advised to only purchase monitors that have been validated according to standard protocols (see above), and their upper arm circumference should be measured so that they can be advised if they need a large cuff. They should be told that readings should be taken when they are sitting quietly after resting for 5 minutes, with the arm supported on a flat surface, such that the upper arm is supported at the level of the heart. The patient’s back should be supported, and both feet should be flat on the floor. The cuff should be positioned so that its mid-portion lies over the brachial artery. Most patients find it easiest to measure BP in the non-dominant arm, and this should be encouraged unless there is a marked difference between the two arms, which is relatively rare in the absence of obstructive arterial disease (31). The patient should not have indulged within the 30 minutes preceding the measurement in activities such as smoking, drinking coffee, or exercising, which are likely to affect the blood pressure. It is recommended that at least 2 and preferably 3 readings be taken at one time, and the value for each reading written down, unless the device has a memory which stores the readings automatically. The interval between readings can be as little as 1 minute (32). Readings should routinely be taken first thing in the morning (preferably before taking medications) and at night before going to bed. The frequency of readings can be determined by the physician. Patients should not be encouraged to take readings at other times, such as when they think they are under stress or that their BP is high. Patients need not routinely keep diaries, but it may be helpful to record if they missed taking their medications.
Patients should be advised that the variability of readings is high, and that individual high or low readings have little, if any, significance.
Once a monitor has been purchased, it is recommended that the patient should bring it into the office, both to verify the patient’s technique and the accuracy of the device. This procedure should be repeated annually. Unlike aneroid and mercury devices, however, it has been found that the accuracy of the measurement of the cuff pressure does not deteriorate over time with oscillometric monitors (33).
Contra-indications to HBPM
There are some patients in whom HBPM is contra-indicated. The oscillometric method may not work well in patients who have atrial fibrillation or other arrhythmias such as frequent ectopic beats. In such patients it may be worth checking the ability of a monitor to measure BP in the clinic by comparing the monitor readings against those taken with the auscultatory method.
Some patients may become obsessional about taking readings. The inherent variability of BP means that there will inevitably be some high readings, which in anxious patients may exacerbate their anxiety, leading to further increases of BP, and effectively setting up a vicious cycle. In such patients frequent checking of their BP should be discouraged, and in extreme cases discontinued altogether.
Information Provided by HPBM
Although office BP measurement has been the foundation of the diagnosis and management of hypertension, when the Korotkoff sound technique is used, there are many sources of inaccuracy (e.g., noisy environment, impaired hearing, soft Korotkoff sounds, leaky bulb etc.) (34). Additionally, office BPs have been reported to be less reliable when compared to both home and ambulatory measurements; they have also been found to vary depending on the health care provider conducting the measurement, and subject to terminal digit preference (observer tendency to record measurement using certain digits, e.g., 0 in the units position, more frequently than other digits, e.g., 7) (35–41). Importantly, office measurements are associated with white coat hypertension and the risk of false positive diagnoses of hypertension and needless prescription of medications (42;43).
HBPM as an alternative to the office BP reading can no longer be overlooked as a significant adjunct to assessment and treatment of individuals with hypertension. The following paragraphs will review data about the quality and type of increased information that HBPM can provide health care providers.
Information is Reliable and Reproducible
One of the advantages of HBPM is that large numbers of readings can be used to define a patient’s BP level. Stergiou et al. (44) compared the reproducibility of BP measured in the office (5 visits within 3 months), in the home (6 workdays within 2 weeks) and by ABPM (twice, 2 weeks apart). Reproducibility was quantified using the standard deviation of the differences between repeated measurements. The researchers found that home BP provided the lowest standard deviation of the differences (6.9/4.7 mm Hg for systolic and diastolic pressures), compared with clinic (11.0/6.6 mm Hg) and ambulatory pressures (8.3/5.6 mmHg) and therefore have superior reproducibility. The reason why home readings are more stable than ABPM readings may be because the conditions in which they are taken are less variable.
Long term reproducibility was examined in a sample of 136 untreated subjects who measured their BP at home at least three times on at least 3 days in each of two 4-week periods separated by one year (45). Two clinic BPs were also obtained from subjects at each of two health examinations also separated by one year. The mean differences between the first and second home BP (0.8 ± 7.7 mm Hg for SBP and 0.9 ± 5.5 mm Hg for DBP) were significantly smaller than those for the clinic BP (−3.9 ± 13.8 mm Hg for SBP and −3.1 ± 10.2 for DBP) (p<.001, for both comparisons). These findings suggest that home BP measurements are more reproducible over time than office BP.
Another aspect of the reliability of HBPM is how accurate patients are in reporting the readings displayed by the monitors. This issue has been looked at by providing patients with monitors that, unknown to the patients, have memory. When patients’ reported readings are compared with those stored in the memory it has been found that there is often poor agreement between them. In one study 20% of readings were reported with an error of more than 10 mmHg, and the error rate was higher in patients with less well controlled hypertension (46). In another there was a consistent tendency for high readings to be under-reported (47). Thus patients may tend to make their home readings look better than they really are, and for this reason monitors with memory are to be encouraged.
The Number of HBP Measurements Needed to Ensure a Reliable Estimate of the True BP
The reproducibility of HBP measurements is heavily dependent on the number of measurements that are averaged. One study demonstrated that the maximal reduction in the standard deviation of the mean difference between the average values of two HBPM sessions is obtained when the average value is based on at least 30 readings (three measurements per day for 10 days) (48). Others have suggested no further improvement is obtained by increasing the number above five (49), and that the improvement in the measurement precision is obtained with at least six home measurements (50;51).
There is some agreement that correlations with ABP are more reliable if the first day’s home BP readings are discarded (52;53). Two recent analyses have recommended taking between 8 and 15 readings in total (53;54), and we recommend following the last set of ESH guidelines to take at least 2 morning and 2 evening readings every day for 1 week (16), but to discard the readings of the first day, which gives a total of 12 readings on which to make clinical decisions.. Getting multiple readings is particularly important for the initial diagnosis of hypertension, but the same procedure is also recommended to be performed at intervals in patients whose condition is thought to be stable, and who require long term follow-up. Patients should be instructed to record all the readings that they take.
Information about the “True BP” Level
BP fluctuates continuously in a 24-hour period, and the variability is influenced by neural, mechanical, and humoral factors (55;56). Patient related factors, for example hurrying to get to a clinic visit, or impatience over waiting to be seen, are also associated with BP variability. BP readings in the office tend to reflect the patient’s status at the moment, and may not be a true representation of the BP outside the office (57). It is difficult to determine true BP level based on 1 or 2 BP measurements at the time of an office visit. HBPM is a simple and inexpensive way for obtaining a large number of readings, representative of usual BPs over long periods of time, that are unaffected by the white coat effect (the increase of BP that occurs during an office visit) or other factors influencing variability that are present in the office (58). Patterns of BP rather than isolated measurements can be important in confirming the diagnosis of hypertension. For patients found hypertensive in the office, high BPs measured at home may confirm the diagnosis, while low HBP levels may indicate a need for further assessment with ambulatory BP measurement for identification of white coat hypertension (59).
A recent development in the measurement of clinic BP is the introduction of automated oscillometric devices which can take multiple (2–6) readings in the clinic in the absence of a physician. They have the potential advantage over traditional clinic measurement that they reduce the white coat effect (hence they are consistently lower than physicians’ readings) (60), and are closer to the daytime average measured with ABPM (61). Data are lacking for comparisons with HBPM.
Another technique that has been used by patients to monitor their BP out of the office is the use of automated devoices in malls and supermarkets. These devices may be inaccurate, and their use is not encouraged.
Information about BP at Different Times of the Day
The pattern of BP change over the day may vary considerably from one patient to another, depending on their daily routine. Thus in Japanese studies, the evening pressure tends to be lower than in the morning, which has been related to the fact that Japanese often take baths in the evenings, after which the BP is reduced (62). Other studies have found that evening readings are higher (63;64). The morning pressure may be higher if the patient has drunk alcohol the night before (65), or has sleep apnea (66). Antihypertensive treatment may also have a major influence (67). There is some evidence that the morning pressure may be a better predictor of risk than the evening pressure (68;69). For these reasons, it is generally recommended that patients should take readings both in the early morning and at night. The main limitation of home monitors in comparison with 24 hour ambulatory monitors is that nighttime readings cannot be taken. However, monitors are being developed that can be programmed to take a limited number of readings during the night.
HBPM for diagnosing hypertension
The diagnosis of hypertension may be expedited by HBPM, particularly in individuals with Stage 1 hypertension where the elevation of BP is relatively modest (typically those without diabetes, chronic kidney disease, or target organ damage). Often individuals with white coat hypertension may make multiple office visits over a prolonged period of months before the diagnosis of hypertension is established. Home BP is usually lower than office BP (as a result of the white coat effect), and may suggest a diagnosis of white coat hypertension. But in about 10% of patients it may be higher, indicating a possible diagnosis of masked hypertension (70). As described below, there is increasing evidence that HBP may give a better prediction of risk than office BP, so any discrepancies between office and HBP should be taken seriously.
Evaluation of White Coat Hypertension and the White Coat Effect
National hypertension guideline committees from the US (2), Europe (16), Canada (71),(16) and Japan (17) have all endorsed the use of HBPM to confirm or refute the diagnosis of white coat hypertension, which is defined as high BP occurring only in a medical care setting, and which has been reported in as many as 20% of patients in whom hypertension has been diagnosed using office BP (72–74). The phenomenon that leads to it is called the white coat effect, which is usually defined as the difference between the office BP and the BP measured at home or during the day using ABPM, and which has been attributed to anxiety, a hyperactive alerting response, or a conditioned response (42). The white coat effect is typically positive, and is present in the majority of hypertensive patients, but in some patients with low office BP it may be negative (HBP higher than office BP). If the HBP is normal (<135/85 mmHg) a diagnosis of white coat hypertension may be considered.
White coat hypertension is more common in the elderly, and is generally associated with a relatively benign prognosis that is similar to what is seen in truly normotensive subjects, as shown by several prognostic studies comparing office BP and ABP (75;76). However, with longer term follow-up (e.g. 6–11 years) there have been reports of higher CVD event rates that are similar to those seen in patients with sustained hypertension (77;78). The implication of these results is that out-of-office monitoring (HBPM and/or ABPM) should be conducted long-term in all patients diagnosed with white coat hypertension.
White coat hypertension cannot be diagnosed reliably on clinical examination alone. The average BP levels obtained by multiple home readings and those recorded by an ambulatory monitor while the patient is awake are very close and both are lower than BPs measured in the office (37). In a study of 247 untreated hypertensive patients, investigators examined the extent to which HBPM can be an alternative to ABPM to diagnose white coat hypertension. Using ABPM as a reference, they found the specificity of HBPM to detect white coat hypertension was 88.6%, and the sensitivity was 68.4%.(79). While home BPs may not be completely without white-coat effects, (80) they may thus serve better as a screen for white coat hypertension than for the final diagnosis. The Ohasama study was the first to show the superior predictive value of home BP over office BP, such that patients with white coat hypertension were at relatively low risk (81). The PAMELA study evaluated prognosis with office, home, and ABP over a 11 year follow-up (78). Although they found that patients with high OBP and normal HBP or ABP (i.e. white coat hypertension) were at increased risk, the thresholds were different. Thus the systolic BP level that would confer a risk of CV death over an 11 year period of 10% was 179 mm Hg for office BP, 163 mmHg for home BP, and 157 mmHg for daytime ABP (82). This is consistent with the recommendation that a lower cut-off level should be used for home BP than office BP.
Algorithm for the Use of HBPM in Clinical Practice
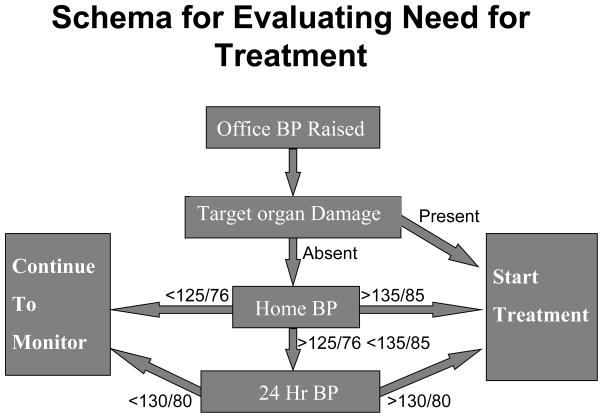
An algorithm that uses both HBPM as an initial screening test and ABPM to make the definitive diagnosis has been proposed by a panel of the American Society of Hypertension (13) and by the First International Consensus Conference for Self-Blood Pressure Monitoring (83), as shown in a modified version in Figure 2. The rationale for this is that the exclusive reliance on office BP for making therapeutic decisions may lead to both under-treatment and over-treatment in individual patients both because of the inherent variability of BP and the white coat effect. As originally proposed, this algorithm would only be applied to patients who have a persistently high office BP (>140/90 mmHg), but it might also be applicable to those with high normal BP (e.g. a patient who has had some readings above 140/90, but on rechecking has a slightly lower level), in whom masked hypertension may be suspected. And in patients with diabetes or kidney disease it may be used if the office BP is 130/80 mmHg or higher. In patients who have evidence of target organ damage that is thought to be the result of hypertension it may be decided to start treatment on the basis of the high office BP, although HBPM is still valuable for monitoring the response to treatment. The rationale here is that numerous studies have shown that even subclinical markers of organ damage such as microalbuminuria or left ventricular hypertrophy have been shown to increase CVD risk, as reviewed in the recent European guidelines on the management of hypertension (84), which may justify more aggressive treatment.
Figure 2.
Schema for evaluating BP status of hypertensive patients, which can be used in patients in whom the decision to start treatment may be uncertain on the basis of the office BP, which may be just above or below the cut-off point defining adequate control. HBPM may be used to aid the diagnosis, if necessary in conjunction with ABPM.
In those in whom the decision to start treatment remains unclear, HBPM is an appropriate next step, with the goal of obtaining a minimum of 12 readings taken both in the morning and at night over a period of 7 days. If the average value is >135/85 mmHg there is a high probability (85%) that the ABP will also be high (85), and a decision to start treatment can be made. And if the home BP is less than 125/76 mmHg the probability of missing a diagnosis of true hypertension is quite low (85). Because BP varies with time, whichever method of measurement is used, a diagnosis of white coat hypertension is not cast in stone, and all patients in whom the diagnosis is made require long term monitoring of BP, for which HBPM is ideally suited.
Evaluation of Masked Hypertension
HBPM may also be useful in detecting “masked hypertension,” also known as reverse white-coat hypertension or ”isolated home” or ”isolated ambulatory” hypertension. Masked hypertension occurs when a patient’s office BP is less than 140/90 mm Hg but ambulatory or home readings are in the hypertensive range (typically >135/85 mmHg) (86). It conveys the same cardiovascular risk as sustained hypertension, therefore it is important that it is detected (87;88).
The prevalence of masked hypertension may be about 10% in the general population (81;89) (87), but at the present time there is no consensus as to how it should be detected or treated in people who have not been diagnosed as hypertensive. However, in patients with treated hypertension that is thought to be well controlled (i.e. an office BP <140/90 mmHg) it may be equally common. In the SHEAF study (Self-Measurement of Blood Pressure at Home in the Elderly: Assessment and Follow-up) of 4939 elderly treated hypertensive patients being followed in family practices in France, the prevalence of masked hypertension (defined by an office BP <140/90 plus HBP >135/85 mmHg) was 42% of the patients with a normal office BP (87). In a descriptive study of 438 Turkish patients receiving care in an internal medicine clinic, all patients had their BP measured in the office, by 24-hour ABPM, and by HBPM twice a day for 10 days (90). The prevalence of masked hypertension was lower than 5% until the seventh decade of life, and it was 7.6% in the 7th and 16.6% in the 8th decade of life. There were no significant differences in the prevalence of masked hypertension depending on whether ambulatory or home BPs were used to define it. In the J-HOME study (91) of treated hypertensive patients in Japan, more than 50% of patients with controlled office BP had masked hypertension (HBP >135/85 mmHg). These patients tended to be older, and were more likely to have a past history of coronary heart disease or chronic kidney disease. This high prevalence in patients whose BP appears to be controlled by conventional clinical criteria makes the case that HBPM should be used routinely in treated hypertensive patients.
Evaluation of Prehypertension
About 28% of American adults, or 59 million people, have prehypertension, defined as a BP in the range 120-139/80-89 mmHg (2;11). Since this is normally diagnosed by office BP, some will have white coat hypertension. Regular and consistent monitoring of BP should begin during prehypertension to establish the need for treatment or help establish a firm baseline for determining response and change. Limited information is available on the use of HBPM in this situation, but it is ideally suited to these needs. One study (the Tecumseh study) found that in prehypertensive individuals (n = 735) diagnosed by office readings, home BP (average of 14 readings, 7 days with AM and PM readings) was more predictive than office BP of future BP status after 3 years, even when the same number of measurements were used for both methods (92)
Evaluation of Resistant Hypertension
HBPM may be helpful for evaluating resistant hypertension in patients exhibiting high office BP under antihypertensive therapy. Patients who appear to be refractory to treatment in the office may have adequately controlled home BPs (93), and consequently require less intensification of drug treatment than those whose home BP is also high.
HBPM for predicting cardiovascular risk
HBPM has been shown to be useful in predicting target organ damage, cardiovascular (CVD) mortality and CVD events. In a small study conducted in Italy, Mule compared office, ambulatory and home BP measurements and their relationship to various indices of target organ damage (94). Subjects underwent electrocardiographic (ECG) recordings, echocardiographic studies, and microalbuminuria assays. Neither systolic nor diastolic BP recorded in the office showed a significant correlation with left ventricular mass (LVM) or albumin excretion rate (AER.) However, HBP, especially during the second day of monitoring, correlated significantly with LVM, AER, and global target organ damage.
Several other cross-sectional studies have shown that BP measured at home correlates with hypertensive target organ damage. Kleinert et al. found the degree of left ventricular hypertrophy determined by echocardiography was more strongly correlated to multiple self-measurements than to office BP (23). Abe et al. (95) found the correlation between BP levels and target organ damage for self-measured readings at home and office readings were similar. Hypertensive complications were equally related to home and office BPs (95). Jula et al. compared multiple office and HBPs and ABPs in the clinical evaluation of hypertension using a sample of 239 untreated hypertensive adults (96). They found that office and HBPs predicted albuminuria and left ventricular hypertrophy at least equally to ABPM. Left ventricular mass index correlated slightly more strongly with morning home systolic BP/diastolic BPs than evening readings (r = .46/.43, p<.001 and r = .41/.37, p < .001 for morning and evening BPs)
Other investigators have used cross-sectional designs to evaluate the usefulness of HBPM in diabetics. Researchers examined whether BP elevations in the morning detected by HBPM were more predictive than office BP for microvascular (nephropathy and retinopathy) and macrovascular complications (coronary heart disease and cerebral vascular disease) in type 2 and type 1 diabetic patients (69;97). In both groups, home BP but not office BP was strongly related to nephropathy. There were no significant differences between the groups for the other measures of target organ damage.
Five prospective studies (all with several publications) have compared the prediction of morbid events using both conventional office BP and HBP (Table 1). Three were based on population samples, while two recruited hypertensive patients. Four found that HBP was the stronger predictor of risk. The fifth (Didima) reported that both HBP and OBP predicted risk equally well (98).
Table 1.
Prospective studies relating HBP and OBP to cardiovascular events and mortality
| Study (ref) | Population studied | No. of subjects | HBP schedule | Outcome* | |||
|---|---|---|---|---|---|---|---|
| Days | AM | PM | Total | ||||
| Ohasama (81) | Population | 1789 | 28 | 1 | 0 | 28 | Strokes and mortality predicted better by HBPM |
| SHEAF (99) | Treated hypertensives | 4939 | 4 | 3 | 3 | 24 | CV morbidity and mortality predicted better by HBPM |
| PAMELA (82) | Population | 2051 | 1 | 1 | 1 | 2 | CV and total mortality predicted better by HBPM |
| Belgian (100) | Referred | 391 | 1 | 3 | 0 | 3 | Combined CV events predicted better by HBPM |
| Didima (98) | Population | 662 | 3 | 2 | 2 | 12 | CV events predicted by both HBPM and by office BP |
The first was the population-based Ohasama study, which was conducted in 1,789 subjects over 40 years of age who were followed for a mean of 6.6 years (81). Subjects were asked to measure their BP at home within one hour of waking over a four-week period. The mean number of measures recorded was 20.8+8.3. As part of annual screening visits two consecutive measures of BP were recorded by a nurse or technician after two minutes of rest. When HBPM and BPs taken during annual screening were included in a Cox regression model, only home systolic BPs were significantly related to cardiovascular mortality risk. (multiple home systolic BP RH=1.012, p=0.048; screening systolic BP RH=1.000. p=0.972; multiple diastolic BP RH=1.013, p=0.414; screening diastolic BP RH=1.006, p=0.642.) Moreover, the average of two home BP measures showed a stronger relationship to mortality than the screening BPs taken by nurses and technicians.
More recently, the Ohasama data have been examined to determine the predictive value of HBPM on the risk of TIA, and hemorrhagic and ischemic stroke. (101). Of the 1,789 patients in the original study, mean duration of follow-up was 10.6 years. Home BP values were linearly related to risks for total, hemorrhagic, and ischemic stroke. A 10 mmHg elevation in home systolic BP was associated with 29%, 32%, and 30% increases in the risk of total, hemorrhagic, and ischemic strokes, respectively. Finally, home BP values showed a significantly greater relation to the risk of both hemorrhagic and ischemic stroke than screening BP values. (p<0.02). In another analysis Ohkubo and colleagues found that the predictive value of stroke risk increased for all measures of home BP but was greatest when at least 14 measurements were obtained (102). The original reports were based on readings taken in the morning, but a later analysis included evening readings, and found that both measures predicted strokes, but morning readings were superior in patients taking antihypertensive medications (68). The Ohasama study also included ABPM, and has reported that the average BP during the first 2 hours after waking is an independent predictor of risk (103). These findings emphasize the importance of taking BP readings first thing in the morning.
The second prospective study was the SHEAF study, a 3-year prospective cohort study designed to determine the prognostic value of HBPM compared to office measures in an older population (>60 years) with hypertension seen in general practice settings in France (99). Treated patients with hypertension were followed in two phases: Phase 1 included an evaluation of office and home BP over one month, and Phase 2 included a 3-year observational phase without specific recommendations with regard to the management of hypertension. Phase 1 office measures included triplicate measures on each of two visits. HBPM was done over a four-day period with three consecutive measurements taken in the morning, and repeated in the evening. At the end of follow-up, neither method of measurement was significantly related to CVD events or mortality. However, using a Cox model to control for predictors such as age, CVD history and smoking status, HBPM was predictive of cardiovascular events. Each 10-mmHg increment of systolic BP measured at home increased the risk of a cardiovascular event by 17.2%, and each 5 mmHg increase in diastolic BP increased the risk by 11.7%. Conversely, when the model was applied to office measures controlling for the same predictors, there was no significant increase in CVD events. In patients with masked hypertension (i.e. normal office but raised home BP, who comprised 9% of the total sample), the risk was increased (hazard ratio 2.06), and much higher than in patients with high office and normal home BP (hazard ratio 1.18).
The third study was PAMELA, a population based survey of 2051 Italian subjects who were evaluated with HBPM (2 readings- one in the morning and one in the evening), OBP (3 readings taken with a sphygmomanometer on each of 2 visits), and ABPM (82). About half of the subjects were hypertensive. Over a 10 year follow-up, there were 186 deaths. All three measures of BP predicted mortality. The steepest association between BP and outcomes was with the nighttime BP, but this may be attributed to the fact that nighttime BP shows much less variation than other measures. The goodness of fit, which is a better measure of the strength of the relationship was strongest for the home BP. In a subsequent publication (78) it was reported that elevation of any of the three measures of BP was associated with increased risk. Thus a high home BP should not be ignored, even if other measures are normal.
The fourth study was conducted in Belgium, and compared the prognostic significance of office and home BP both measured by a physician (who visited the patients’ homes), and ambulatory BP in a sample of 391 adults at least 60 years of age who were being seen in a primary care setting (100). Home and office examinations were performed within 2 weeks of one another. Health outcomes (i.e., aggregate of stroke, myocardial infarction and cardiovascular death) were determined after a median follow-up of 10.9 years. Home BP, and daytime and night time ambulatory BP predicted cardiovascular events, independent of office BP. BP measured by the primary care physician in the office was not independently predictive of future cardiovascular events. Diastolic but not systolic home BP added prognostic precision to daytime and night time ambulatory BP. In sum, the prognostic value of BP measured in the patient’s home was at least equal to that of daytime ambulatory BP. This study is of particular interest because it suggests that the relatively poor predictive value of office BP in comparison with home BP is not because of the confounding effects of the physician, but rather to the medical setting itself.
The fifth study is a long-term (8.2 years) follow-up of 662 subjects in the Didima Study (98), which is a population–based study of the inhabitants of Didima, a village in Greece. The average age was 54 years, and hypertension was diagnosed in 28%, of whom 55% were on antihypertensive drug treatment. Office BP was evaluated on 2 days (3 readings each day) by the village family physician. Home BP was taken as duplicate readings morning and evening for 3 days. The main finding was that both the office and the home BP predicted CVD events, but neither was clearly superior. After adjustment for age and gender the hazard ratio for a 1 mmHg increase of systolic BP was 1.016 (CI 1.004–1.029, p =0.01) for home BP, and 1.021 (CI 1.009–1.034, p= 0.001) for office BP. When fully adjusted (including history of CVD, antihypertensive treatment, diabetes, and smoking) neither measure of systolic BP predicted events. For diastolic pressure the office BP was superior to the home BP, and was the only measure to predict events after fully adjusting for covariates (hazard ratio 1.034, CI 1.008–1.061, p=0.01). The authors concluded that the confidence intervals were too wide to draw firm conclusions about the relative importance of the two methods for predicting risk.
A sixth study performed in Kahoku, a rural town in Japan, on 1186 elderly people (mean age 74) reported a U-shaped relationship between HBP and mortality (evaluated from death certificates) (104). There was no comparison with office BP, however, so it is not included in the Table.
Three longitudinal studies have examined the ability of HBPM to predict the progression of renal disease. One found that systolic home BP was a stronger predictor of end-stage renal disease (ESRD) and death than office BP among 217 Veterans with chronic kidney disease who had a median follow-up of 3.5 years (105). The second followed 77 diabetic patients for 6 years, and concluded that HBP was a better predictor of progression of diabetic nephropathy when compared to office BP measurements (106). The third used a sample of 113 hypertensive patients with non-diabetic chronic kidney disease who were followed for 3 years, and found that HBP measured in the morning was a better predictor of the decline in glomerular filtration rate (107).
These studies thus present a very consistent picture showing that HBPM can give a better prediction of cardiovascular risk than office BP (Class IIa; Level of Evidence A).
Information about BP Control
HPBM has the ability to provide information about BP control outside the office setting. Using data (n = 3400) from the J-Home Study (Japan Home versus Office BP Measurement Evaluation), investigators examined the characteristics of BP control based on home and office measurement (108). Although 42% of the sample had their BP controlled by office BP criteria (<140/90mmHg), only 34% also had HBP control (<135/85 mmHg). Other investigators have also demonstrated the value of HBPM in determining BP control outside the office (109–111). The SHEAF study described above found that the 9% of patients with normal office BP but elevated home BP (i.e. masked hypertension) had twice the risk of CVD events as the group in whom both office and home BP were controlled (99).
Use of HBPM to Guide and Evaluate Treatment
HBPM may provide important information about the responsiveness of individuals to anti-hypertensive treatment. In the Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation (SAMPLE) investigators compared three measures of BP (office, ambulatory, and home) to changes in BP resulting from treatment with an ACE inhibitor on regression of left ventricular hypertrophy (112). Improvements in left ventricular mass, an intermediate measure of target organ damage, were predicted best by both ambulatory BP and home BP while no changes were correlated with the changes in office BP. Thus, this trial showed the benefit of the use of HBPM to monitor the response to treatment, with important physiologic implications.
Findings about adjusting antihypertensive treatment on the basis of home BPs are mixed. Two studies have compared the effects of treating according to HBP as compared to office BP. In a blinded randomized controlled trial (TOHP- Treatment based on Home or Office blood Pressure) that compared the use of office BP versus HBPM to adjust hypertension treatment, more participants in the home measurement group had their antihypertensive treatment stopped (25.6% vs 11.3% in the office measurement group, p < .001), but had higher final office and 24 hour ambulatory BP compared to the office measurement group (113;114). A second study, with a very similar design, was the HOMERUS (Home Versus Office Measurement, Reduction of Unnecessary Treatment Study), in which 430 patients with uncontrolled hypertension were randomized to HBPM or usual care. Their physicians were blinded as to their treatment group, and were provided with the BP levels measured either in the office or by HBPM. In both cases the target BP was <140/90 mmHg. At the end of one year the patients in the HBPM group were on less antihypertensive medications. The office BPs were the same in both groups, but the 24 hour ambulatory BP was significantly higher in the HBPM group.
Thus in both studies, treatment based on HBPM appeared to lead to less intensive drug treatment, and thus less tight BP control. However, the differences between the two groups in both studies can be explained by the fact that home BP tends to be lower than office BP, although the target BP level was the same for both groups. It remains unclear whether the HBPM group was under-treated, but the study did provide evidence for the feasibility of basing treatment on home readings.
Studies of the effects of placebo drugs have found that they have little effect on HBP, in contrast to their much bigger effect on OBP (49). By having patients take readings both in the early morning and in the evening the adequacy of BP control throughout the day (and the trough: peak ratio) can be assessed (115). Thus HBPM may be regarded as the method of choice for monitoring the effects of antihypertensive treatment.
Use of HBPM as an intervention for improving medication adherence and BP control
Although most of the attention paid to HBPM is its value as a diagnostic tool, there is increasing evidence that it may also serve as an intervention to improve BP control. Success with behavioral or lifestyle interventions in patients with chronic conditions is often improved by encouraging the patient to become actively involved in their care, which may include self-monitoring. In the case of obesity, 75% of people who are successful with long-term weight loss report weighting themselves regularly (116).
Effects on medication adherence
If HBPM does improve BP control, a potential mechanism is by improved medication adherence, which is supported by recent evidence. Ogedegbe & Schoenthaler reviewed eleven randomized controlled trials that tested the effects of HBPM on medication adherence in various settings including non-clinical sites (117). Nine of the eleven trials reviewed were complex interventions that tested the effects of HBPM in combination with other adherence-enhancing strategies such as patient education (118;119), counseling on medication adherence by nurses, pharmacists or through telephone-linked system (120–122), use of timed-medication reminders (123), monthly home visits (124), and nurse case management (125). Fifty-four percent of the trials (6 out of 11) reported statistically significant improvement in medication adherence attributed to HBPM; and the intervention effects were greatest in trials that tested HBPM along with other adherence-enhancing strategies. Because of the heterogeneity of the adherence measures used the authors could not perform a meta-analysis of the intervention effects. However, when the intervention effects were categorized based on the type of medication adherence measure employed in each of the individual trials, all three studies that employed objective electronic monitoring of medication adherence reported positive intervention effects (123;125;126), three of five trials that utilized pill counts reported significant improvement in medication adherence (120;122;127), whereas all the studies that utilized self-report measures or pharmacy refill data reported negative findings (118;119;121;124;128). The authors concluded that the data on the effects of HBPM on patients’ medication-taking behavior is mixed; and that HBPM should be considered as a useful adherence-enhancing strategy especially when used in combination with other approaches such as patient counseling, patient reminders and use of nurse case mangers. Not included in this systematic review, was a Spanish study which tested the effect of HBPM compared to usual care in improving medication adherence assessed with electronic monitoring. Among the 200 study participants with newly-diagnosed or uncontrolled hypertension, 92% of the intervention group were compliant (i.e. took at least 80–100% with prescribed antihypertensive medications) compared to only 74% of the control group (P = 0.0001) (128).
Effects on BP Control
There is also evidence that HBPM is associated with better BP control. A meta-analysis of 18 randomized controlled trials that compared HBPM with usual care found that HBPM resulted in better BP control (129). While these BP effects were small, (2.2/1.9 mm Hg), the implications from a prognostic standpoint and as a population-based strategy are significant. Taken together, these findings suggest that HBPM on its own will not necessarily result in better BP control, but it has the potential to do so if the data are communicated regularly to the health care providers and appropriate action taken. Further study is needed in this area.
The need for HBPM in special populations
The elderly
It is well established that the white coat effect tends to be greater in older than younger patients. Since there are also potential hazards of excessive BP reduction in older people, the case for using out-of-office monitoring such as HBPM is very strong. The difference between the office and home BP (the white coat effect) increases progressively with age, so that office BP tends to overestimate the out-of-office BP more in older than in younger people (130). The variability of systolic home BP readings also increases with age (130). HBPM can also be used to detect orthostatic BP changes if readings are taken both sitting and standing.
Diabetics
BP control is one of the most important aspects of managing patients with diabetes (131), and as in non-diabetic patients, the home BP is superior to the office BP for predicting the 24 hour BP level (132). In one study the HBP was not consistently lower than the office BP (51). It is not uncommon for home BP to be elevated (>130/80 mmHg) even when office BP is controlled (133). In the J-HOME study 7% of diabetic patients with an office BP below the target level (<130/80 mmHg) had elevated home BP (>130/80) (109). It has been reported that home BP, particularly when measured in the morning, correlates better with target organ damage such as diabetic nephropathy than office BP (69). In this study, two thirds of patients with normal office BP had elevated home BP in the morning hours. So far, only one study has examined the ability of out-of-office BP monitoring in diabetics to predict cardiovascular outcomes (134), and as in non-diabetics, ambulatory BP monitoring predicted risk independently of office BP. One study has examined the role of home BP monitoring (in conjunction with glucose monitoring and nurse case management, and found a small but significant reduction of BP (3.4/1.9 mmHg) when compared with the control group (135). There are at present no official guidelines for the home BP level equivalent to an office BP of 130/80 mmHg in diabetic patients, although one study used 125/75 mmHg (51).
Although there is less evidence for the benefits of HBPM in patients with diabetes, what data exists is entirely consistent with what has been observed in those without it, and since there is strong evidence that aggressive reduction of BP is more effective in patients with diabetes in lowering CVD risk, a strong case can be made for the wider use of HBPM in patients with diabetes. The International Diabetes Federation has advocated its use (136), but the American Diabetes Association has remained silent on this issue.
Pregnancy
The accurate measurement of BP during pregnancy is one of the most important aspects of prenatal care, and pre-eclampsia, which is the commonest cause of maternal and fetal death, can develop quite rapidly. The situation in pregnancy is essentially dynamic: BP first falls, and then rises, so the best way of detecting an abnormal pattern that presages pre-eclampsia may be to monitor its changes very frequently throughout the course of pregnancy. Thus the earliest manifestation of pre-eclampsia is a failure to decrease, or a premature increase, of BP during the second trimester. HBPM is theoretically ideal for monitoring changes of BP during pregnancy, since it is the best technique for providing multiple readings recorded at the same time of day over prolonged periods of time (137). Several monitors have been validated for use in pregnant women (138). Although some studies have been done to show that HBPM is practical (139), and has the potential to reduce clinic visits (140), it has yet to be shown to what extent it will improve the evaluation and management of hypertension during pregnancy.
White coat hypertension is not uncommon, and may lead to unnecessary early termination of pregnancy (141). This should be detectable using HBPM.
Chronic Kidney Disease
Hypertension is highly prevalent in patients with chronic kidney disease, and also in the dialysis population, but the BP is very variable, and measurements made in dialysis centers give a poor prediction of clinical outcomes (142). HBPM has been advocated in these patients, but so far has been little used (143). Despite the fact that arterial stiffness is greatly increased in such patients oscillometric monitors may still be accurate in patients with ESRD. (30;144;145). HBPM has been shown to be superior to measurements made in the dialysis unit for predicting ambulatory hypertension (146).
Children
There is increasing attention being paid to the issue of hypertension in children, particularly because, with the epidemic of obesity, it is likely that its prevalence will increase, and guidelines for its evaluation were published in 2004 (147). The phenomenon of white coat hypertension occurs in children just as in adults (148), so it makes sense to use out-of-office monitoring in addition to clinic measurements. So far, there are relatively few studies of HBPM in children. One useful study was performed by Stergiou et al (149) in 55 children aged 6 to18, of whom 26 were hypertensive by office BP criteria. There were strong correlations between office and home BP (0.73 for systolic and 0.57 for diastolic pressure), and also between home BP and ABP (0.72/0.66). In the hypertensive children the systolic home BP was lower than both office and ABP, while in normotensive children the ABP was higher than both the office and home BP. The authors concluded that home BP is difficult to interpret in children. Another study found that home BP was better than office BP at predicting ABP in children with renal disease (150). Thus, HBPM appears to be of great potential value in children when the proper cuff size is used, although more studies are needed in this area.
Cost-Effectiveness of HBPM
The potential for HBPM to be cost-effective for the diagnosis and management of hypertension has received little attention. In principle, there are two types of situation where it is used: first, for the diagnosis of hypertension and hence the need for treatment, for which monitoring need only be done for a limited period of time; and second, for the evaluation of treatment, for which long-term monitoring is appropriate. Other potential advantages for use of HBPM are a reduced need for office visits, but with increased need for alternative communication by telephone or telemetry, and more accurate assessment of over-treatment and the opportunity of reducing medication in some patients.
In contrast to HBPM, it has been shown that use of ABPM can be cost-effective when applied to the diagnosis of hypertension (specifically white coat hypertension) (151;152). If HBPM and ABPM were fully equivalent with regard to detection of white coat hypertension, then any difference in cost between the two methods would be a basis for choosing the one which costs less. Currently Medicare reimburses ABPM for patients with suspected white coat hypertension. This requires the patient meeting the following criteria: (a) office BP > 140/90 mm Hg on at least three separate office visits with two separate measurements made at each visit; (b) at least two BP measurements taken outside the office which are < 140/90 mm Hg; and (c) no evidence of end-organ damage. The charges allowed by CMS for ABPM in the US to confirm the diagnosis of white coat hypertension vary from ≈$70 to ≈$105 (Data from CMS site). This reimbursement (CPT code 93784) includes both the monitoring procedure for ≥ 24 hours, per se, and an interpretation provided by the physician.
There is no recognized CPT code for HBPM (without the memory and computational equivalents to Ambulatory Monitoring) and no systematic basis for how reimbursement might be developed. However, several known costs and likely factors allow for an argument that HBPM be considered for reimbursement if incorporated into a systematic plan for management of individual hypertensive patients. These are summarized below.
Cost of Home BP Devices
Many devices for HBPM are available for purchase by consumers who want to take their own BP or measure that of others in their household or at screening sites. Devices are available at drug stores and many other sources. Purchase through web-sites is firmly established, and was reviewed in 2005 (153). Prices vary from <$50 to ≈$100 (sources: web-sites for CVS/pharmacy- www.cvs.com, Rite Aid Drugstore- www.drugstore.com, and Walgreens-www.walgreens.com). Lower priced units have aneroid sensors without any memory storage, use hand pumped bulbs for compression and require stethoscopes so that the patient is fully responsible for all elements of taking and recording each measurement. By contrast, higher priced devices have electric powered cuff pumps (battery and/or wall outlet), oscillometric detectors, printers and or memory storage which may include time-date stamp. It is recommended that the best devices for HBPM have electric inflation of cuffs, oscillometric detection and memory (54). These recommendations are based on two concerns: 1) errors that may be introduced by self inflation of the cuff (154) and 2) selection bias that may affect the recording and reporting of pressures if patients choose the values to report (47) (155). Thus, the out-of-pocket cost to a patient for purchase of a recommended device for HBPM will be in the range of $80 to $100 unless reimbursement is provided from that patient’s health insurance provider or the cost is offset by an incentive, such as a tax-free purchase. Buying a large adult cuff, which is not standard, may add to the overall cost.
As described above, the use of HBPM is growing rapidly in the US, and many patients are buying units without being prescribed by physicians. A small study of 13 randomly selected subjects using an intensive interview method found a wide range of ideas about hypertension and its treatment. Most welcomed the opportunity to perform HBPM, but others preferred management by the doctor alone (156). A one year experience with HBPM combined with telephone transmission to a central server and reports to treating physicians found that initial enthusiasm for HBPM was followed by a fall-off of use, so that only 50% preferred to continue HBPM for the second year (157). A survey comparing patients’ attitudes to different methods of getting accurate BP measurements found that HBPM was preferred over other methods (which included ABPM and measurement by either the doctor or nurse in the office) (158). Will patients want to pay for HBPM as an add-on for management? Studies on ‘willingness to pay’ in the context of telemedicine indicate that hypertensive patients are very ‘cost-sensitive’ in making decisions about what they say they will pay for in contrast to those with heart failure who state that they will pay more out-of-pocket for a ‘telemedicine’ visit. A survey by structured questionnaire using ‘contingent valuation’ found that for a telemedicine visit charge of $20, 30% of those with hypertension would accept the charge, whereas 45–50% of those with chronic heart failure would accept that charge (159).
Costs and savings related to implementation and use of HBPM
In theory, incorporation of HBPM into treatment of hypertension may appear to save cost of care (160). A study from Japan where a large fraction of the population have home BP devices predicts that a substantial reduction in cost for management of hypertension might be realized (161). Savings could come from reduced need for office visits with replacement by phone calls, as has been reported (162). Several studies have demonstrated that effective control of hypertension can be achieved when patients using HBPM can communicate with their providers (either trained nurse clinicians or physicians) to adjust medication as needed to achieve goals for treatment (125;163–165). The significance of apparent control of hypertension using HBPM with regard to prevention of cardiovascular morbidity and mortality is not established. In the study described above which compared HBPM with office management for hypertension and used the same BP goal for both office and home assessment (113), the cost of treatment (medication) was slightly lower for the HBPM group because less medication was required for control, but the target level of BP (a diastolic pressure of 80–89 mmHg) was higher than generally recommended. These reports lend support to the simple view that HBPM can reduce costs for treatment of hypertension (reduced visits and, perhaps, less medication) while increasing or, at least maintaining the effectiveness of treatment for prevention of cardiovascular disease, given the relatively low cost of purchase for a home pressure device. A large multi-center trial (HOMERUS) has been performed to compare cost of treatment for office management with an HBPM strategy (166;167). The effect of using HBPM was to reduce the amount of medications prescribed, which even after allowing for the cost of the monitors, resulted in a net cost saving. However, the results are difficult to interpret because the same target BP was used for both groups, and the HBPM group ended up with a higher 24 hour BP.
Other Cost Considerations
There are several hidden or off-setting factors that should be taken into account when calculating the actual costs for use of HBPM. First, there are costs related to the necessary validation of each device and training of each patient in proper use for measurement of BP and recording and/or transmission of measurements, which are not well established. Next, there are costs related to the review of HBPM data, and advice to patients regarding change in treatment. There is need for some calculation of equivalency to assure reimbursement for the provider, should office visits be replaced by an HBPM strategy that still takes up time and resource for the provider. Here, differences in medical care systems may be relevant. Those who practice in fee-for-service modes may be reluctant to give up the reimbursements related to office visits unless some incentive is evident. By contrast, those with high volume capitated practices may welcome a strategy that reduces office visits but reimburses for hypertensive patients enrolled and managed by HBPM. Going further, it might be suggested that providers expanding use of HBPM be given incentives for this effort, should outcome studies justify this approach.
It should be recognized that the long-term cost of care for hypertension is dominated by costs for drug treatment, rather than for visits to providers or testing (168–170). However, costs for the 1st year of management tend to be higher than for subsequent years (more tests, visits). Drug choices then determine the greatest fraction of costs, so that over a 5 year period the cost for treatment of a patient may vary from $1700–$3000. In general, emphasis on guideline-based drug selection (diuretics and beta blockers up front) is associated with lower combined treatment costs (168;170). Thus, use of HBPM to reduce cost of treatment will be most effective when implemented to detect white coat hypertension and reduce need for drug treatment, as has been shown for ABPM (151).
The impact of HBPM for overall cost of management for hypertensives in community practice who are placed on drug treatment is less certain. If telemedicine methods are used, what will the costs be for receiving and processing information? Who will pay for such services? Can the methods be made so efficient that there is minimal demand for time by the provider? What financial incentives are available to support providers for their responsibilities? These questions pose the need for research in the health care systems that link patients with hypertension to physicians and practices via the various financial structures that pay for medical care (171). Without such research, the actual impact of HBPM on cost-effectiveness for prevention of cardiovascular disease cannot be calculated.
Part II. Action Plan
Given the amount of accumulated evidence about the value of HBPM, it is time to make HBPM a part of routine management of hypertensive patients, especially those with diabetes, coronary heart disease, chronic kidney disease, suspected non-adherence, or a substantial white coat effect. The following table (Table 2) provides recommendations (13;16;32;34;54;55;71;83;172–176) for its use.
Table 2.
Summary of Recommendations for HBPM
| Procedure | Recommendation |
|---|---|
| Technical aspects of BP measurement | Measure BP:
|
| BP Monitor |
|
| Training of Patients |
|
| Target BP Goal |
|
| Frequency and schedule of measurement |
Initial Values (when patients begin SBPM at home):
|
Additionally, since HBPM is part of evidenced-based care it should be reimbursed. Regular use of HBPM will improve the quality and cost of delivering care to the 72 millions with hypertension and should lead to improved control of hypertension. Reimbursement is critically important to hypertensive patients and to their providers. Cost should not be a barrier to patients receiving the documented benefits of HBPM. Reimbursement will improve access to recommended health care for the impoverished, isolated, medically vulnerable and/or disadvantaged minority groups. Improved access may contribute to reductions in hypertension-related disparities among disproportionately affected groups.
It is recommended that patients be reimbursed for the purchase of a monitor prescribed by their health care provider (physician and/or nurse practitioner), and that providers be reimbursed for services related to HBPM (i.e., initial patient education regarding correct HBPM technique; yearly or as needed assessments to validate that individuals self-measure their blood pressures accurately; interpretation of BPs stored in the monitor’s memory; in-person, telephone and/or email consultation to deliver medical advice based analysis of BP reports generated from monitor). Monitors should be renewable after 5 years, or if they are shown to be inaccurate.
Need for future studies
There are a number of areas where there is a need for future studies using HBPM. These include:
Measurement of nighttime BP. There is increasing evidence that the nighttime BP has important prognostic significance. HBPM devices are being developed which can be preprogrammed to take readings during the night.
Use of HBPM in conjunction with office BP for making diagnostic and therapeutic decisions.
Use of HBPM for improving BP control in treated patients.
Use of HBPM in patients with diabetes. Tight BP control is of paramount importance in patients with diabetes, but the use of HBPM has not been adequately explored.
Use of HBPM in pregnancy. HBPM is ideally suited to detecting early increases of BP that herald pre-eclampsia.
Use of HBPM in children. The decision to start treatment is particularly difficult in children, and HBPM may help to establish the need for this.
Cost-effectiveness of HBPM. While HBPM has the potential of saving costs while improving BP control, few studies have evaluated this systematically.
Executive Summary.
Home blood pressure monitoring (HBPM) overcomes many of the limitations of traditional office BP measurement, and is both cheaper and easier to perform than ambulatory BP monitoring (ABPM). Monitors that use the oscillometric method are currently available that are accurate, reliable, easy to use, and relatively inexpensive. An increasing number of patients are using them regularly to check their BP at home, but although this has been endorsed by national and international guidelines, detailed recommendations for their use have been lacking. There is a rapidly growing literature showing that measurements taken by patients at home are often lower than readings taken in the office, and closer to the average BP recorded by 24 hour ambulatory monitors, which is the BP that best predicts cardiovascular risk. Because of the larger numbers of readings that can be taken by HBPM than in the office, and the elimination of the white coat effect (the increase of BP during an office visit), home readings are more reproducible than office readings, and show better correlations with measures of target organ damage. And prospective studies that have used multiple home readings to express the true BP have found that home BP predicts risk better than office BP (Class IIa; Level of Evidence A).
This Call-to-Action paper makes the following recommendations:
It is recommended that HBPM should become a routine component of BP measurement in the majority of patients with known or suspected hypertension.
Patients should be advised to purchase oscillometric monitors that measure BP on the upper arm with an appropriate cuff size, and which have been shown to be accurate according to standard international protocols. They should be shown how to use them by their healthcare providers.
Two to three readings should be taken while resting in the seated position, both in the morning and at night, over a period of 1 week. A total of 12 readings or more is recommended for making clinical decisions.
HBPM is indicated in patients with newly diagnosed or suspected hypertension, in whom it may distinguish between white coat and sustained hypertension. If the results are equivocal ABPM may help to establish the diagnosis.
In patients with prehypertension HBPM may be useful for detecting masked hypertension.
HBPM is recommended for evaluating the response to any type of antihypertensive treatment, and may improve adherence.
The target HBPM goal for treatment is <135/85 mmHg, or <130/80 in high risk patients.
HBPM is useful in the elderly, in whom both BP variability and the white coat effect are increased.
HBPM is of value in patients with diabetes, in whom tight BP control is of paramount importance.
Other populations in whom HBPM may be beneficial include pregnant women, children and patients with kidney disease.
HBPM has the potential to improve the quality of care while reducing costs, and should be reimbursed.
Reference List
- 1.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000: a rising tide. Hypertension. 2004;44(4):398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB. Prevalence and implications of uncontrolled systolic hypertension. Drugs Aging. 2003;20(4):277–286. doi: 10.2165/00002512-200320040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 6.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH, Jr, Folsom AR. Risk Factors for Ischemic Stroke Subtypes. The Atherosclerosis Risk in Communities Study. Stroke. 2006 doi: 10.1161/01.STR.0000239694.19359.88. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB. Current status of the epidemiology of heart failure. Curr Cardiol Rep. 1999;1(1):11–19. doi: 10.1007/s11886-999-0035-7. [DOI] [PubMed] [Google Scholar]
- 8.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol. 2003;14(11):2934–2941. doi: 10.1097/01.asn.0000095249.99803.85. [DOI] [PubMed] [Google Scholar]
- 9.Lawes CM, Vander HS, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part II: estimates of attributable burden. J Hypertens. 2006;24(3):423–430. doi: 10.1097/01.hjh.0000209973.67746.f0. [DOI] [PubMed] [Google Scholar]
- 10.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49(1):69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 11.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien E, Waeber B, Parati G, Staessen J, Myers MG. Blood pressure measuring devices: recommendations of the European Society of Hypertension. BMJ. 2001;322(7285):531–536. doi: 10.1136/bmj.322.7285.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pickering T. Recommendations for the use of home (self) and ambulatory blood pressure monitoring. American Society of Hypertension Ad Hoc Panel. Am J Hypertens. 1996;9(1):1–11. doi: 10.1016/0895-7061(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 15.Williams B, Poulter NR, Brown MJ, Davis M, McInnes GT, Potter JF, Sever PS, McG TS. Guidelines for management of hypertension: report of the fourth working party of the British Hypertension Society, 2004-BHS IV. J Hum Hypertens. 2004;18(3):139–185. doi: 10.1038/sj.jhh.1001683. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21(5):821–848. doi: 10.1097/00004872-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, Otsuka K, Kawano Y, Shimada K, Hayashi H, Tochikubo O, Miyakawa M, Fukiyama K. Japanese society of hypertension (JSH) guidelines for self-monitoring of blood pressure at home. Hypertens Res. 2003;26(10):771–782. doi: 10.1291/hypres.26.771. [DOI] [PubMed] [Google Scholar]
- 18.1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 19.The 2006 Gallup study of the hypertension market. Princeton, NJ: Multi-Sponsor Surveys inc; 2006. Ref Type: Report. [Google Scholar]
- 20.Cuspidi C, Meani S, Lonati L, Fusi V, Magnaghi G, Garavelli G, Palumbo G, Pini C, Vaccarella A, Parati G, Leonetti G, Zanchetti A. Prevalence of home blood pressure measurement among selected hypertensive patients: results of a multicenter survey from six hospital outpatient hypertension clinics in Italy. Blood Press. 2005;14(4):251–256. doi: 10.1080/08037050500210765. [DOI] [PubMed] [Google Scholar]
- 21.Tisler A, Dunai A, Keszei A, Fekete B, Othmane TH, Torzsa P, Logan AG. Primary-care physicians’ views about the use of home/self blood pressure monitoring: nationwide survey in Hungary. J Hypertens. 2006;24(9):1729–1735. doi: 10.1097/01.hjh.0000242396.15097.f3. [DOI] [PubMed] [Google Scholar]
- 22.Bald M, Hoyer PF. Measurement of blood pressure at home: survey among pediatric nephrologists. Pediatr Nephrol. 2001;16(12):1058–1062. doi: 10.1007/s004670100027. [DOI] [PubMed] [Google Scholar]
- 23.Kleinert HD, Harshfield GA, Pickering TG, Devereux RB, Sullivan PA, Marion RM, Mallory WK, Laragh JH. What is the value of home blood pressure measurement in patients with mild hypertension? Hypertension. 1984;6(4):574–578. doi: 10.1161/01.hyp.6.4.574. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell PL, Parlin RW, Blackburn H. Effect of vertical displacement of the arm on indirect blood-pressure measurement. NEJM. 1964;271:72–74. doi: 10.1056/NEJM196407092710204. [DOI] [PubMed] [Google Scholar]
- 25.Sesler JM, Munroe WP, McKenney JM. Clinical evaluation of a finger oscillometric blood pressure device. DICP. 1991;25(12):1310–1314. doi: 10.1177/106002809102501204. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien E, Pickering T, Asmar R, Myers M, Parati G, Staessen J, Mengden T, Imai Y, Waeber B, Palatini P, Gerin W. Working Group on Blood Pressure Monitoring of the European Society of Hypertension International Protocol for validation of blood pressure measuring devices in adults. Blood Press Monit. 2002;7(1):3–17. doi: 10.1097/00126097-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Gerin W, Schwartz AR, Schwartz JE, Pickering TG, Davidson KW, Bress J, O’Brien E, Atkins N. Limitations of current validation protocols for home blood pressure monitors for individual patients. Blood Press Monit. 2002;7(6):313–318. doi: 10.1097/00126097-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 28.van Popele NM, Bos WJ, de Beer NA, Der Kuip DA, Hofman A, Grobbee DE, Witteman JC. Arterial stiffness as underlying mechanism of disagreement between an oscillometric blood pressure monitor and a sphygmomanometer. Hypertension. 2000;36(4):484–488. doi: 10.1161/01.hyp.36.4.484. [DOI] [PubMed] [Google Scholar]
- 29.van Ittersum FJ, Wijering RM, Lambert J, Donker AJ, Stehouwer CD. Determinants of the limits of agreement between the sphygmomanometer and the SpaceLabs 90207 device for blood pressure measurement in health volunteers and insulin-dependent diabetic patients. J Hypertens. 1998;16(8):1125–1130. doi: 10.1097/00004872-199816080-00007. [DOI] [PubMed] [Google Scholar]
- 30.Thompson AM, Eguchi K, Reznik ME, Shah SS, Pickering TG. Validation of an oscillometric home blood pressure monitor in an end-stage renal disease population and the effect of arterial stiffness on its accuracy. Blood Press Monit. 2007;12(4):227–232. doi: 10.1097/MBP.0b013e328108f544. [DOI] [PubMed] [Google Scholar]
- 31.Eguchi K, Yacoub M, Jhalani J, Gerin W, Schwartz JE, Pickering TG. How consistent are blood pressure differences between the left and right arms? Arch Int Med. 2006 doi: 10.1001/archinte.167.4.388. In Press. [DOI] [PubMed] [Google Scholar]
- 32.Brook RD. Home blood pressure: accuracy is independent of monitoring schedules. Am J Hypertens. 2000;13(6 Pt 1):625–631. doi: 10.1016/s0895-7061(99)00273-3. [DOI] [PubMed] [Google Scholar]
- 33.Coleman AJ, Steel SD, Ashworth M, Vowler SL, Shennan A. Accuracy of the pressure scale of sphygmomanometers in clinical use within primary care. Blood Press Monit. 2005;10(4):181–188. doi: 10.1097/01.mbp.0000168398.87167.c2. [DOI] [PubMed] [Google Scholar]
- 34.O’Brien E, Beevers G, Lip GY. ABC of hypertension: Blood pressure measurement. Part IV-automated sphygmomanometry: self blood pressure measurement. BMJ. 2001;322(7295):1167–1170. doi: 10.1136/bmj.322.7295.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.James GD, Pickering TG, Yee LS, Harshfield GA, Riva S, Laragh JH. The reproducibility of average ambulatory, home, and clinic pressures. Hypertension. 1988;11(6 Pt 1):545–549. doi: 10.1161/01.hyp.11.6.545. [DOI] [PubMed] [Google Scholar]
- 36.La Batide-Alanore A, Chatellier G, Bobrie G, Fofol I, Pierre-Francois P. Comparison of nurse- and physician-determined clnic blood pressure levels in patients referred to a hypertension clinic: implications for subsequent management. J Hypertens. 2000;18:391–398. doi: 10.1097/00004872-200018040-00006. [DOI] [PubMed] [Google Scholar]
- 37.Brueren MM, Schouten HJ, de Leeuw PW, van Montfrans GA, van Ree JW. A series of self-measurements by the patient is a reliable alternative to ambulatory blood pressure measurement. Br J Gen Pract. 1998;48(434):1585–1589. [PMC free article] [PubMed] [Google Scholar]
- 38.Bruce NG, Shaper AG, Walker M, Wannamethee G. Observer bias in blood pressure studies. J Hypertens. 1988;6(5):375–380. [PubMed] [Google Scholar]
- 39.Stergiou GS, Voutsa AV, Achimastos AD, Mountokalakis TD. Home self-monitoring of blood pressure: is fully automated oscillometric technique as good as conventional stethoscopic technique? Am J Hypertens. 1997;10(4 Pt 1):428–433. [PubMed] [Google Scholar]
- 40.Wingfield D, Cooke J, Thijs L, Staessen JA, Fletcher AE, Fagard R, Bulpitt CJ. Terminal digit preference and single-number preference in the Syst-Eur trial: influence of quality control. Blood Press Monit. 2002;7(3):169–177. doi: 10.1097/00126097-200206000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Nietert PJ, Wessell AM, Feifer C, Ornstein SM. Effect of terminal digit preference on blood pressure measurement and treatment in primary care. Am J Hypertens. 2006;19(2):147–152. doi: 10.1016/j.amjhyper.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Pickering TG, Gerin W, Schwartz AR. What is the white-coat effect and how should it be measured? Blood Press Monit. 2002;7(6):293–300. doi: 10.1097/00126097-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Stergiou GS, Skeva II, Baibas NM, Kalkana CB, Roussias LG, Mountokalakis TD. Diagnosis of hypertension using home or ambulatory blood pressure monitoring: comparison with the conventional strategy based on repeated clinic blood pressure measurements. J Hypertens. 2000;18(12):1745–1751. doi: 10.1097/00004872-200018120-00007. [DOI] [PubMed] [Google Scholar]
- 44.Stergiou GS, Baibas NM, Gantzarou AP, Skeva II, Kalkana CB, Roussias LG, Mountokalakis TD. Reproducibility of home, ambulatory, and clinic blood pressure: implications for the design of trials for the assessment of antihypertensive drug efficacy. Am J Hypertens. 2002;15(2 Pt 1):101–104. doi: 10.1016/s0895-7061(01)02324-x. [DOI] [PubMed] [Google Scholar]
- 45.Sakuma M, Imai Y, Nagai K, Watanabe N, Sakuma H, Minami N, Satoh H, Abe K. Reproducibility of home blood pressure measurements over a 1-year period. Am J Hypertens. 1997;10(7 Pt 1):798–803. doi: 10.1016/s0895-7061(97)00117-9. [DOI] [PubMed] [Google Scholar]
- 46.Johnson KA, Partsch DJ, Rippole LL, McVey DM. Reliability of self-reported blood pressure measurements. Arch Intern Med. 1999;159(22):2689–2693. doi: 10.1001/archinte.159.22.2689. [DOI] [PubMed] [Google Scholar]
- 47.Mengden T, Hernandez Medina RM, Beltran B, Alvarez E, Kraft K, Vetter H. Reliability of reporting self-measured blood pressure values by hypertensive patients. Am J Hypertens. 1998;11(12):1413–1417. doi: 10.1016/s0895-7061(98)00241-6. [DOI] [PubMed] [Google Scholar]
- 48.Chatellier G, Day M, Bobrie G, Menard J. Feasibility study of N-of-1 trials with blood pressure self-monitoring in hypertension. Hypertension. 1995;25(2):294–301. doi: 10.1161/01.hyp.25.2.294. [DOI] [PubMed] [Google Scholar]
- 49.Imai Y, Ohkubo T, Hozawa A, Tsuji I, Matsubara M, Araki T, Chonan K, Kikuya M, Satoh H, Hisamichi S, Nagai K. Usefulness of home blood pressure measurements in assessing the effect of treatment in a single-blind placebo-controlled open trial. J Hypertens. 2001;19(2):179–185. doi: 10.1097/00004872-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Chatellier G, Dutrey-Dupagne C, Vaur L, Zannad F, Genes N, Elkik F, Menard J. Home self blood pressure measurement in general practice. The SMART study. Self-measurement for the Assessment of the Response to Trandolapril [see comments] Am J Hypertens. 1996;9(7):644–652. doi: 10.1016/0895-7061(96)00018-0. [DOI] [PubMed] [Google Scholar]
- 51.Mazze RS, Simonson GD, Robinson RL, Kendall DM, Idrogo MA, Adlis SA, Boyce KS, Dunne CJ, Anderson RL, Bergenstal RM. Characterizing blood pressure control in individuals with Type 2 diabetes: the relationship between clinic and self-monitored blood pressure. Diabet Med. 2003;20(9):752–757. [PubMed] [Google Scholar]
- 52.Stergiou GS, Skeva II, Zourbaki AS, Mountokalakis TD. Self-monitoring of blood pressure at home: how many measurements are needed? J Hypertens. 1998;16(6):725–731. doi: 10.1097/00004872-199816060-00002. [DOI] [PubMed] [Google Scholar]
- 53.Verberk WJ, Kroon AA, Kessels AG, Lenders JW, Thien T, van Montfrans GA, Smit AJ, de Leeuw PW. The optimal scheme of self blood pressure measurement as determined from ambulatory blood pressure recordings. J Hypertens. 2006;24(8):1541–1548. doi: 10.1097/01.hjh.0000239289.87141.b6. [DOI] [PubMed] [Google Scholar]
- 54.Verberk WJ, Kroon AA, Kessels AG, de Leeuw PW. Home blood pressure measurement: a systematic review. J Am Coll Cardiol. 2005;46(5):743–751. doi: 10.1016/j.jacc.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 55.Reims H, Fossum E, Kjeldsen SE, Julius S. Home blood pressure monitoring. Current knowledge and directions for future research. Blood Press. 2001;10(5–6):271–287. doi: 10.1080/080370501753400584. [DOI] [PubMed] [Google Scholar]
- 56.Kario K. Morning surge and variability in blood pressure: a new therapeutic target? Hypertension. 2005;45(4):485–486. doi: 10.1161/01.HYP.0000158313.57142.3f. [DOI] [PubMed] [Google Scholar]
- 57.Myers MG, Tobe SW, McKay DW, Bolli P, Hemmelgarn BR, McAlister FA. New algorithm for the diagnosis of hypertension. Am J Hypertens. 2005;18(10):1369–1374. doi: 10.1016/j.amjhyper.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Verdecchia P. Using out of office blood pressure monitoring in the management of hypertension. Curr Hypertens Rep. 2001;3(5):400–405. doi: 10.1007/s11906-001-0057-z. [DOI] [PubMed] [Google Scholar]
- 59.Herpin D, Pickering T, Stergiou G, de Leeuw P, Germano G. Consensus Conference on Self-blood pressure measurement. Clinical applications and diagnosis. Blood Press Monit. 2000;5(2):131–135. [PubMed] [Google Scholar]
- 60.Myers MG, Valdivieso MA. Use of an automated blood pressure recording device, the BpTRU, to reduce the “white coat effect” in routine practice. Am J Hypertens. 2003;16(6):494–497. doi: 10.1016/s0895-7061(03)00058-x. [DOI] [PubMed] [Google Scholar]
- 61.Beckett L, Godwin M. The BpTRU automatic blood pressure monitor compared to 24 hour ambulatory blood pressure monitoring in the assessment of blood pressure in patients with hypertension. BMC Cardiovasc Disord. 2005;5(1):18. doi: 10.1186/1471-2261-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawabe H, Saito I. Influence of nighttime bathing on evening home blood pressure measurements: how long should the interval be after bathing? Hypertens Res. 2006;29(3):129–133. doi: 10.1291/hypres.29.129. [DOI] [PubMed] [Google Scholar]
- 63.Stergiou GS, Thomopoulou GC, Skeva II, Mountokalakis TD. Home blood pressure normalcy: the DIDIMA Study. J Hypertens. 1999;17(Suppl 3):S25. doi: 10.1016/s0895-7061(99)00266-6. Abstract. [DOI] [PubMed] [Google Scholar]
- 64.Kok RH, Beltman FW, Terpstra WF, Smit AJ, May JF, de Graeff PA, Meyboom-de Jong B. Home blood pressure measurement: reproducibility and relationship with left ventricular mass. Blood Press Monit. 1999;4(2):65–69. [PubMed] [Google Scholar]
- 65.Ishikawa J, Kario K, Eguchi K, Morinari M, Hoshide S, Ishikawa S, Shimada K. Regular alcohol drinking is a determinant of masked hypertension detected by home blood pressure monitoring in medicated hypertensive patients with well-controlled clinic blood pressure: The Jichi Morning Hypertension Research (J-MORE) study. Hypertens Res. 2006;29:679–686. doi: 10.1291/hypres.29.679. [DOI] [PubMed] [Google Scholar]
- 66.Lavie-Nevo K, Pillar G. Evening-morning differences in blood pressure in sleep apnea syndrome: effect of gender. Am J Hypertens. 2006;19(10):1064–1069. doi: 10.1016/j.amjhyper.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 67.Imai Y, Nishiyama A, Sekino M, Aihara A, Kikuya M, Ohkubo T, Matsubara M, Hozawa A, Tsuji I, Ito S, Satoh H, Nagai K, Hisamichi S. Characteristics of blood pressure measured at home in the morning and in the evening: the Ohasama study. J Hypertens. 1999;17(7):889–898. doi: 10.1097/00004872-199917070-00004. [DOI] [PubMed] [Google Scholar]
- 68.Asayama K, Ohkubo T, Kikuya M, Obara T, Metoki H, Inoue R, Hara A, Hirose T, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Prediction of stroke by home “morning” versus “evening” blood pressure values: the Ohasama study. Hypertension. 2006;48(4):737–743. doi: 10.1161/01.HYP.0000240332.01877.11. [DOI] [PubMed] [Google Scholar]
- 69.Kamoi K, Miyakoshi M, Soda S, Kaneko S, Nakagawa O. Usefulness of home blood pressure measurement in the morning in type 2 diabetic patients. Diabetes Care. 2002;25(12):2218–2223. doi: 10.2337/diacare.25.12.2218. [DOI] [PubMed] [Google Scholar]
- 70.Stergiou GS, Salgami EV, Tzamouranis DG, Roussias LG. Masked hypertension assessed by ambulatory blood pressure versus home blood pressure monitoring: is it the same phenomenon? Am J Hypertens. 2005;18(6):772–778. doi: 10.1016/j.amjhyper.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 71.Hemmelgarn BR, McAlister FA, Grover S, Myers MG, McKay DW, Bolli P, Abbott C, Schiffrin EL, Honos G, Burgess E, Mann K, Wilson T, Penner B, Tremblay G, Milot A, Chockalingam A, Touyz RM, Tobe SW. The 2006 Canadian Hypertension Education Program recommendations for the management of hypertension: Part I--Blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2006;22(7):573–581. doi: 10.1016/s0828-282x(06)70279-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reeves RA. The rational clinical examination. Does this patient have hypertension? How to measure blood pressure. JAMA. 1995;%19;273(15):1211–1218. doi: 10.1001/jama.273.15.1211. [DOI] [PubMed] [Google Scholar]
- 73.Pickering TG. Blood pressure measurement and detection of hypertension [see comments] Lancet. 1994;344(8914):31–35. doi: 10.1016/s0140-6736(94)91053-7. [DOI] [PubMed] [Google Scholar]
- 74.McAlister FA, Straus SE. Evidence based treatment of hypertension. Measurement of blood pressure: an evidence based review. BMJ. 2001;322(7291):908–911. doi: 10.1136/bmj.322.7291.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognosis of “masked” hypertension and “white-coat” hypertension detected by 24-h ambulatory blood pressure monitoring 10-year follow-up from the Ohasama study. J Am Coll Cardiol. 2005;46(3):508–515. doi: 10.1016/j.jacc.2005.03.070. [DOI] [PubMed] [Google Scholar]
- 76.Verdecchia P, O’Brien E, Pickering T, Staessen JA, Parati G, Myers M, Palatini P. When can the practicing physician suspect white coat hypertension? Statement from the Working Group on Blood Pressure Monitoring of the European Society of Hypertension. Am J Hypertens. 2003;16(1):87–91. doi: 10.1016/s0895-7061(02)03150-3. [DOI] [PubMed] [Google Scholar]
- 77.Verdecchia P, Reboldi GP, Angeli F, Schillaci G, Schwartz JE, Pickering TG, Imai Y, Ohkubo T, Kario K. Short- and long-term incidence of stroke in white-coat hypertension. Hypertension. 2005;45(2):203–208. doi: 10.1161/01.HYP.0000151623.49780.89. [DOI] [PubMed] [Google Scholar]
- 78.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-term risk of mortality associated with selective and combined elevation in office, home, and ambulatory blood pressure. Hypertension. 2006;47(5):846–853. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 79.Hond ED, Celis H, Fagard R, Keary L, Leeman M, O’Brien E, Vandenhoven G, Staessen JA. Self-measured versus ambulatory blood pressure in the diagnosis of hypertension. J Hypertens. 2003;21(4):717–722. doi: 10.1097/00004872-200304000-00014. [DOI] [PubMed] [Google Scholar]
- 80.Den Hond E, Celis H, Vandenhoven G, O’Brien E, Staessen JA. Determinants of white-coat syndrome assessed by ambulatory blood pressure or self-measured home blood pressure. Blood Press Monit. 2003;8(1):37–40. doi: 10.1097/00126097-200302000-00008. [DOI] [PubMed] [Google Scholar]
- 81.Ohkubo T, Imai Y, Tsuji I, Nagai K, Kato J, Kikuchi N, Nishiyama A, Aihara A, Sekino M, Kikuya M, Ito S, Satoh H, Hisamichi S. Home blood pressure measurement has a stronger predictive power for mortality than does screening blood pressure measurement: a population-based observation in Ohasama, Japan. J Hypertens. 1998;16(7):971–975. doi: 10.1097/00004872-199816070-00010. [DOI] [PubMed] [Google Scholar]
- 82.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111(14):1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 83.Asmar R, Zanchetti A. Guidelines for the use of self-blood pressure monitoring: a summary report of the First International Consensus Conference. Groupe Evaluation & Measure of the French Society of Hypertension. J Hypertens. 2000;18(5):493–508. doi: 10.1097/00004872-200018050-00001. [DOI] [PubMed] [Google Scholar]
- 84.Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, Grassi G, Heagerty AM, Kjeldsen SE, Laurent S, Narkiewicz K, Ruilope L, Rynkiewicz A, Schmieder RE, Boudier HA, Zanchetti A. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;25(6):1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 85.Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17(11 Pt 1):1017–1022. doi: 10.1016/j.amjhyper.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 86.McKay DW, Myers MG, Bolli P, Chockalingam A. Masked hypertension: a common but insidious presentation of hypertension. Can J Cardiol. 2006;22(7):617–620. doi: 10.1016/s0828-282x(06)70285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bobrie G, Genes N, Vaur L, Clerson P, Vaisse B, Mallion JM, Chatellier G. Is “isolated home” hypertension as opposed to “isolated office” hypertension a sign of greater cardiovascular risk? Arch Intern Med. 2001;161(18):2205–2211. doi: 10.1001/archinte.161.18.2205. [DOI] [PubMed] [Google Scholar]
- 88.Bjorklund K, Lind L, Zethelius B, Andren B, Lithell H. Isolated ambulatory hypertension predicts cardiovascular morbidity in elderly men. Circulation. 2003;107(9):1297–1302. doi: 10.1161/01.cir.0000054622.45012.12. [DOI] [PubMed] [Google Scholar]
- 89.Sega R, Trocino G, Lanzarotti A, Carugo S, Cesana G, Schiavina R, Valagussa F, Bombelli M, Giannattasio C, Zanchetti A, Mancia G. Alterations of cardiac structure in patients with isolated office, ambulatory, or home hypertension: Data from the general population (Pressione Arteriose Monitorate E Loro Associazioni [PAMELA] Study) Circulation. 2001 Sep 18;104(12):1385–1392. doi: 10.1161/hc3701.096100. [DOI] [PubMed] [Google Scholar]
- 90.Helvaci MR, Seyhanli M. What a high prevalence of white coat hypertension in society! Intern Med. 2006;45(10):671–674. doi: 10.2169/internalmedicine.45.1650. [DOI] [PubMed] [Google Scholar]
- 91.Oikawa T, Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Komai R, Murai K, Hashimoto J, Totsune K, Imai Y. Characteristics of resistant hypertension determined by self-measured blood pressure at home and office blood pressure measurements: the J-HOME study. J Hypertens. 2006;24(9):1737–1743. doi: 10.1097/01.hjh.0000242397.53214.27. [DOI] [PubMed] [Google Scholar]
- 92.Nesbitt SD, Amerena JV, Grant E, Jamerson KA, Lu H, Weder A, Julius S. Home blood pressure as a predictor of future blood pressure stability in borderline hypertension. The Tecumseh Study. Am J Hypertens. 1997;10(11):1270–1280. doi: 10.1016/s0895-7061(97)00267-7. [DOI] [PubMed] [Google Scholar]
- 93.Denolle T, Waeber B, Kjeldsen S, Parati G, Wilson M, Asmar R. Self-measurement of blood pressure in clinical trials and therapeutic applications. Blood Press Monit. 2000;5(2):145–149. [PubMed] [Google Scholar]
- 94.Mule G, Caimi G, Cottone S, Nardi E, Andronico G, Piazza G, Volpe V, Federico MR, Cerasola G. Value of home blood pressures as predictor of target organ damage in mild arterial hypertension. J Cardiovasc Risk. 2002;9(2):123–129. doi: 10.1177/174182670200900208. [DOI] [PubMed] [Google Scholar]
- 95.Abe H, Yokouchi M, Saitoh F, Deguchi F, Kimura G, Kojima S, Yoshimi H, Ito K, Kuramochi M, Ikeda M. Hypertensive complications and home blood pressure: comparison with blood pressure measured in the doctor’s office. J Clin Hypertens. 1987;3(4):661–669. [PubMed] [Google Scholar]
- 96.Jula A, Puukka P, Karanko H. Multiple clinic and home blood pressure measurements versus ambulatory blood pressure monitoring. Hypertension. 1999;34(2):261–266. doi: 10.1161/01.hyp.34.2.261. [DOI] [PubMed] [Google Scholar]
- 97.Kamoi K, Imamura Y, Miyakoshi M, Kobayashi C. Usefulness of home blood pressure measurement in the morning in type 1 diabetic patients. Diabetes Care. 2003;26(8):2473–2475. doi: 10.2337/diacare.26.8.2473. [DOI] [PubMed] [Google Scholar]
- 98.Oikawa T, Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Komai R, Murai K, Hashimoto J, Totsune K, Imai Y. Characteristics of resistant hypertension determined by self-measured blood pressure at home and office blood pressure measurements: the J-HOME study. J Hypertens. 2006;24(9):1737–1743. doi: 10.1097/01.hjh.0000242397.53214.27. [DOI] [PubMed] [Google Scholar]
- 99.Bobrie G, Chatellier G, Genes N, Clerson P, Vaur L, Vaisse B, Menard J, Mallion JM. Cardiovascular prognosis of “masked hypertension” detected by blood pressure self-measurement in elderly treated hypertensive patients. JAMA. 2004;291(11):1342–1349. doi: 10.1001/jama.291.11.1342. [DOI] [PubMed] [Google Scholar]
- 100.Fagard RH, Van Den BC, De Cort P. Prognostic significance of blood pressure measured in the office, at home and during ambulatory monitoring in older patients in general practice. J Hum Hypertens. 2005;19(10):801–807. doi: 10.1038/sj.jhh.1001903. [DOI] [PubMed] [Google Scholar]
- 101.Ohkubo T, Asayama K, Kikuya M, Metoki H, Obara T, Saito S, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. Prediction of ischaemic and haemorrhagic stroke by self-measured blood pressure at home: the Ohasama study. Blood Press Monit. 2004;9(6):315–320. doi: 10.1097/00126097-200412000-00009. [DOI] [PubMed] [Google Scholar]
- 102.Ohkubo T, Asayama K, Kikuya M, Metoki H, Hoshi H, Hashimoto J, Totsune K, Satoh H, Imai Y. How many times should blood pressure be measured at home for better prediction of stroke risk? Ten-year follow-up results from the Ohasama study. J Hypertens. 2004;22(6):1099–1104. doi: 10.1097/00004872-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 103.Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hara A, Hirose T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance of night-time, early morning, and daytime blood pressures on the risk of cerebrovascular and cardiovascular mortality: the Ohasama Study. J Hypertens. 2006;24(9):1841–1848. doi: 10.1097/01.hjh.0000242409.65783.fb. [DOI] [PubMed] [Google Scholar]
- 104.Metoki H, Ohkubo T, Kikuya M, Asayama K, Obara T, Hara A, Hirose T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Prognostic significance of night-time, early morning, and daytime blood pressures on the risk of cerebrovascular and cardiovascular mortality: the Ohasama Study. J Hypertens. 2006;24(9):1841–1848. doi: 10.1097/01.hjh.0000242409.65783.fb. [DOI] [PubMed] [Google Scholar]
- 105.Agarwal R, Andersen MJ. Prognostic importance of clinic and home blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69(2):406–411. doi: 10.1038/sj.ki.5000081. [DOI] [PubMed] [Google Scholar]
- 106.Rave K, Bender R, Heise T, Sawicki PT. Value of blood pressure self-monitoring as a predictor of progression of diabetic nephropathy. J Hypertens. 1999;17(5):597–601. doi: 10.1097/00004872-199917050-00002. [DOI] [PubMed] [Google Scholar]
- 107.Suzuki H, Nakamoto H, Okada H, Sugahara S, Kanno Y. Self-measured systolic blood pressure in the morning is a strong indicator of decline of renal function in hypertensive patients with non-diabetic chronic renal insufficiency. Clin Exp Hypertens. 2002;24(4):249–260. doi: 10.1081/ceh-120004229. [DOI] [PubMed] [Google Scholar]
- 108.Ohkubo T, Obara T, Funahashi J, Kikuya M, Asayama K, Metoki H, Oikawa T, Takahashi H, Hashimoto J, Totsune K, Imai Y. Control of blood pressure as measured at home and office, and comparison with physicians’ assessment of control among treated hypertensive patients in Japan: First Report of the Japan Home versus Office Blood Pressure Measurement Evaluation (J-HOME) study. Hypertens Res. 2004;27(10):755–763. doi: 10.1291/hypres.27.755. [DOI] [PubMed] [Google Scholar]
- 109.Obara T, Ohkubo T, Kikuya M, Asayama K, Metoki H, Inoue R, Oikawa T, Murai K, Komai R, Horikawa T, Hashimoto J, Totsune K, Imai Y. The current status of home and office blood pressure control among hypertensive patients with diabetes mellitus: The Japan Home Versus Office Blood Pressure Measurement Evaluation (J-HOME) study. Diabetes Res Clin Pract. 2006 doi: 10.1016/j.diabres.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 110.Llisterri JL, Gil VF, Rodriguez G, Orozco D, Garcia A, Merino J. Interest of home blood pressure measurements (HBPM) to establish degree of hypertensive control. Blood Press. 2003;12(4):220–224. doi: 10.1080/08037050310015476. [DOI] [PubMed] [Google Scholar]
- 111.Hitzenberger G, Magometschnigg D. Blood pressure characteristics of hypertensive patients in Austria as determined by self-monitoring (SCREEN-II) Blood Press. 2003;12(3):134–138. [PubMed] [Google Scholar]
- 112.Mancia G, Zanchetti A, Agabiti-Rosei E, Benemio G, De Cesaris R, Fogari R, Pessina A, Porcellati C, Rappelli A, Salvetti A, Trimarco B, Agebiti-Rosei E, Pessino A. Ambulatory blood pressure is superior to clinic blood pressure in predicting treatment-induced regression of left ventricular hypertrophy. SAMPLE Study Group. Study on Ambulatory Monitoring of Blood Pressure and Lisinopril Evaluation [published erratum appears in Circulation 1997 Aug 5; 96(3):1065] Circulation. 1997;95(6):1464–1470. doi: 10.1161/01.cir.95.6.1464. [DOI] [PubMed] [Google Scholar]
- 113.Staessen JA, Den Hond E, Celis H, Fagard R, Keary L, Vandenhoven G, O’Brien ET. Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291(8):955–964. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 114.Den Hond E, Staessen JA, Celis H, Fagard R, Keary L, Vandenhoven G, O’Brien ET. Antihypertensive treatment based on home or office blood pressure--the THOP trial. Blood Press Monit. 2004;9(6):311–314. doi: 10.1097/00126097-200412000-00008. [DOI] [PubMed] [Google Scholar]
- 115.Menard J, Chatellier G, Day M, Vaur L. Self-measurement of blood pressure at home to evaluate drug effects by the trough: peak ratio. J Hypertens Suppl. 1994;12(8):S21–S25. [PubMed] [Google Scholar]
- 116.Klem ML, Wing RR, McGuire MT, Seagle HM, Hill JO. A descriptive study of individuals successful at long-term maintenance of substantial weight loss. Am J Clin Nutr. 1997;66(2):239–246. doi: 10.1093/ajcn/66.2.239. [DOI] [PubMed] [Google Scholar]
- 117.Ogedegbe G, Schoenthaler A. A systematic review of the effects of home blood pressure monitoring on medication adherence. J Clin Hypertens (Greenwich) 2006;8(3):174–180. doi: 10.1111/j.1524-6175.2006.04872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Binstock ML, Franklin KL. A comparison of compliance techniques on the control of high blood pressure. Am J Hypertens. 1988;1(3 Pt 3):192S–194S. doi: 10.1093/ajh/1.3.192s. [DOI] [PubMed] [Google Scholar]
- 119.Zarnke KB, Feagan BG, Mahon JL, Feldman RD. A randomized study comparing a patient-directed hypertension management strategy with usual office-based care. Am J Hypertens. 1997;10(1):58–67. doi: 10.1016/s0895-7061(96)00305-6. [DOI] [PubMed] [Google Scholar]
- 120.Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, Torgerson J, Carey K. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. Amer J Hypertens. 1996;9:285–292. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 121.Mehos BM, Saseen JJ, MacLaughlin EJ. Effect of pharmacist intervention and initiation of home blood pressure monitoring in patients with uncontrolled hypertension. Pharmacotherapy. 2000;20(11):1384–1389. doi: 10.1592/phco.20.17.1384.34891. [DOI] [PubMed] [Google Scholar]
- 122.Ogbuokiri JE. Self-monitoring of blood pressures in hypertensive subjects and its effects on patient compliance. Drug Intell Clin Pharm. 1980;14(6):424–427. doi: 10.1177/106002808001400606. [DOI] [PubMed] [Google Scholar]
- 123.McKenney JM, Munroe WP, Wright JT., Jr Impact of an electronic medication compliance aid on long-term blood pressure control. J Clin Pharmacol. 1992;32(3):277–283. doi: 10.1002/j.1552-4604.1992.tb03837.x. [DOI] [PubMed] [Google Scholar]
- 124.Johnson AL, Taylor DW, Sackett DL, Dunnett CW, Shimizu AG. Self-recording of blood pressure in the management of hypertension. Can Med Assoc J. 1978;119(9):1034–1039. [PMC free article] [PubMed] [Google Scholar]
- 125.Rudd P, Miller NH, Kaufman J, Kraemer HC, Bandura A, Greenwald G, Debusk RF. Nurse management for hypertension. A systems approach. Am J Hypertens. 2004;17(10):921–927. doi: 10.1016/j.amjhyper.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 126.Vaur L, Dubroca II, Dutrey-Dupagne C, Genes N, Chatellier G, Bouvier-d’Yvoire M, Elkik F, Menard J. Superiority of home blood pressure measurements over office measurements for testing antihypertensive drugs. Blood Press Monit. 1998;3(2):107–114. [PubMed] [Google Scholar]
- 127.Haynes RB, Sackett DL, Gibson ES, Taylor DW, Hackett BC, Roberts RS, Johnson AL. Improvement of medication compliance in uncontrolled hypertension. Lancet. 1976;1(7972):1265–1268. doi: 10.1016/s0140-6736(76)91737-2. [DOI] [PubMed] [Google Scholar]
- 128.Bailey B, Carney SL, Gillies AA, Smith AJ. Antihypertensive drug treatment: a comparison of usual care with self blood pressure measurement. J Hum Hypertens. 1999;13(2):147–150. doi: 10.1038/sj.jhh.1000758. [DOI] [PubMed] [Google Scholar]
- 129.Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004;329(7458):145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Imai Y, Satoh H, Nagai K, Sakuma M, Sakuma H, Minami N, Munakata M, Hashimoto J, Yamagishi T, Watanabe N, Yabe T, Nishiyama A, Nakatsuka H, Koyama H, Abe K. Characteristics of a community-based distribution of home blood pressure in Ohasama in northern Japan. J Hypertens. 1993;11:1441–1449. doi: 10.1097/00004872-199312000-00017. [DOI] [PubMed] [Google Scholar]
- 131.Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, Menard J, Rahn KH, Wedel H, Westerling S. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group Lancet. 1998;351(9118):1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 132.Masding MG, Jones JR, Bartley E, Sandeman DD. Assessment of blood pressure in patients with Type 2 diabetes: comparison between home blood pressure monitoring, clinic blood pressure measurement and 24-h ambulatory blood pressure monitoring. Diabet Med. 2001;18(6):431–437. doi: 10.1046/j.1464-5491.2001.00513.x. [DOI] [PubMed] [Google Scholar]
- 133.Mazze RS, Robinson R, Simonson G, Idrogo M, Simpson B, Kendall D, Bergenstal R. Undetected, uncontrolled blood pressure in type 2 diabetes: self-monitored blood pressure profiles. Blood Press. 2004;13(6):335–342. doi: 10.1080/08037050410004774. [DOI] [PubMed] [Google Scholar]
- 134.Eguchi K, Hoshide S, Ishikawa J, Ishikawa S, Pickering TG, Schwartz JE, Shimada K, Kario K. Ambulatory awake blood pressure is a better marker than clinic blood pressure in predicting cardiovascular events in patients with type 2 diabetes. 2007. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shea S, Weinstock RS, Starren J, Teresi J, Palmas W, Field L, Morin P, Goland R, Izquierdo RE, Wolff LT, Ashraf M, Hilliman C, Silver S, Meyer S, Holmes D, Petkova E, Capps L, Lantigua RA. A randomized trial comparing telemedicine case management with usual care in older, ethnically diverse, medically underserved patients with diabetes mellitus. J Am Med Inform Assoc. 2006;13(1):40–51. doi: 10.1197/jamia.M1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hypertension in people with Type 2 diabetes: knowledge-based diabetes-specific guidelines. Diabet Med. 2003;20:972–987. doi: 10.1046/j.1464-5491.2003.01021.x. [DOI] [PubMed] [Google Scholar]
- 137.Pickering TG. Reflections in hypertension. How should blood pressure be measured during pregnancy? J Clin Hypertens (Greenwich) 2005;7(1):46–49. doi: 10.1111/j.1524-6175.2005.04088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Waugh JJ, Halligan AW, Shennan AH. Ambulatory monitoring and self-monitoring of blood pressure during pregnancy. Blood Press Monit. 2000;5(1):3–10. doi: 10.1097/00126097-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 139.Mooney P, Dalton KJ, Swindells HE, Rushant S, Cartwright W, Juett D. Blood pressure measured telemetrically from home throughout pregnancy. Am J Obstet Gynecol. 1990;163(1 Pt 1):30–36. doi: 10.1016/s0002-9378(11)90660-7. [DOI] [PubMed] [Google Scholar]
- 140.Ross-McGill H, Hewison J, Hirst J, Dowswell T, Holt A, Brunskill P, Thornton JG. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG. 2000;107(2):217–221. doi: 10.1111/j.1471-0528.2000.tb11692.x. [DOI] [PubMed] [Google Scholar]
- 141.Bellomo G, Narducci PL, Rondoni F, Pastorelli G, Stangoni G, Angeli G, Verdecchia P. Prognostic value of 24-hour blood pressure in pregnancy. JAMA. 1999;282(15):1447–1452. doi: 10.1001/jama.282.15.1447. [DOI] [PubMed] [Google Scholar]
- 142.Pickering TG. Target blood pressure in patients with end-stage renal disease: evidence-based medicine or the emperor’s new clothes? J Clin Hypertens (Greenwich) 2006;8(5):369–375. doi: 10.1111/j.1524-6175.2005.05123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andersen MJ, Khawandi W, Agarwal R. Home blood pressure monitoring in CKD. Am J Kidney Dis. 2005;45(6):994–1001. doi: 10.1053/j.ajkd.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 144.Thompson AM, Pickering TG. The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int. 2006;70(6):1000–1007. doi: 10.1038/sj.ki.5001695. [DOI] [PubMed] [Google Scholar]
- 145.Semret M, Zidehsarai M, Agarwal R. Accuracy of oscillometric blood pressure monitoring with concurrent auscultatory blood pressure in hemodialysis patients. Blood Press Monit. 2005;10(5):249–255. doi: 10.1097/01.mbp.0000172713.28029.84. [DOI] [PubMed] [Google Scholar]
- 146.Agarwal R, Andersen MJ, Bishu K, Saha C. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69(5):900–906. doi: 10.1038/sj.ki.5000145. [DOI] [PubMed] [Google Scholar]
- 147.National High Blood Pressure Working Group on high blood pressure in children and adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 148.Sorof JM, Poffenbarger T, Franco K, Portman R. Evaluation of white coat hypertension in children: importance of the definitions of normal ambulatory blood pressure and the severity of casual hypertension. Am J Hypertens. 2001;14(9 Pt 1):855–860. doi: 10.1016/s0895-7061(01)02180-x. [DOI] [PubMed] [Google Scholar]
- 149.Stergiou GS, Alamara CV, Kalkana CB, Vaindirlis IN, Stefanidis CJ, Dacou-Voutetakis C, Mountokalakis TD. Out-of-office blood pressure in children and adolescents: disparate findings by using home or ambulatory monitoring. Am J Hypertens. 2004;17(10):869–875. doi: 10.1016/j.amjhyper.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 150.Wuhl E, Hadtstein C, Mehls O, Schaefer F. Home, clinic, and ambulatory blood pressure monitoring in children with chronic renal failure. Pediatr Res. 2004;55(3):492–497. doi: 10.1203/01.PDR.0000106863.90996.76. [DOI] [PubMed] [Google Scholar]
- 151.Krakoff LR. Cost-effectiveness of ambulatory blood pressure: a reanalysis. Hypertension. 2006;47(1):29–34. doi: 10.1161/01.HYP.0000197195.84725.66. [DOI] [PubMed] [Google Scholar]
- 152.McGrath BP. Ambulatory blood pressure monitoring. Med J Aust. 2002;176(12):588–592. doi: 10.5694/j.1326-5377.2002.tb04590.x. [DOI] [PubMed] [Google Scholar]
- 153.Graves JW. A survey of validated automated home blood pressure monitors available for the Internet shopper. Blood Press Monit. 2005;10(2):103–107. doi: 10.1097/00126097-200504000-00009. [DOI] [PubMed] [Google Scholar]
- 154.Veerman DP, Van Montfrans GA, Wieling W. Effects of cuff inflation on self-recorded blood pressure. Lancet. 1990;335:451–453. doi: 10.1016/0140-6736(90)90676-v. [DOI] [PubMed] [Google Scholar]
- 155.Myers MG. Self-measurement of blood pressure at home: the potential for reporting bias. Blood Press Monit. 1998;3 (Suppl 1):S19–S22. [Google Scholar]
- 156.Rickerby J, Woodward J. Patients’ experiences and opinions of home blood pressure measurement. J Hum Hypertens. 2003;17(7):495–503. doi: 10.1038/sj.jhh.1001582. [DOI] [PubMed] [Google Scholar]
- 157.Port K, Palm K, Viigimaa M. Daily usage and efficiency of remote home monitoring in hypertensive patients over a one-year period. J Telemed Telecare. 2005;11(Suppl 1):34–36. doi: 10.1258/1357633054461705. [DOI] [PubMed] [Google Scholar]
- 158.Little P, Barnett J, Barnsley L, Marjoram J, Fitzgerald-Barron A, Mant D. Comparison of acceptability of and preferences for different methods of measuring blood pressure in primary care. BMJ. 2002;325(7358):258–259. doi: 10.1136/bmj.325.7358.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bradford WD, Kleit A, Krousel-Wood MA, Re RM. Comparing willingness to pay for telemedicine across a chronic heart failure and hypertension population. Telemed J E Health. 2005;11(4):430–438. doi: 10.1089/tmj.2005.11.430. [DOI] [PubMed] [Google Scholar]
- 160.Rickerby J. The role of home blood pressure measurement in managing hypertension: an evidence-based review. J Hum Hypertens. 2002;16(7):469–472. doi: 10.1038/sj.jhh.1001423. [DOI] [PubMed] [Google Scholar]
- 161.Funahashi J, Ohkubo T, Fukunaga H, Kikuya M, Takada N, Asayama K, Metoki H, Obara T, Inoue R, Hashimoto J, Totsune K, Kobayashi M, Imai Y. The economic impact of the introduction of home blood pressure measurement for the diagnosis and treatment of hypertension. Blood Press Monit. 2006;11(5):257–267. doi: 10.1097/01.mbp.0000217996.19839.70. [DOI] [PubMed] [Google Scholar]
- 162.Soghikian K, Casper SM, Fireman BH, Hunkeler EM, Hurley LB, Tekawa IS, Vogt TM. Home blood pressure monitoring. Effect on use of medical services and medical care costs. Med Care. 1992;30(9):855–865. [PubMed] [Google Scholar]
- 163.Canzanello VJ, Jensen PL, Schwartz LL, Worra JB, Klein LK. Improved blood pressure control with a physician-nurse team and home blood pressure measurement. Mayo Clin Proc. 2005;80(1):31–36. doi: 10.1016/S0025-6196(11)62954-6. [DOI] [PubMed] [Google Scholar]
- 164.Mengden T, Ewald S, Kaufmann S, vor dem EJ, Uen S, Vetter H. Telemonitoring of blood pressure self measurement in the OLMETEL study. Blood Press Monit. 2004;9(6):321–325. doi: 10.1097/00126097-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 165.Rogers MA, Small D, Buchan DA, Butch CA, Stewart CM, Krenzer BE, Husovsky HL. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med. 2001 Jun 5;134(11):1024–1032. doi: 10.7326/0003-4819-134-11-200106050-00008. [DOI] [PubMed] [Google Scholar]
- 166.Verberk WJ, Kroon AA, Kessels AG, Dirksen C, Nelemans PJ, Lenders JW, Thien TA, van Montfrans GA, Smit AJ, de Leeuw PW. Home versus Office blood pressure MEasurements: Reduction of Unnecessary treatment Study: rationale and study design of the HOMERUS trial. Blood Press. 2003;12(5–6):326–333. doi: 10.1080/08037050310022405. [DOI] [PubMed] [Google Scholar]
- 167.Verberk WJ, Kroon AA, Lenders JW, Kessels AG, van Montfrans GA, Smit AJ, van der Kuy PH, Nelemans PJ, Rennenberg RJ, Grobbee DE, Beltman FW, Joore MA, Brunenberg DE, Dirksen C, Thien T, de Leeuw PW. Self-measurement of blood pressure at home reduces the need for antihypertensive drugs: a randomized, controlled trial. Hypertension. 2007;50(6):1019–1025. doi: 10.1161/HYPERTENSIONAHA.107.094193. [DOI] [PubMed] [Google Scholar]
- 168.Fischer MA, Avorn J. Economic implications of evidence-based prescribing for hypertension: can better care cost less? JAMA. 2004;291(15):1850–1856. doi: 10.1001/jama.291.15.1850. [DOI] [PubMed] [Google Scholar]
- 169.Odell TW, Gregory MC. Cost of hypertension treatment. J Gen Intern Med. 1995;10(12):686–688. doi: 10.1007/BF02602764. [DOI] [PubMed] [Google Scholar]
- 170.Ramsey SD, Neil N, Sullivan SD, Perfetto E. An economic evaluation of the JNC hypertension guidelines using data from a randomized controlled trial. Joint National Committee. J Am Board Fam Pract. 1999;12(2):105–114. doi: 10.3122/jabfm.12.2.105. [DOI] [PubMed] [Google Scholar]
- 171.Krakoff LR. Systems for care of hypertension in the United States. J Clin Hypertens (Greenwich) 2006;8(6):420–426. doi: 10.1111/j.1076-7460.2006.05385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mengden T, Chamontin B, Phong CN, Luis Palma GJ, Chanudet X. User procedure for self-measurement of blood pressure. First International Consensus Conference on Self Blood Pressure Measurement. Blood Press Monit. 2000;5(2):111–129. [PubMed] [Google Scholar]
- 173.Myers MG. Blood pressure measurement and the guidelines: a proposed new algorithm for the diagnosis of hypertension. Blood Press Monit. 2004;9(6):283–286. doi: 10.1097/00126097-200412000-00002. [DOI] [PubMed] [Google Scholar]
- 174.Myers MG. Reporting bias in self-measurement of blood pressure. Blood Press Monit. 2001;6(4):181–183. doi: 10.1097/00126097-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 175.Celis H, Den Hond E, Staessen JA. Self-measurement of blood pressure at home in the management of hypertension. Clin Med Res. 2005;3(1):19–26. doi: 10.3121/cmr.3.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.American College of Physicians. Automated ambulatory blood pressure and self-measured blood pressure monitoring devices: their role in the diagnosis and management of hypertension. Ann Int Med. 1993;118:889–892. doi: 10.7326/0003-4819-118-11-199306010-00010. [DOI] [PubMed] [Google Scholar]