Abstract
Neuronal networks generating rhythmic activity as an emergent property are common throughout the nervous system. Some are responsible for rhythmic behaviours, as is the case for the spinal cord locomotor networks; however, for others the function is more subtle and usually involves information processing and/or transfer. An example of the latter is sympathetic nerve activity, which is synchronized into rhythmic bursts in vivo. This arrangement is postulated to offer improved control of target organ responses compared to tonic nerve activity. Traditionally, oscillogenic circuits in the brainstem are credited with generating these rhythms, despite evidence for the persistence of some frequencies in spinalized preparations. Here, we show that rhythmic population activity can be recorded from the intermediolateral cell column (IML) of thoracic spinal cord slices. Recorded in slices from 10- to 12-day-old rats, this activity was manifest as 8–22 Hz oscillations in the field potential and was spatially restricted to the IML. Oscillations often occurred spontaneously, but could also be induced by application of 5-HT, α-methyl 5-HT or MK212. These agents also significantly increased the strength of spontaneous oscillations. Rhythmic activity was abolished by TTX and attenuated by application of gap junction blockers or by antagonists of GABAA receptors. Together these data indicate that this rhythm is an emergent feature of a population of spinal neurons coupled by gap junctions. This work questions the assumption that sympathetic rhythms are dependent on supraspinal pacemaker circuits, by highlighting a surprisingly strong rhythmogenic capability of the reduced sympathetic networks of the spinal cord slice.
Key words: oscillation, sympathetic nervous system, serotonin, spontaneous activity, gap junctions, GABA
Abbreviations: aCSF, artificial cerebrospinal fluid; Cx36, connexin 36; GABA, gamma amino butyric acid; Hb9, homeobox gene 9; HERG, human Ether-a-go-go Related Gene; mRNA, messenger RNA; IML, intermediolateral cell column; IQ, interquartile; SEM, standard error of the mean; SPNs, sympathetic preganglionic neurons
In many areas of the central nervous system, the synchronized and rhythmic discharge of neuronal networks is considered to be an important aspect of information processing (Llinas, 1988). Such discharges have been observed in various in vitro CNS slice preparations (Gillette, 1986; Llinas and Yarom, 1986; Whittington et al., 1995), demonstrating that local neuronal assemblies are capable of generating synchronized and rhythmic activities, even when isolated from the circuits in which they would normally be embedded. Brain slice preparations have proved invaluable as a means of probing the mechanisms underlying the generation of coordinated population rhythms as they permit the manipulation of the neuronal environment, provide enhanced stability and increase the opportunity of identifying cell types contributing to the rhythms by enabling the combination of field potential and single neuronal recordings.
In the sympathetic nervous system in vivo, synchronized activity is observed in the form of rhythmic population bursts of sympathetic postganglionic neuronal activity (see (Malpas, 1998). It has been shown that these rhythmic bursts lead to enhanced target organ responses when compared with a similar mean level of continuous activity (Hardebo, 1992; Kishi et al., 1998). It is generally considered that the networks which generate these rhythms are located upstream of the sympathetic ganglia, in the brainstem (see review by Barman and Gebber, 2000). Nevertheless, “2–6 Hz” and “10 Hz” rhythms have been observed in sympathetic neuronal discharges of spinalized animals (Allen et al., 1993; Gootman and Cohen, 1981; Kubota et al., 1995; McCall and Gebber, 1975; Ootsuka et al., 1995) and sympathetic rhythmic activity can be generated in an isolated spinal cord preparation (Su, 1999; Su et al., 2003). Thus, a role for the sympathetic networks of the spinal cord, which are centred primarily in the intermediolateral cell column (IML), also seems likely. Importantly, a “1 Hz” sympathetic rhythm (“T rhythm”) can be generated following application of 5-hydroxytryptamine (5-HT) to the spinal cord of spinalized rats (Marina et al., 2006). This is of particular interest since there is a dense 5-HT-containing innervation of the IML (Minson et al., 1990)—denser, in fact, than that to any other spinal cord region. Furthermore, 5-HT enhances rhythmic activity in a variety of neuronal networks, including the locomotor central pattern generator (see review by Schmidt and Jordan, 2000), the inferior olive (Sugihara et al., 1995; although the opposite was observed by Placantonakis et al., 2000) and the brainstem respiratory network (Holtman and King, 1994; Manzke et al., 2003; Onimaru et al., 1998; Pena and Ramirez, 2002; Richter et al., 2003).
It appears that spinal cord circuits are capable of generating synchronized rhythmic sympathetic activity and that 5-HT may enhance or induce this activity. However, the mechanisms and neuronal types contributing to these rhythms have yet to be determined. Given the proven utility of brain slice preparations for similarly deconstructing oscillators in the forebrain (e.g. Gloveli et al., 2005), spinal cord slices would seem to be the most appropriate tool to this end. The spinal cord slice has been routinely used for patch clamp recordings from sympathetic preganglionic neurons (SPNs) and presympathetic interneurons, which can be targeted visually (see Deuchars et al., 2001, 2005). However before applying these techniques to study the mechanisms underlying coordinated rhythmic sympathetic activity at the cellular level, it is appropriate to determine whether such network activity can be generated in the spinal cord slice by first recording field potentials.
Consequently, we have developed a novel use of the spinal cord slice preparation to demonstrate that rhythmic population discharges can be recorded in the IML, which is known from many previous anatomical and functional studies to comprise mainly sympathetic preganglionic neuronal somata and presympathetic interneurons (Deuchars, 2007; Gilbey and Spyer, 1993). We also provide insights into the mechanisms underpinning this rhythmicity. The discovery of this capability of spinal cord slices to generate rhythmogenic network activity in the IML is a precursor to more detailed studies of the neuronal circuits responsible.
Experimental procedures
Ethical approval
All experimental procedures conform to the UK Animals (Scientific Procedures) Act 1986 and to ethical standards set out by the University of Leeds Ethical Review Committee.
General procedures
10–12 day old Wistar rats (n=89) of either sex were deeply anaesthetized with urethane (2 g/kg of body weight intraperitoneally). Animals were transcardially perfused with ice cold sucrose artificial cerebrospinal fluid (sucrose aCSF) containing (in mM): sucrose (215), NaHCO3 (13), glucose (5), KCl (1.5), NaH2PO4 (1.5), MgSO4.7H2O (1) and CaCl2 (1), then humanely killed by decapitation. The thoracic spinal cord was dissected out and 500 μm transverse slices cut using a Vibraslice (Campden Instruments). Slices were stored at room temperature submerged in a holding chamber of aCSF containing (in mM): NaCl (124), NaHCO3 (26), glucose (10), KCl (3), NaH2PO4 (2.5), MgSO4.7H2O (2), CaCl2 (2), equilibrated with 5 % CO2/ 95% O2.
Extracellular recordings
Slices were allowed to equilibrate for at least 20 min in a custom built “interface” recording chamber, superfused with gassed aCSF at 30–35 °C. To keep slices moist, humidified 95 % O2/ 5% CO2 was circulated in the recording chamber. A glass micropipette (2–4 μm tip diameter) was positioned in the IML, filled with aCSF supplemented with 0.02% Rhodamine dye. Signals were amplified ×10 by an Axoclamp2B amplifier (Axon Instruments, USA), and a further ×1000 amplification was achieved with two Neurolog NL106 (Digitimer, UK) amplifier modules. Signals were low-pass filtered (<35 Hz), notch filtered for 50 Hz mains noise (Humbug, Digitimer), digitized (CED1401Plus, Cambridge Electronic Design, UK) and captured at 5 kHz on a PC running Spike2 software (Cambridge Electronic Design, UK).
Extracellular field potential recordings were made, at depths of approximately 100 μm below the surface, from the IML of 157 transverse spinal cord slices (which retain the mediolateral dendrites that are predominant in SPNs at this age (Markham and Vaughn, 1991)) from 89 neonatal rats. Extracellular IML activity had peak amplitudes of 20–60 μV which did not vary appreciably during the course of a recording.
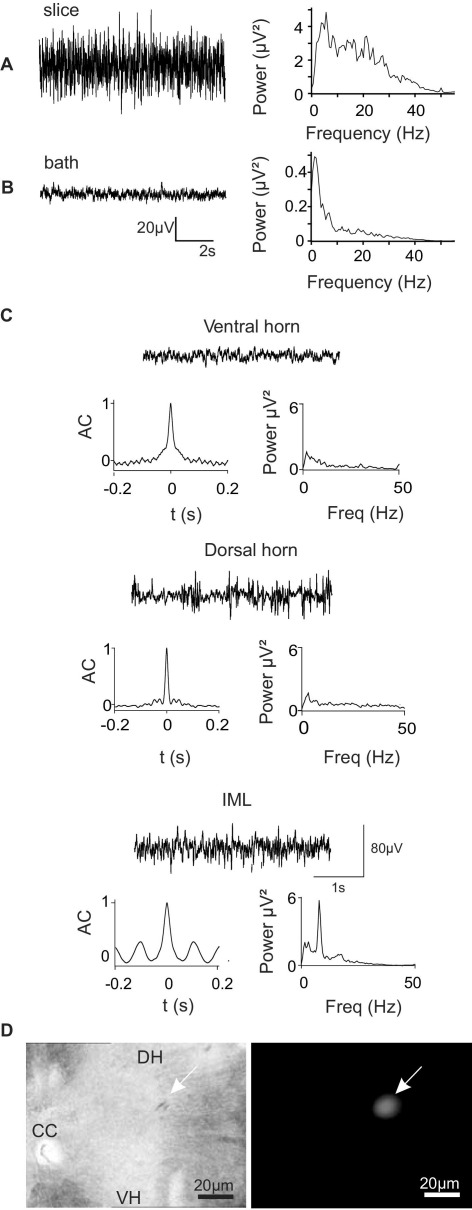
Background noise levels were determined by comparing traces captured with the electrode placed in the IML (Fig. 1A) and in the bath (Fig. 1B). The amplitude of the activity was much lower with the electrode in the bath (<10 μV), as expected. In addition, power spectral analysis (right panels in Fig. 1A, B) revealed that the frequency components of the two traces were different: the background noise consisted mainly of a small peak at 3 Hz, whereas a larger proportion of higher frequencies was present in the neuronal trace. To enable post hoc verification that the recording electrode was correctly positioned, rhodamine dye was deposited at the recording site (Fig. 1D).
Fig. 1.
Verification of the experimental set-up for capturing extracellular traces. (A) Typical recording from the IML of a spinal cord slice. Left: extracellular recording. Right: power spectrum of recorded activity with a range of frequencies between 5 and 30 Hz. (B) Placing the electrode in the bathing solution shows the level of background noise. Note the difference in scale. (C) Comparison of extracellular activity recorded in the same slice from three different regions: lamina IX of ventral horn, Lamina II of dorsal horn and the IML. Each trace is accompanied by the autocorrelogram (left) and power spectrum (right). Oscillations were present only in the IML. AC, autocorrelation coefficient. (D) Rhodamine dye at the recording site shows correct positioning in the IML. Images show the same area of spinal cord revealing rhodamine fluorescence (right), or structure of the cord (left). Arrow indicates the dorsolateral border of the IML.
Data analysis
All analysis was carried out using Spike2. DC offset was removed from traces before analysis. Power spectra (Fast Fourier Transform (FFT) with Hanning window, FFT size=8192 and resolution=0.6 Hz) and autocorrelograms were generated from 1 min of activity. The field potentials were considered to be rhythmic if there was a peak in the power spectrum and the first autocorrelogram peak to the right of the central peak indicated a correlation coefficient of greater than 0.1. The principal frequency of rhythmic activity was assessed from the highest peak in the power spectrum. The degree of rhythmicity (“area power”) was quantified by measuring the area under the power spectrum in a 3 Hz band surrounding the highest peak. Note that due to this analysis protocol, slices that lacked rhythmic discharges nevertheless had an area power considerably greater than zero because a degree of activity in the frequency band of interest is to be expected at all times. If the frequency of the highest peak changed following drug treatment, the power under this new peak was calculated, not the power at the original frequency. If no power spectrum peak was present (for example, after blockade of oscillations with a drug), then area power was measured from the same 3 Hz range as in control conditions.
Statistical comparisons involving area power were carried out with Wilcoxon matched pairs signed rank test because these data do not fit a Normal distribution. All other comparisons were made using paired Student's T tests. Results were considered significant when P<0.05. Frequency data are presented as mean±standard error of the mean (SEM), whereas area power data are presented as median and interquartile (IQ) range.
Drugs
Stock solutions of drugs (apart from 5-HT which was made up fresh on the day) were diluted in aCSF on the day of the experiment and superfused over the slice without recirculation. Drugs were obtained from Tocris Bioscience (UK) except where stated: 5-hydroxytryptamine hydrochloride (5-HT, Sigma-Aldrich, UK), αmethyl-5-hydroxytryptamine maleate (αme5-HT), 6-Chloro-2-(1-piperazinyl)pyrazine hydrochloride (MK212), Potassium (2S,4aS,6aR,6aS,6bR,8aR,10S,12aS,14bR)-10-hydroxy-2,4a,6a,6b,9,9,12a-7 heptamethyl-13-oxo-3,4,5,6,6a,7,8,8a,10,11,12,14b-dodecahydro-1H-picene-2-carboxylate (18β-glycyrrhetinic acid, stock in 50% ethanol; Sigma-Aldrich, UK), (AS)-rel-a-(2R)-2-peperidinyl-2,8-bis(trifluoromethyl)-4-quinolinemethanol monohydrochloride (mefloquine), N-[2-[[3-(Dimethylamino)propyl]thio]phenyl]-3-phenyl-2-propenamide hydrochloride (cinanserin), tetrodotoxin citrate (TTX), bicuculline hydrochloride (bicuculline).
Results
Spontaneous rhythmic activity recorded from the IML of spinal cord slices
Power spectral and autocorrelation analysis of IML activity revealed spontaneous rhythmic field potential oscillations in 48/164 (29.3%) slices tested. The rhythmic discharges were manifest as a peak in the power spectrum in the range 7.5–22 Hz (mean 13.5±0.7 Hz) and as a sinusoidal autocorrelogram (e.g. Fig. 2B). Oscillation frequency did not drift appreciably during the recording, even though different slices showed a range of frequencies. This rhythmic activity typically persisted for the duration of a recording, which could be in excess of 3 h.
Fig. 2.
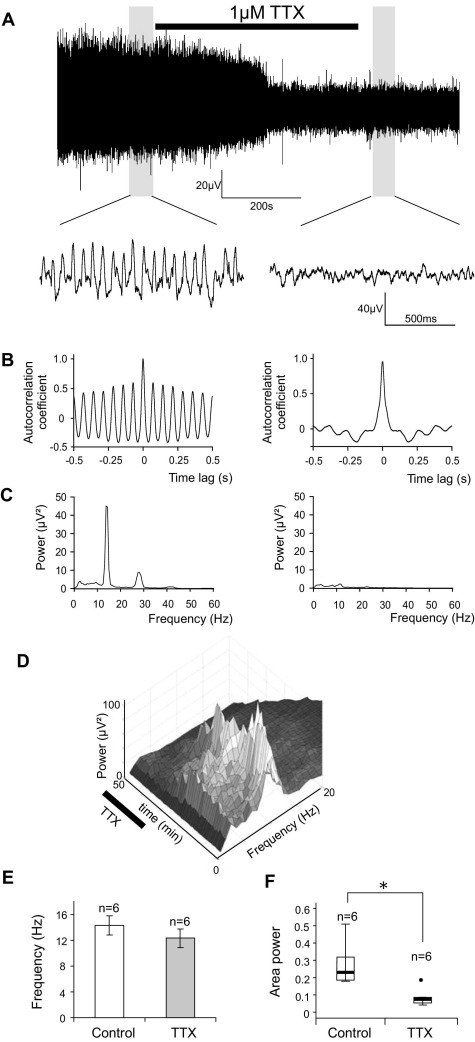
IML oscillations are abolished by the Na+ channel blocker TTX. (A) Low-pass filtered (<35 Hz) extracellular recording from the IML of a spinal cord slice. Application of 1 μM TTX reduced the activity in the slice. The trace did not recover upon removal of TTX. Grey regions were subjected to the analyses presented below. A segment of data from each of the grey regions is presented on a faster timescale below to show the oscillation more clearly. (B) Autocorrelograms of the grey regions, showing that the activity was self similar (sinusoidal plot) before, but not after, application of TTX. (C) Power spectra of the grey regions reveal the presence of a rhythm at 13 Hz, and a small harmonic at approximately twice this frequency. Both peaks were abolished by TTX. (D) Surface plot showing a series of power spectra constructed at regular time intervals from the data in (A). TTX first reduced then abolished the main frequency peak over a period of approximately 30 s. (E) Bar chart where frequency was measured at the latest time in the recording that a power spectrum peak could still be observed. Oscillation frequency was not affected by TTX. (F) Box plot shows the median (thick horizontal line), interquartile range (box) and range (T bars) of oscillation power. Filled circle represents an individual outlier. Oscillation power was significantly reduced by TTX (n=6). * P<0.05, Wilcoxon matched pairs signed rank test.).
Of the slices that did not exhibit spontaneous rhythmic activity, a further 18 previously non-rhythmic slices could be pharmacologically manipulated with 5-HT agonists to induce this activity (see below). The other slices were not able to generate spontaneous or 5-HT-induced oscillations (see discussion) and therefore were not considered any further.
In six slices where robust rhythmic oscillatory activity was recorded from the IML, moving the electrode to regions outside the IML resulted in a loss of rhythmic activity in all slices tested. These regions included the motor nuclei (lamina IX) of the ventral horn (n=3) or superficial dorsal horn (n=3), (Fig. 1C) and suggests that this particular rhythm was specific to the IML (see discussion). The positioning of the electrode in the IML was verified by deposition of rhodamine from the electrode at the end of recording (Fig. 1D).
TTX abolishes field oscillations
In six slices tested, spontaneous field oscillations were abolished within 10 min of 1 μM TTX exposure. This was seen as the disappearance of the peak in the power spectrum and the flattening of the sinusoidal morphology of the autocorrelogram (Fig. 2). Consequently, TTX significantly reduced the power of IML oscillations, from a median value of 45.8 (IQ range 31.6–67.1 μV2) to 4.6 (IQ range 1.7–6.2; P=0.028, n=6; Fig. 2F), representing an average 89.8% reduction from control. The frequency of the oscillations (measured at the latest time the power spectrum peak was still detectable) was unaffected by TTX (14.3±1.5 Hz in control conditions vs. 12.3±1.4 Hz in TTX, P=0.201, n=6; Fig. 2E). This is apparent in the waterfall plot in Fig. 2D, where the rhythm fades without any preceding change in frequency.
Gap junction channel blockers reduce IML rhythmic activity
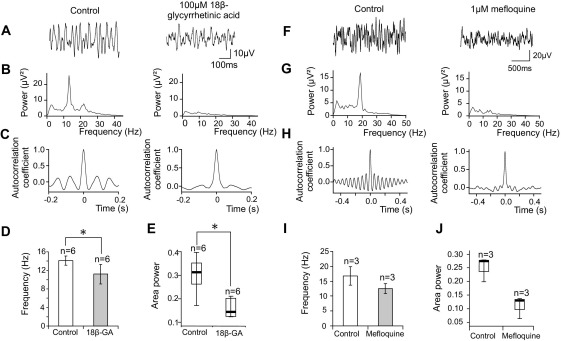
Sympathetic preganglionic neurons in the IML express the gap junction channel subunit connexin 36 (Cx36, Marina et al., 2008) and fire synchronous action potentials in slices as a result of electrotonic coupling (Logan et al., 1996). We therefore sought to determine whether direct electrical coupling contributed to the rhythmic population activity in our slice preparation. The gap junction blocker 18β-glycyrrhetinic acid (100 μM, verified as effective at this concentration at blocking spikelets, the hallmark of gap junctions in recordings from single SPNs; data not shown) was continuously bath applied to six spontaneously oscillating slices (Fig. 3A–E). 18β-glycyrrhetinic acid reduced the area power of spontaneous oscillations by an average of 68.2% (median power was 42.9 [IQ range 26.9–56.1] μV2 in control and 5.0 [IQ range 4.8–10.6] μV2 in 18β-glycyrrhetinic acid, P=0.028, n=6; Fig. 3E). Rhythmic activity was completely abolished in three of these six slices. In addition, the oscillation frequency (measured immediately before abolition of the power spectrum peak, where necessary) was significantly reduced by 18β-glycyrrhetinic acid from 14.1±1.0 Hz in control conditions to 11.2±1.5 Hz in the presence of the drug (P=0.02, n=6; Fig. 3D). Another gap junction blocker, carbenoxolone (100 μM, a concentration at which spontaneous spikelets were reduced in current clamped SPNs, not shown), had a similar effect (data not shown), reducing the power of IML oscillations by an average of 69.8%. Area power was reduced from 5.5 (IQ range 3.6–110.5) μV2 to 1.4 (IQ range 1.0–36.6) μV2 (P=0.068, n=4) by this drug; however, this reduction did not quite reach statistical significance, possibly due to the range of ongoing area powers observed. Oscillation frequency, measured just before abolition of the power spectrum peak, was not affected (15.2±2.3 Hz in control to 16.7±4.1 Hz in carbenoxolone, P=0.47).
Fig. 3.
IML oscillations are sensitive to gap junction blockers. (A–C) illustrate a single experiment. (A) Low-pass filtered (<35 Hz) extracellular recordings. The gap junction blocker 18β-glycyrrhetinic acid (100 μM) reduced the amplitude of activity in the IML. (B) Power spectral analysis of the raw traces showing that a 13 Hz oscillation in IML activity was abolished by 18β-glycyrrhetinic acid. (C) The autocorrelogram becomes flattened following gap junction blockade, indicating that the degree of rhythmicity has been reduced by this drug. (D) Bar chart illustrating the change in mean frequency of IML oscillations following treatment with 18β-glycyrrhetinic acid (18β-GA). Error bars show SEM. Oscillation frequency was significantly reduced by this agent. (E) Box plot (as for Fig. 2) of oscillation power before and after application of 18β-glycyrrhetinic acid. The median power was significantly reduced by this drug. (F–H) illustrate a second experiment. (F) Low-pass filtered (<35 Hz) extracellular recordings. The Cx36-selective gap junction blocker mefloquine (1 μM) reduced the amplitude of IML activity. (G) Power spectra of the traces in (D) show that a 19 Hz oscillation in this slice was abolished by mefloquine. (H) Autocorrelograms of the same data segments illustrate that the degree of rhythmicity was reduced by mefloquine. (I) Bar chart showing the effect of mefloquine on the mean frequency of IML oscillations. Error bars show SEM. (J) Box plot showing the distribution of oscillation power before and after mefloquine treatment. There was a trend towards a reduction of both power (J) and frequency (I) by mefloquine but this was not statistically significant. * P<0.05, paired Student's T test (D) or Wilcoxon matched pairs signed ranks test (E).
Finally, we also tested the effects of mefloquine, a gap junction blocker which has been reported to block Cx36-containing gap junction channels more effectively than those with alternative subunit compositions (Cruikshank et al., 2004). Bath application of 1 μM mefloquine, a concentration also verified to reduce spikelet activity in SPNs (not shown) abolished spontaneous oscillations in 2/3 slices (Fig. 3F–J), and reduced the power from 33.2 to 5.8 μV2 in the remaining slice. The mean reduction in area power produced by mefloquine was 76.4% (median 33.2 [IQ range 20.2–40.0] under control conditions, 3.9 [IQ range 3.6–4.9] in mefloquine, P=0.109, n=3; Fig. 3J). Oscillation frequency (measured just before abolition of the power spectrum peak, where necessary) was not significantly changed by mefloquine, although a decrease from 16.7±3.1 Hz to 12.5±1.7 Hz (P=0.153, n=3; Fig. 3I) was observed following application of this drug.
The GABAA receptor antagonist bicuculline attenuates oscillations
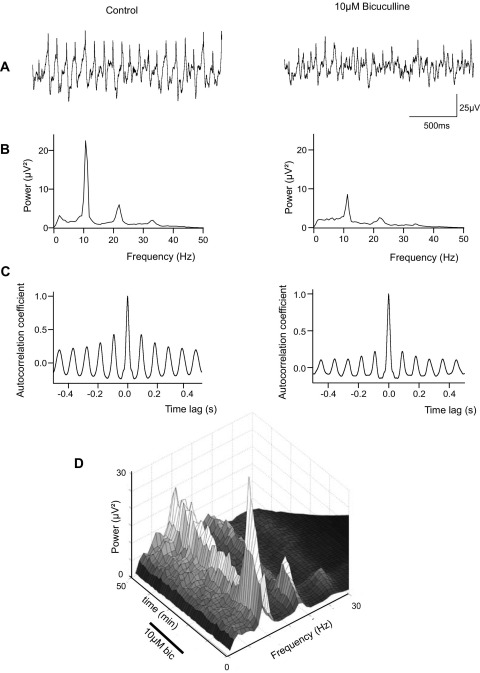
To test whether inhibitory GABAergic synaptic transmission is required for rhythm generation in the IML, bicuculline, a blocker of GABAA receptors, was applied to spontaneously oscillating slices. 10 μM bicuculline significantly reduced the power of ongoing oscillations from a median value of 19.1 (IQ range 5.1–35.1) μV2 to 3.6 (IQ range 0.8–4.4) μV2 (average 65.1% reduction—Fig. 4A–C; P=0.043, n=5). This effect was reversed upon washout (Fig. 4D). The frequency of the oscillation was not significantly changed by bicuculline (11.9±2.0 Hz in bicuculline vs. 10.1±1.2 Hz under control conditions, P=0.149, n=5). These data strongly implicate GABAergic neurotransmission in the maintenance of spontaneous IML oscillations.
Fig. 4.
Blockade of GABAA receptors with bicuculline attenuates IML oscillations. (A) Extracellular oscillations are present in control conditions but are reduced by 10 μM bicuculline. (B) Power spectra of extracellular activity. A large 10 Hz peak is present in control conditions, but is reduced after treatment with bicuculline. (C) Autocorrelograms of the same segment of data analysed in (B). The activity is less strongly correlated following treatment with bicuculline. (D) Surface plot of oscillation power over time showing the time course of inhibition of oscillations by bicuculline and the subsequent recovery upon removal of the drug.
Effects of 5-HT and 5-HT2 receptor agonists on rhythmic activity
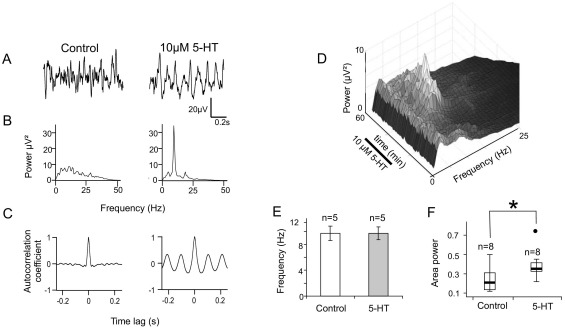
10μM 5-HT was applied to eight slices which were demonstrated to be capable of oscillating, of which five were spontaneously rhythmically active and three were non-rhythmic at the time 5-HT was applied. Intermittent application of 5-HT over 30 min using a 5-min-on, 5-min-off protocol resulted in an increase in the area power at the rhythm frequency in all eight slices (Fig. 5). Intermittent, rather than continuous, application of 5-HT was employed because this type of protocol was more successful at enhancing inspiratory nerve burst amplitude than continuous application of 5-HT to a brainstem-spinal cord preparation (Lovett-Barr et al., 2006). The increase in area power was from a median value of 4.1 (IQ range 2.1–10.4) μV2 to 14.2 (IQ range 8.7–34.4) μV2 (average 544.7% increase—Fig. 5F; n=8, P=0.012). The 5-HT-induced rhythmic activity lasted at least 10 min and often in excess of 1 h. 5-HT-induced oscillations fell within the normal range for spontaneous oscillations, averaging 9.9±1.8 Hz. This is not significantly different to the frequency of spontaneous oscillations (P=0.095). In the five slices which exhibited spontaneous oscillations, the oscillation frequency was not changed by 5-HT treatment (9.7±1.1 Hz vs. 9.7±1.0 Hz, P=0.655, Fig. 5E).
Fig. 5.
5-HT induces oscillatory activity in the IML. (A) Low-pass filtered (<35 Hz) extracellular voltage recording from the IML of a single spinal cord slice before (left) and after (right) application of 10 μM 5-HT. Rhythmic oscillations appear during 5-HT application. (B) Power spectral analysis of a 1 min segment of control (left) and 5-HT (right) activity showing a prominent peak at 9.4 Hz. (C) Autocorrelograms showing that the activity is self-similar (rhythmic) only after 5-HT is applied. (D) Surface plot of power spectra taken at consecutive time points from a different slice, showing the development and decline of an 11.9 Hz oscillation over time. (E) Bar chart of the mean data showing that the mean oscillation frequency was not affected by 5-HT (n=5). Error bars=SEM. (F) Box plot of oscillation power (as for Fig. 2). 10 μM 5-HT significantly increased the power of the oscillation. * P<0.05, Wilcoxon matched pairs signed rank test.
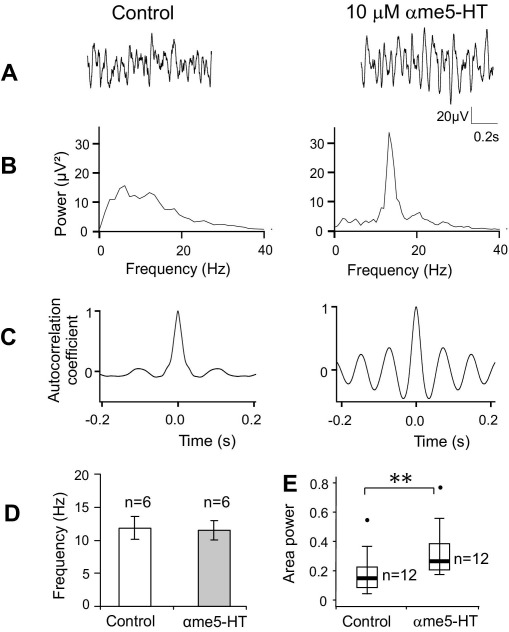
Selective agonists were also used to determine the receptor subtype which contributed to the effects of 5-HT. 10 μM αme5-HT, an agonist of 5-HT2 receptors, mimicked the effects of 5-HT. When applied to slices which were demonstrated to be capable of rhythmic activity, αmethyl5-HT increased area power in all 12 slices, from a median value of 4.9 (IQ range 2.1–12.9) μV2 to 13.3 (IQ range 5.4–21.6) μV2 (average 574.0% increase; Fig. 6; P=0.005). Six of these slices were spontaneously active when the drug was applied, whereas the other six were not. Similar to the effects of 5-HT, αme5-HT did not significantly change spontaneous oscillation frequency (11.7±1.9 Hz during control, 11.3±1.4 Hz during αmethyl5-HT application, P=0.595; Fig. 6D). In addition, the mean frequency of rhythmic activity induced by αme5-HT was very similar to that of spontaneous oscillations (13.0±2.2 Hz, P=0.617).
Fig. 6.
The 5-HT2 agonist αme5-HT increases rhythmic activity in the IML. (A) Voltage recordings from the IML showing effects of 10 μM αme5-HT. (B) Power spectra of the activity in (A) showing the presence of a peak at 13.1 Hz only after addition of αme5-HT. (C) Autocorrelograms showing that the activity is self-similar after αme5-HT. (D) Bar chart showing that the mean frequency of IML oscillations was not affected by αme5-HT (n=6). (E) Box plot (as Fig. 2) showing that oscillation power was significantly increased by αme5-HT (n=12). ** P<0.01, Wilcoxon matched pairs signed rank test.
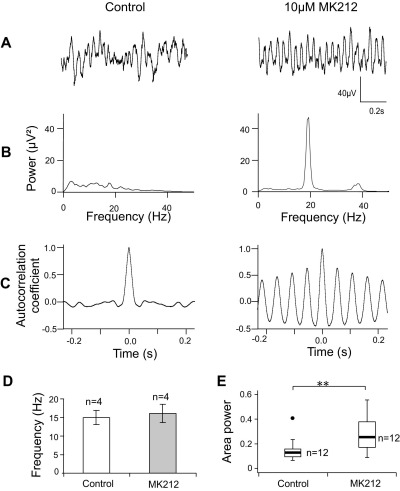
Since αme5-HT does not discriminate between the three subtypes of 5-HT2 receptor, and mRNA for 5-HT2C is high in the IML (Fonseca et al., 2001), the action of the selective 5-HT2C agonist MK212 was tested. 10 μM MK212 was continuously bath applied to 12 slices deemed capable of generating rhythmic activity, of which four were spontaneously rhythmic. The area power of IML oscillations was significantly enhanced in all 12 slices (Fig. 7). The median control power in these experiments was 12.0 (IQ range 6.0–16.0) μV2, rising to 18.7 (IQ range 10.7–50.2) μV2 (average increase of 167.0%, P=0.006, n=12; Fig. 7E) measured after at least 8 min in MK212. Again, the frequency of spontaneous rhythmic discharges was unaffected (15.0±1.9 Hz in control, 16.1±2.5 Hz in MK212, P=0.288, n=4; Fig. 7D).
Fig. 7.
The 5-HT2C agonist MK212 induces oscillatory activity in the IML. (A) Extracellular recordings where activity becomes oscillatory following 10 μM MK212. (B) Power spectra of the activity traces in (A) with a large peak at around 20 Hz and a smaller harmonic peak at twice the frequency. (C) Autocorrelograms of same activity. (D) Bar chart shows the lack of effect of MK212 on the frequency of the ongoing activity (n=4). (E) Box plot (as Fig. 2) showing that MK212 significantly increased the power of IML oscillations (n=13). ** P=0.006, Wilcoxon matched pairs signed rank test.
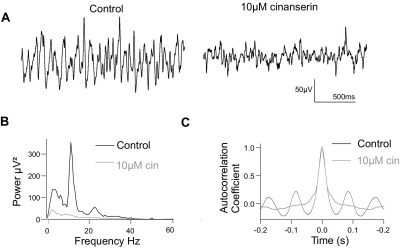
To determine the contribution, if any, of 5-HT receptors to the spontaneous oscillations, the 5-HT2 receptor antagonist cinanserin was continuously bath applied to three spontaneously oscillating slices. 10 μM cinanserin reduced IML oscillations in all three slices, with a mean reduction in power of 33.3% (from 0.355±0.079 to 0.236±0.060, n=3; Fig. 8). Cinanserin did not significantly affect oscillation frequency: the mean frequency in control conditions was 11.1±1.2 Hz, and in cinanserin the frequency was 9.8±1.8 Hz, n=3.
Fig. 8.
Spontaneous IML oscillations were reduced by the 5-HT antagonist cinanserin. (A) Extracellular recording from the IML where rhythmic spontaneous oscillations were reduced by 10 μM cinanserin. (B) Power spectra of the activity in (A) showing the peak in the power is reduced by cinanserin. (C) Autocorrelograms of activity, showing that the activity was self similar (sinusoidal plot) before, but not after, application of cinanserin.
Discussion
In this study we have recorded field potentials, through electrodes placed in the IML, that have (often spontaneous) rhythmical discharge characteristics. We therefore suggest that the neuronal networks present in a spinal cord slice preparation are sufficient to generate and maintain rhythmic sympathetic activity, challenging the current view that brainstem circuits are the key contributors to the patterning of rhythmic sympathetic nerve bursts. Rhythmic activity was abolished by application of TTX, indicating that neuronal firing plays a critical role in generating these rhythmic field potentials. We have also observed that IML rhythmic activity is disrupted by gap junction blockers and bicuculline. Furthermore, rhythmic activity could be enhanced or induced by agonists that activate 5-HT receptors, including those selective for 5-HT2C receptors, an effect similar to that observed in vivo or in spinalized preparations.
It should be noted that these network IML rhythms are a result of ensemble neuronal activity (probably including SPNs and interneurons in this region) and as such are distinct from the membrane potential fluctuations (similarly termed “oscillations”) in single cell recordings such as those reported in Hb9-positive interneurons involved in locomotion (Wilson et al., 2005) or indeed SPNs (Spanswick and Logan, 1990). However, this is not to say that these intracellular events reported by Logan's group do not correlate with, or indeed contribute to, our ensemble activity—indeed, these events are gap junction-mediated potentials (spikelets) (Logan et al., 1996) and thus they are a probable target of our gap junction blockers. Nevertheless, it is clear that our network oscillations are not simply trains of synchronized spikelets summating in the extracellular milieu—the spontaneous spikelet frequency is much too low in spinal cord slices (mean value 1 Hz), and spikelet frequency is increased by application of 5-HT (Pickering et al., 1994), whereas our network oscillations are increased in power by this drug but their frequency remains unaffected.
Insights into the mechanism of IML network oscillations: the role of gap junctions, sodium channels and GABAergic interneurons
Spontaneous IML rhythms were reduced in power or abolished by gap junction blockers. It is well known that the drugs used here can have non-specific actions (Coker et al., 2000; McArdle et al., 2006), such as an inhibitory effect of carbenoxolone on Ca2+ channels (Vessey et al., 2004) or mefloquine on hERG K+ channels (Traebert et al., 2004); although with an IC50 of 2.6 μM, higher than the dose used here. Mefloquine and 18 β-glycyrrhetinic acid can also block volume-regulated anion channels (Maertens et al., 2000; Ye et al., 2009) and since other blockers of these channels may also have an effect at glycine receptors (Scain et al., 2010), there is a chance that these blockers may exert an action at glycine receptors. However, the fact that three different drugs exerted similar effects suggests it is their shared action at gap junctions, rather than other possible non-specific effects, which is responsible for attenuating the oscillations. This is further supported by the observation that all three gap junction blockers used disrupt gap junction-mediated spikelets in SPNs at these concentrations, without obvious effects on other firing properties of the neurons. Although further experiments may prove unequivocally that these effects are mediated solely by an action on gap junctions, this indicates that synchronization of neuronal activity by gap junctions may be an important mechanism underpinning this rhythmic IML activity. At least some of the junctions involved may contain the subunit Cx36 because mefloquine, a blocker with selectivity for Cx36-containing gap junctions, replicated the effects of general gap junction blockade. A similar role of Cx36-containing gap junctions in the inferior olive (Placantonakis et al., 2006), suprachiasmatic nucleus (Long et al., 2005), and hippocampus (Buhl et al., 2003) has been confirmed in Cx36-knockout mice. For two reasons, it is likely that the gap junctions involved in IML rhythm generation are located between SPNs. Firstly, gap junction coupling tightly synchronizes subthreshold and suprathreshold activity between pairs of SPNs (Logan et al., 1996), but gap junction coupling between interneurons or between SPN-interneuron pairs has not been observed. Secondly, Cx36 immunoreactivity has been localized to the dendritic membrane of SPNs, but not to interneurons, in the IML (Marina et al., 2008).
In common with other CNS networks (e.g. see Somogyi and Klausberger, 2005), spontaneous IML oscillations were also attenuated by bicuculline, a GABAA receptor antagonist, suggesting that spontaneously active GABAergic interneurons also contribute to the network rhythms. This is relevant to sympathetic control since GABAergic interneurons within the central autonomic area synapse directly onto SPNs (Deuchars et al., 2005) and provide ongoing phasic inhibitory input in the shape of inhibitory postsynaptic potentials (IPSPs) recorded in spinal cord slices. Therefore, antagonism of the effects of these interneurons is the likely mechanism underlying the effects of bicuculline on oscillation power. These inhibitory interneurons may act to pace or reinforce the activity generated in the coupled networks, as originally observed in the hippocampus (Cobb et al., 1995).
IML rhythms were abolished by the voltage-gated Na+ channel blocker TTX in all experiments, indicating that action potential propagation is a vital component of the oscillations. Taken together with the results of gap junction and GABAA receptor blockade, these data suggest that IML field oscillations are the emergent property of a network of neurons that is dependent on synaptic properties and patterns of connections between neurons.
Role of 5-HT in IML rhythms
5-HT acts as a modulator of IML rhythmic oscillations, able both to enhance the power of existing rhythms on every occasion tested and induce rhythms in otherwise quiescent slices. This effect was mimicked by αme5-HT and MK212, suggesting this is mediated at least in part by 5-HT2 receptors. It is likely that the mechanisms underlying the spontaneous and 5-HT-induced rhythms are similar since the frequencies of spontaneous and 5-HT-induced rhythms are not significantly different and 5-HT does not significantly change the frequency of ongoing rhythms even when it enhances the power. Moreover, results from the slices where cinanserin reduced the power of the oscillation provide evidence that, in these cases, at least a proportion of the spontaneous oscillations is mediated by activation of 5-HT2 receptors.
Where 5-HT or αmethyl5-HT were unable to elicit rhythmic activity in hitherto quiescent slices, it is likely that these slices were either not viable (i.e. not healthy enough to generate complex oscillations), did not contain enough of the circuitry necessary for generating rhythmic activity, or may oscillate in the presence of drugs other than those of interest to us here. Since the spontaneous activity level of many slices was only marginally above background noise levels, it was difficult to be certain of the viability of slices which did not display oscillations. We therefore report the existence of these slices for transparency, and with the admission that we cannot say for sure whether they were the result of sub-optimal slice preparation or maintenance, or whether some may have been alive but intrinsically unable to oscillate. Possible causes of the latter would include the “ladder and rung” morphology (Petras and Cummings, 1972) of the IML, in which small clusters of SPN somata are separated by highly variable gaps of 50–150 μm (our unpublished estimates in 11-day-old rats) consisting mainly of dendrites. The number of clusters per 500 μm slice is therefore likely to vary, perhaps resulting in some slices containing too few SPNs to generate oscillatory activity.
Previous studies support the notion that 5-HT is an important modulator of rhythmic sympathetic activity. For example, the 10 Hz rhythm in sympathetic nerve discharge is selectively enhanced by intravenous administration of a 5-HT2 receptor agonist and eliminated by a 5-HT2 antagonist, and these effects are mimicked by exciting or inhibiting serotonergic neurons in the medullary raphe respectively (Orer et al., 1996). Furthermore, microinjections of 5-HT into the upper thoracic IML increased sympathetic outflow to brown adipose tissue (Madden and Morrison, 2006). Significantly, 5-HT acting at a spinal site induces at least two distinct sympathetic rhythms (Marina et al., 2006; Stafford et al., 2006).
The effects of 5-HT on IML rhythmic activity were reproduced by the selective 5-HT2C receptor agonist MK212, suggesting that the facilitation is at least partially mediated by this receptor. Indeed, 5-HT2C mRNA is abundant throughout the spinal cord, including the IML (Fonseca et al., 2001). However, a role for additional 5-HT receptor subtypes cannot be ruled out.
Implications for sympathetic function
The rhythmic activity reported here was confined to the IML region of the spinal cord since, in all slices tested where spontaneous activity was present in this region, moving the electrode to other areas such as the ventral or dorsal horn resulted in loss of this activity. This suggests that, although both dorsal and ventral horn regions are capable of generating rhythmic activity if driven pharmacologically (Asghar et al., 2005; Nakayama et al., 2004), this particular spontaneous or 5-HT-induced rhythm is specific to the IML. It is therefore likely that it is generated locally within the IML, and represents the summed network activity of the SPNs and/or sympathetic interneurons in this region.
The presence of rhythmic activity in the IML of a spinal cord slice strongly supports previous suggestions that rhythmic sympathetic activity can be generated by the spinal cord (see introduction). The frequency of IML oscillations in the current study was in the range 7.5–22 Hz. In vivo, the “10 Hz” rhythm occurs in a similar, but much more restricted, frequency band (8–12 Hz; Cohen and Gootman, 1970; Green and Heffron, 1967). Thus, it might be speculated that the IML oscillations in the current study represent a stripped-down version of the 10 Hz rhythm. In support of this idea, although it is known that at least a component of the 10 Hz rhythm is generated in the brainstem, (Barman and Gebber, 2000), 10 Hz discharges can also be generated within the spinal cord (Kubota et al., 1995; McCall and Gebber, 1975; Ootsuka et al., 1995) and the 10 Hz rhythm is dependent on the activity of 5-HT2 receptors (Orer et al., 1996). It is important to consider the age of the animals used in this study. However, since the closest in vivo correlate of these oscillations, the 10 Hz rhythm, occurs in both young kittens and swine (Hundley et al., 2001; Sica et al., 1990) as well as adult animals, it seems likely that our oscillations will accurately reflect those observed in adults. The generation of 10 Hz discharges by the spinal cord is poorly understood, so this theory remains speculative. Nevertheless, our preparation will facilitate testing of this hypothesis in future studies. Further, it will enable the contribution of individual cell types to network oscillations to be determined, since intracellular sharp electrode or patch clamp recordings may be performed in 500 μm slices in conjunction with field recordings.
Conclusion
Our data demonstrate that rhythmic field potential oscillations can be recorded from the IML of neonatal rat spinal cord slices, and this activity is spontaneous in a proportion of slices. These findings suggest that the rhythmogenic capabilities of the sympathetic circuits of the spinal cord have been underestimated, and that spinal oscillators could potentially play a role in generating the physiologically-important sympathetic nerve bursts that have hitherto been credited mainly to supraspinal networks.
5-HT acting at 5-HT2 receptors modulates IML oscillations, inducing rhythmic network activity in at least a proportion of quiescent slices, and significantly enhancing spontaneous rhythmic activity in all slices tested. Action potential propagation is required for the oscillations to occur, and both gap junctions (probably between SPNs) and GABAergic interneurons are important components of the spontaneous rhythmogenic network. IML oscillations in slices represent a valuable paradigm for studying the rhythmogenic capabilities of the spinal cord.
Acknowledgments
We thank Dan Haggarty and Prem Jesudason for their contribution to some of the preliminary findings. We also thank Brenda Frater for her skilled technical contribution. Michelle Pierce was funded by the MRC, whose generosity we acknowledge. We are very grateful to the British Heart Foundation (Grant No. PG/2001119 and PG08/120/26338, SAD and JD) and RCUK for their generous support.
References
- Allen A.M., Adams J.M., Guyenet P.G. Role of the spinal cord in generating the 2- to 6-Hz rhythm in rat sympathetic outflow. Am J Physiol. 1993;264:R938–R945. doi: 10.1152/ajpregu.1993.264.5.R938. [DOI] [PubMed] [Google Scholar]
- Asghar A.U., Cilia La Corte P.F., LeBeau F.E., Al Dawoud M., Reilly S.C., Buhl E.H., Whittington M.A., King A.E. Oscillatory activity within rat substantia gelatinosa in vitro: a role for chemical and electrical neurotransmission. J Physiol. 2005;562:183–198. doi: 10.1113/jphysiol.2004.076398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman S.M., Gebber G.L. “Rapid” rhythmic discharges of sympathetic nerves: sources, mechanisms of generation, and physiological relevance. J Biol Rhythms. 2000;15(5):365–379. doi: 10.1177/074873000129001468. [DOI] [PubMed] [Google Scholar]
- Buhl D.L., Harris K.D., Hormuzdi S.G., Monyer H., Buzsaki G. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb S.R., Buhl E.H., Halasy K., Paulsen O., Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Cohen M.I., Gootman P.M. Periodicities in efferent discharge of splanchnic nerve of the cat. Am J Physiol. 1970;218:1092–1101. doi: 10.1152/ajplegacy.1970.218.4.1092. [DOI] [PubMed] [Google Scholar]
- Coker S.J., Batey A.J., Lightbown I.D., Diaz M.E., Eisner D.A. Effects of mefloquine on cardiac contractility and electrical activity in vivo, in isolated cardiac preparations, and in single ventricular myocytes. Br J Pharmacol. 2000;129:323–330. doi: 10.1038/sj.bjp.0703060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank S.J., Hopperstad M., Younger M., Connors B.W., Spray D.C., Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars S.A. Multi-tasking in the spinal cord—do “sympathetic” interneurones work harder than we give them credit for? J Physiol. 2007;580:723–729. doi: 10.1113/jphysiol.2007.129429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuchars S.A., Brooke R.E., Frater B., Deuchars J. Properties of interneurones in the intermediolateral cell column of the rat spinal cord: role of the potassium channel subunit Kv31. Neuroscience. 2001;106:433–446. doi: 10.1016/s0306-4522(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Deuchars S.A., Milligan C.J., Stornetta R.L., Deuchars J. GABAergic neurons in the central region of the spinal cord: a novel substrate for sympathetic inhibition. J Neurosci. 2005;25:1063–1070. doi: 10.1523/JNEUROSCI.3740-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca M.I., Ni Y.G., Dunning D.D., Miledi R. Distribution of serotonin 2A, 2C and 3 receptor mRNA in spinal cord and medulla oblongata. Brain Res Mol Brain Res. 2001;89:11–19. doi: 10.1016/s0169-328x(01)00049-3. [DOI] [PubMed] [Google Scholar]
- Gilbey M.P., Spyer K.M. Essential organization of the sympathetic nervous system. Baillieres Clin Endocrinol Metab. 1993;7:259–278. doi: 10.1016/s0950-351x(05)80177-6. [DOI] [PubMed] [Google Scholar]
- Gillette M.U. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Gloveli T., Dugladze T., Saha S., Monyer H., Heinemann U., Traub R.D., Whittington M.A., Buhl E.H. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562:131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootman P.M., Cohen M.I. Sympathetic rhythms in spinal cats. J Auton Nerv Syst. 1981;3:379–387. doi: 10.1016/0165-1838(81)90076-x. [DOI] [PubMed] [Google Scholar]
- Green J.H., Heffron P.F. Observations on the origin and genesis of a rapid sympathetic rhythm. Arch Int Pharmacodyn Ther. 1967;169:403–411. [PubMed] [Google Scholar]
- Hardebo J.E. Influence of impulse pattern on noradrenaline release from sympathetic nerves in cerebral and some peripheral vessels. Acta Physiol Scand. 1992;144:333–339. doi: 10.1111/j.1748-1716.1992.tb09302.x. [DOI] [PubMed] [Google Scholar]
- Holtman J.R., Jr, King K.A. Effect of activation of 5-HT1A receptors at the ventral medulla on phrenic nerve activity. Eur J Pharmacol. 1994;253:307–310. doi: 10.1016/0014-2999(94)90208-9. [DOI] [PubMed] [Google Scholar]
- Hundley B.W., Sica A.L., Gootman P.M. Rhythmicities in sympathetic discharge: a signal of cardiorespiratory integration in developing animals. Ann N Y Acad Sci. 2001;940:416–430. doi: 10.1111/j.1749-6632.2001.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Kishi E., Ootsuka Y., Rong W., Terui N. Functional significance of the 10 Hz rhythmic discharges in sympathetic nerves. Clin Exp Pharmacol Physiol. 1998;25(6):464–467. doi: 10.1111/j.1440-1681.1998.tb02236.x. [DOI] [PubMed] [Google Scholar]
- Kubota A., Ootsuka Y., Xu T., Terui N. The 10-Hz rhythm in the sympathetic nerve activity of cats, rats and rabbits. Neurosci Lett. 1995;196:173–176. doi: 10.1016/0304-3940(95)11868-w. [DOI] [PubMed] [Google Scholar]
- Llinas R., Yarom Y. Oscillatory properties of guinea-pig inferior olivary neurones and their pharmacological modulation: an in vitro study. J Physiol. 1986;376:163–182. doi: 10.1113/jphysiol.1986.sp016147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R.R. The intrinsic electrophysiological properties of mammalian neurons—insights into central nervous-system function. Science. 1988;242:1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Logan S.D., Pickering A.E., Gibson I.C., Nolan M.F., Spanswick D. Electrotonic coupling between rat sympathetic preganglionic neurones in vitro. J Physiol. 1996;495(Pt 2):491–502. doi: 10.1113/jphysiol.1996.sp021609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M.A., Jutras M.J., Connors B.W., Burwell R.D. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- Lovett-Barr M.R., Mitchell G.S., Satriotomo I., Johnson S.M. Serotonin-induced in vitro long-term facilitation exhibits differential pattern sensitivity in cervical and thoracic inspiratory motor output. Neuroscience. 2006;142:885–892. doi: 10.1016/j.neuroscience.2006.06.036. [DOI] [PubMed] [Google Scholar]
- Madden C.J., Morrison S.F. Serotonin potentiates sympathetic responses evoked by spinal NMDA. J Physiol. 2006;577:525–537. doi: 10.1113/jphysiol.2006.116574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens C., Wei L., Droogmans G., Nilius B. Inhibition of volume-regulated and calcium-activated chloride channels by the antimalarial mefloquine. J Pharmacol Exp Ther. 2000;295:29–36. [PubMed] [Google Scholar]
- Malpas S.C. The rhythmicity of sympathetic nerve activity. Prog Neurobiol. 1998;56(1):65–96. doi: 10.1016/s0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Manzke T., Guenther U., Ponimaskin E.G., Haller M., Dutschmann M., Schwarzacher S., Richter D.W. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Marina N., Becker D.L., Gilbey M.P. Immunohistochemical detection of connexin36 in sympathetic preganglionic and somatic motoneurons in the adult rat. Auton Neurosci. 2008;139:15–23. doi: 10.1016/j.autneu.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina N., Taheri M., Gilbey M.P. Generation of a physiologic sympathetic motor rhythm in the rat following spinal application of 5-HT. J Physiol. 2006;571:441–450. doi: 10.1113/jphysiol.2005.100677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham J.A., Vaughn J.E. Migration patterns of sympathetic preganglionic neurons in embryonic rat spinal cord. J Neurobiol. 1991;22:811–822. doi: 10.1002/neu.480220803. [DOI] [PubMed] [Google Scholar]
- McArdle J.J., Sellin L.C., Coakley K.M., Potian J.G., Hognason K. Mefloquine selectively increases asynchronous acetylcholine release from motor nerve terminals. Neuropharmacology. 2006;50:345–353. doi: 10.1016/j.neuropharm.2005.09.011. [DOI] [PubMed] [Google Scholar]
- McCall R.B., Gebber G.L. Brain stem and spinal synchronization of sympathetic nervous discharge. Brain Res. 1975;89:139–143. doi: 10.1016/0006-8993(75)90141-9. [DOI] [PubMed] [Google Scholar]
- Minson J., Chalmers J., Drolet G., Kapoor V., Llewellyn-Smith I., Mills E., Morris M., Pilowsky P. Central serotonergic mechanisms in cardiovascular regulation. Cardiovasc Drugs Ther. 1990;4(Suppl 1):27–32. doi: 10.1007/BF00053423. [DOI] [PubMed] [Google Scholar]
- Nakayama K., Nishimaru H., Kudo N. Rhythmic motor activity in thin transverse slice preparations of the fetal rat spinal cord. J Neurophysiol. 2004;92(1):648–652. doi: 10.1152/jn.01029.2003. [DOI] [PubMed] [Google Scholar]
- Onimaru H., Shamoto A., Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch Eur J Physiol. 1998;435(4):485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Ootsuka Y., Xu T., Terui N. The spinally mediated 10-Hz rhythm in the sympathetic nerve activity of cats. J Auton Nerv Syst. 1995;54:89–103. doi: 10.1016/0165-1838(94)00194-o. [DOI] [PubMed] [Google Scholar]
- Orer H.S., Clement M.E., Barman S.M., Zhong S., Gebber G.L., McCall R.B. Role of serotonergic neurons in the maintenance of the 10-Hz rhythm in sympathetic nerve discharge. Am J Physiol. 1996;270:R174–R181. doi: 10.1152/ajpregu.1996.270.1.R174. [DOI] [PubMed] [Google Scholar]
- Pena F., Ramirez J.M. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petras J.M., Cummings J.F. Autonomic neurons in the spinal cord of the Rhesus monkey: a correlation of the findings of cytoarchitectonics and sympathectomy with fiber degeneration following dorsal rhizotomy. J Comp Neurol. 1972;146:189–218. doi: 10.1002/cne.901460205. [DOI] [PubMed] [Google Scholar]
- Pickering A.E., Spanswick D., Logan S.D. 5-Hydoxytryptamine evokes depolarizations and membrane potential oscillations in rat sympathetic preganglionic neurones. J Physiol. 1994;480(Pt 1):109–121. doi: 10.1113/jphysiol.1994.sp020345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placantonakis D.G., Bukovsky A.A., Aicher S.A., Kiem H.P., Welsh J.P. Continuous electrical oscillations emerge from a coupled network: a study of the inferior olive using lentiviral knockdown of connexin36. J Neurosci. 2006;26:5008–5016. doi: 10.1523/JNEUROSCI.0146-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placantonakis D.G., Schwarz C., Welsh J.P. Serotonin suppresses subthreshold and suprathreshold oscillatory activity of rat inferior olivary neurones in vitro. J Physiol. 2000;524(Pt 3):833–851. doi: 10.1111/j.1469-7793.2000.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D.W., Manzke T., Wilken B., Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Scain A.L., Le C.H., Allain A.E., Muller E., Rigo J.M., Meyrand P., Branchereau P., Legendre P. Glycine release from radial cells modulates the spontaneous activity and its propagation during early spinal cord development. J Neurosci. 2010;30:390–403. doi: 10.1523/JNEUROSCI.2115-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B.J., Jordan L.M. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull. 2000;53:689–710. doi: 10.1016/s0361-9230(00)00402-0. [DOI] [PubMed] [Google Scholar]
- Sica A.L., Siddiqi Z.A., Gandhi M.R., Condemi G. Evidence for central patterning of sympathetic discharge in kittens. Brain Res. 1990;530:349–352. doi: 10.1016/0006-8993(90)91310-d. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanswick D., Logan S.D. Spontaneous rhythmic activity in the intermediolateral cell nucleus of the neonate rat thoracolumbar spinal cord in vitro. Neuroscience. 1990;39:395–403. doi: 10.1016/0306-4522(90)90276-a. [DOI] [PubMed] [Google Scholar]
- Stafford S.A., Tang K., Coote J.H. Activation of lumbosacral 5-HT2C receptors induces bursts of rhythmic activity in sympathetic nerves to the vas deferens in male rats. Br J Pharmacol. 2006;148:1083–1090. doi: 10.1038/sj.bjp.0706814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C.K. Rhythmic sympathetic nerve discharges in an in vitro neonatal rat brain stem-spinal cord preparation. J Appl Physiol. 1999;87:1066–1074. doi: 10.1152/jappl.1999.87.3.1066. [DOI] [PubMed] [Google Scholar]
- Su C.K., Phoon S.L., Yen C.T. Identification of active thoracic spinal segments responsible for tonic and bursting sympathetic discharge in neonatal rats. Brain Res. 2003;966:288–299. doi: 10.1016/s0006-8993(02)04227-0. [DOI] [PubMed] [Google Scholar]
- Sugihara I., Lang E.J., Llinas R. Serotonin modulation of inferior olivary oscillations and synchronicity: a multiple-electrode study in the rat cerebellum. Eur J Neurosci. 1995;7:521–534. doi: 10.1111/j.1460-9568.1995.tb00657.x. [DOI] [PubMed] [Google Scholar]
- Traebert M., Dumotier B., Meister L., Hoffmann P., Dominguez-Estevez M., Suter W. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484:41–48. doi: 10.1016/j.ejphar.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Vessey J.P., Lalonde M.R., Mizan H.A., Welch N.C., Kelly M.E., Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- Whittington M.A., Traub R.D., Jefferys J.G. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- Wilson J.M., Hartley R., Maxwell D.J., Todd A.J., Lieberam I., Kaltschmidt J.A., Yoshida Y., Jessell T.M., Brownstone R.M. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z.C., Oberheim N., Kettenmann H., Ransom B.R. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia. 2009;57:258–269. doi: 10.1002/glia.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]