Summary
Centrosome separation, critical for bipolar spindle formation and subsequent chromosome segregation during mitosis, occurs via distinct prophase and prometaphase pathways [1–3]. Kinesin-5 (Eg5), a microtubule (MT) motor, pushes centrosomes apart during bipolar spindle assembly [4]; its suppression results in monopolar spindles and mitotic arrest [5, 6]. Forces that antagonize Eg5 in prophase are unknown. Here we identify a new force generating mechanism mediated by the guanine nucleotide exchange factor (GEF) Tiam1, dependent on its ability to activate the GTPase Rac. We reveal that Tiam1 and Rac localize to centrosomes during prophase and prometaphase, and Tiam1, acting through Rac, ordinarily retards centrosome separation. Importantly, both Tiam1-depleted cells in culture and Rac1-deficient epithelial cells in vivo escape the mitotic arrest induced by Eg5 suppression. Moreover, Tiam1-depleted cells transit more slowly through prometaphase and display increased chromosome congression errors. Significantly, Eg5 suppression in Tiam1-depleted cells rectifies not only their increased centrosome separation but also their chromosome congression errors and mitotic delay. These findings identify Tiam1-Rac signaling as the first antagonist of centrosome separation during prophase, demonstrate its requirement in balancing Eg5-induced forces during bipolar spindle assembly in vitro and in vivo, and show that proper centrosome separation in prophase facilitates subsequent chromosome congression.
Keywords: CELLBIO
Highlights
► Tiam1-Rac signaling is the first antagonist of centrosome separation in prophase ► Tiam1-Rac signaling antagonizes Eg5 in bipolar spindle formation ► Tiam1-Rac signaling is required for optimal chromosome congression ► Regulated centrosome separation in prophase facilitates chromosome congression
Results and Discussion
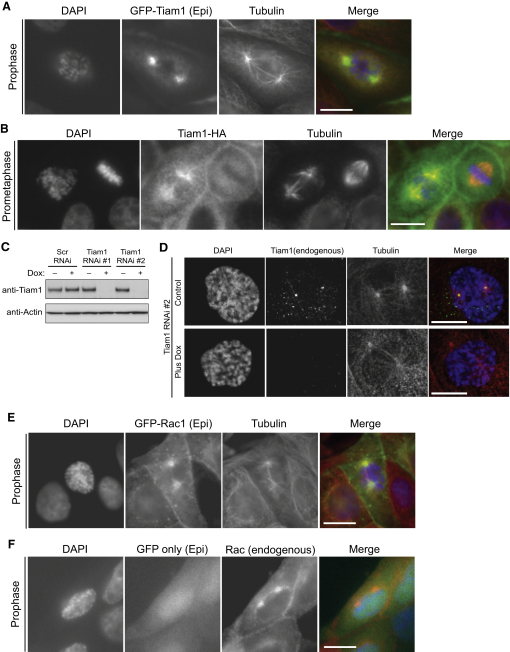
Tiam1 is involved in a number of cellular processes including migration and the regulation of cell-cell adhesions and survival [7]. AlthoughTiam1 has been implicated in cellular proliferation [8], its role during the cell cycle is unknown. In MDCK II cells expressing either GFP-tagged Tiam1 or HA-tagged Tiam1, we were intrigued to observe a signal not only at cell-cell adhesions (as previously described [9]) but also at the two microtubule (MT) asters surrounding the centrosomes. This localization was apparent both before nuclear envelope breakdown (NEBD) in prophase cells (Figure 1A and Figure S1A, available online) and after NEBD in prometaphase cells (Figure 1B and Figure S1B) and was dependent on MTs because it was lost after nocodazole treatment (data not shown). However, in metaphase cells, Tiam1 localization to centrosomal regions was no longer detected (right-hand cell, Figure 1B).
Figure 1.
Tiam1 and Rac Localize to Centrosomal Regions during Early Mitosis
(A and B) MDCK II cells stably expressing (A) GFP-tagged Tiam1 or (B) HA-tagged Tiam1 were fixed and stained where indicated with anti-HA (green), anti-tubulin (red), and DAPI (blue). GFP-tagged Tiam1 was detected solely with epifluorescence from the GFP tag (green). A single z plane at the level of MT asters is shown. Scale bars represent 15 μm.
(C) MDCK II cells carrying a doxycycline-inducible system with either two separate shRNA targets to Tiam1 (RNAi #1, RNAi #2) or a scrambled control shRNA (Scr RNAi) were treated with doxycycline (dox) where indicated as described in Supplemental Experimental Procedures. Levels of Tiam1 and actin were detected by immunoblotting.
(D) Control and Tiam1-depleted (RNAi#2; plus dox) MDCK II cells were fixed and stained with anti-Tiam1 (green), anti-tubulin (red), and DAPI (blue). A single z plane at the level of MT asters is shown. Scale bars represent 10 μm.
(E and F) MDCK II cells stably expressing GFP-Rac1 (E) or a GFP-tag only (F) were fixed and stained where indicated with anti-tubulin or anti-Rac (red) and DAPI (blue). The GFP tag (green) was detected with epifluorescence. Scale bars represent 15 μm.
See also Figure S1.
To investigate the potential function of Tiam1 at centrosomes, we generated MDCK II cells expressing doxycycline-inducible short hairpin interfering RNAs (shRNAs) to Tiam1 (two independent targets: RNAi #1 and RNAi #2) or control scrambled shRNA (Scr). Upon doxycycline addition, endogenous Tiam1 protein was depleted from cells containing either Tiam1 shRNA target (Figure 1C). In subcellular fractions enriched for centrosomes, endogenous Tiam1 cosedimented with the centrosomal marker γ-tubulin and was depleted from these fractions after Tiam1 shRNA expression (Figures S1C and S1D). Moreover, immunofluorescence analysis confirmed that endogenous Tiam1 localized to centrosomal regions in early mitosis (Figures 1D and S1E); this signal was depleted after Tiam1 shRNA expression (Figure 1D).
Because Tiam1 is a Rac-specific activator, we determined where Rac localizes in early mitosis. GFP-tagged Rac was detected at cell-cell adhesions as shown previously [10]; however, it also colocalized with the two MT asters in prophase (Figure 1E) and prometaphase cells (Figure S1F). Endogenous Rac showed a similar localization (Figure 1F and Figure S1G). Hence, Tiam1 and Rac localize to centrosomal regions in early mitosis.
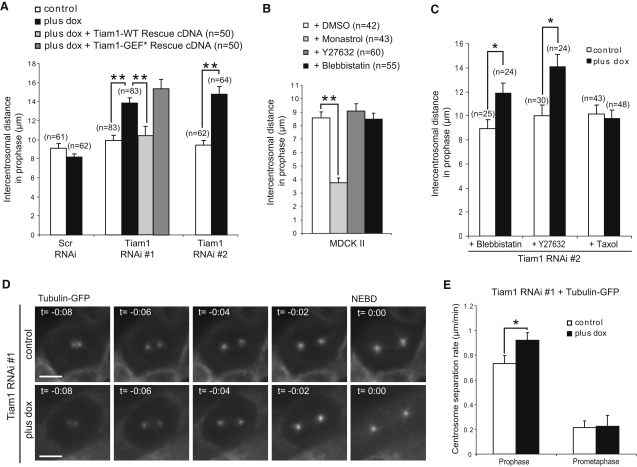
During prophase, duplicated centrosomes begin to separate around the nuclear envelope before NEBD (prophase pathway), in preparation to form a bipolar spindle in prometaphase [1]. We hypothesized that Tiam1 signaling might function to regulate centrosome movement during prophase. Hence, we measured centrosome separation in control and Tiam1-depleted prophase cells. Strikingly, the intercentrosomal distance increased by over 40% in Tiam1-depleted cells (Figure 2A). This defect was efficiently rescued by expressing RNAi-resistant wild-type Tiam1 (Figure 2A) but not the GEF mutant Tiam1 (Q1191A, K1195A [11]), which is unable to activate Rac (Figure 2A). Treatment with a specific Rac inhibitor (NSC-23766 [12]) also increased centrosome separation in prophase cells (Figure S2A). After Tiam1 depletion, a similar increase in centrosome separation was seen in mouse P1 cells (Figures S2B and S2C). Thus, Tiam1 functions via Rac to oppose centrosome separation in prophase.
Figure 2.
Tiam1-Rac Signaling Regulates Centrosome Separation during Early Stages of Mitosis
(A) Control MDCK II cells, those depleted of Tiam1 (RNAi#1 and #2; plus dox), or those re-expressing RNAi#1-resistant Tiam1 (either WT or with a deactivating mutation in the GEF domain [GEF∗]) were fixed and costained with anti-α-tubulin, anti-γ-tubulin (or anti-pericentrin), and DAPI. The distance between centrosomes was measured in 3D for the indicated number of prophase cells.
(B) Control MDCK II cells treated with vehicle (DMSO), Monastrol (200 μM for 4 hr), Y27632 (20 μM for 25 mins), or blebbistatin (50 μM for 25 min) were fixed and stained as in panel (A). The distance between centrosomes in prophase cells was measured in 3D.
(C) Control MDCK II cells or those depleted of Tiam1 (RNAi#2; plus dox) were treated with Y27632 or blebbistatin (as described in B) or taxol (120 nM for 6 hr), fixed and stained as in (A). The distance between centrosomes in prophase cells was measured in 3D.
(D and E) Control and Tiam1-depleted (RNAi #1; plus dox) cells expressing GFP-tagged β-tubulin were analyzed by fluorescence time-lapse microscopy. (D) Representative still images (Maximal-intensity projections) show control and Tiam1-depleted (RNAi#1; plus dox) cells initiating centrosome separation during prophase, where time 0:00 = NEBD. Scale bars represent 10 μm. (E) The distance between centrosomes at each time-point was measured in 3D throughout prophase and prometaphase for at least 22 control and Tiam1-depleted (RNAi#1; plus dox) cells; the centrosome separation rate was calculated as described in Supplemental Experimental Procedures.
In all cases, data are presented as the mean + SEM. ∗p < 0.05, ∗∗p < 0.001. See also Figure S2.
Relatively little is known about centrosome separation during prophase, with conflicting evidence for the involvement of Eg5 [13, 14]. Treatment with the Eg5-specific inhibitor Monastrol [6] led to a significant decrease in the intercentrosomal distance of prophase MDCK II cells (Figure 2B); similar results were obtained with a second Eg5-specific inhibitor S-trityl-L-cysteine [5] (STLC, data not shown). Tiam1 also localizes to the cortex in prophase cells (Figure 1A and Figure S1A), and cortical movements driven by Myosin-II activity (regulated by Rho kinase [ROCK]) are required after NEBD for centrosome separation via astral MT connections [1]. To gain mechanistic insight into the regulation of centrosome separation in prophase, we treated cells with inhibitory concentrations of blebbistatin (Myosin-II inhibitor) or Y-27632 (ROCK inhibitor) (Figures S2D and S2E). However, neither treatment affected centrosome separation in control prophase cells (Figure 2B) or the increased intercentrosomal distance in Tiam1-depleted prophase cells (Figure 2C). In contrast, low concentrations of Taxol, a MT-stabilizing agent, although not effecting centrosome separation in control cells, prevented the increased centrosomal separation of Tiam1-depleted prophase cells (Figure 2C). These data suggest that in prophase, centrosome separation is driven by Eg5 but is unlikely to involve cortical movements. Further, the effects of Tiam1 depletion on centrosome separation, although probably independent of the cortex, may be mediated through microtubule stabilization, although how is currently unclear. A role for Tiam1 in stabilizing MTs during front-rear polarization in cell migration has previously been reported [15]. We detected no change in the localization of Eg5 in Tiam1-depleted cells (data not shown), suggesting that this is independent of Tiam1.
Using time-lapse fluorescence microscopy of cells expressing β-Tubulin-GFP, we followed centrosome movement in individual cells through prophase. After Tiam1 depletion, there was no significant difference in the period between the onset of centrosome separation and NEBD compared with controls (Figure 2D). However, the centrosome separation rate in prophase was significantly increased in Tiam1-depleted cells compared to controls (Figures 2D and 2E). Further, Tiam1-depleted cells maintained their increased centrosome separation after NEBD (Figure S2F). Consistent with this, the intercentrosomal distance in prometaphase from fixed samples was also significantly increased after Tiam1 depletion (Figure S2G) or Rac inhibition (Figure S2H). However, the rate of centrosome separation during prometaphase was markedly reduced compared to prophase and was unaffected by Tiam1 depletion (Figure 2E). To investigate further the role of Tiam1 in centrosome separation in prometaphase, we arrested cells in a monopolar prometaphase state with Monastrol, then released them to allow mitosis to proceed. We found that centrosomes in Tiam1-depleted cells separate significantly further 10 min after washout compared to controls; however, at 20 min the increase was not significant (Figure S2I). Thus, Tiam1-Rac affects centrosome separation apparently independently of the nuclear envelope and seemingly more potently when centrosomes are in close proximity. This property may account for the greater influence that inhibition of this pathway exerts during prophase compared to prometaphase.
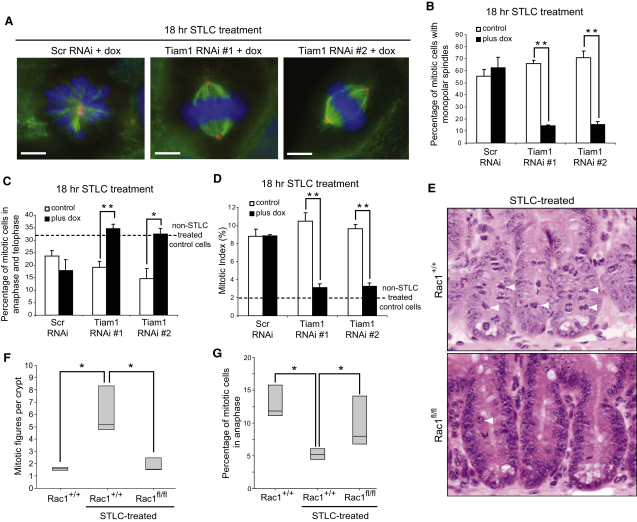
To examine whether Tiam1 antagonizes Eg5 during bipolar spindle formation, we treated control and Tiam1-depleted cells for 18 hr with STLC or Monastrol at concentrations that reduce but do not abolish Eg5 activity [13]. In controls, Eg5 suppression resulted in mainly monopolar mitotic spindles (Figure 3A): ∼60% in STLC-treated cells (Figure 3B) and ∼70% in Monastrol-treated cells (Figure S3A). However, after Tiam1 depletion, mitotic spindles were mainly bipolar (Figure 3A), with significantly fewer monopolar spindles: ∼15% in STLC-treated (Figure 3B) and ∼30% in Monastrol-treated cells (Figure S3A).
Figure 3.
Tiam1-Rac Signaling Antagonizes Eg5 Function during Bipolar Spindle Formation In Vitro and In Vivo
(A–D) Control and Tiam1-depleted (RNAi #1 and RNAi#2; plus dox) cells were treated with STLC (1 μM for 18 hr) and fixed. Samples were costained with anti-α-tubulin (green), anti-γ-tubulin (red), and DAPI (blue).
(A) Single-plane images of representative dox-treated shRNA cell lines are shown. (B) The percentage of mitotic cells with monopolar spindles (>120 cells), (C) the percentage of mitotic cells in anaphase and telophase (>120 cells), or (D) the mitotic index (from 550–1100 cells) was determined by manual counting. The dotted lines (C–D) indicate the normal percentage of mitotic cells in anaphase and telophase (C), as well as the normal mitotic index (D), observed in Eg5-inhibitor-untreated control cells. Data are presented as the mean of at least three separate experiments + SEM. ∗p < 0.05, ∗∗p < 0.01. Scale bars represent 5 μm.
(E–G) AhCre Rac1+/+ (Rac1+/+) and AhCre Rac1fl/fl (Rac1fl/fl) mice were treated with β-napthoflavone to induce Cre expression. (E) H&E staining of intestinal crypts 3 days after Cre induction from Rac1+/+ and Rac1fl/fl mice treated with STLC for 3 hr, as described in Supplemental Experimental Procedures, is shown. Example mitotic figures are highlighted with white arrowheads (note presence of multiple arrested mitotic figures in wild-type [Rac1+/+] but not Rac1-deficient [Rac1fl/fl] intestines treated with STLC). (F and G) Box plots represent (F) the number of mitotic figures per intestinal crypt and (G) the percentage of mitotic cells in anaphase, in either control Rac1+/+ mice or Rac1+/+ and Rac1fl/fl mice after treatment with STLC, as described in the Supplemental Experimental Procedures (box represents the lower and upper quartiles; line represents the median). ∗p < 0.05, Mann Whitney test. See also Figure S3.
We next confirmed that the bipolar spindles formed in Tiam1-depleted cells, under conditions of Eg5 suppression, were functional and able to segregate chromosomes later in mitosis. In control cells, the proportion of mitotic cells in anaphase or telophase significantly reduced from over 32% to ∼20% after STLC (Figure 3C) and ∼12% after Monastrol treatment (Figure S3B). Conversely, in Tiam1-depleted cells, Eg5 suppression had little effect on the proportion of mitotic cells in anaphase or telophase: ∼32% after STLC (Figure 3C) and over 25% after Monastrol treatment (Figure S3B). Consistent with this, in controls the mitotic index increased by over 4.5-fold after Eg5 suppression (Figure 3D and Figure S3C) as a result of the monopolar spindles activating the spindle assembly checkpoint (SAC), thereby delaying mitosis. However, Tiam1 depletion prevented the accumulation of mitotic cells after Eg5 suppression (Figure 3D and Figure S3C).
To determine whether Eg5 function is antagonized by Rac signaling in vivo, we utilized conditional Rac1 knockout mice [16], focusing on the intestinal epithelium. Epithelial cells within intestinal crypts are highly proliferative and arrest rapidly after administration of various drugs [17]; this arrest is visualized via an accumulation of mitotic figures within the intestinal crypt. We first conditionally deleted Rac1 within the intestinal epithelium (as described in the Supplemental Experimental Procedures). Three days after Cre induction [18], the intestinal epithelium from AhCre Rac1fl/fl mice showed loss of Rac1 (Figures S3D and S3E). Deletion of Rac1 at this stage did not affect proliferation (data not shown) or cellularity (Figure S3F). We next treated mice with 25 mg/kg STLC and investigated mitotic arrest after 3 hr. In wild-type mice, STLC treatment induced a significant mitotic arrest (Figures 3E and 3F; Rac1+/+ control versus Rac1+/+ STLC-treated). Remarkably, Rac1 deletion completely rescued the induction of mitotic arrest (Figures 3E and 3F; Rac1+/+ STLC-treated versus Rac1fl/fl STLC-treated). Moreover, in wild-type mice the proportion of mitotic cells in anaphase was significantly reduced after STLC treatment, indicating an arrest prior to this stage (Figure 3G). This reduction was significantly prevented after Rac1 deletion (Figure 3G). Taken together, these data indicate that Tiam1-Rac signaling antagonizes Eg5 function, both in vitro and in vivo; in their absence mitotic cells can assemble bipolar spindles and complete mitosis under conditions of reduced Eg5 activity.
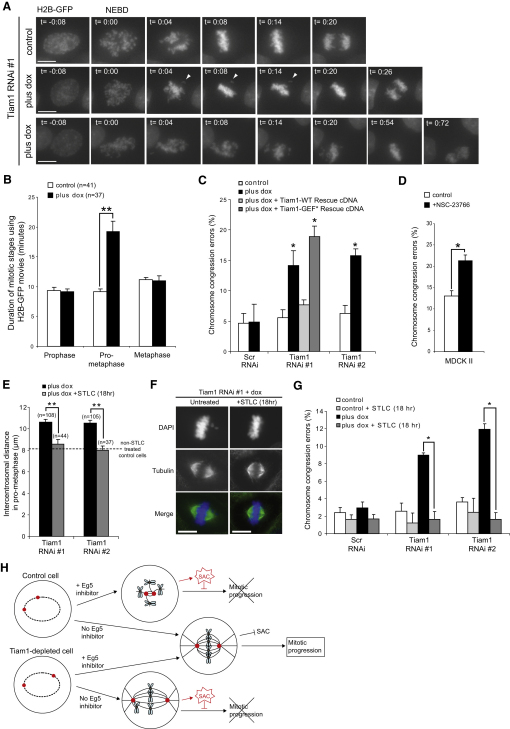
We tested whether Tiam1 depletion induced further mitotic defects by using time-lapse fluorescence microscopy of cells expressing Histone-2B-GFP. In controls, chromosomes efficiently congressed to the metaphase plate after NEBD and rapidly entered anaphase (Figure 4A). In over 60% of Tiam1-depleted cells, however, chromosomes spent over 15 min aligning on the metaphase plate (compared to less than 5% of controls), before eventually aligning and entering anaphase (Figure 4A). The duration of early mitotic stages, calculated from numerous movies, indicated that Tiam1 depletion specifically increased prometaphase duration (Figure 4B).
Figure 4.
Regulation of Centrosome Separation by Tiam1-Rac Signaling Is Required for Proper Chromosome Congression
(A and B) Control and Tiam1-depleted (RNAi #1; plus dox) cells expressing GFP-tagged Histone-2B (H2B-GFP) were analyzed with fluorescence time-lapse microscopy as described in the Supplemental Experimental Procedures. (A) Maximal-intensity projections of example still images from control and two Tiam1-depleted cells displaying intermediate (middle panel) or severe (lower panel) chromosome congression defects, where time 0:00 = first frame after NEBD. Arrowheads in the middle panel indicate a single unaligned chromosome. Scale bars represent 10 μm. (B) The durations of prophase, prometaphase, and metaphase were determined from time-lapse movies.
(C) Control MDCK II cells, those depleted of Tiam1 (RNAi#1 and #2; plus dox), or those re-expressing RNAi#1-resistant Tiam1 (either WT or GEF mutant [GEF∗]) were fixed and stained with anti-α-tubulin and DAPI. Cells with chromosomes clearly aligned at the metaphase plate were scored for congression errors as described in the Supplemental Experimental Procedures (45–300 cells/rep, n ≥ 3).
(D) Parental MDCK II cells synchronized by double thymidine block (as described in the Supplemental Experimental Procedures) and released for 6–8 hr into Rac inhibitor (NSC-23776) or mock treatment were fixed, stained, and analyzed as in (C) (45–300 cells/rep, n ≥ 3).
(E) Tiam1-depleted (RNAi#1 and #2; plus dox) cells were left untreated or were treated with STLC (1 μM for 18 hr), fixed, and costained with anti-α-tubulin, anti-γ-tubulin (or anti-pericentrin), and DAPI. The distance between centrosomes was measured in 3D from prometaphase cells. The dotted line represents the normal intercentrosomal distance observed in untreated control cells.
(F and G) Control or Tiam1-depleted cells were treated with STLC (1 μM for 18 hr) or left untreated. Samples were fixed and stained with anti-α-tubulin and DAPI. (F) Example single-plane images are shown, as well as (G) chromosome congression defects assessed as in (C), from control or Tiam1-depleted (RNAi#1 and #2; plus dox) cells treated with STLC or left untreated (45–300 cells/rep, n ≥ 3). In all cases data are presented as mean + SEM, ∗p < 0.05, ∗∗p < 0.001.
(H) Model: Eg5 suppression in control cells leads to monopolar spindles and SAC activation. Conversely, Tiam1 depletion increases centrosome separation in early mitosis, leading to chromosome congression errors and SAC activation. However, Eg5 suppression within Tiam1-depleted cells restores centrosome separation to that of control Eg5 inhibitor-untreated mitotic cells, allowing efficient chromosome congression and progression through mitosis.
See also Figure S4.
Analysis of fixed mitotic cells revealed that after Tiam1 depletion, congression errors were over 2.5-fold higher than controls (Figure 4C; see Figure S4A for example images). Similar results were obtained after Tiam1 depletion in P1 cells (data not shown). Importantly, this chromosome congression defect was efficiently rescued by expressing RNAi-resistant wild-type Tiam1, but not GEF mutant Tiam1 (Figure 4C). Moreover, cells treated with Rac inhibitor also displayed significantly increased congression errors compared to controls (Figure 4D; see Figure S4B for example images), consistent with a role for Rac downstream of Tiam1. We confirmed that these congression errors observed in Tiam1-depleted cells were due to an increased prometaphase duration (Figure S4C). Hence, cells with compromised Tiam1-Rac signaling fail to efficiently align chromosomes to the metaphase plate, resulting in a prolonged prometaphase. This is probably due to maintained activation of the SAC, which delays mitosis until all chromosome kinetochores are captured [19].
Subsequently, we determined whether the increased centrosome separation of Tiam1-depleted cells leads to the congression errors. Using time-lapse fluorescence microscopy of individual mitotic cells expressing β-Tubulin-GFP, we confirmed that in the presence of STLC, the majority of Tiam1-depleted mitotic cells formed bipolar spindles (Figure S4D); furthermore, the intercentrosomal distance in prometaphase was now similar to that found in untreated controls (Figure 4E). Significantly, correcting the balance of forces during bipolar spindle formation completely rescued the chromosome congression errors resulting from Tiam1 depletion (Figures 4F and 4G) and restored normal mitotic progression (Figures S4E and S4F). Together, these data indicate that the defects in chromosome congression induced by Tiam1 depletion are due to the increased centrosome separation.
In conclusion, we have shown that Tiam1, acting through Rac, is a critical mediator of forces during bipolar spindle assembly. Tiam1-Rac signaling counteracts Eg5 during bipolar spindle formation in vitro and in vivo. To our knowledge, this is the first signaling module that retards centrosome separation during prophase in mammalian cells and the first demonstration of an increased rate of centrosome separation in prophase leading to chromosome congression defects. Importantly, we show that correcting the balance of forces, by attenuating Eg5 activity in Tiam1-depleted cells, completely rescues the defects in chromosome congression, allowing cells to satisfy the SAC and progress through mitosis normally (for a model see Figure 4H). There are numerous other pathways that promote [1, 3, 20] and oppose [13, 21] centrosome separation during bipolar spindle formation. This considerable overlap is not surprising given the importance of the bipolar mitotic spindle to the survival of eukaryotic cells, providing a number of fail-safes that decrease the risk of chromosome segregation errors during cell division.
Acknowledgments
We thank F. Sanchez, A. Welman, G. Lacaud and V. Allan for kindly providing plasmids; A. Welman for help with the single-vector inducible shRNA vector; and V. Tybulewicz for the Rac1 conditional knockout mice. We are grateful to I. Hagan, A. McAinsh, A. Hurlstone, S. Woolner, K. Labib, C. Morrow, S. Castillo-Lluva, and other members of the Cell Signalling Group for critical reading of the manuscript and helpful discussions. Thanks also go to T. Watanabe, K. Kaibuchi, J. Chernoff, F. Martin-Belmonte and F. Kozielski for technical advice. We are also grateful to S. Bagley for his help with microscopy. This work was supported by Cancer Research UK (CR-UK), predominantly grant number C147/A6058.
Published online: March 25, 2010
Footnotes
Supplemental Information includes four figures and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/j.cub.2010.02.033.
Supplemental Information
References
- 1.Rosenblatt J., Cramer L.P., Baum B., McGee K.M. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–372. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- 2.Rosenblatt J. Spindle assembly: Asters part their separate ways. Nat. Cell Biol. 2005;7:219–222. doi: 10.1038/ncb0305-219. [DOI] [PubMed] [Google Scholar]
- 3.Toso A., Winter J.R., Garrod A.J., Amaro A.C., Meraldi P., McAinsh A.D. Kinetochore-generated pushing forces separate centrosomes during bipolar spindle assembly. J. Cell Biol. 2009;184:365–372. doi: 10.1083/jcb.200809055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawin K.E., LeGuellec K., Philippe M., Mitchison T.J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992;359:540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- 5.DeBonis S., Skoufias D.A., Lebeau L., Lopez R., Robin G., Margolis R.L., Wade R.H., Kozielski F. In vitro screening for inhibitors of the human mitotic kinesin Eg5 with antimitotic and antitumor activities. Mol. Cancer Ther. 2004;3:1079–1090. [PubMed] [Google Scholar]
- 6.Mayer T.U., Kapoor T.M., Haggarty S.J., King R.W., Schreiber S.L., Mitchison T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science. 1999;286:971–974. doi: 10.1126/science.286.5441.971. [DOI] [PubMed] [Google Scholar]
- 7.Mertens A.E., Roovers R.C., Collard J.G. Regulation of Tiam1-Rac signalling. FEBS Lett. 2003;546:11–16. doi: 10.1016/s0014-5793(03)00435-6. [DOI] [PubMed] [Google Scholar]
- 8.Malliri A., van der Kammen R.A., Clark K., van der Valk M., Michiels F., Collard J.G. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature. 2002;417:867–871. doi: 10.1038/nature00848. [DOI] [PubMed] [Google Scholar]
- 9.Woodcock S.A., Rooney C., Liontos M., Connolly Y., Zoumpourlis V., Whetton A.D., Gorgoulis V.G., Malliri A. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol. Cell. 2009;33:639–653. doi: 10.1016/j.molcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Nakagawa M., Fukata M., Yamaga M., Itoh N., Kaibuchi K. Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 2001;114:1829–1838. doi: 10.1242/jcs.114.10.1829. [DOI] [PubMed] [Google Scholar]
- 11.Tolias K.F., Bikoff J.B., Burette A., Paradis S., Harrar D., Tavazoie S., Weinberg R.J., Greenberg M.E. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–538. doi: 10.1016/j.neuron.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y., Dickerson J.B., Guo F., Zheng J., Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanenbaum M.E., Macůrek L., Galjart N., Medema R.H. Dynein, Lis1 and CLIP-170 counteract Eg5-dependent centrosome separation during bipolar spindle assembly. EMBO J. 2008;27:3235–3245. doi: 10.1038/emboj.2008.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharp D.J., McDonald K.L., Brown H.M., Matthies H.J., Walczak C., Vale R.D., Mitchison T.J., Scholey J.M. The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J. Cell Biol. 1999;144:125–138. doi: 10.1083/jcb.144.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegtel D.M., Ellenbroek S.I., Mertens A.E., van der Kammen R.A., de Rooij J., Collard J.G. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr. Biol. 2007;17:1623–1634. doi: 10.1016/j.cub.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 16.Walmsley M.J., Ooi S.K., Reynolds L.F., Smith S.H., Ruf S., Mathiot A., Vanes L., Williams D.A., Cancro M.P., Tybulewicz V.L. Critical roles for Rac1 and Rac2 GTPases in B cell development and signaling. Science. 2003;302:459–462. doi: 10.1126/science.1089709. [DOI] [PubMed] [Google Scholar]
- 17.Pritchard D.M., Potten C.S., Hickman J.A. The relationships between p53-dependent apoptosis, inhibition of proliferation, and 5-fluorouracil-induced histopathology in murine intestinal epithelia. Cancer Res. 1998;58:5453–5465. [PubMed] [Google Scholar]
- 18.Ireland H., Kemp R., Houghton C., Howard L., Clarke A.R., Sansom O.J., Winton D.J. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: Effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Musacchio A., Salmon E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- 20.Tanenbaum M.E., Macůrek L., Janssen A., Geers E.F., Alvarez-Fernández M., Medema R.H. Kif15 cooperates with eg5 to promote bipolar spindle assembly. Curr. Biol. 2009;19:1703–1711. doi: 10.1016/j.cub.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 21.Woolner S., O'Brien L.L., Wiese C., Bement W.M. Myosin-10 and actin filaments are essential for mitotic spindle function. J. Cell Biol. 2008;182:77–88. doi: 10.1083/jcb.200804062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.