Abstract
Using the Manco-Johnson instrument in a derivation cohort of 107 children with/without central venous catheters, upper extremity (UE) physical findings of post-thrombotic syndrome were absent and pain score was zero in all but one child. Inter-rater reliability in an independent validation cohort (n=38) of children with/without UE DVT was 97-100%.
Keywords: Post-thrombotic syndrome, Children, Outcome measurement, Validation, Deep venous thrombosis
Post-thrombotic syndrome (PTS) is an important long-term sequela of DVT in children, which involves obstructed and/or refluxed venous blood flow of the venous circulation draining the upper or lower extremities (UE, LE) [1]. It is characterized by a variety of signs and symptoms constituting a spectrum of clinical severity, including pain, swelling, collateral venous formation, venous stasis dermatitis, and frank ulceration [2]. Despite the frequency of UE DVT in pediatric registries and cohort studies, no validated, standardized outcome measure currently exists for UE PTS in children. Consequently, the understanding of pediatric PTS has been limited by variability in outcome measurement across published studies. Validation data have been published [3] for LE PTS outcome measurement using the Manco-Johnson instrument. In the present work, we investigated the validity of this instrument for UE PTS in children, via a cross-sectional derivation cohort/validation cohort design.
Methods
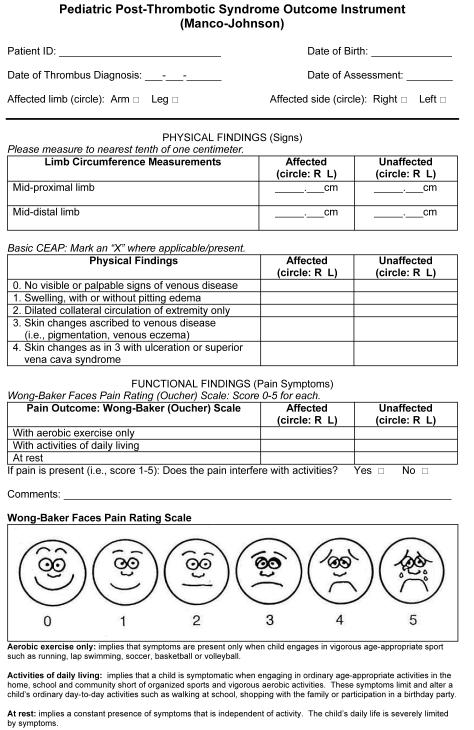
Following local institutional review board approval (COMIRB protocol #02-904), the items of the Manco-Johnson instrument (Figure) were assessed in a cross-sectional study consisting first of a derivation cohort of healthy children (n=78) and children with presence/history of unilateral central venous catheter (CVC; n=29) placed in the upper venous system, including Mediport (n=25) or peripherally-inserted central catheter (n=4). The Manco-Johnson instrument combines the “basic CEAP” component of the chronic venous insufficiency scale developed by the American Venous Forum [4] with the Wong-Baker “faces” scale for pediatric pain assessment [5]. Pediatric pain assessment utilizes parental report for age <7 years, and self-report for age ≥ 7 years (in the absence of developmental delay or cognitive impairment).
Figure.
Manco-Johnson instrument for pediatric PTS outcome measurement.
Inclusion criteria for the derivation cohort consisted of age 12 months to 21 years. Exclusion criteria in the healthy child group included major medical illness, personal history of venous thromboembolism (VTE), and first-degree family history of VTE before age 55 years. Healthy subjects were grouped as follows: preschool (12 mo - <6 y); school age (6 - <13 y); adolescent (13 - 21 y). Exclusion criteria for the CVC group were the same as for the healthy child group, except major medical illness was permissible, and history of CVC malfunction was an additional exclusion.
Upper limits of normal values for the contralateral difference in mid-limb circumference were calculated in each age group in the derivation cohort as [median + (1.5*interquartile range)] according to the method of Tukey and coworkers [6], given non-parametric distributions of data. Distributions of the difference in contralateral circumference were compared among age groups by Kruskal-Wallis test.
Inter-rater reliability was evaluated for all items of the Manco-Johnson instrument via two mutually-blinded, trained examiners in a mixed validation cohort (n=38) consisting of an independent group of healthy children and patients with history of UE DVT. In the case of contralateral difference in limb circumference in the derivation cohort, abnormality was defined by a value exceeding 1.0 cm. Inter-rater reliability was measured as percent agreement. Based upon prior data for inter-reliability of the instrument in LE PTS [3], and using a 95% confidence interval approach, a target sample size of 38 was determined for the validation study, in order to achieve 80% power in detecting a percent agreement no lower than 90%.
Longitudinal data on preliminary application of the instrument in children with acute UE DVT followed for a minimum of six months post-event (n=14) were obtained via an institution-based prospective inception cohort study for pediatric VTE (COMIRB protocol #05-0339).
Results
Investigation of the Manco-Johnson instrument among healthy children in the derivation cohort revealed that contralateral differences in upper limb circumferences did not significantly differ by age group (upper arm: P=0.89; lower arm: P=0.51). The upper limit of normal for contralateral difference in upper limb circumference was 1.0 cm for both mid-forearm and mid-upper arm measurements, independent of age group (Table). None of these children had physical findings of chronic venous insufficiency and none reported chronic pain that limited physical activity.
Table.
UE findings for Manco-Johnson instrument items in a derivation cohort (n=78) of healthy children, by age group.
| Age group | Age range | n | Median (IQR) contralateral difference in mid-upper arm circumference (cm) |
Median (IQR) contralateral difference in mid-forearm circumference (cm) |
|---|---|---|---|---|
| Preschool | 12 mo-< 6 y | 30 | 0.2 (0.5) | 0.3 (0.5) |
| School age | 6 y-< 13 y | 28 | 0.1 (0.4) | 0.0 (0.5) |
| Adolescent | 13 y-21 y | 20 | 0.2 (0.5) | 0.0 (0.6) |
Abbreviations: UE, upper extremity; PTS, post-thrombotic syndrome; n, number of subjects; IQR, inter-quartile range.
Data for contralateral differences in UE circumference measurements are summarized. PTS findings of venous stasis dermatitis, venous stasis ulcers, chronic UE pain that limits aerobic activities, chronic UE pain that limits activities of daily living, and chronic UE pain at rest were all absent.
Because the presence of a CVC is a prevalent risk factor for UE DVT, we next evaluated this instrument in a cohort (n=29) of children with history or current presence of a unilateral CVC in the upper venous system, with no known history of VTE or CVC malfunction. The upper limit of normal for contralateral difference in mid-forearm and mid-upper arm circumference was again determined to be 1.0 cm. None of these children with CVCs had physical findings and only one child (3%) reported chronic pain that limited physical activity (specifically, aerobic exercise). Median time from CVC insertion to PTS assessment in this cohort was 9 months (range: 1-230 months).
In the validation cohort, inter-rater reliability (percent agreement) was 97% for significant (i.e., >1.0 cm) contralateral difference in mid-forearm circumference, and 100% for all remaining items of the instrument. Preliminary application in a prospective series of children with acute UE DVT revealed cumulative incidences of all-PTS (i.e., either abnormal physical findings OR chronic pain that limits physical activity) of 29% (4/14) at six months and 30% (3/10) at one year; cumulative incidences of physically- and-functionally-significant PTS (i.e., abnormal physical findings AND pain that limits physical activity) were 14% (2/14) at six months and 20% (2/10) at one year.
Discussion
The present study establishes the validity of the Manco-Johnson instrument for UE PTS in children. It demonstrates that PTS finding by physical examination and pain report are absent among healthy children and rare among children with a history or current presence of a CVC who have no history of VTE. Furthermore, it shows excellent inter-rater reliability of the instrument when performed by appropriately trained examiners.
The determination of 1.0 cm as the upper limit of normal for contralateral differences in mid-forearm and mid-upper arm circumference is concordant with recent findings by Boulden and group [7]. In our derivation cohort, 10% of children had circumference differences greater than 3% and 4% for the mid-upper arm and mid-forearm, respectively. Percent differences of this magnitude equated to a raw difference of only 0.7 cm in circumference. Furthermore, our finding that contralateral difference in UE circumference in children does not appreciably vary with age also concurs with observations by Boulden et al [7].
Discrepancies in findings for normal variation in contralateral UE circumferences between our study and other published data may exist, due to differences in anatomic landmarks employed or other aspects of measurement technique. Present findings for inter-rater reliability of the Manco-Johnson instrument are germane to trained examiners. An online training module for the use of this instrument is available on the Kids-DOTT clinical trial website [8]. It should also be noted that the limited numbers of subjects in the validation cohort described here (although appropriately powered) and the prospective series of children with UE DVT may engender some imprecision in both the observed inter-rater reliability of the instrument and the frequency of PTS following UE DVT in children.
Notwithstanding these caveats, the present work in the UE, together with prior published data in the LE, demonstrate the validity of the Manco-Johnson instrument for PTS outcome measurement in children. Broader use of the Manco-Johnson instrument is warranted in prospective studies of pediatric venous thrombosis.
Acknowledgments
Supported by a Career Development Award from the National Institutes of Health, National Heart Lung and Blood Institute (1K23HL084055-01A1 to N.G.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
REFERENCES
- 1.Kahn SR, Ginsberg J. The post-thrombotic syndrome: current knowledge, controversies, and directions for future research. Blood Rev. 2002;16:155–165. doi: 10.1016/s0268-960x(02)00008-5. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg NA. Long-term outcomes of venous thrombosis in children. Curr Opin Hematol. 2005;12:370–376. doi: 10.1097/01.moh.0000160754.55131.14. [DOI] [PubMed] [Google Scholar]
- 3.Goldenberg NA, Durham JD, Knapp-Clevenger R, et al. A thrombolytic regimen for high-risk deep venous thrombosis may substantially reduce the risk of postthrombotic syndrome in children. Bood. 2007;110:45–53. doi: 10.1182/blood-2006-12-061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutherford RB, Padberg FT, Comerota AJ, et al. Venous severity score: an adjunct to venous outcome assessment. J Vasc Surg. 2000;31:1307–1312. doi: 10.1067/mva.2000.107094. [DOI] [PubMed] [Google Scholar]
- 5.Wong DL, Baker CM. Pain in children: comparison of assessment scales. Pediatr Nurs. 1988;14:9–17. [PubMed] [Google Scholar]
- 6.Hoaglin DC, Iglewicz B, Tukey JW. Performance of some resistant rules for outlier labeling. J Amer Stat Assoc. 1986;81:991. [Google Scholar]
- 7.Boulden BM, Crary SE, Buchanan GR, et al. Determination of pediatric norms for assessment of upper venous system post-thrombotic syndrome. J Thromb Haemost. 2007;5:1077–1079. doi: 10.1111/j.1538-7836.2007.02445.x. [DOI] [PubMed] [Google Scholar]
- 8. [Accessed 6 May 2010]; www.kids-dott.net.