Abstract
Protein-energy wasting (PEW) is one of the strongest risk factors of adverse outcomes in patients with chronic kidney disease (CKD) including those with end stage renal disease (ESRD) who undergo maintenance dialysis treatment. One important determinant of PEW in this patient population is an inadequate amount of protein and energy intake. Compounding the problem are the many qualitative nutritional deficiencies that arise because of the altered dietary habits of dialysis patients. Many of these alterations are iatrogenically induced, and albeit well intentioned, they could induce unintended harmful effects. In order to determine the best possible diet in ESRD patients, one must first understand the complex interplay between the quantity and quality of nutrient intake in these patients, and their impact on relevant clinical outcomes. We review available studies examining the association of nutritional intake with clinical outcomes in ESRD, stressing the complicated and often difficult-to-study interrelationship between quantitative and qualitative aspects of nutrient intake in nutritional epidemiology. The currently recommended higher protein intake of 1.2 g/kg/day may be associated with a higher phosphorus and potassium burden and with worsening hyperphosphatemia and hyperkalemia, whereas dietary control of phosphorus and potassium by restricting protein intake may increase the risk of PEW. We assess the relevance of associative studies by examining the biologic plausibility of underlying mechanisms of action and emphasize areas in need of further research.
Patients with end stage renal disease (ESRD) on dialysis experience exceptionally high mortality rates, mainly from causes related to cardiovascular disease (CVD) and infections.(1) Multiple novel risk factors have been invoked to explain the large excess mortality seen in ESRD patients, but measures of nutritional status have invariably emerged as some of the strongest predicators of adverse outcomes in this patient population.(2–9)
In order to alleviate the nomenclatural confusion arising from this complexity an expert panel has recently recommended the use of the term protein-energy wasting (PEW) to incorporate all the different aspects of malnutrition and other metabolic or nutritional derangements such as inflammation in patients with CKD.(10) Based on this definition a diagnosis of PEW can be made by using the following criteria: (1) biochemical measures (serum albumin, prealbumin, transferrin and cholesterol); (2) measures of body mass (body mass index [BMI], unintentional weight loss and total body fat), (3) measures of muscle mass (total muscle mass, mid-arm muscle circumference and creatinine appearance); (4) measures of dietary intake (dietary protein and energy intake) and (5) integrative nutritional scoring systems (subjective global assessment of nutrition and malnutrition-inflammation score).
The impact of PEW on outcomes is considered to be complex, and in spite of the strong associations with mortality it remains unclear if some or all aspects of it are a direct cause of the poor outcomes, or if they are merely surrogate markers of other clinical conditions portending a poor survival.(11) Because of this complexity the various aspects of PEW have to be carefully studied in order to determine which, if any of them could be considered as truly causally related to adverse outcomes, and to thus determine which of them should be considered as targets of interventional clinical trials. Of the five major PEW criteria nutrient intake is most difficult to study, as the direct assessment of what and how much a person ingests over extended periods of time can only be feasibly done in small numbers of subjects and under carefully controlled circumstances. To bypass such impracticalities the use of surrogate markers of nutrient intake has become wide spread in clinical practice and has also allowed for the studying of this parameter on a broader scale in research studies.
We have reviewed epidemiological aspects of nutrient intake in ESRD patients, focusing on observational studies to describe the outcomes associated with deficiencies in nutritional intake in this patient population.
Nutrient intake in ESRD
The ingestion of food is a basic activity that is meant to provide the body with macro- and micronutrients needed in order to maintain tissue growth and structure and to provide fuel for energy-requiring processes. Anorexia-induced inadequate nutrient intake is an important cause of malnutrition in CKD and ESRD patients,(12–16) and a decline in protein and calorie intake becomes gradually manifest typically once the glomerular filtration rate declines to approximately <25–38 ml/min.(15) The causes of anorexia in CKD and ESRD are multiple.(17) The retention of uremic toxins and various comorbid conditions can lead to lowered appetite and a decrease in protein and energy intake, which is often compounded by the ill-advised imposition of various dietary restrictions.
Complicating the problem is the concomitant increase in protein and energy requirements in advanced CKD and ESRD due to the combined catabolism-inducing effects of dialytic therapies and various metabolic alterations such as metabolic acidosis. In order to maintain an even or positive nitrogen balance dialysis patients are advised to ingest a daily amount of protein of approximately 1.1–1.3 g/kg/day (18–21) and a daily amount of energy of approximately 35 kcal/kg/day.(22) In contrasting to these ideal levels derived from experimental studies, the actual protein intake of patients receiving maintenance hemodialysis (MHD) or peritoneal dialysis (PD) appears to be approximately 0.95–1.0 g/kg/day(12–14;16;23) and the actual energy intake is approximately 23–28 kcal/kg/day,(13;14;16) suggesting an inadequate average protein and energy intake in dialysis patients. Such deficient intake, be it due to diminished appetite or other pathophysiological or psychosocial constellations, can be causally linked to the development of PEW. Nevertheless, it remains unclear to what extent the resultant lower-than-ideal protein and/or energy intake can be causally linked to increased mortality and morbidity.
Outcomes associated with quantitative deficiencies in nutrient intake
Due to the difficulties in obtaining direct information about protein and energy intake, there is a paucity of data about the outcomes associated with these variables. Anorexia, a major reason for the low nutrient intake in ESRD has been associated with 4-times higher risk of death and with increased morbidity in a study of 344 maintenance hemodialysis (MHD) patients in the US(24); these findings were recently replicated by a similar study in 233 European MHD patients (25)(Table 1). However, reduced appetite can only be considered an indirect measure of dietary nutrient intake. Studies examining risks associated with dietary protein and/or calorie intake obtained from diet diaries have been few in number, small in size and failed to provide conclusive evidence on the risks associated with these parameters (Table 1).(26;27) Evidence from these studies needs to be interpreted with caution as their small size makes these studies susceptible to type II statistical errors.
Table 1.
Observational studies examining associations between the amount of dietary protein or calorie intake and clinical outcomes in patients with end stage renal disease on maintenance dialysis therapy.
| Study | Patient population | Predictor | Results | Comment |
|---|---|---|---|---|
| Acchiardo et al, 1983(31) | N=120, MHD | PCR and pre-dialysis BUN | Higher morbidity and mortality associated with lower PCR and pre-dialysis BUN. | Exclusively non-diabetic patients. |
| Harter, 1983(35) | N=160, MHD | PCR | Increased treatment (dialysis) failure in group with lower PCR. | Randomized controlled trial of dialysis dose (National Cooperative Dialysis Study). |
| Teehan et al, 1990(37) | N=51, CAPD | PCR | Increase in hospitalization associated with lower PCR. | Single center study. |
| Blake et al, 1991(33) | N=76, CAPD | nPCR | No association between nPCR and mortality. | None of the components of the urea kinetic model correlated with outcomes. |
| Raja et al, 1992(39) | N=88, MHD | nPCR | Lowest morbidity in the group with Kt/V > 1.0 and PCR > 1.0 g/kg/day. | Examined the concomitant effect of KT/V and nPCR. |
| Davies et al, 1995(34) | N=97, CAPD | Protein intake, calorie intake, PCR | Dietary protein and calorie intake, but not PCR were associated with higher mortality in univariate, but not multivariate analyses. | Adjustment for comorbid conditions rendered protein and calorie intake non-significant. |
| Herselman et al, 2000(27) | N=37, MHD | Dietary protein and energy intake, PCR | No association with all-cause or infectious morbidity over 26 months. | Small, single center study. Other markers of nutritional status showed significant association with outcome. |
| Kalantar-Zadeh et al, 2003(36) | N=122, MHD with KT/V>1.20 | nPCR | Lower nPCR associated with high higher mortality and hospitalization rates. | Lower serum albumin, TIBC and creatinine showed similar associations. |
| Kalantar-Zadeh et al, 2004(24) | N=331, MHD | Appetite (self-rated) | Diminished appetite associated with higher rates of mortality and hospitalizations, and higher levels of inflammatory markers. | Prospective cohort. |
| Beddhu et al, 2005(30) | N=5,059, incident MHD | Total and dietary protein intake (derived from BUN and urine urea clearance) | 18% increase in hazard of death for patients in the lowest quartile of TPI compared to highest quartile. Lower DPI associated with lower risk of death. | Data from USRDS. Lower TPI also associated with lower serum albumin, urine creatinine and BMI. |
| Araujo et al, 2006(26) | N=344, incident MHD | Protein and energy intake assessed by food diary. | Protein intake <1.0 g/kg/d, and energy intake <25 kcal/kg/d associated with worse survival in univariate analysis. | Low energy intake remained associated with mortality after multivariable adjustment. Single center study. |
| Shinaberger et al, 2006(38) | N=53,933, MHD | nPCR and change in nPCR | Best survival associated with nPCR of 1.0 to 1.4 g/kg/day. A decrease in nPCR associated with increased mortality and an increase in nPCR associated with decreased mortality. | All patients enrolled in DaVita dialysis units between 2001 and 2003. |
| Carrero et al, 2008(25) | N=233, MHD | Appetite | Poor appetite associated with higher mortality. | Poor appetite also correlated with other markers of malnutrition and inflammation. |
An indirect way to assess dietary protein intake is by measuring urinary urea appearance, assuming that the amount of excreted urea in urine is mainly determined by the amount of dietary protein intake.(28;29) A large observational study of 5,059 incident MHD patients examined the association between total dietary protein intake (calculated from urine urea nitrogen reported on Medicare Form 2728) and mortality, and found a significant association between lower total protein intake and increased mortality (Table 1).(30) This study also highlighted the difficulties in examining the impact of the normalized amount of protein intake (defined as total protein intake divided by body weight), which is a reflection not only of protein intake but also of the various other conditions affecting body weight. In spite of the significant association between lower total protein intake and higher mortality this study found that the association between normalized protein intake and mortality was in fact reversed (lower normalized protein intake was associated with lower mortality).(30)
The advent of urea kinetic modeling in dialysis patients has made it possible, albeit with certain limitations, to indirectly assess on a large scale daily protein intake, expressed as normalized protein nitrogen appearance (nPNA, also known as normalized protein catabolic rate or nPCR). Two important conditions have to be met for the validity of nPNA: the metabolic stability of the studied individual (not being overtly catabolic or anabolic) and his/her closed status (no urinary loss of urea). Stable maintenance dialysis patients with minimal residual urine usually meet these criteria.
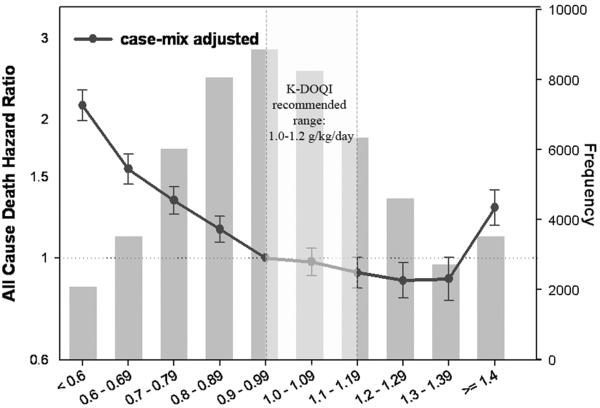
Data gathered from large clinical trials and especially from the databases of large independent dialysis chains has allowed for large scale assessment of outcomes associated with estimates of dietary protein intake. Such studies have invariably linked a lower nPCR to higher mortality and morbidity in a variety of patient populations (Table 1).(30–39) The largest such study examined 53,933 MHD patients receiving chronic dialysis between 2001 and 2003.(38) In this study, patients with time-varying nPNA of 1.0–1.4 g/kg/day experienced the lowest mortality rates. Interestingly, higher mortality was associated both with nPNA levels of <1.0 g/kg/day and >1.4 g/kg/day (Figure 1). Possible explanations for the higher mortality seen in those with the highest nPNA levels were outcome-associated confounding effects of body weight in smaller patients, the toxic effect of a very high-protein diet, a highly catabolic state caused by inflammation, or residual confounding by a behavior pattern of poor compliance in those with the highest levels of protein intake.
Figure 1.
Case-mix adjusted hazard ratios for all-cause death associated with various levels of normalized protein nitrogen appearance in 53,933 maintenance hemodialysis patients receiving chronic dialysis between 2001 and 2003. Based on data from Reference 38.
Another interesting finding of this study was a strong association between a decrease in nPNA over time and increased mortality, and a slightly less pronounced, but still significant association between an increase in nPNA over time and decreased mortality. As the reasons for the temporal changes in nPNA were not evident in this observational study these latter findings require experimental confirmation before one can conclude that an increase in protein intake can cause improved survival.
A significant drawback of the evidence derived from observational studies assessing associations between nPNA and outcomes is the imperfect nature of this variable as a marker of protein intake. Measured nPNA incorporates the estimated permeability of the dialyzer and is also affected by the accuracy of measured blood and dialysate flow rates.(36) Furthermore, as protein intake fluctuates from day to day due to changes in intake and catabolism,(40) the monthly assessment of nPNA may not be an accurate reflection of true average protein intake, especially if the patient's protein metabolism is not in equilibrium. Further inaccuracies are incurred because of difficulties with assessment of the volume of distribution of urea in obese, malnourished, or edematous patients,(41) and because of overestimation of nPNA caused by delayed equilibrium with subsequent urea rebound after dialysis, which can vary according to patient and dialysis procedure characteristics.(42)
In summary, the evidence from observational studies suggests a robust association between lower nPNA (nPCR) and adverse outcomes in ESRD patients. Much less data are available on outcomes associated with actual protein and energy intake. Self-reported appetite appears to be a very robust predictor of mortality and morbidity, but data on this variable are also scant.
Consequences of qualitative deficiencies in nutritional intake
The aforementioned studies of dietary protein and energy intake described associations with outcomes related to the amount of the studied nutritional characteristics. It is important to recognize, though that in addition to the amount of protein and energy intake there are several other nutritional characteristics that involve the quality, rather than the quantity of consumed food which could affect the health and well being of patients with ESRD. These include deficiencies in various micronutrients (vitamins and trace elements) and imbalances of macronutrients stemming from incorrect dietary habits or prescriptions.
Deficiencies in many micronutrients may be linked to PEW-related cardiovascular and infectious mortality in ESRD.(11) The lack of arginine, glutamine, zinc, vitamin B6 (pyridoxine), vitamin C, folic acid and levocarnitine may all adversely affect various aspects of immune function and could be instrumental in the high infectious mortality seen in ESRD.(43–50) Cardiovascular outcomes may also be affected by diet characteristics, as an atherogenic diet is imposed upon most individuals with CKD.(51;52) Due to the difficulty of maintaining adequate energy intake on low protein, low potassium diets, patients may tend to rely more on food sources containing high amounts of atherogenic fat. A recent comparative study based on food frequency questionnaires indicated that MHD patients consume significantly lower amounts of potassium, dietary fiber, vitamin C and certain cardioprotective carotenoids.(52) Such patients appear to have a lower intake of dietary nutrients including minerals and vitamins, but a higher intake of cholesterol. Most advanced CKD patients are exposed to traditional restrictions in potassium intake, which may result in reduced fruit and vegetable intake, leaving meat and other high fat foods as the main sources of calories.(52)
In spite of the plausibility of the aforementioned mechanisms of action, the long term consequences of the many qualitative nutritional deficiencies involving micronutrients and trace elements in ESRD remain unknown. Large scale observational studies have not been conducted to determine outcomes associated with the individual deficiencies, and clinical trials involving the supplementation of such nutritional elements are also lacking.
The desire to achieve adequate quantities of protein and energy intake could in itself have unintended consequences if not accompanied by careful planning and supervision to assure that the macronutrient content of the patients' food is appropriate. Higher protein intake can result in increased potassium and phosphorus intake, with resultant increase in the serum levels of these elements. While higher serum levels of both potassium and phosphorus have been associated with adverse outcomes in MHD patients,(53;54) there is a paucity of data regarding the direct association between the dietary intake of these elements and clinical outcomes. Recent results based on data obtained from food frequency questionnaires in 224 MHD patients enrolled in the Nutritional and Inflammatory Evaluation (NIED) study have indicated that higher amounts of both potassium and phosphorus intake are associated with increased all-cause mortality even after adjustments for markers of nutritional status including protein intake (K Kalantar-Zadeh, personal communication).
Another recent study using food frequency questionnaires in 160 kidney transplant recipients indicated that a Mediterranean dietary pattern is associated with a reduced risk of metabolic syndrome in these patients.(55) The long term outcomes of such a diet were not examined in this study and the relevance of these findings to MHD patients is questionable.
The above results underscore a dilemma posed by the interplay between qualitative and quantitative changes of diet in ESRD, where a (desirable) increase in the quantity of protein and energy can result in the (undesirable) increase in the intake of several potentially harmful elements. This is perhaps best exemplified by changes in dietary prescriptions that concomitantly affect protein and phosphorus intake. On the one hand dietary restrictions meant to alleviate increases in serum phosphorus may result in diminished dietary protein intake and could consequently cause or worsen PEW; on the other hand prescriptions meant to increase dietary protein intake in patients with existing PEW could lead to higher phosphorus intake and worsening hyperphosphatemia.
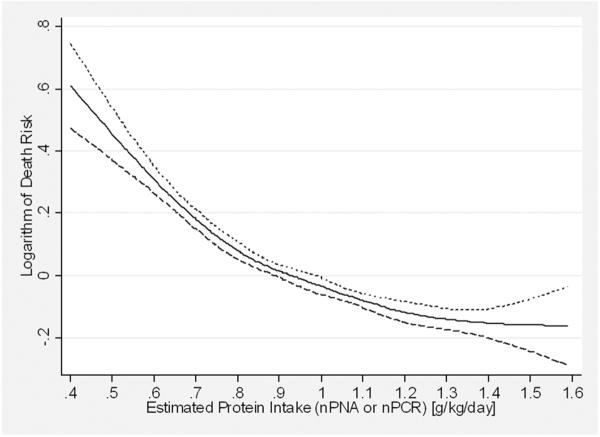
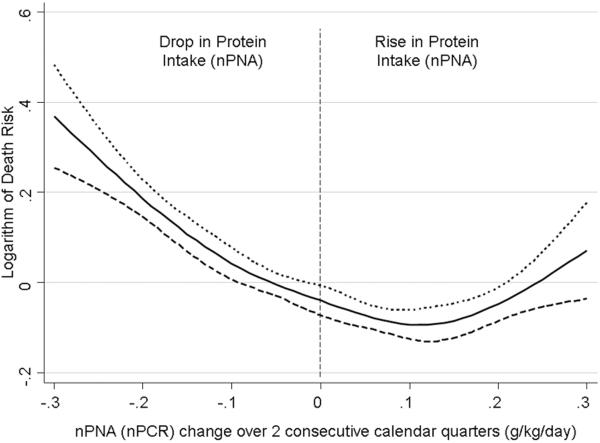
The potential effects of such dietary measures were highlighted in a recent epidemiologic study of 30,075 prevalent MHD patients,(56) which described the risk of death associated with concomitant changes over 6 months in serum phosphorus and protein intake (measured by nPNA). When examined individually both lower protein intake (Figure 2) and a decrease in protein intake over 6 months (Figure 3) were associated with higher mortality in this study. Similar analyses for serum phosphorus indicated that higher serum phosphorus and an increase in serum phosphorus over 6 months were also individually associated with higher mortality. When the two variables (protein intake and serum phosphorus) were examined concomitantly, the best outcomes were found in patients whose decrease in serum phosphorus was accompanied by increased protein intake and worst did those whose phosphorus and nPNA both decreased. Higher mortality was seen in patients whose serum phosphorus and nPNA both increased over 6 months, followed by those whose phosphorus increased but whose nPNA decreased. A limitation of this study was that the reasons for the changes in serum phosphorus and nPNA were unknown and may have been only partially related to dietary intake.
Figure 2.
Multivariable-adjusted hazard ratios for all-cause mortality over a 3 year observation period associated with baseline levels of dietary protein intake (represented by the normalized protein equivalent of total nitrogen appearance) in 30,075 prevalent maintenance hemodialysis patients. Adapted from Reference 56, with permission.
Figure 3.
Multivariable-adjusted hazard ratios for all-cause mortality over a 3 year observation period associated with changes in levels of dietary protein intake (represented by the normalized protein equivalent of total nitrogen appearance) over a six month time period in 30,075 prevalent maintenance hemodialysis patients. Adapted from Reference 56, with permission.
While the foregoing results have to be interpreted with caution, they could indicate that the risk of controlling serum phosphorus by restricting dietary protein intake may outweigh the benefit of controlled serum phosphorus and may lead to greater mortality. Clinical trials will be needed to assess the impact of phosphorus-control measures with neutral or positive impact on protein intake, such as the use of phosphate binders or the implementation of dietary restriction through curbing non-protein containing sources of phosphorus.
Conclusions
Deficiencies in protein and energy intake are an important determinant of PEW in ESRD, and are associated with significantly higher mortality and morbidity. Qualitative dietary changes could add to the burden of diet-related morbidity and mortality through complex mechanisms involving cardiovascular and immunologic mechanisms, but their role in such outcomes is less well defined. Epidemiological studies suggest that interventions targeting an optimal protein and energy intake could have a substantial benefit in ESRD patients. Such interventions will also have to address the complex qualitative changes brought about by increased protein and energy intake. Due to the complex nature of PEW it is also possible that deficient nutritional intake is a surrogate marker of more advanced morbid conditions, without a direct causal effect on adverse outcomes. A causal effect of nutritional intake on outcomes can only be proven by prospective randomized clinical trials. Due to the significant impact of PEW on mortality in ESRD such trials are eagerly awaited.
Acknowledgments
Funding source: KKZ is supported by grant 1R01DK078106-01 from the NIDDK of the NIH.
Reference List
- 1.U.S. Renal Data System . USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2006. [Google Scholar]
- 2.Beddhu S, Kaysen GA, Yan G, Sarnak M, Agodoa L, Ornt D, Cheung AK. Association of serum albumin and atherosclerosis in chronic hemodialysis patients. Am J Kidney Dis. 2002;40:721–727. doi: 10.1053/ajkd.2002.35679. [DOI] [PubMed] [Google Scholar]
- 3.Iseki K, Kawazoe N, Fukiyama K. Serum albumin is a strong predictor of death in chronic dialysis patients. Kidney Int. 1993;44:115–119. doi: 10.1038/ki.1993.220. [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kilpatrick RD, Kuwae N, McAllister CJ, Alcorn H, Jr., Kopple JD, Greenland S. Revisiting mortality predictability of serum albumin in the dialysis population: time dependency, longitudinal changes and population-attributable fraction. Nephrol Dial Transplant. 2005;20:1880–1888. doi: 10.1093/ndt/gfh941. [DOI] [PubMed] [Google Scholar]
- 5.Lowrie EG, Lew NL. Death risk in hemodialysis patients: the predictive value of commonly measured variables and an evaluation of death rate differences between facilities. Am J Kidney Dis. 1990;15:458–482. doi: 10.1016/s0272-6386(12)70364-5. [DOI] [PubMed] [Google Scholar]
- 6.Kuwae N, Kopple JD, Kalantar-Zadeh K. A low lymphocyte percentage is a predictor of mortality and hospitalization in hemodialysis patients. Clin Nephrol. 2005;63:22–34. doi: 10.5414/cnp63022. [DOI] [PubMed] [Google Scholar]
- 7.Pifer TB, McCullough KP, Port FK, Goodkin DA, Maroni BJ, Held PJ, Young EW. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Reddan DN, Klassen PS, Szczech LA, Coladonato JA, O'Shea S, Owen WF, Jr., Lowrie EG. White blood cells as a novel mortality predictor in haemodialysis patients. Nephrol Dial Transplant. 2003;18:1167–1173. doi: 10.1093/ndt/gfg066. [DOI] [PubMed] [Google Scholar]
- 9.Johnson DW, Wiggins KJ, Armstrong KA, Campbell SB, Isbel NM, Hawley CM. Elevated white cell count at commencement of peritoneal dialysis predicts overall and cardiac mortality. Kidney Int. 2005;67:738–743. doi: 10.1111/j.1523-1755.2005.67135.x. [DOI] [PubMed] [Google Scholar]
- 10.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, Massy Z, Mitch W, Pineda E, Stenvinkel P, Trevinho-Becerra A, Wanner C. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 11.Kovesdy CP, Kalantar-Zadeh K. Why is protein-energy wasting associated with mortality in chronic kidney disease? Semin Nephrol. 2009;29:3–14. doi: 10.1016/j.semnephrol.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cianciaruso B, Brunori G, Kopple JD, Traverso G, Panarello G, Enia G, Strippoli P, De VA, Querques M, Viglino G. Cross-sectional comparison of malnutrition in continuous ambulatory peritoneal dialysis and hemodialysis patients. Am J Kidney Dis. 1995;26:475–486. doi: 10.1016/0272-6386(95)90494-8. [DOI] [PubMed] [Google Scholar]
- 13.Dwyer JT, Cunniff PJ, Maroni BJ, Kopple JD, Burrowes JD, Powers SN, Cockram DB, Chumlea WC, Kusek JW, Makoff R, Goldstein DJ, Paranandi L. The hemodialysis pilot study: nutrition program and participant characteristics at baseline. The HEMO Study Group. J Ren Nutr. 1998;8:11–20. doi: 10.1016/s1051-2276(98)90032-2. [DOI] [PubMed] [Google Scholar]
- 14.Kluthe R, Luttgen FM, Capetianu T, Heinze V, Katz N, Sudhoff A. Protein requirements in maintenance hemodialysis. Am J Clin Nutr. 1978;31:1812–1820. doi: 10.1093/ajcn/31.10.1812. [DOI] [PubMed] [Google Scholar]
- 15.Kopple JD, Berg R, Houser H, Steinman TI, Teschan P. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of Diet in Renal Disease (MDRD) Study Group. Kidney Int Suppl. 1989;36(Suppl 27):S184–S194. [PubMed] [Google Scholar]
- 16.Schoenfeld PY, Henry RR, Laird NM, Roxe DM. Assessment of nutritional status of the National Cooperative Dialysis Study population. Kidney Int Suppl. 1983;23(Suppl 13):S80–S88. [PubMed] [Google Scholar]
- 17.Bergstrom J. Mechanisms of uremic suppression of appetite. J Ren Nutr. 1999;9:129–132. doi: 10.1053/JREN00900129. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. Am J Kidney Dis. 2000;35:s1–s140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 19.Borah MF, Schoenfeld PY, Gotch FA, Sargent JA, Wolfsen M, Humphreys MH. Nitrogen balance during intermittent dialysis therapy of uremia. Kidney Int. 1978;14:491–500. doi: 10.1038/ki.1978.154. [DOI] [PubMed] [Google Scholar]
- 20.Kopple JD, Shinaberger JH, Coburn JW, Sorensen MK, Rubini ME. Optimal dietary protein treatment during chronic hemodialysis. Trans Am Soc Artif Intern Organs. 1969;15:302–308. [PubMed] [Google Scholar]
- 21.Toigo G, Aparicio M, Attman PO, Cano N, Cianciaruso B, Engel B, Fouque D, Heidland A, Teplan V, Wanner C. Expert working group report on nutrition in adult patients with renal insufficiency (Part 2 of 2) Clin Nutr. 2000;19:281–291. doi: 10.1054/clnu.2000.0129. Abstract. [DOI] [PubMed] [Google Scholar]
- 22.Kopple JD, Monteon FJ, Shaib JK. Effect of energy intake on nitrogen metabolism in nondialyzed patients with chronic renal failure. Kidney Int. 1986;29:734–742. doi: 10.1038/ki.1986.59. [DOI] [PubMed] [Google Scholar]
- 23.Lindholm B, Bergstrom J. Nutritional aspects on peritoneal dialysis. Kidney Int Suppl. 1992;38:S165–S171. [PubMed] [Google Scholar]
- 24.Kalantar-Zadeh K, Block G, McAllister CJ, Humphreys MH, Kopple JD. Appetite and inflammation, nutrition, anemia, and clinical outcome in hemodialysis patients. Am J Clin Nutr. 2004;80:299–307. doi: 10.1093/ajcn/80.2.299. [DOI] [PubMed] [Google Scholar]
- 25.Carrero JJ, Qureshi AR, Axelsson J, Avesani CM, Suliman ME, Kato S, Barany P, Snaedal-Jonsdottir S, Alvestrand A, Heimburger O, Lindholm B, Stenvinkel P. Comparison of nutritional and inflammatory markers in dialysis patients with reduced appetite. Am J Clin Nutr. 2007;85:695–701. doi: 10.1093/ajcn/85.3.695. [DOI] [PubMed] [Google Scholar]
- 26.Araujo IC, Kamimura MA, Draibe SA, Canziani ME, Manfredi SR, Avesani CM, Sesso R, Cuppari L. Nutritional parameters and mortality in incident hemodialysis patients. J Ren Nutr. 2006;16:27–35. doi: 10.1053/j.jrn.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 27.Herselman M, Moosa MR, Kotze TJ, Kritzinger M, Wuister S, Mostert D. Protein-energy malnutrition as a risk factor for increased morbidity in long-term hemodialysis patients. J Ren Nutr. 2000;10:7–15. doi: 10.1016/s1051-2276(00)90017-7. [DOI] [PubMed] [Google Scholar]
- 28.Subar AF, Kipnis V, Troiano RP, Midthune D, Schoeller DA, Bingham S, Sharbaugh CO, Trabulsi J, Runswick S, Ballard-Barbash R, Sunshine J, Schatzkin A. Using intake biomarkers to evaluate the extent of dietary misreporting in a large sample of adults: the OPEN study. Am J Epidemiol. 2003;158:1–13. doi: 10.1093/aje/kwg092. [DOI] [PubMed] [Google Scholar]
- 29.Kopple JD, Gao XL, Qing DP. Dietary protein, urea nitrogen appearance and total nitrogen appearance in chronic renal failure and CAPD patients. Kidney Int. 1997;52:486–494. doi: 10.1038/ki.1997.358. [DOI] [PubMed] [Google Scholar]
- 30.Beddhu S, Ramkumar N, Pappas LM. Normalization of protein intake by body weight and the associations of protein intake with nutritional status and survival. J Ren Nutr. 2005;15:387–397. doi: 10.1053/j.jrn.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Acchiardo SR, Moore LW, Latour PA. Malnutrition as the main factor in morbidity and mortality of hemodialysis patients. Kidney Int Suppl. 1983;24(Suppl 16):S199–S203. [PubMed] [Google Scholar]
- 32.Allen KL, Miskulin D, Yan G, Dwyer JT, Frydrych A, Leung J, Poole D. Association of nutritional markers with physical and mental health status in prevalent hemodialysis patients from the HEMO study. J Ren Nutr. 2002;12:160–169. doi: 10.1053/jren.2002.33512. [DOI] [PubMed] [Google Scholar]
- 33.Blake PG, Sombolos K, Abraham G, Weissgarten J, Pemberton R, Chu GL, Oreopoulos DG. Lack of correlation between urea kinetic indices and clinical outcomes in CAPD patients. Kidney Int. 1991;39:700–706. doi: 10.1038/ki.1991.84. [DOI] [PubMed] [Google Scholar]
- 34.Davies SJ, Russell L, Bryan J, Phillips L, Russell GI. Comorbidity, urea kinetics, and appetite in continuous ambulatory peritoneal dialysis patients: their interrelationship and prediction of survival. Am J Kidney Dis. 1995;26:353–361. doi: 10.1016/0272-6386(95)90657-6. [DOI] [PubMed] [Google Scholar]
- 35.Harter HR. Review of significant findings from the National Cooperative Dialysis Study and recommendations. Kidney Int Suppl. 1983;23(Suppl 13):S107–S112. [PubMed] [Google Scholar]
- 36.Kalantar-Zadeh K, Supasyndh O, Lehn RS, McAllister CJ, Kopple JD. Normalized protein nitrogen appearance is correlated with hospitalization and mortality in hemodialysis patients with Kt/V greater than 1.20. J Ren Nutr. 2003;13:15–25. doi: 10.1053/jren.2003.50005. [DOI] [PubMed] [Google Scholar]
- 37.Teehan BP, Schleifer CR, Brown JM, Sigler MH, Raimondo J. Urea kinetic analysis and clinical outcome on CAPD. A five year longitudinal study. Adv Perit Dial. 1990;6:181–185. [PubMed] [Google Scholar]
- 38.Shinaberger CS, Kilpatrick RD, Regidor DL, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Longitudinal associations between dietary protein intake and survival in hemodialysis patients. Am J Kidney Dis. 2006;48:37–49. doi: 10.1053/j.ajkd.2006.03.049. [DOI] [PubMed] [Google Scholar]
- 39.Raja RM, Ijelu G, Goldstein M. Influence of Kt/V and protein catabolic rate on hemodialysis morbidity. A long-term study. ASAIO J. 1992;38:M179–M180. doi: 10.1097/00002480-199207000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Kloppenburg WD, Stegeman CA, de Jong PE, Huisman RM. Relating protein intake to nutritional status in haemodialysis patients: how to normalize the protein equivalent of total nitrogen appearance (PNA)? Nephrol Dial Transplant. 1999;14:2165–2172. doi: 10.1093/ndt/14.9.2165. [DOI] [PubMed] [Google Scholar]
- 41.Canaud B, Leblanc M, Garred LJ, Bosc JY, Argiles A, Mion C. Protein catabolic rate over lean body mass ratio: a more rational approach to normalize the protein catabolic rate in dialysis patients. Am J Kidney Dis. 1997;30:672–679. doi: 10.1016/s0272-6386(97)90492-3. [DOI] [PubMed] [Google Scholar]
- 42.Stegeman CA, Huisman RM, de RB, Joostema A, de Jong PE. Determination of protein catabolic rate in patients on chronic intermittent hemodialysis: urea output measurements compared with dietary protein intake and with calculation of urea generation rate. Am J Kidney Dis. 1995;25:887–895. doi: 10.1016/0272-6386(95)90571-5. [DOI] [PubMed] [Google Scholar]
- 43.Hulsewe KW, van Acker BA, von Meyenfeldt MF, Soeters PB. Nutritional depletion and dietary manipulation: effects on the immune response. World J Surg. 1999;23:536–544. doi: 10.1007/pl00012344. [DOI] [PubMed] [Google Scholar]
- 44.Souba WW. Nutritional support. N Engl J Med. 1997;336:41–48. doi: 10.1056/NEJM199701023360107. [DOI] [PubMed] [Google Scholar]
- 45.Alexander JW. Immunoenhancement via enteral nutrition. Arch Surg. 1993;128:1242–1245. doi: 10.1001/archsurg.1993.01420230070011. [DOI] [PubMed] [Google Scholar]
- 46.Kimmel PL, Phillips TM, Lew SQ, Langman CB. Zinc modulates mononuclear cellular calcitriol metabolism in peritoneal dialysis patients. Kidney Int. 1996;49:1407–1412. doi: 10.1038/ki.1996.198. [DOI] [PubMed] [Google Scholar]
- 47.Erten Y, Kayatas M, Sezer S, Ozdemir FN, Ozyigit PF, Turan M, Haberal A, Guz G, Kaya S, Bilgin N. Zinc deficiency: prevalence and causes in hemodialysis patients and effect on cellular immune response. Transplant Proc. 1998;30:850–851. doi: 10.1016/s0041-1345(98)00075-x. [DOI] [PubMed] [Google Scholar]
- 48.Casciato DA, McAdam LP, Kopple JD, Bluestone R, Goldberg LS, Clements PJ, Knutson DW. Immunologic abnormalities in hemodialysis patients: improvement after pyridoxine therapy. Nephron. 1984;38:9–16. doi: 10.1159/000183270. [DOI] [PubMed] [Google Scholar]
- 49.Dobbelstein H, Korner WF, Mempel W, Grosse-Wilde H, Edel HH. Vitamin B6 deficiency in uremia and its implications for the depression of immune responses. Kidney Int. 1974;5:233–239. doi: 10.1038/ki.1974.28. [DOI] [PubMed] [Google Scholar]
- 50.DeSimone C, Famularo G, Tzantzoglou S, Trinchieri V, Moretti S, Sorice F. Carnitine depletion in peripheral blood mononuclear cells from patients with AIDS: effect of oral L-carnitine. AIDS. 1994;8:655–660. doi: 10.1097/00002030-199405000-00012. [DOI] [PubMed] [Google Scholar]
- 51.Dolson GM. Do potassium deficient diets and K+ removal by dialysis contribute to the cardiovascular morbidity and mortality of patients with end stage renal disease? Int J Artif Organs. 1997;20:134–135. [PubMed] [Google Scholar]
- 52.Kalantar-Zadeh K, Kopple JD, Deepak S, Block D, Block G. Food intake characteristics of hemodialysis patients as obtained by food frequency questionnaire. J Ren Nutr. 2002;12:17–31. doi: 10.1053/jren.2002.29598. [DOI] [PubMed] [Google Scholar]
- 53.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 54.Kovesdy CP, Regidor DL, Mehrotra R, Jing J, McAllister CJ, Greenland S, Kopple JD, Kalantar-Zadeh K. Serum and dialysate potassium concentrations and survival in hemodialysis patients. Clin J Am Soc Nephrol. 2007;2:999–1007. doi: 10.2215/CJN.04451206. [DOI] [PubMed] [Google Scholar]
- 55.Nafar M, Noori N, Jalali-Farahani S, Hosseinpanah F, Poorrezagholi F, Ahmadpoor P, Samadian F, Firouzan A, Einollahi B. Mediterranean diets are associated with a lower incidence of metabolic syndrome one year following renal transplantation. Kidney Int. 2009;76:1199–1206. doi: 10.1038/ki.2009.343. [DOI] [PubMed] [Google Scholar]
- 56.Shinaberger CS, Greenland S, Kopple JD, Van WD, Mehrotra R, Kovesdy CP, Kalantar-Zadeh K. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88:1511–1518. doi: 10.3945/ajcn.2008.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]