Abstract
Southeastern Europe is a centre of European biodiversity, but very little is known about factors causing the observed richness. Here, we contribute to fill this gap by reconstructing the spatio-temporal diversification of the cytologically variable and taxonomically intricate complex of Veronica chamaedrys (Plantaginaceae s.l.), growing in open forests, forest edges and grasslands, with flow cytometry, molecular markers (AFLPs, plastid DNA sequences) and morphometry. Our results show that both diploid and tetraploid cytotypes are widespread, but diploids predominate on the southern Balkan Peninsula. Plastid sequences suggest a first split into three main lineages in the mid-Pleistocene and a continuous diversification during the last 0.4 my. Two of the identified plastid lineages coincide with geographically distinct AFLP clusters. Altogether, the genetic data suggest forest refugia on the southern-most Balkan Peninsula (Greece), in Bulgaria, Istria (Croatia and Slovenia) and maybe the southeastern Carpathians (Romania). Morphometric and genetic data show little congruence with current taxonomy.
Keywords: Veronica chamaedrys, Balkan Peninsula, Southeastern Europe, Polyploidy, Genome size, AFLP, Plastid DNA, Morphometrics
1. Introduction
It is long and widely acknowledged that southeastern Europe, and especially the Balkan Peninsula, is a centre of European biodiversity (Turrill, 1929). One early recognised factor is the refugial character of the Balkan Peninsula with a high proportion of relic taxa, even if in many cases the claimed relic status still needs to be confirmed. Much less is, however, known about diversification processes and their spatio-temporal patterns on the Balkan Peninsula especially at lower taxonomic levels, i.e., within and among closely related species. This is partly due to the fact that in molecular studies the Balkan Peninsula is often neglected, such as in large-scale phylogeographical studies, where only a few (e.g., Taberlet, 1994; Santucci et al., 1998; Trewick et al., 2002) or no samples were included (Dumolin-Lapègue et al., 1997; Petit et al., 2002). Detailed studies focussing on the Balkan Peninsula are few and mostly deal with vertebrates (Podnar et al., 2004; Kryštufek et al., 2007; Sotiropoulos et al., 2007; Ursenbacher et al., 2008), butterflies (Schmitt et al., 2006) or mountain plants (Frajman and Oxelman, 2007; Stefanović et al., 2008; Albach et al., 2009).
Southeastern Europe is recognised as a prime refugium for temperate European forest vegetation during the cold stages of the Pleistocene, together with the Iberian and Apennine Peninsulas (Comes and Kadereit, 1998; Taberlet et al., 1998; Gömöry et al., 1999; Hewitt, 2000; Hampe et al., 2003; Petit et al., 2003; Magri et al., 2006; Médail and Diadema, 2009). On the Balkan Peninsula, Pleistocene glaciation was restricted to the high massifs (Hughes et al., 2007; Milivojević et al., 2008), but as the climate was drier and more continental than at present (Horvat et al., 1974), survival of tree species was likely restricted to small areas with favourable conditions—“refugia within refugia”—as has been hypothesised for the Iberian Peninsula (Gómez and Lunt, 2007). These forest refugia were previously assumed to have been restricted to the southern tips of the Southern European peninsulas (Horvat et al., 1974; Hewitt, 2000), but recent studies found evidence for survival of tree species significantly further north than previously assumed (Stewart and Lister, 2001).
In southeastern Europe refugia have been suggested for numerous temperate tree species. Refugia of the beech (Fagus sylvatica) were suggested in Istria and adjacent areas on the Dalmatian coast, southern Bulgaria to northwestern Greece and maybe parts of the Carpathian arc (Magri et al., 2006). Possible refugia for the hornbeam (Carpinus betulus) were proposed in Romania and northern Greece (Grivet and Petit, 2003). Caucasian and European ash (Fraxinus angustifolia and F. excelsior) survived in at least two possible refugial areas, a western one in the Dinaric Alps and an eastern one stretching from the Rhodopes to the Carpathians (Heuertz et al., 2006). The hypothesis of several unconnected forest refugia during the Pleistocene is also supported by the understory vegetation that is much more diverse, regionally differentiated, and richer in endemics than in forests in central and northern Europe (Meusel and Jäger, 1992; Willner et al., 2009). However, the hypothesis of multiple forest refugia in southeastern Europe has never been tested with a herbaceous species in a phylogeographic framework.
A major force in plant evolution and diversification is polyploidy (Ramsey and Schemske, 1998, 2002; Wendel, 2000), which may in many cases be the result of secondary contact of populations differentiated in phases of allopatry, e.g., during restriction to different refugia (e.g., Petit et al., 1999). Polyploidy is recognised as an important mode of diversification by, for instance, promoting adaptation to new ecological niches or conferring reproductive isolation, which may eventually lead to speciation (Otto and Whitton, 2000). While allopolyploids may differ conspicuously from their diploid progenitors in morphology and physiology, autopolyploids that arise from the crosses within or between populations of a single species (Ramsey and Schemske, 1998) are often more difficult to distinguish on the basis of morphology alone (Levin, 1983, 2002). Recent cytogeographical studies not only indicate a higher incidence of autopolyploidy than previously thought, but also that autopolyploids often co-exist with their diploid parental populations (Husband and Sabara, 2004; Kron et al., 2007; Kolář et al., 2009). Despite the widely recognised importance of polyploidization in plant diversification and speciation, very little is known about its contribution to the high diversity on the Balkan Peninsula.
A good system to investigate diversification patterns on the Balkan Peninsula in the contexts of putative differentiation due to isolation in refugia and of polyploidy is the Veronica chamaedrys group (Plantaginaceae s.l.). Although it is widely distributed from western Europe to western Siberia, the Caucasus and Syria (Riek, 1935; Walters and Webb, 1972) and has a rather broad ecological amplitude (Dale and Causton, 1992a,b,c,d), it is a characteristic and widespread element of southeastern European forest vegetation, growing at forest margins and open forests dominated by, e.g., oaks, hornbeam or beech, in grasslands, thickets and hedges (Walters and Webb, 1972). This perennial herb is outbreeding (Goyder, 1983) with at least central European genotypes being self-incompatible (Albach, unpublished) and has the ability of clonal growth (Boutin and Harper, 1991). The V. chamaedrys group is a member of V. subgenus Chamaedrys section Chamaedrys subsection Chamaedrys (Albach et al., 2008) and comprises V. ch. subspp. chamaedrys, chamaedryoides and micans as well as V. krumovii, V. micrantha, V. orbelica and V. vindobonensis (Albach et al., 2004), whose phylogenetic relationships have not been resolved so far (Albach, 2006). With the exception of V. micrantha, which is endemic to the northwestern and central-western Iberian Peninsula (Martínez Ortega et al., 2009), all taxa occur in southeastern Europe and V. ch. subsp. chamaedryoides, V. krumovii and V. orbelica are restricted to that area. Apart from morphological differences concerning, among others, indumentum characters, these taxa do also differ karyologically. In particular, V. ch. subsp. chamaedrys is mainly tetraploid (Fischer, 1970, 1973b; Mirek and Fischer, 1986) with only a few diploids recorded from southern Austria (Fischer, 1973a), whereas V. ch. subsp. chamaedryoides and subsp. micans, as well as V. krumovii, V. orbelica and V. vindobonensis were suggested to be exclusively diploid (Fischer, 1970, 1973b, 1974; Peev, 1972; Strid and Franzén, 1984; Mirek and Fischer, 1986). Since traditional cytotaxonomy, which is often based on a few chromosome counts only, may grossly underestimate the actual intricacy of polyploid complexes in general and of cytotype distribution patterns in particular (e.g., Suda et al., 2004), the association of ploidy level and taxonomy in the V. chamaedrys group remains to be tested, in particular if diploid and tetraploid taxa within the same genetic group are spatially segregated.
Here, we explore diversification patterns within the cytologically polymorphic V. chamaedrys group in southeastern Europe employing genetic (plastid sequences and AFLP fingerprints), ploidy level and morphometric data. Specifically, we want to assess the distribution of cytotypes in this region to test the hypothesis that polyploids are more frequent at higher latitudes, were range shifts of taxa due to climatic oscillations were more pronounced than in the South. We also want to infer mode and minimum number of polyploidization events to test (i) whether polyploids originated via autopolyploidy, as frequently observed in angiosperms (Otto, 2007), or via allopolyploidy, as possible after secondary contact of once geographically isolated diploid lineages (Petit et al., 1999) and (ii) whether polyploid cytotypes originated once or multiple times. Furthermore, we want to test whether the phylogeographical pattern of the woodland herb V. chamaedrys group agrees with those of tree species found in the same vegetation types. Finally, we want to assess whether and to which extent current taxonomy reflects genetically and/or morphometrically defined lineages.
2. Materials and methods
2.1. Plant material
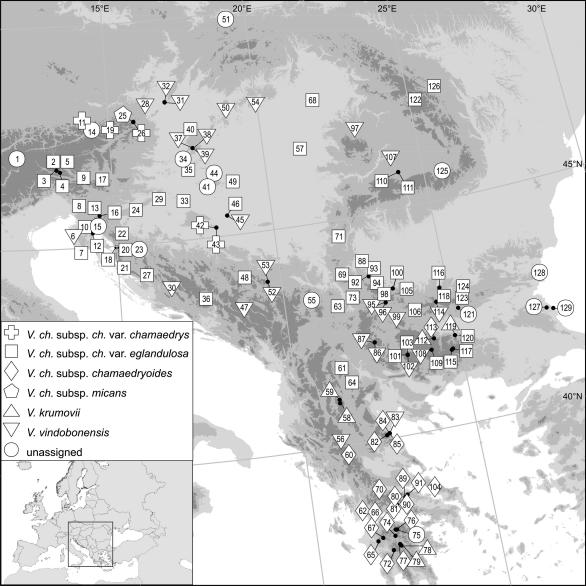
In the summers of 2006 and 2007 the V. chamaedrys group was sampled in 121 sample sites (in the following referred to as “populations”). Leaf material was collected and immediately stored in silica gel. Voucher specimens are deposited at the Faculty Centre of Biodiversity, University of Vienna, Austria (herbarium WU; voucher numbers given in Table 1). Plants were determined by Manfred A. Fischer based on Fischer (1970, 1973b, 1974, 1991), Mirek and Fischer (1986) and Peev (1972, 1995) as well as on personal experience. A detailed description of taxa delimitation as used in our study (in particular subsuming V. orbelica and V. ch. subsp. chamaedrys var. eglandulosa, Table 1) is given in Appendix 1, an overview over the characters used for determination in Appendix 2.
Table 1.
Population number, taxon, collection information, co-ordinates, ploidy, cpDNA haplotype and GenBank accession numbers of 129 sampled populations of the Veronica chamaedrys group from southeastern Europe. Populations that could not be assigned to a taxon are indicated with “–”. cha, V. chamaedrys subsp. chamaedrys var. chamaedrys; coi, V. chamaedrys subsp. chamaedryoides; egl, V. chamaedrys subsp. chamaedrys var. eglandulosa; kru, V. krumovii; mic, V. chamaedrys subsp. micans; kru, V. krumovii; vin, V. vindobonensis. In the column “GenBank accession no.” the first number refers to the trnH-psbA spacer sequence, the second to the rps16-trnK spacer and the third to the rpl32-trnL spacer.
| Pop. No. | Taxon | Locality (voucher number)⁎ | Altitude (m a.s.l.) | Co-ordinates (E, N) | Ploidy | Haplotype | GenBank Accession No. |
|---|---|---|---|---|---|---|---|
| 1 | — | A: Mayerhofen (Bardy_ch1) | 1650 | 11°54′56″, 47°03′58″ | 4x | h27 | HM370828, HM370699, HM370570 |
| 2 | egl | A: Obervellach (Bardy_ch2) | 850 | 13°09′34″, 46°56′18″ | 4x | h33 | HM370829, HM370700, HM370571 |
| 3 | egl | A: Greifenburg (Bardy_ch3) | 1525 | 13°11′53″, 46°46′10″ | 4x | h27 | HM370830, HM370701, HM370572 |
| 4 | egl | A: Lake Weißensee (Bardy_ch4) | 1000 | 13°18′25″, 46°43′08″ | 4x | h28 | HM370831, HM370702, HM370573 |
| 5 | egl | A: Mt. Maltaberg (Bardy_ch5) | 1250 | 13°30′52″, 46°57′54″ | 4x | h33 | HM370832, HM370703, HM370574 |
| 6 | vin | HR: Poreč (Bardy_ch6) | 140 | 13°39′39″, 45°15′31″ | 2x | h23 | HM370833, HM370704, HM370575 |
| 7 | egl | HR: Pula (Bardy_ch7) | 60 | 13°51′50″, 44°53′51″ | 2x | h23 | HM370834, HM370705, HM370576 |
| 8o | egl | SLO: Predmeja (Frajman and Schönwetter 11129) | 970 | 13°52′31″, 45°57′12″ | 4x | h52 | HM370835, HM370706, HM370577 |
| 9 | egl | A: St. Egyden (Bardy_ch9) | 640 | 14°04′10″, 46°35′01″ | 4x | h52 | HM370836, HM370707, HM370578 |
| 10 | egl | HR: Lanišće (Bardy_ch10) | 470 | 14°05′49″, 45°24′48″ | 2x | h26 | HM370837, HM370708, HM370579 |
| 11 | cha | A: Sengsengebirge (Bardy_ch11) | 1400 | 14°12′14″, 47°48′27″ | 4x | h27 | HM370838, HM370709, HM370580 |
| 12 | egl | HR: Opatija (Bardy_ch12) | 270 | 14°16′05″, 45°19′21″ | 4x | h23 | HM370839, HM370710, HM370581 |
| 13 | egl | SLO: Pekel pri Borovnici (Frajman and Schönwetter 11113) | 345 | 14°22′18″, 45°53′26″ | 4x | h53 | HM370840, HM370711, HM370582 |
| 14 | — | A: Rosenau am Hengstpaß (no voucher) | 800 | 14°23′00″, 47°42′00″ | 4x | h27 | HM370841, HM370712, HM370583 |
| 15 | — | HR: Trstenik mire (no voucher) | 965 | 14°27′29″, 45°29′19″ | 4x | h56 | HM370842, HM370713, HM370584 |
| 16 | egl | SLO: Vrhnika pri Ložu (Frajman 12022) | 590 | 14°30′42″, 45°41′69″ | 4x | h23 | HM370843, HM370714, HM370585 |
| 17 | egl | A: Bad Eisenkappel (Bardy_ch17) | 1600 | 14°40′44″, 46°31′00″ | 4x | h35 | HM370844, HM370715, HM370586 |
| 18 | egl | HR: Vratnik pass (Bardy_ch18) | 750 | 14°59′9″, 44°58′43″ | 4x | h24 | HM370845, HM370716, HM370587 |
| 19 | cha | A: Mt. Hochschwab (Bardy_ch19) | 1510 | 15°04′37″, 47°35′19″ | 4x | h33 | HM370846, HM370717, HM370588 |
| 20 | egl | HR: Žuta Lokva (Bardy_ch20) | 440 | 15°06′41″, 44°57′08″ | 4x | h25 | HM370847, HM370718, HM370589 |
| 21o | egl | HR: Baške Oštarije (Frajman and Schönwetter 12063) | 1150 | 15°10′40″, 44°30′20″ | 4x | h52 | HM370848, HM370719, HM370590 |
| 22 | egl | HR: Ogulin (Bardy_ch22) | 330 | 15°11′12″, 45°16′21″ | 4x | h24 | HM370849, HM370720, HM370591 |
| 23 | — | HR: Plitvička jezera lakes (no voucher) | 700 | 15°36′43″, 44°52′29″ | 4x | h24 | HM370850, HM370721, HM370592 |
| 24 | egl | HR: Zagreb (Bardy_ch24) | 230 | 15°41′09″, 45°46′42″ | 4x | h55 | HM370851, HM370722, HM370593 |
| 25 | mic | A: Mt. Schneeberg (Bardy_ch25) | 1600 | 15°49′58″, 47°45′14″ | 2x | h30 | HM370852, HM370723, HM370594 |
| 26 | cha | A: Mt. Schneeberg (Bardy_ch26) | 1700 | 15°50′19″, 47°45′27″ | 4x | h24 | HM370853, HM370724, HM370595 |
| 27o | egl | HR: Gračac (Bardy_ch27) | 560 | 15°52′08″, 44°17′48″ | 4x | h24 | HM370854, HM370725, HM370596 |
| 28 | vin | A: Mödling (Bardy_ch28) | 300 | 16°16′40″, 48°04′49″ | 2x | h38 | HM370855, HM370726, HM370597 |
| 29 | egl | HR: Križevci (Bardy_ch29) | 130 | 16°27′30″, 45°58′38″ | 4x | h23 | HM370856, HM370727, HM370598 |
| 30 | vin | BiH: Mt. Troglav (Bardy_ch30) | 16°36′06″, 43°56′07″ | 2x | h39 | HM370857, HM370728, HM370599 | |
| 31 | vin | A: Mt. Hundsheimer Berg (Frajman and Schönwetter 11096) | 340 | 16°56′14″, 48°08′21″ | 2x | h55 | HM370858, HM370729, HM370600 |
| 32 | vin | A: Mt. Hundsheimer Berg (Englisch_ch32) | 450 | 16°56′20″, 48°07′57″ | 2x | h43 | HM370859, HM370730, HM370601 |
| 33 | egl | HR: Bilo Gora (Bardy_ch33) | 210 | 17°14′19″, 45°52′36″ | 4x | h23 | HM370860, HM370731, HM370602 |
| 34 | — | H: Nemesvita (Bardy_ch34) | 190 | 17°21′56″, 46°49′25″ | 4x | h33 | HM370861, HM370732, HM370603 |
| 35 | egl | H: Balatonboglar (Bardy_ch35) | 140 | 17°30′02″, 46°34′16″ | 4x | h34 | HM370862, HM370733, HM370604 |
| 36 | egl | BiH: Mt. Čvrsnica (Surina and Modrić_ch36) | 1530 | 17°37′36″, 43°39′06″ | 4x | h54 | HM370863, HM370734, HM370605 |
| 37 | vin | H: Ajka (Bardy_ch37) | 420 | 17°42′30″, 47°02′45″ | 2x | h43 | HM370864, HM370735, HM370606 |
| 38 | vin | H: Ajka (Bardy_ch38) | 420 | 17°42′30″, 47°02′45″ | 2x | h39 | HM370865, HM370736, HM370607 |
| 39 | vin | H: Ajka (Bardy_ch38) | 420 | 17°42′30″, 47°02′45″ | 4x | h55 | HM370866, HM370737, HM370608 |
| 40 | egl | H: Sokorópátka (Bardy_ch40) | 230 | 17°42′33″, 47°28′27″ | 4x | h55 | HM370867, HM370738, HM370609 |
| 41 | — | H: Pécs (Bardy_ch41) | 200 | 18°03′33″, 46°08′32″ | 4x | h56 | HM370868, HM370739, HM370610 |
| 42 | cha | HR: Slavonski Brod (Bardy_ch42) | 170 | 18°11′35″, 45°12′40″ | 4x | h31 | HM370869, HM370740, HM370611 |
| 43 | cha | HR: Slavonski Brod (Bardy_ch42) | 170 | 18°11′35″, 45°12′40″ | 2x | h55 | HM370870, HM370741, HM370612 |
| 44 | — | H: Dombóvár (Bardy_ch44) | 220 | 18°19′27″, 46°25′52″ | 4x | h33 | HM370871, HM370742, HM370613 |
| 45 | vin | HR: Vladislavci (Bardy_ch45) | 90 | 18°34′48″, 45°26′58″ | 2x | h37 | HM370872, HM370743, HM370614 |
| 46 | egl | HR: Vladislavci (Bardy_ch45) | 90 | 18°34′48″, 45°26′58″ | 4x | h33 | HM370873, HM370744, HM370615 |
| 47 | vin | BiH: Maglić (Bardy_ch47) | 1700 | 18°45′10″, 43°17′52″ | 2x | h29 | HM370874, HM370745, HM370616 |
| 48o | egl | SRB: Podromanija (Bardy_ch48) | 1070 | 18°53′19″, 44°01′27″ | 4x | h6 | HM370875, HM370746, HM370617 |
| 49o | egl | H: Baja (Bardy_ch49) | 140 | 18°53′20″, 46°11′41″ | 4x | h23 | HM370876, HM370747, HM370618 |
| 50 | vin | H: Esztergom (Bardy_ch50) | 110 | 18°57′18″, 47°47′32″ | 2x | h47 | HM370877, HM370748, HM370619 |
| 51 | — | PL: Andrychow (no voucher) | 348 | 19°20′15″, 49°50′45″ | 4x | h33 | HM370878, HM370749, HM370620 |
| 52 | vin | SRB: Kremna (Bardy_ch52) | 780 | 19°34′40″, 43°51′43″ | 2x | h52 | HM370879, HM370750, HM370621 |
| 53 | vin | SRB: Kremna (Bardy_ch52) | 780 | 19°34′40″, 43°51′43″ | 4x | h11 | HM370880, HM370751, HM370622 |
| 54 | vin | H: Gyöngyö (Bardy_ch54) | 390 | 19°57′44″, 47°50′19″ | 2x | h50 | HM370881, HM370752, HM370623 |
| 55 | — | SRB: Mt. Kapaonik (Bardy_ch55) | 1770 | 20°50′08″, 43°18′55″ | 4x | h5 | HM370882, HM370753, HM370624 |
| 56 | vin | GR: Smolikas (Frajman and Schönwetter 11638) | 1400 | 21°03′48″, 40°06′09″ | 2x | h66 | HM370883, HM370754, HM370625 |
| 57o | egl | H: Békéscsaba (Bardy_ch57) | 130 | 21°12′04″, 46°43′6″ | 4x | h32 | HM370884, HM370755, HM370626 |
| 58 | kru | MK: Nižepole—Orlove bari (Frajman and Schönwetter 11634) | 1800–2150 | 21°12′35″, 40°57′01″ | 2x | h60 | HM370885, HM370756, HM370627 |
| 59 | kru | MK: Kopanke (Frajman and Schönwetter 11636) | 1700–1890 | 21°12′45″, 41°01′43″ | 2x | h59 | HM370886, HM370757, HM370628 |
| 60 | coi | GR: Metsovo (Calvo et al. JC0881)a | 1618 | 21°13′39″, 39°47′32″ | 2x | h67 | HM370887, HM370758, HM370629 |
| 61 | egl | MK: Begovo pole (Frajman and Schönwetter 11698) | 1800–2200 | 21°24′50″, 41°43′16″ | 4x | h1 | HM370888, HM370759, HM370630 |
| 62 | coi | GR: Kato Kerasovo (Bardy_ch62) | 230 | 21°26′07″, 38°31′06″ | 2x | h76 | HM370889, HM370760, HM370631 |
| 63 | egl | SRB: Bojnik (Bardy_ch63) | 480 | 21°35′36″, 43°05′25″ | 2x | h70 | HM370890, HM370761, HM370632 |
| 64 | egl | MK: Pletvar (Frajman and Schönwetter 11682) | 1000 | 21°39′18″, 41°21′58″ | 2x | h64 | HM370891, HM370762, HM370633 |
| 65 | coi | GR: Hani Ponopoulou (Bardy_ch65) | 620 | 21°39′47″, 37°48′19″ | 2x | h77 | HM370892, HM370763, HM370634 |
| 66 | coi | GR: Vlachomandra (Bardy_ch66) | 200–230 | 21°42′06″, 38°27′05″ | 2x | h74 | HM370893, HM370764, HM370635 |
| 67 | coi | GR: Lampia (Bardy_ch67) | 860–880 | 21°48′45″, 37°51′47″ | 2x | h78 | HM370894, HM370765, HM370636 |
| 68o | egl | H: Debrecen (Bardy_ch68) | 130 | 21°52′02″, 47°45′02″ | 4x | h51 | HM370895, HM370766, HM370637 |
| 69o | egl | SRB: Mt. Rtanj (Frajman and Schönwetter 11374) | 1000–1560 | 21°53′30″, 43°46′30″ | 4x | h9 | HM370896, HM370767, HM370638 |
| 70 | coi | GR: Aghios Georgios (Bardy_ch70) | 630–640 | 21°55′24″, 38°56′10″ | 2x | h68 | HM370897, HM370768, HM370639 |
| 71 | egl | SRB: Dobra (Bardy_ch71) | 140 | 21°58′36″, 44°37′50″ | 4x | h45 | HM370898, HM370769, HM370640 |
| 72 | coi | GR: Moní Philosóphou (Bardy_ch72) | 680 | 22°02′35″, 37°33′30″ | 2x | h72 | HM370899, HM370770, HM370641 |
| 73 | egl | SRB: Mt. Suva planina (Bardy_ch73) | 940 | 22°05′24″, 43°13′45″ | 4x | h12 | HM370900, HM370771, HM370642 |
| 74 | coi | GR: Klitoria (Bardy_ch74) | 490 | 22°09′04″, 37°51′47″ | 2x | h79 | HM370901, HM370772, HM370643 |
| 75 | — | GR: Kalavrita (Aedo et al. MA760495)b | 1980 | 22°11′22″, 37°59′50″ | 4x | h65 | HM370902, HM370773, HM370644 |
| 76 | coi | GR: River Styx (Bardy_ch76) | 1250 | 22°13′42″, 37°59′41″ | 2x | h76 | HM370903, HM370774, HM370645 |
| 77 | coi | GR: Ostrakina (Bardy_ch77) | 1500 | 22°14′29″, 37°40′09″ | 4x | h62 | HM370904, HM370775, HM370646 |
| 78 | kru | GR: Ostrakina (Bardy_ch78) | 1350–1420 | 22°16′11″, 37°38′00″ | 4x | h62 | HM370905, HM370776, HM370647 |
| 79 | kru | GR: Ostrakina (Bardy_ch78) | 1350–1420 | 22°16′11″, 37°38′00″ | 2x | h61 | HM370906, HM370777, HM370648 |
| 80 | coi | GR: Poulani (Bardy_ch80) | 1110–1130 | 22°19′36″, 38°43′17″ | 2x | h76 | HM370907, HM370778, HM370649 |
| 81 | coi | GR: Amfissa (Bardy_ch81) | 870–890 | 22°20′39″, 38°31′54″ | 2x | h76 | HM370908, HM370779, HM370650 |
| 82 | coi | GR: Olimbos (Frajman and Schönwetter 11675) | 1200–1500 | 22°24′08″, 40°04′53″ | 2x | h63 | HM370909, HM370780, HM370651 |
| 83 | vin | GR: Litochoro (Frajman and Schönwetter 11660) | 580 | 22°28′44″, 40°06′54″ | 2x | h63 | HM370910, HM370781, HM370652 |
| 84 | coi | GR: Litochoro (Frajman and Schönwetter 11659) | 580 | 22°28′44″, 40°06′54″ | 2x | h67 | HM370911, HM370782, HM370653 |
| 85 | coi | GR: Litochoro (Frajman and Schönwetter 11658) | 370 | 22°29′28″, 40°06′49″ | 2x | h67 | HM370912, HM370783, HM370654 |
| 86 | vin | MK: Mt. Rujen (Frajman and Schönwetter 11708) | 1800–2252 | 22°30′55″, 42°09′29″ | 2x | h13 | HM370913, HM370784, HM370655 |
| 87 | vin | MK: Mt. Rujen (Frajman and Schönwetter 11708) | 1800–2252 | 22°30′55″, 42°09′29″ | 4x | h1 | HM370914, HM370785, HM370656 |
| 88o | egl | BG: Vidin vis Gradec (Bardy_ch88) | 50 | 22°33′57″, 43°59′51″ | 4x | h4 | HM370915, HM370786, HM370657 |
| 89 | coi | GR: Amfiklia (Bardy_ch89) | 280 | 22°36′41″, 38°40′07″ | 2x | h73 | HM370916, HM370787, HM370658 |
| 90 | coi | GR: Mt. Parnassos (Bardy_ch90) | 1400 | 22°37′11″, 38°30′18″ | 2x | h73 | HM370917, HM370788, HM370659 |
| 91 | coi | GR: Regini (Bardy_ch91) | 370 | 22°41′07″, 38°42′43″ | 2x | h73 | HM370918, HM370789, HM370660 |
| 92 | egl | BG: Belogradčik (Bardy_ch92) | 450 | 22°41′11″, 43°38′09″ | 4x | h2 | HM370919, HM370790, HM370661 |
| 93o | egl | BG: Vidin (Bardy_ch93) | 100 | 22°52′60″, 43°47′35″ | 4x | h1 | HM370920, HM370791, HM370662 |
| 94o | egl | BG: Montana (Bardy_ch94) | 325 | 22°53′40″, 43°27′33″ | 4x | h57 | HM370921, HM370792, HM370663 |
| 95 | vin | BG: Godec (Bardy_ch95) | 650 | 23°03′09″, 42°59′32″ | 2x | h36 | HM370922, HM370793, HM370664 |
| 96 | vin | BG: Godec (Bardy_ch95) | 650 | 23°03′09″, 42°59′32″ | 4x | h15 | HM370923, HM370794, HM370665 |
| 97 | vin | RO: Fildu de Jos (Bardy_ch97) | 350 | 23°04′55″, 46°55′31″ | 2x | h44 | HM370924, HM370795, HM370666 |
| 98o | egl | BG: Petrohan pass (Bardy_ch98) | 1320 | 23°07′20″, 43°07′15″ | 4x | h20 | HM370925, HM370796, HM370667 |
| 99 | vin | BG: Mt. Vitosha (Bardy_ch99) | 1650 | 23°17′00″, 42°34′00″ | 2x | h21 | HM370926, HM370797, HM370668 |
| 100o | egl | BG: Vraca (Bardy_ch100) | 250 | 23°20′52″, 43°16′14″ | 4x | h3 | HM370927, HM370798, HM370669 |
| 101o | egl | BG: Mt. Vihren (Bardy_ch101) | 2049 | 23°24′50″, 41°45′27″ | 4x | h58 | HM370928, HM370799, HM370670 |
| 102 | vin | BG: Bansko (Frajman and Schönwetter 11326) | 1900 | 23°24′59″, 41°45′36″ | 2x | h13 | HM370929, HM370800, HM370671 |
| 103o | egl | BG: Mt. Vihren (Sternburg_ch103) | 1900–1930 | 23°25′00″, 41°45′00″ | 4x | h14 | HM370930, HM370801, HM370672 |
| 104 | coi | GR: Manoudi (Bardy_ch104) | 110 | 23°28′37″, 38°46′12″ | 2x | h75 | HM370931, HM370802, HM370673 |
| 105o | egl | BG: Vraca (Bardy_ch105) | 600 | 23°45′39″, 43°12′31″ | 2x | h8 | HM370932, HM370803, HM370674 |
| 106o | egl | BG: Saranci (Frajman and Schönwetter 11275) | 760 | 23°52′56″, 42°42′47″ | 4x | h17 | HM370933, HM370804, HM370675 |
| 107 | vin | RO: Valea Lunga (Bardy_ch107) | 400 | 24°03′08″, 46°08′31″ | 2x | h49 | HM370934, HM370805, HM370676 |
| 108 | vin | BG: Batak (Bardy_ch108) | 1450 | 24°08′44″, 41°45′50″ | 2x | h22 | HM370935, HM370806, HM370677 |
| 109o | egl | BG: Batak (Bardy_ch108) | 1450 | 24°08′44″, 41°45′50″ | 4x | h1 | HM370936, HM370807, HM370678 |
| 110o | egl | RO: Sibiu (Bardy_ch110) | 550 | 24°12′24″, 45°48′36″ | 4x | h48 | HM370937, HM370808, HM370679 |
| 111o | egl | RO: Sibiu (Bardy_ch111) | 550 | 24°12′24″, 45°48′36″ | 4x | h48 | HM370938, HM370809, HM370680 |
| 112 | kru | BG: Peštera (Bardy_ch112) | 800 | 24°16′47″, 42°00′09″ | 2x | h41 | HM370939, HM370810, HM370681 |
| 113 | coi | BG: Peštera (Bardy_ch113) | 700 | 24°16′47″, 42°00′09″ | 2x | h69 | HM370940, HM370811, HM370682 |
| 114 | vin | BG: Trojanski pass (Bardy_ch114) | 1550 | 24°33′57″, 42°47′28″ | 2x | h39 | HM370941, HM370812, HM370683 |
| 115o | egl | BG: Trojanski pass (Bardy_ch115) | 1350 | 24°43′60″, 41°40′29″ | 4x | h1 | HM370942, HM370813, HM370684 |
| 116o | egl | BG: Loveč (Bardy_ch116) | 200 | 24°44′38″, 43°05′09″ | 4x | h18 | HM370943, HM370814, HM370685 |
| 117o | egl | BG: Sokelovtsi (Bardy_ch117) | 1570 | 24°46′25″, 41°41′45″ | 4x | h1 | HM370944, HM370815, HM370686 |
| 118o | egl | BG: Trojan (Bardy_ch118) | 500 | 24°47′01″, 42°54′34″ | 4x | h17 | HM370945, HM370816, HM370687 |
| 119 | kru | BG: Manastir Sveta Petka (Bardy_ch119) | 525 | 24°54′49″, 41°58′42″ | 2x | h42 | HM370946, HM370817, HM370688 |
| 120 | egl | BG: Manastir Sveta Petka (Bardy_ch120) | 525 | 24°54′49″, 41°58′42″ | 4x | h1 | HM370947, HM370818, HM370689 |
| 121 | — | BG: Kazanlăk (no voucher) | 450 | 25°10′33″, 42°33′39″ | 4x | h19 | HM370948, HM370819, HM370690 |
| 122o | egl | RO: Vatra Dornei (Bardy_ch122) | 875 | 25°13′41″, 47°19′52″ | 4x | h51 | HM370949, HM370820, HM370691 |
| 123o | egl | BG: Šipka pass (Bardy_ch123) | 1250 | 25°19′24″, 42°44′52″ | 4x | h16 | HM370950, HM370821, HM370692 |
| 124o | egl | BG: Veliko Tărnova (Bardy_ch124) | 350 | 25°27′52″, 43°00′25″ | 4x | h7 | HM370951, HM370822, HM370693 |
| 125 | — | RO: Braşov (Bardy_ch125) | 900–950 | 25°35′34″, 45°38′02″ | 2x | h46 | HM370952, HM370823, HM370694 |
| 126o | egl | RO: Gura Humorului (Bardy_ch126) | 500 | 25°56′05″, 47°32′06″ | 4x | h40 | HM370953, HM370824, HM370695 |
| 127 | — | BG: Tsarevo (Albach_2007_45) | 247 | 27°45′58″, 42°07′04″ | 4x | h10 | HM370954, HM370825, HM370696 |
| 128 | — | BG: Goritsa (Albach_2007_21) | 70 | 27°49′13″, 42°55′01″ | 4x | h10 | HM370955, HM370826, HM370697 |
| 129 | — | BG: Ahtopol (Albach_2007_52) | 50 | 27°57′15″, 42°04′15″ | 4x | h71 | HM370956, HM370827, HM370698 |
Voucher specimen deposited at the Universidad de Salamanca (SALA).
Voucher specimen deposited at the Real Jardin Botanico de Madrid (MA).
Population corresponds to V. orbelica.
A, Austria; BG, Bulgaria; BiH, Bosnia and Herzegovina; GR, Greece, H, Hungary; HR, Croatia; MK, FYR of Macedonia; RO, Romania; SLO, Slovenia; SRB, Serbia.
2.2. Flow cytometry
DNA ploidy levels were estimated for five individuals per population. Flow cytometry was conducted with silica gel dried material following the protocol of Baranyi and Greilhuber (1996) with propidium iodide staining using a CyFlow ML (Partec GmbH, Münster, Germany) equipped with a green laser (Cobolt Samba 532 nm, Cobolt AB, Solna, Sweden) and using Pisum sativum cultivar ‘Kleine Rheinländerin’ as internal standard.
2.3. Molecular methods
Total genomic DNA was extracted from silica gel dried tissue (ca. 10 mg) of one individual per population. In eight populations with mixed cytotypes, one individual per cytotype was analysed and will in the following be referred to with a separate population identifier: this concerns populations 38/39, 42/43, 45/46, 52/53, 78/79, 86/87, 95/96 and 108/109 (Fig. 1 and Table 1). Extraction followed the CTAB-protocol of Doyle and Doyle (1987) with a few modifications: after precipitation with isopropanol and subsequent centrifugation, the DNA pellet was washed in 70% ethanol, dried at 37 °C and re-suspended in TE-buffer. The quality of the extracted DNA was checked on 1% TAE-agarose gels.
Fig. 1.
Sampled populations of taxa of the Veronica chamaedrys group in southeastern Europe. Details of the collected populations are given in Table 1.
The AFLP procedure followed Vos et al. (1995) with the modifications described in Schönswetter et al. (2009). Initially, selective primers were screened using 23 primer combinations. The five final primer combinations for the selective PCR (fluorescent dye in brackets) were EcoRI (6-FAM)-ACT/MseI-CTA, EcoRI (VIC)-ACC/MseI-CAA, EcoRI (NED)-ACC/MseI-CTT, EcoRI (6-FAM)-ACT/MseI-CAT and EcoRI (VIC)-AGG/MseI-CTC. Five microlitres each of 6-FAM, NED and VIC labelled selective PCR products were combined and purified using Sephadex G-50 Fine (GE Healthcare Bio-Sciences, Uppsala, Sweden) applied to a Multi Screen-HV plate (Millipore, Molsheim, France). 1.2 μl of the elution product were mixed with 10 μl formamide (Applied Biosystems, Foster City, CA, USA) and 0.1 μl GeneScan 500 ROX (Applied Biosystems) and run on an ABI 3130x automated capillary sequencer (Applied Biosystems). Twenty individuals were replicated to calculate the error rate and to allow non-reproducible fragments to be excluded from the analysis. Raw AFLP data were aligned with the internal size standard using ABI Prism GENESCAN 3.7.1 (Applied Biosystems), and imported into GENOGRAPHER 1.6.0 (available at http://hordeum.oscs.montana.edu/genographer) for scoring. The error rate was calculated as the ratio of mismatches (scoring of 0 vs. 1) over matches (1 vs. 1) in AFLP profiles of replicated individuals (Bonin et al., 2004).
Three regions of the plastid genome, the trnH-psbA spacer (primers trnH-F, psbA-R; Tate and Simpson, 2003), the rps16-trnK spacer (primers rps16x2F2, trnK(UUU)x1; Shaw et al., 2007) and the rpl32-trnL spacer (primers rpl32-F, trnL (UAG); Shaw et al., 2007), were sequenced from one individual per population. PCR conditions for all three regions were 5 min at 95 °C followed by 30 cycles of 1 min at 95 °C, 1 min at 50 °C and 3 min at 65 °C, followed by 7 min at 65 °C. Reaction volumes of 24 μl included 8 μl REDTaq ReadyMix PCR Reaction Mix (Sigma–Aldrich, Vienna, Austria), 2 μl of each primer (10 μM), 8 μl of H2O and 4 μl of 1:10 diluted template DNA of unknown concentration. The PCR products were cleaned with Exonuclease I and Calf Intestine Alkaline Phosphatase (Fermentas, St. Leon-Rot, Germany) according to the manufacturer’s instructions. All reactions were carried out on a GeneAmp 9700 thermocycler (Applied Biosystems). BigDye Terminator chemistry (Applied Biosystems) was used according to the manufacturer’s instructions for cycle sequencing following electrophoresis on a 3130xl Genetic Analyzer capillary sequencer (Applied Biosystems). Sequences were edited with SEQMAN II 5.05 (DNAStar, Madison, WI, USA) and manually aligned using BIOEDIT 7.0.4.1 (Hall, 1999). All sequences were deposited in GenBank (Table 1). Sequences from the three plastid regions were concatenated based on the assumption that the plastid forms a single linkage group.
2.4. Data analyses
Genetic structure of AFLP data was inferred using the population mixture analysis implemented in BAPS 5.2 (Bayesian Analysis of Populations Structure; (Corander et al., 2003); http://www.abo.fi./fak/mnf/mate/jc/software/baps.html). This program, which can handle dominant markers like AFLPs under the module “clustering with linked loci” (Corander and Tang, 2007), treats both the frequencies of the markers and the number of genetically divergent groups as random variables. Stochastic optimisation is used to infer the mode of the posterior distribution. As our data set included diploid as well as tetraploids individuals, the following strategy was adopted. A mixture analysis of the diploid individuals only was conducted with the maximal number of groups (K) set to 2–10. Each run was replicated 10 times and the results were averaged according to the resultant likelihood scores. Results of the mixture analysis of diploid individuals (excluding two individuals which could not be unambiguously assigned to a single gene pool, see Section 3) were used to define “training” clusters for subsequent assignment of tetraploid individuals (“admixture based on pre-defined populations”). Admixture coefficients were estimated using 500 iterations, and the significance of these coefficients was estimated by employing the simulation strategy described by Corander and Marttinen (2006) using 50 reference individuals and 10 iterations each. An Unweighted Pair Group Method with Arithmetic mean (UPGMA) tree was inferred based on Kullback–Leibler distances (Kullback and Leibler, 1951) among clusters as implemented in BAPS 5.2. A Neighbour-Joining (NJ) analysis based on a matrix of Nei–Li distances (Nei and Li, 1979) including 2000 pseudo-replicates was conducted with TREECON 1.3b (Van de Peer and De Wachter, 1997). Using the program SPLITSTREE4 version 4.6 (Huson and Bryant, 2006), a NeighbourNet was constructed based on the same distance matrix. A Principal Co-ordinate Analysis (PCoA) based on a matrix of Jaccard distances among individuals was conducted using NTSYS-PC 2.0 (Rohlf, 1998).
Phylogenetic analysis was conducted using the approach implemented in BEAST 1.4.8 (Drummond and Rambaut, 2007), as this allows taking into account the genealogical uncertainty due to the stochastic nature of the coalescence process. As inversions introduce substitutional changes which actually are due to a structural mutation, these have been inverted prior to all analyses. The best-fit substitution model was determined using the Akaike Information Criterion (AIC) as implemented in MODELTEST 3.6 (Posada and Crandall, 1998). As the set of models until the cumulative Akaike weight exceeded 0.95 included often non-nested models with at least two substitution rates, we finally used a GTR+Γ model subsuming the proportion of invariable sites in the gamma distribution and using Jeffrey’s priors for the substitution model parameters.
Since a strict clock model was rejected (Bayes factors <−13; calculated with Tracer 1.4 available from http://tree.bio.ed.ac.uk/software/tracer/), rate evolution was modelled in a relaxed clock framework using a lognormal distribution (Drummond et al., 2006) with uniform distributions for mean and standard deviation of 0–100 and 0–10, respectively. Due to the lack of external calibrations, we used a strong prior on the substitution rate, derived from previously published substitution rates for plastid regions (Yamane et al., 2003; Smith et al., 2008), and modelled it with a normal distribution with a mean of 4 × 10−3 substitutions per site per million years and a wide standard deviation of half the mean. After initial analyses, the root was constrained to a maximum age of 10 my. We used the Bayesian Skyline Plot (Drummond et al., 2005) as the most general demographic model, as it also allows fluctuations in population size to be detected. Using different group intervals (m = 3, 5, 10) gave very similar results (absolute Bayes factors <2.4), and only those with m = 3 are shown. Stationarity of the Markov chain, which was run for 3 × 107 generations with sampling every 1000th generation, was determined using TRACER 1.4. The first 10% of sampled generations was discarded as burn-in, after which all effective sample size (ESS) values were greater than 290. A second run was conducted to confirm convergence of the Markov chain on the stationary distribution. All parameter estimates were based on these two runs combined (54,000 sampling points). A chronogram was constructed from the majority rule consensus tree calculated using PAUP 4.0b10 (Swofford, 2001) with node heights being the median values of the age estimates determined with FIGTREE 1.2.3 (available from http://tree.bio.ed.ac.uk/).
A statistical parsimony haplotype network was constructed using TCS 1.21 (Clement et al., 2000). For this analysis, insertions/deletions longer than one base pair as well as inversions were re-coded as single step mutations, and then sequence gaps were treated as a fifth character state. Mononucleotide repeats of varying length were excluded, since these are prone to homoplasy at larger geographic scales (Ingvarsson et al., 2003).
In order to compare the within-group differentiation of the lineages identified by BEAST, we calculated π, the mean number of pairwise differences (Tajima, 1983) and its variance (Tajima, 1993) with Arlequin 3.11 (Excoffier et al., 2005). Population expansions of the four cpDNA lineages were tested using mismatch distribution with a unimodal distribution indicating population expansion (Rogers and Harpending, 1992). Agreement between the observed and the expected distribution under a sudden-expansion model was tested in ARLEQUIN 3.11 (Excoffier et al., 2005) via the sum of squared differences, which, if significant at p ⩽ 0.05, indicates deviation from the expansion model (Schneider and Excoffier, 1999). Significance was assessed via a non-parametric bootstrapping procedure with 10,000 replicates.
2.5. Morphometry
In 98 of the 129 sampled populations one or two flowering or fruiting individuals, whose phenological stage allowed scoring of more than half of the morphometric characters, were available for morphometric analysis. Five sampled locations (38/39, 42/43, 52/53, 78/79 and 86/87) included two cytotypes that could not be distinguished in the morphometric analysis and were subsequently treated as polymorphic in ploidy level. One qualitative and nineteen quantitative characters (including one ratio), including those deemed diagnostic for intraspecific or specific taxa in the V. chamaedrys group, were scored. Unless stated differently, length characters were measured in mm, and densities in number per mm2; leaf characters were scored on the leaf pair subtending the lowermost inflorescences while the stem indumentum was determined from the internodium below the lowermost inflorescences. Qualitative characters were scored once, whereas quantitative characters other than leaf dimensions were measured five times and averaged. Of quantitative characters that were highly correlated (p < 0.001) only the one with the fewest missing characters was retained for the final analysis, thus resulting in twelve quantitative characters (Table 2; data matrix in Appendix 3).
Table 2.
Morphological characters or ratios of characters employed in a morphometric analysis of 98 populations of the Veronica chamaedrys group from southeastern Europe.
| Abbreviation | Morphological character or ratio |
|---|---|
| KG | Calyx hairs glandular (1) or not (0) |
| KHD | Density of hairs on the calyx lobes |
| KHL | Length of hairs on the calyx lobes |
| LLW | Length/maximum width of lamina |
| LP | Length of the petiole |
| LS | Length of the style |
| LT | Number of teeth on one half of the lamina |
| LTB | Vertical distance from first tooth to the base of the lamina (negative if lamina base is rounded to cuneate, positive if base is cordate) |
| LTLW | Length/width of lamina tooth at maximum width line |
| LUBD | Density of hairs on the upper side of the lamina next to the apex of the lamina |
| LUBL | Length of hairs on the upper side of the lamina next to the apex of the lamina |
| SRD | Density of hairs between the two opposite lines of hairs on the stem |
| SRL | Length of hairs on the two opposite lines of hairs on the stem |
The seven excluded characters were (1) the length of the stem that was correlated with the number of teeth on the lamina (LT), (2) the length of the petiole one leaf pair above and (3) one leaf pair below the leaf pair subtending the lowermost inflorescence, both correlated with the length of the petiole subtending the lowermost inflorescence (LP), (4) the length and (5) density of hairs on the upper side of the lamina next to the base of the lamina and (6) the length and (7) density of hairs on the lower side of the lamina next to the apex of the lamina, which were correlated with the length (LUBL) and density (LUBD) of hairs on the upper side of the lamina next to the apex of the lamina, respectively.
Of the remaining twelve quantitative characters (the qualitative character was treated separately) a matrix of pairwise Gower distances (S15; Legendre and Legendre, 1998) was calculated with R Package 3.0 (Legendre and Vaudor, 1991) and served as basis for a Principal Co-ordinate Analysis (PCoA) using NTSYS-PC 2.0 (modules DCENTER and EIGEN; Rohlf, 1998). To check for correspondence of the morphological characters with the first two principle co-ordinates, a test for association between paired samples using Spearman’s rho was carried out.
Additionally, a linear discriminant analysis of the twelve quantitative characters was carried out using R 2.7.2 (R Development Core Team, 2008). As pre-defined groups we used the Eastern, Western and Southern Groups as inferred from AFLP analyses; individuals pertaining to the Krumovii Group (see Section 3) were excluded. Missing values were replaced by the average values of the respective group (Eastern Group, 6%; Western Group, 7%; Southern Group, 1%). In order to ascertain characters differentiating the Southern Group from the remaining samples, we conducted a second discriminant analysis with these two groups only. The results of the coefficients that contributed most to the linear discriminator were virtually identical with the coefficients of the first discriminator resulting from the analysis with three groups and are thus not shown.
To test for significant morphological differentiation between the Southern Group and the remaining samples (see Section 3), t-tests were undertaken and subsequently boxplots produced for all characters studied. The latter analyses were conducted with R 2.7.2 (R Development Core Team, 2008).
3. Results
3.1. Flow cytometry
Flow cytometry analyses yielded histograms with mean CVs of G1 peaks of the sample and internal standard of 9.1% and 4.2%, respectively. DNA ploidy levels (Suda et al., 2006) inferred from measured genome sizes revealed that DNA diploids (for simplicity in the following referred to as “diploids”) and DNA tetraploids (referred to as “tetraploids”) were present throughout most of the study area (Fig. 2). However, tetraploids were more frequent in the North of the distribution area, resulting in a significant association of cytotypes with latitude (Spearman’s rho = 0.337, p < 0.001). In contrast, no altitudinal separation could be detected (rho = −0.029, p = 0.756).
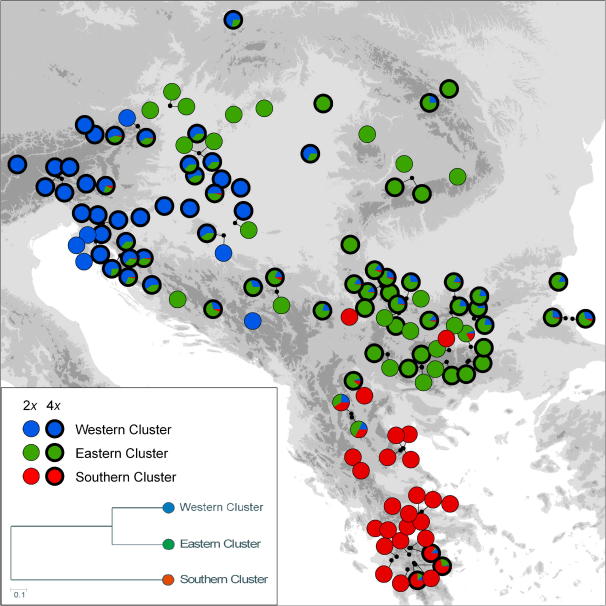
Fig. 2.
Distribution of ploidy level (diploid, thin outline; tetraploid, thick outline) and of three genetic clusters derived from BAPS analysis of AFLP markers in 129 populations of the Veronica chamaedrys group from southeastern Europe. The insert shows a UPGMA tree of Kullback–Leibler distances among clusters. Population identifiers are given in Fig. 1.
3.2. AFLPs
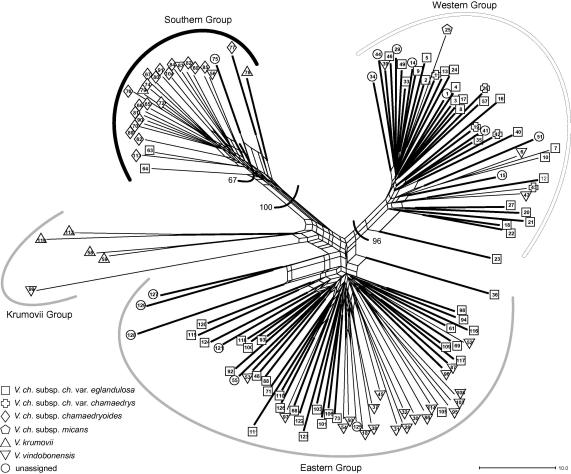
We scored 468 AFLP fragments ranging from 101 to 498 base pairs. The error rate amounted to 1.6% and was thus well within the range deemed acceptable by Bonin et al. (2004). Mixture analysis including only diploid individuals identified three clusters (data not shown). Subsequent admixture analysis assigned most individuals to a single cluster; only two individuals (from populations 58 to 59) were admixed and were excluded from the “training clusters”. The subsequent admixture analysis including diploid as well as tetraploid individuals (Fig. 2) identified three clusters, hereinafter called Southern, Western and Eastern Cluster, each containing both diploid and tetraploid individuals. Allele distributions of the Western and the Eastern Cluster were more similar to each other than they were to the Southern Cluster (insert in Fig. 2). Admixture was encountered among all clusters and mostly concerned tetraploid individuals, albeit not all tetraploids were admixed, while only three diploid individuals were admixed. The three clusters were geographically somewhat separated: The Western Cluster was most prominent in the northwest, the Eastern Cluster in the east and the Southern Cluster in the south, but especially Western and Eastern Clusters were widely geographically overlapping (Fig. 2). In the NeighbourNet (Fig. 3), two groups (Southern and Western Group) corresponding to two clusters resolved by the admixture analysis were separated along strongly weighted splits. The remaining populations fell into the weakly differentiated Eastern and Krumovii Group, the latter being positioned between the Southern and the Eastern Group. All groups comprised more than one taxonomic entity, and V. vindobonensis was found in all four groups. In the Neighbour-Joining analysis (not shown) both the Southern and the Western Group received high bootstrap support (100 BS and 96 BS, respectively). Neither the Krumovii nor the Eastern Group had bootstrap support >50% in agreement with the lack of strongly weighted splits.
Fig. 3.
NeighbourNet diagram of AFLP data constructed for 129 populations of the Veronica chamaedrys group from southeastern Europe. Splits with weight <0.5 have been omitted to aid legibility. Numbers along branches are bootstrap values above 50% derived from a Neighbour-Joining analysis and are given for major branches only. Bold branches mark tetraploid individuals.
3.3. cpDNA sequencing
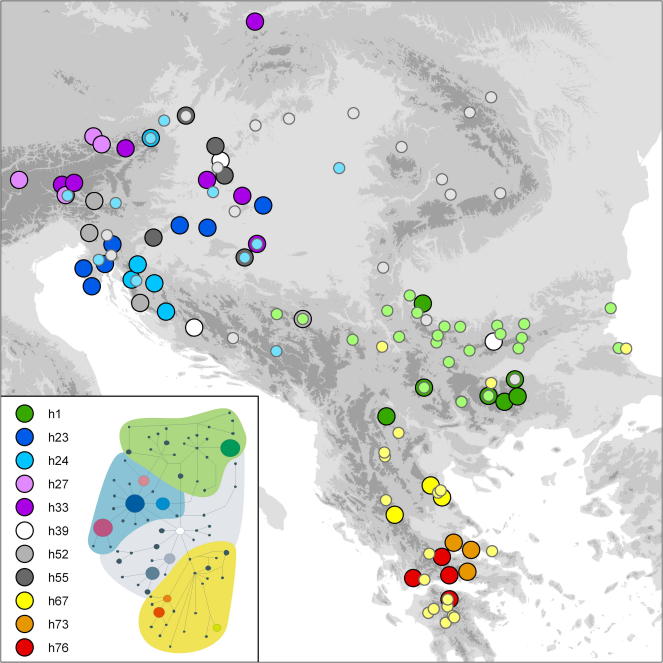
The trnH-psbA sequences were 295–304 bp, the rps16-trnK sequences 701–709 bp and the rpl32-trnL sequences 843–867 bp long; the length of the concatenated alignment was 1978 bp. Concatenating the sequences yielded 79 haplotypes (Table 1). Phylogenetic analysis using BEAST (Fig. 4) revealed three major clades, from here on referred to as Southern, Widespread and Eastern + Western Lineage, and haplotype h71 found in a tetraploid individual. The oldest differentiation among the lineages (age given as median and its 95% highest posterior density interval) occurred 0.81 (0.22–3.01) mya. The Southern and the Widespread Lineage (posterior probabilities of 0.82 and 0.97, respectively) diversified nearly simultaneously 0.40 (0.10–1.55) and 0.37 (0.10–1.36) mya, respectively. The Southern Lineage (h59–h70, h72–h79) was distributed on the southern Balkan Peninsula (Greece and northerly adjacent areas; Fig. 5). The Widespread Lineage (h39–h58) extended from the southeastern Alps (Austria, Slovenia) to the Bulgarian Stara Planina and was the sole lineage in the Carpathians (Romania). A deep phylogenetic split was evident in the Eastern + Western Lineage (posterior probability 0.84). The Eastern and the Western Lineages, which separated 0.47 (0.14–1.72) mya, started to diversify at 0.33 (0.09–1.20) and 0.29 (0.07–1.05) mya, respectively. The Western Lineage (h23–h38) extended from the southeastern Alps (Austria, Slovenia) to the northern Dinaric Mountains (Croatia, Bosnia and Herzegovina) and the eastern Pannonian Plain (Hungary), the Eastern Lineage (h1–h22) from western Serbia to Bulgaria. Both lineages geographically overlapped with the Widespread Lineage. Rapid population expansion for all lineages was inferred with the Bayesian Skyline Analysis (Fig. 4) to have occurred about 0.05 mya.
Fig. 4.
Plastid DNA haplotype diversity encountered in 129 populations of the Veronica chamaedrys group from southeastern Europe. (Left) Simplified majority rule consensus tree from relaxed clock Bayesian analysis with the software BEAST. Node heights correspond to median ages (see text for details); nodes with age estimates younger than 0.04 my are not shown and are thus not distinguishable from unresolved lineages. Numbers along branches are Bayesian posterior probabilities; identical haplotypes or unresolved polytomies are collapsed as triangles, their vertical extension being proportional to the number of individuals. The bar depicts the ploidy level of a lineage (light grey, diploid; dark grey, tetraploid). The insert at the bottom shows the Bayesian Skyline Plot (median and 95% high posterior density limits). (Right) Statistical parsimony network of plastid DNA haplotypes. Haplotypes that were not sampled are shown with small open circles. Outline shading corresponds to the three main AFLP groups presented in Fig. 3; individuals of the Krumovii Group are marked with an asterisk. Di- and tetraploids have thin and thick outlines, respectively. The four main lineages identified by Bayesian analysis (at the left) are indicated.
Fig. 5.
Geographical distribution of plastid DNA haplotypes encountered in 129 populations of the Veronica chamaedrys group from southeastern Europe. In the insert, the most frequently sampled haplotypes (>2 occurrences) are listed, and their position is indicated in the statistical parsimony network onto which the four main lineages identified by BEAST are overlaid (green, Eastern Lineage; blue, Western Lineage; grey, Widespread Lineage; yellow, Southern Lineage). The map shows the geographic distribution of the eleven most frequent haplotypes (big dots) as well as the rarer ones (small dots, coloured as the overlay in the insert).
In contrast to the clearly divergent Southern Lineage, differentiation among the Widespread, Eastern and Western Lineages identified by BEAST was only weakly reflected in the parsimony network (Fig. 4). Relatively few haplotypes were frequent and many were found in a single population only. The most frequent haplotypes were found in three separate regions (Fig. 5): (i) central to southern Greece (h67, h73 and h76), (ii) Bulgaria and westerly adjacent areas (h1), and (iii) northern Croatia to eastern Austria and southern Poland (h23, h24, h27, h33 and h55); the only exception of this pattern were the haplotypes h39 and h52 from the Widespread Lineage, which occurred in the West as well as in the East. Most haplotypes corresponding to the Western Lineage were closely related and maximally three mutational steps apart. Haplotype lineages were only partly congruent with AFLP-derived clusters (BAPS) or groups (NeighbourNet). The Southern Lineage formed a concise group in the network and comprised all individuals from the Southern Group as well as two haplotypes corresponding to the Krumovii Group (h59 and h60) and haplotype h71, which belongs to the Eastern Group. With the exception of haplotypes h36–h38 sampled in populations pertaining to the Eastern Group, the Western Lineage comprised mainly haplotypes of the Western Group, albeit not all. The Eastern Lineage included exclusively haplotypes which occurred in the Eastern Group, whereas the Widespread Lineage contained mostly haplotypes of the Eastern Group plus four sampled in the Western Group (h52, h53, h55 and h56). Haplotypes sampled in individuals from the Krumovii Group were spread over the network and were partly internal to those corresponding to the Southern Lineage and partly related to those of the Eastern as well as the Widespread Lineages (Fig. 4).
The mean number of pairwise differences (π) calculated for sets of haplotypes of the Western, Eastern, Widespread and Southern Lineages amounted to 2.13 ± 1.21 (mean ± variance), 4.06 ± 2.08, 4.85 ± 2.45 and 6.34 ± 3.09, respectively. The mismatch distributions showed a unimodal distribution only for the Eastern and Western Lineages, the distribution of the Widespread Lineage was close to unimodal, and that of the Southern Lineage was multimodal; only the Eastern distribution was significant (p = 0.02).
3.4. Morphometry
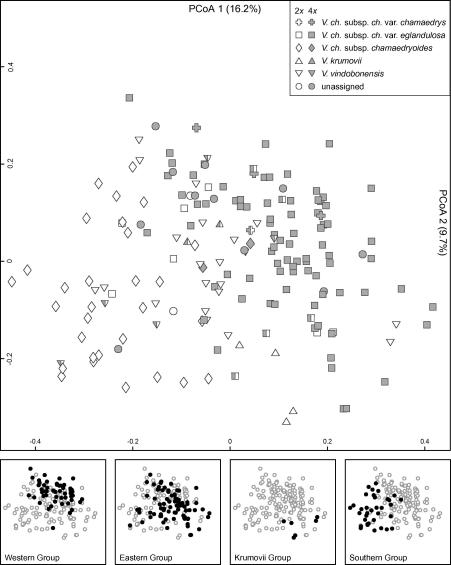
The PCoA of the morphometric data (Fig. 6) showed a weak segregation of di- and tetraploids along the first factor (16.8%; second factor: 10.3%), but failed to reflect the taxonomic grouping. There was, however, a weak clinal separation mainly along the first factor with V. chamaedrys subsp. chamaedryoides and subsp. chamaedrys var. eglandulosa being most distant and subsp. chamaedrys var. chamaedrys as well as V. vindobonensis and V. krumovii being intermediate. The first factor was positively correlated with different length characters (e.g., length of hairs on different organs (LUBL; SRL; KHL)), the distance between the first tooth and the lamina base (LTB), the length of the style (LS) and the number of teeth on the lamina (LT), but negatively correlated with the densities of hairs (KHD; LUBD; SRD), the length-to-width ratio of the tooth at the broadest part of the lamina (LTLW) or the length of the petiole (LP). The second factor showed positive correlation with the length of the petiole (LP) and with the length-to-width ratio of the lamina (LLW), but was negatively correlated with the density of hairs on different organs (KHD; LUBD), with the length-to-width ratio of the tooth at the broadest part of the lamina (LTLW), the length of the style (LS), as well as the number of teeth on the lamina (LT) (Appendix 4). Differentiation along the first axis was also detected when the only qualitative character, i.e., presence of glandular hairs on the calyx (KG), was plotted (plot not shown); importantly, all individuals of subsp. chamaedryoides had a glandular hairy calyx. When displaying the genetic groups obtained from the NeighbourNet of the AFLP data onto the morphometric plot, individuals of the Southern, Western and Krumovii Groups were separated but the Eastern Group blurred the separation (Fig. 6).
Fig. 6.
Principal Co-ordinate Analysis of a matrix of pairwise Gower distances based on 12 morphological characters scored for 187 individuals from 98 populations of the Veronica chamaedrys group from southeastern Europe. Symbols of the upper graph correspond to taxa as used in Fig. 1; diploid individuals are white, tetraploids are shaded in grey. Sampling sites with both cytotypes are marked both grey and white. The graphs at the bottom show the correspondence with the four AFLP groups as indicated in Fig. 3.
The linear discriminant analysis (Appendix 5) showed a similar pattern as the PCoA, but separation of the groups was more pronounced. The proportion of among-group variance explained by the first linear discriminator was 67%, that of the second linear discriminator 33%. The first discriminator separated the Southern Group from the Western and Eastern Group, which showed considerable overlap along the second discriminator. The coefficients that contributed most to the first discriminator were the length of hairs on the two opposite lines of hairs (SRL, 2.00) and the ratio length to maximum width of lamina (LLW, 1.35) as well as the ratio length to width of lamina tooth at maximum width (LTLW, −1.02). The coefficients that contributed most to the second discriminator were the length of hairs on the calyx lobes (KHL, 2.41), the length of hairs on the upper side of the lamina next to the apex of the lamina (LUBL, −1.19), the length of the petiole (LP, 0.84) and the ratio length to maximum width of lamina (LLW, 0.69).
Significant differences between the Southern Group and the remaining samples (excluding the Krumovii Group, for details, see Section 4) overlapped only partly with the strength of the coefficients contributing to the first discriminator (details in Appendix 6). Altogether, individuals of the Southern Group had denser but shorter hairs and the teeth at the margin of the lamina were longer and narrower than in the Western and the Eastern Groups.
4. Discussion
4.1. Distribution of cytotypes within the V. chamaedrys group in southeastern Europe
Di- and tetraploid cytotypes within the V. chamaedrys group occurred all over southeastern Europe, as has been assumed previously (Mirek and Fischer, 1986). There was, however, a significant association with latitude in the study area as diploids were predominant in the south, whereas in the north both di- and tetraploids occurred (Fig. 2). Previous studies revealed similar patterns of cytotype distribution across Europe. Autotetraploids of Rorippa amphibia (Luttikhuizen et al., 2007), for example, occur in northern Europe, whereas diploids grow in Central and Western Europe. A similar pattern was also found in Plantago media in which diploids had small fragmented distributions in southern and southeastern Europe but tetraploids were more widespread and continuously distributed over the continent (Van Dijk and Bakx-Schotman, 1997).
The cytotypes of the V. chamaedrys group did neither show altitudinal segregation (Table 1), as observed in Lotus corniculatus s.l. (Gauthier et al., 1998) or Taraxacum sect. Ruderalia (Calame and Felber, 2000), nor was there obvious habitat differentiation between the cytotypes, as has been shown for Anthoxanthum alpinum (Felber-Girard et al., 1996), Claytonia virginica (Lewis and Suda, 1976), Dactylis glomerata (Lumaret et al., 1987) or Senecio carniolicus (Hülber et al., 2009). Our results are in concert with studies on Solidago altissima, where autopolyploids co-exist with diploids in a broad zone of overlap (Halverson et al., 2008), or Ranunculus adoneus with no ecological differentiation between di- and tetraploids (Baack and Stanton, 2005).
Tetraploid individuals of the V. chamaedrys group were mainly of autopolyploid origin and evolved several times independently within each genetic group. This is supported by the unambiguous assignment of tetraploid individuals to clusters including also diploids (Fig. 2), the NeighbourNet diagram uniting di- and tetraploid individuals with heavily weighted splits (Fig. 3), as well as the co-occurrence of di- and tetraploids in plastid lineages (Figs. 4 and 5). Our results are thus adding to an ample body of evidence that multiple origins of polyploids are rather the rule than the exception (e.g., Doyle et al., 1990; Soltis et al., 2003; Albach, 2007). Tetraploids mainly evolved within various northern genetic lineages, and only a few originated within the southern refugium (Figs. 2–4). Although the exact causes remain elusive, we speculate that the more stable conditions on the southern Balkans (Tzedakis et al., 2002) may have prevented the successful establishment of polyploids. More pronounced climatic oscillations on the northern Balkan Peninsula and adjacent areas in the north, in contrast, triggered massive range shifts of forest vegetation that also concerned the V. chamaedrys group and were probably a driving force allowing tetraploid establishment.
Whereas our data strongly support prevalence of autopolyploidy in the V. chamaedrys group in southeastern Europe, they are inconclusive with respect to the role of allopolyploidy. The admixed state of many tetraploid individuals in the Bayesian clustering analysis (Fig. 2) may indicate that they originated by crossings between divergent diploid lineages. Alternatively, these crosses may as well have occurred at the tetraploid level, in line with the close geographical proximity of non-admixed tetraploid populations. In any event it needs to be pointed out that the NeighbourNet, which is a method of choice for uncovering reticulate relationships (Huson and Bryant, 2006), only identified two tetraploid individuals (populations 23 and 36; Fig. 3) as being intermediate between the Western and Eastern Groups.
4.2. Spatio-temporal evolution of the V. chamaedrys group
Slowly mutating, maternally inherited (Zhang et al., 2003) plastid DNA sequence data (Figs. 4 and 5) and rapidly evolving, nearly entirely nuclear-derived (Bussell et al., 2005) AFLP marker (Figs. 2 and 3) do not yield congruent patterns of spatio-temporal evolution of the V. chamaedrys group, most probably because both marker systems trace differentiation processes taking place at different time horizons. Integrating information obtained from both marker systems, however, allows reconstructing a detailed scenario from the earliest diversification in the mid-Pleistocene over the relatively constant diversification during the last 0.4 my (Fig. 4) to the allopatric differentiation accompanying isolation in refugia during the Last Glacial Maximum and postglacial secondary contact leading to admixture of gene pools (Fig. 2).
The oldest traceable phylogeographic pattern in the V. chamaedrys group is the simultaneous differentiation of three plastid DNA lineages in the mid-Pleistocene (Fig. 4). A fourth lineage is constituted by haplotype h71 sampled in population 129 from the Bulgarian Black Sea coast. Since this sample is not differentiated in the AFLP data set (Figs. 2 and 3) and our sampling from the southeastern-most Balkan Peninsula is very scarce, we here only point out the possibility of a divergent plastid lineage probably distributed along the southern coast of the Black Sea. Reconstructing the palaeo-distribution of the three main lineages is straightforward for only one of them. The Southern Lineage is restricted to the south of the Balkan Peninsula (Fig. 5) except for two isolated occurrences in southern Serbia (population 63) and central Bulgaria (population 113). In contrast to the other lineages, the multimodal mismatch distribution indicates distributional stasis and lack of population expansion. The distinctness of the genetic entity from the southern Balkans including its two northern outliers is corroborated by AFLP data (Figs. 2 and 3), and the nearly perfect congruence between the data sets with contrasting inheritance strongly suggests that hybridisation with other entities was at least rare. It remains to be tested whether the observed integrity has only historical reasons or if also ecological or crossing reasons are involved. The mountain ranges of northern Greece were glaciated (e.g., Hughes et al., 2007) and probably acted as strong barriers, contributing to the genetic isolation of the Southern Lineage/Group. Accordingly, the southern-most Balkan Peninsula is well-known as one of the centres of endemicity in Europe (Tan et al., 2001).
The distributions of the Widespread and Eastern + Western Lineages (Fig. 4) overlap throughout their entire distribution area from the Alps to the Rhodope Mountains and both lineages sometimes co-occur in immediate vicinity (e.g., populations 52 and 53; Fig. 5). Our data do not allow discriminating if both lineages either co-occurred in sympatry (“ancient polymorphism”) after their split or if they regained secondary contact after an initial allopatric phase. The Carpathians, however, were obviously never reached by the Western + Eastern Lineage and were colonised by the Widespread Lineage in the course of the first diversification event at about 0.4 my (Fig. 4), as evidenced by the presence of micro-lineages that have their most internal haplotypes in the Carpathians (h44–h46 and h48–h51; Figs. 4 and 5).
The Eastern + Western Lineage was disrupted into the vicariant Western and Eastern Lineages at about 0.47 my (Fig. 4). The two entities occur from the Alps and the Western Hungarian Plains to the northern Dinaric Mountains and from the Stara Planina to the Rhodope Mountains, respectively (Fig. 5). Diversification within the two lineages started roughly simultaneously at about 0.29 and 0.33 my ago (Fig. 4). Longitudinal vicariance is also seen in the AFLP data (Figs. 2 and 3). The Eastern Group is distributed from the Hungarian Plain and the Carpathians to the Stara Planina and the Rhodope Mountains. It comprises the entire Eastern Lineage and most haplotypes of the Widespread Lineage (Fig. 5). In contrast to the other main AFLP groups, the Eastern Group lacks strongly weighted splits and bootstrap support (Fig. 3). It is unclear if the lack of divergence is a consequence of the relatively recent contact of two formerly differentiated genetic entities corresponding to the Widespread and Eastern plastid Lineages.
The northwestern genetic entities encountered in the V. chamaedrys group, the Western Lineage and the Western Group, are roughly congruent and occur from the Alps and the Hungarian Plain to the Dinaric Mountains. The Western Lineage possesses a set of closely related haplotypes some of which were sampled frequently (Figs. 4 and 5), and thus exhibits the lowest value of pairwise differences, amounting to only one third of that of the Southern Lineage. All this suggests either a comparatively young age or a stronger bottleneck within the contemporary distribution range. The Western AFLP group comprises mostly tetraploid individuals (Fig. 2); diploids were scattered throughout the range but were most frequent in Istria.
Allopatric differentiation during the Last Glacial Maximum presumably shaped the pattern seen in the AFLP data (Figs. 2 and 3). It is mainly governed by three refugia in the southern, northwestern and eastern parts of southeastern Europe whose existence was already previously proposed for associated tree species based on macrofossil charcoal, pollen evidence and phylogeographical studies. (1) The southern Balkan Peninsula (Pindus mountain range and central Greece) provided buffered climatic oscillations (Tzedakis et al., 2002) and refugia of tree and shrub species such as beech and hornbeam were hypothesised (Grivet and Petit, 2003; Magri et al., 2006), overlapping with the Southern Cluster/Group. (2) On the eastern Balkan Peninsula (Eastwood, 2004) and the Carpathians (Willis and van Andel, 2004), refugia for beech, hornbeam and ash were proposed (Grivet and Petit, 2003; Heuertz et al., 2006; Magri et al., 2006) within the distribution area of the Eastern Cluster/Group. (3) Refugia for beech and ash (Heuertz et al., 2006; Magri et al., 2006) have been suggested on the northwestern Balkan Peninsula and adjacent areas, namely Istria, the Pannonian Basin and the valley of River Danube in Austria (Willis and van Andel, 2004), roughly congruent with the extent of the Western Group/Cluster. Generally, the forest refugia were either separated by glaciated mountain massifs as the climatic snow line was about 1000 m lower than today (e.g., Bognar and Prugovečki, 1997; Reuther et al., 2007), or by Artemisia-steppes prevailing in the lowlands (Willis, 1994).
Triggered by most probably postglacial climatic amelioration, the V. chamaedrys group underwent massive range expansions as suggested by the Bayesian Skyline Plot (Fig. 4) for ca. 0.05 mya. As substitution rates derived from phylogenetic studies with usually deeper time coverage (older than 1–2 my) will often be gross underestimates of more recent substitution rates (Ho et al., 2005) the obtained age estimate will be biased towards older ages; thus the rapid expansion may have occurred after the Last Glacial Maximum. Subsequently, as suggested by the admixture analysis (Fig. 2) but not by the NeighbourNet diagram (Fig. 3), secondary contact between Western and Eastern Groups partially broke down previously existing genetic differentiation by hybridisation involving frequent independent polyploidization (Fig. 2).
4.3. Taxonomic considerations
Our data show that current taxonomy does not reflect genetic lineages (Figs. 2–5). Furthermore, there is no tight association between taxa and ploidy level, as previously suggested, but diploids and polyploids are found in all recognised taxa as well as in all major genetic lineages (Fig. 4). Morphometric analysis does not recover current taxonomic entities, either (Fig. 6). Therefore, our data suggest changes to the currently used taxonomic framework of the V. chamaedrys group. The only entity that is congruently identified by different data types and thus merits taxonomic recognition is V. ch. subsp. chamaedryoides from the southern Balkan Peninsula. It is mainly (but not exclusively, see Fig. 3) diploid and its genetic distinctness was confirmed by both the AFLP and the cpDNA data sets (Figs. 2, 3, 5). Although subsp. chamaedryoides is only weakly separated in the PCoA plot of the morphometric data (Fig. 6), the Southern Group is relatively well separated from Eastern and Western Groups in the discriminant analysis (Appendix 5). Using only slightly overlapping characters (Appendix 6), subsp. chamaedryoides can thus be separated from the other taxa. Specifically, indumentum characters as well as the shape of the teeth and the lamina can be used to distinguish subsp. chamaedryoides (Appendix 6). The identifiability of this taxon is evidenced by the fact that about 80% of the voucher specimens have been correctly identified in the initial phase of our study.
The two widespread taxa V. ch. subsp. chamaedrys and V. vindobonensis both comprise diploid and tetraploid cytotypes (Fig. 3) and could neither be differentiated by molecular data (Figs. 2–5) nor by PCoA of morphometric data (Fig. 6)—even in the discriminant analysis Eastern and Western Groups overlap strongly (Appendix 5). Additionally, in contrast to the northwestern distribution edge of V. vindobonensis in eastern Austria (Fischer et al., 2008), the vegetation of the sampling sites did not reveal obvious habitat differentiation between these entities and both entities were found from wet to dry habitats, in open forests or meadows (K. Bardy, personal observations). Although the AFLP data (Fig. 3) suggested two genetic entities, these can neither be paralleled with morphometric data nor are they congruent with a priori determinations of voucher specimens (Figs. 3 and 6). Although we cannot entirely exclude the possibility that morphological differential characters might still be found, we favour to treat these two lineages as informal phylogeographical groups within V. ch. subsp. chamaedrys, which thus becomes a morphologically and cytologically variable taxon comprising diploids as well as mainly autotetraploid derivatives.
Although V. orbelica was described as diploid (Peev, 1972), we found only tetraploid individuals morphologically resembling this taxon. As continuous variation of characters connects V. orbelica (Peev, 1972) and V. ch. subsp. chamaedrys var. eglandulosa (Mirek and Fischer, 1986; see Appendix 1), and it is not genetically divergent, either, V. orbelica should be included in subsp. chamaedrys var. eglandulosa. Similarly, genetic indistinctness and the lack of morphometric differentiation of V. ch. subsp. micans suggest merging this taxon with subsp. chamaedrys.
Veronica krumovii morphologically resembles V. ch. subsp. chamaedrys (Fig. 6) but differs by glandular hairs of varying length scattered over the whole plant. According to the AFLP data (Fig. 3), the majority of populations appears to be intermediate between the Southern and the Eastern Group, and may actually be hybrids between subsp. chamaedrys and chamaedryoides as circumscribed here. Plants morphologically belonging to V. krumovii also occur disjunctly in southern Greece (Fig. 1), suggesting that the erratically occurring, but conspicuous diagnostic character is under simple genetic control and consequently of no taxonomic value.
Acknowledgments
Financial support by the Austrian Science Fund (project P 18598-B03 to M.A. Fischer) is gratefully acknowledged. We thank the following colleagues for helping with collecting: H. Bardy; M. Bardy-Durchhalter; D. Dimitrova; I. Djukic; T. Englisch; S. Ertl; B. Frajman; B. Friedmann; M. Martinez Ortega; A. Müllner; A. Stachurska-Swakon; M. Staudinger; M. Sternburg and B. Surina. Eva M. Temsch helped with the flow cytometry, Verena Klejna was an indispensable help in the lab, and Andreas Berger performed morphometric measurements. We thank M. Martinez Ortega and an anonymous reviewer for their comments, which helped improving the paper.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ympev.2010.06.025.
Appendix A. Supplementary data
References
- Albach D.C. Evolution of Veronica (Plantaginaceae) on the Balkan Peninsula. Phytol. Balcan. 2006;12:231–244. [Google Scholar]
- Albach D.C. Amplified fragment length polymorphisms and sequence data in the phylogenetic analysis of polyploids: multiple origins of Veronica cymbalaria (Plantaginaceae) New Phytol. 2007;176:481–498. doi: 10.1111/j.1469-8137.2007.02172.x. [DOI] [PubMed] [Google Scholar]
- Albach D.C., Martínez Ortega M.M., Fischer M.A., Chase M.W. Evolution of Veroniceae: a phylogenetic perspective. Ann. Mo. Bot. Gard. 2004;91:275–302. [Google Scholar]
- Albach D.C., Martínez Ortega M.M., Delgado L., Weiss-Schneeweiss H., Özgökce F., Fischer M.A. Chromosome numbers in Veroniceae (Plantaginaceae): review and several new counts. Ann. Mo. Bot. Gard. 2008;45:543–566. [Google Scholar]
- Albach D.C., von Sternburg M., Scalone R., Bardy K.E. Phylogenetic analysis and differentiation of Veronica subgenus Stenocarpon in the Balkan Peninsula. Bot. J. Linn. Soc. 2009;159:616–636. [Google Scholar]
- Baack E.J., Stanton M.L. Ecological factors influencing tetraploid speciation in snow buttercups (Ranunculus adoneus): niche differentiation and tetraploid establishment. Evolution. 2005;59:1936–1944. [PubMed] [Google Scholar]
- Baranyi M., Greilhuber J. Flow cytometric and Feulgen densitometric analysis of genome size variation in Pisum. Theor. Appl. Genet. 1996;92:297–307. doi: 10.1007/BF00223672. [DOI] [PubMed] [Google Scholar]
- Bognar A., Prugovečki I. Glaciation traces in the area of Risnjak Mountain Massif. Geol. Croat. 1997;50:269–278. [Google Scholar]
- Bonin A., Bellemain E., Eidesen P.B., Pompanon F., Brochmann C., Taberlet P. How to track and assess genotyping errors in population genetic studies. Mol. Ecol. 2004;13:3261–3273. doi: 10.1111/j.1365-294X.2004.02346.x. [DOI] [PubMed] [Google Scholar]
- Boutin C., Harper J.L. A comparative study of the population dynamics of five species of Veronica in natural habitats. J. Ecol. 1991;79:199–221. [Google Scholar]
- Bussell J.D., Waycott M., Chappill J.A. Arbitrarily amplified DNA markers as characters for phylogenetic inference. Perspect. Plant Ecol. Evol. Syst. 2005;7:3–26. [Google Scholar]
- Calame F.G., Felber F. Distribution of diploid sexual and triploid apomictic dandelions (Taraxacum sect. Ruderalia) along two altitudinal gradients in Switzerland. Bot. Helv. 2000;110:109–114. [Google Scholar]
- Clement M., Posada D., Crandall K.A. TCS: a computer program to estimate gene genealogies. Mol. Ecol. 2000;9:1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Comes H.P., Kadereit J.W. The effect of quaternary climatic changes on plant distribution and evolution. Trends Plant Sci. 1998;3:432–438. [Google Scholar]
- Corander J., Marttinen P. Bayesian identification of admixture events using multilocus molecular markers. Mol. Ecol. 2006;15:2833–2843. doi: 10.1111/j.1365-294X.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- Corander J., Tang J. Bayesian analysis of population structure based on linked molecular information. Math. Biosci. 2007;2005:19–31. doi: 10.1016/j.mbs.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Corander J., Waldmann P., Sillanpää M.J. Bayesian analysis of genetic differentiation between populations. Genetics. 2003;163:367–374. doi: 10.1093/genetics/163.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale M.P., Causton D.R. The ecophysiology of Veronica chamaedrys, V. montana and V. officinalis: I. Light quality and light quantity. J. Ecol. 1992;80:483–492. [Google Scholar]
- Dale M.P., Causton D.R. The ecophysiology of Veronica chamaedrys, V. montana and V. officinalis: II. The interaction of irradiance and water regime. J. Ecol. 1992;80:493–504. [Google Scholar]
- Dale M.P., Causton D.R. The ecophysiology of Veronica chamaedrys, V. montana and V. officinalis: III. Effects of shading on the phenology of biomass allocations—a field experiment. J. Ecol. 1992;80:505–515. [Google Scholar]
- Dale M.P., Causton D.R. The ecophysiology of Veronica chamaedrys, V. montana and V. officinalis: IV. Effects of shading on nutrient allocations—a field experiment. J. Ecol. 1992;80:517–526. [Google Scholar]
- Doyle J.J., Doyle J.L. A rapid DNA isolation procedure for small amounts of fresh leaf tissue. Phytochem. Bull. 1987;19:11–15. [Google Scholar]
- Doyle J.J., Doyle J.L., Brown A.H., Grace J.P. Multiple origins of polyploids in the Glycine tabacina complex inferred from chloroplast DNA polymorphism. Proc. Natl. Acad. Sci. USA. 1990;87:714–717. doi: 10.1073/pnas.87.2.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A.J., Rambaut A., Shapiro B., Pybus O.G. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 2005;22:1185–1192. doi: 10.1093/molbev/msi103. [DOI] [PubMed] [Google Scholar]
- Drummond A.J., Ho S.Y.W., Phillips M.J., Rambaut A. Relaxed phylogenetics and dating with confidence. PLoS Biol. 2006;4:699–710. doi: 10.1371/journal.pbio.0040088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumolin-Lapègue S., Demesure B., Fineschi S., Le Corre V., Petit R.J. Phylogeographic structure of white oaks throughout the European continent. Genetics. 1997;146:1475–1487. doi: 10.1093/genetics/146.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood W.J. East mediterranean vegetation and climate change. In: Griffiths H.I., Kryštufek B., editors. Balkan Biodiversity—Pattern and Process in the European Hotspots. Kluwer Academic Publishers; Dordrecht: 2004. pp. 24–48. [Google Scholar]
- Excoffier L., Laval G., Schneider S. Arlequin ver. 3.0: an integrated software package for population genetics data analysis. Evol. Bioinf. Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Felber-Girard M., Felber F., Buttler A. Habitat differentiation in a narrow hybrid zone between diploid and tetraploid Anthoxanthum alpinum. New Phytol. 1996;133:531–540. [Google Scholar]
- Fischer M. Zur Cytotaxonomie von Veronica chamaedrys L.: I. Subsp. vindobonensis M.FISCHER, eine neue diploide Sippe. Oesterr. Bot. Z. 1970;118:206–215. [Google Scholar]
- Fischer M. Notizen zur Systematik, Chromosomenzahl und Verbreitung einiger Veronica-Sippen in Kärnten. Carinthia II. 1973;163:379–388. [Google Scholar]
- Fischer M.A. Zur Cytotaxonomie von Veronica chamaedrys L. agg.: II. Subsp. micans M. [A.] Fischer, subsp. nova, eine weitere diploide Sippe. Oesterr. Bot. Z. 1973;121:73–79. [Google Scholar]
- Fischer M. Veronica vindobonensis M.FISCHER (Zur Cytotaxonomie von Veronica chamaedrys agg., III) Oesterr. Bot. Z. 1974;122:287–292. [Google Scholar]
- Fischer M.A. Veronica. In: Strid A., Tan K., editors. Mountain Flora of Greece 2. Edinburgh University Press; Edinburgh: 1991. pp. 209–234. [Google Scholar]
- Fischer M.A., Oswald K., Adler W. Biologiezentrum der Oberösterreichischen Landesmuseen; Linz: 2008. Exkursionsflora für Österreich, Liechtenstein und Südtirol. [Google Scholar]
- Frajman B., Oxelman B. Reticulate phylogenetics and phytogeographic structure of Heliosperma (Silenae, Caryophyllaceae) inferred from chloroplast and nuclear DNA sequences. Mol. Phylogenet. Evol. 2007;43:140–155. doi: 10.1016/j.ympev.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Gauthier P., Lumaret R., Bédécarrats A. Genetic variation and gene flow in Alpine diploid and tetraploid populations of Lotus (L. alpinus (D.C.) Schleicher/L. corniculatus L.): 1. Insights from morphological and allozyme markers. Heredity. 1998;80:683–693. [Google Scholar]
- Gómez A., Lunt D.H. Refugia within refugia: patterns of phylogeographic concordance in the Iberian Peninsula. In: Weiss S., editor. Phylogeography of Southern European Refugia. Springer; Dordrecht: 2007. pp. 155–188. [Google Scholar]
- Gömöry D., Paule L., Brus R., Zhelev P., Tomović Z., Gračan J. Genetic differentiation and phylogeny of beech on the Balkan Peninsula. J. Evol. Biol. 1999;12:746–754. [Google Scholar]
- Goyder D.J. Pollination ecology of five species in a limestone community. Watsonia. 1983;14:397–405. [Google Scholar]
- Grivet D., Petit R.J. Chloroplast DNA phylogeography of the hornbeam in Europe: evidence for a bottleneck at the outset of postglacial colonization. Conserv. Genet. 2003;4:47–56. [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999;41:95–98. [Google Scholar]
- Halverson K., Heard S.B., Nason J.D., Stireman J.O. Origins, distribution, and local co-occurence of polyploid cytotypes in Solidago altissima (Asteraceae) Am. J. Bot. 2008;95:50–58. doi: 10.3732/ajb.95.1.50. [DOI] [PubMed] [Google Scholar]
- Hampe A., Arroyo J., Jordano P., Petit R.J. Rangewide phylogeography of a bird-dispersed Eurasian shrub: contrasting Mediterranean and temperate glacial refugia. Mol. Ecol. 2003;12:3415–3426. doi: 10.1046/j.1365-294x.2003.02006.x. [DOI] [PubMed] [Google Scholar]
- Heuertz M., Carnevale S., Fineschi S., Sebastiani F., Hausman J.F., Paule L., Vendramin G.G. Chloroplast DNA phylogeography of European ashes, Fraxinus sp. (Oleaceae): roles of hybridization and life history traits. Mol. Ecol. 2006;15:2131–2140. doi: 10.1111/j.1365-294X.2006.02897.x. [DOI] [PubMed] [Google Scholar]
- Hewitt G.M. The genetic legacy of the quaternary ice ages. Nature. 2000;405:907–913. doi: 10.1038/35016000. [DOI] [PubMed] [Google Scholar]
- Ho S.Y.W., Phillips M.J., Cooper A., Drummond A.J. Time dependency of molecular rate estimates and systematic overestimation of recent divergence times. Mol. Biol. Evol. 2005;22:1561–1568. doi: 10.1093/molbev/msi145. [DOI] [PubMed] [Google Scholar]
- Horvat I., Glavač V., Ellenberg H. Gustav Fischer Verlag; Stuttgart: 1974. Vegetation Südosteuropas. [Google Scholar]
- Hughes P.D., Woodward J.C., Gibbard P.L. Middle Pleistocene cold stage climates in the Mediterranean: new evidence from the glacial record. Earth Planet. Sci. Lett. 2007;253:50–56. [Google Scholar]
- Hülber K., Sonnleitner M., Flatscher R., Berger A., Dobrovsky R., Niessner S., Nigl T., Schneeweiss G.M., Kubešová M., Rauchová J., Suda J., Schönswetter P. Ecological segregation drives fine-scale cytotype distribution of Senecio carniolicus in the Eastern Alps. Preslia. 2009;81:309–319. [PMC free article] [PubMed] [Google Scholar]
- Husband B.C., Sabara H.A. Reproductive isolation between autotetraploids and their diploid progenitors in fireweed, Chamerion angustifolium (Onagraceae) New Phytol. 2004;161:703–713. doi: 10.1046/j.1469-8137.2004.00998.x. [DOI] [PubMed] [Google Scholar]
- Huson D.H., Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Ingvarsson P.K., Ribstein S., Taylor D.R. Molecular evolution of insertions and deletion in the chloroplast genome of Silene. Mol. Biol. Evol. 2003;20:1737–1740. doi: 10.1093/molbev/msg163. [DOI] [PubMed] [Google Scholar]
- Kolář F., Štech M., Trávníček P., Rauchová J., Urfus T., Vít P., Kubešova M., Suda J. Towards resolving the Knautia arvensis agg. (Dipsacaceae) puzzle: primary and secondary contact zones and ploidy segregation at landscape and microgeographic scales. Ann. Bot. 2009;103:963–974. doi: 10.1093/aob/mcp016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kron P., Suda J., Husband B.C. Applications of flow cytometry to evolutionary and population biology. Ann. Rev. Ecol. Evol. Syst. 2007;38:847–876. [Google Scholar]
- Kryštufek B., Buzan E.V., Hutchinson W.F., Hänfling B. Phylogeography of the rare Balkan endemic Martino’s vole, Dinaromys bogdanovi, reveals strong differentiation within the western Balkan Peninsula. Mol. Ecol. 2007;16:1221–1232. doi: 10.1111/j.1365-294X.2007.03235.x. [DOI] [PubMed] [Google Scholar]
- Kullback S., Leibler R.A. On information and sufficiency. Ann. Math. Stat. 1951;22:79–86. [Google Scholar]
- Legendre P., Legendre L. Elsevier; Amsterdam: 1998. Numerical Ecology. [Google Scholar]
- Legendre, P., Vaudor, A., 1991. The R Package: Multidimensional Analysis, Spatial Analysis. Département de Sciences Biologiques, Université de Montréal.
- Levin D.A. Polyploidy and novelty in flowering plants. Am. Nat. 1983;122:1–25. [Google Scholar]
- Levin D.A. Oxford University Press; New York, USA: 2002. The Role of Chromosomal Change in Plant Evolution. [Google Scholar]
- Lewis W.H., Suda Y. Diploids and tetraploids from a single species population: temporal adaption. J. Hered. 1976;67:391–393. [Google Scholar]
- Lumaret R., Guillerm J.L., Delay J., Loutfi A.A.L., Izco J., Jay M. Polyploidy and habitat differentiation in Dactylis glomerata L. from Galicia (Spain) Oecologia. 1987;73:436–446. doi: 10.1007/BF00385262. [DOI] [PubMed] [Google Scholar]
- Luttikhuizen P.C., Stift M., Kuperus P., van Tienderen P.H. Genetic diversity in diploid vs. tetraploid Rorippa amphibia (Brassicaceae) Mol. Ecol. 2007;16:3544–3553. doi: 10.1111/j.1365-294X.2007.03411.x. [DOI] [PubMed] [Google Scholar]
- Magri D., Vendramin G.G., Comps B., Dupanloup I., Geburek T., Gömöry D., Latałowa M., Litt T., Paule L., Roure J.M., Tantau I., van der Knapp W.O., Petit R., de Beaulieu J.-L. A new scenario for the Quaternary history of European beech populations: palaeobotanical evidence and genetic consequences. New Phytol. 2006;171:199–221. doi: 10.1111/j.1469-8137.2006.01740.x. [DOI] [PubMed] [Google Scholar]
- Martínez Ortega M.M., Sánchez J.Á., Rico E. Veronica. In: Benedí C., Rico E., Güemes J., Herrero A., editors. vol. 13. Real Jardín Botánico, CSIC; Madrid: 2009. pp. 360–434. (Flora Iberica—Plantas vasulares de la Peninsula Iberica e Islas Baleares). [Google Scholar]
- Médail F., Diadema K. Glacial refugia influence plant diversity patterns in the Mediterranean Basin. J. Biogeogr. 2009;36:1333–1345. [Google Scholar]
- Meusel H., Jäger E.J. Gustav Fischer; Jena: 1992. Vergleichende Chorologie der zentraleuropäischen Flora. [Google Scholar]
- Milivojević M., Menković L., Ćalić J. Pleistocene glacial relief of the central part of Mt. Prokletije (Albanian Alps) Quat. Int. 2008;190:1112–1122. [Google Scholar]
- Mirek Z., Fischer M. Additions to the ecogeography of Veronica vindobonensis with special reference to Poland. Phyton. 1986;26:107–129. [Google Scholar]
- Nei M., Li W.-H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA. 1979;76:5269–5273. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S.P. The evolutionary consequences of polyploidy. Cell. 2007;131:452–462. doi: 10.1016/j.cell.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Otto S.P., Whitton J. Polyploid incidence and evolution. Ann. Rev. Genet. 2000;34:401–437. doi: 10.1146/annurev.genet.34.1.401. [DOI] [PubMed] [Google Scholar]
- Peev D. New taxa and ploidy levels of some Bulgarian Veronica species. Proc. Bulgarian Acad. Sci. 1972;25:811–814. [Google Scholar]
- Peev D. Velikdenče—Veronica L. In: Kožuharov S.I., Kuzmanov B.A., editors. vol. 10. Drinov; Sofia: 1995. pp. 142–189. (Flora na Republika Bălgarija). [Google Scholar]
- Petit C., Bretagnolle F., Felber F. Evolutionary consequences of diploid–polyploid hybrid zones in wild species. Trends Ecol. Evol. 1999;14:306–311. doi: 10.1016/s0169-5347(99)01608-0. [DOI] [PubMed] [Google Scholar]
- Petit R.J., Csaikl U.M., Bordács S., Burg K., Coart E., Cottrell J., Dam B.v., Deans J.D., Dumolin-Lapègue S., Fineschi S., Finkeldey R., Gillies A., Glaz I., Goicoechea P.G., Jensen J.S., König A.O., Lowe A.J., Madsen S.F., Mátyás G., Munro R.C., Olalde M., Pemonge M.-H., Popescu F., Slade D., Tabbener H., Taurchini D., Vries S.G.M.d., Ziegenhagen B., Kremer A. Chloroplast DNA variation in European white oaks phylogeography and patterns of diversity based on data from over 2600 populations. For. Ecol. Manage. 2002;156:5–26. [Google Scholar]
- Petit R.J., Aguinagalde I., Beaulieu J.-L.d., Bittkau C., Brewer S., Cheddadi R., Ennos R., Fineschi S., Grivet D., Lascoux M., Mohanty A., Müller-Starck G., Demesure-Musch B., Palmé A., Martín J.P., Rendell S., Vendramin G.G. Glacial refugia: hotspots but not melting pots of genetic diversity. Science. 2003;300:1563–1565. doi: 10.1126/science.1083264. [DOI] [PubMed] [Google Scholar]
- Podnar M., Mayer W., Tvrtković N. Mitochondrial phylogeography of the Dalmatian wall lizard, Podarcis melisellensis (Lacertidae) Org. Divers. Evol. 2004;4:307–317. [Google Scholar]
- Posada D., Crandall K. Modeltest: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- R Development Core Team, 2008. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna.
- Ramsey J., Schemske D.W. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annu. Rev. Ecol. Syst. 1998;29:467–501. [Google Scholar]
- Ramsey J., Schemske D.W. Neopolyploidy in flowering plants. Annu. Rev. Ecol. Syst. 2002;33:589–639. [Google Scholar]
- Reuther A.U., Urdea P., Geiger C., Ivy-Ochs S., Niller H.-P., Kubik P.W., Hein K. Late Pleistocene glacial chronology of the Pietrele Valley, Retezat Mountains, Southern Carpathians constrained by 10Be exposure ages and pedological investigations. Quat. Int. 2007;164–165:151–169. [Google Scholar]
- Riek R. Systematische und pflanzengeographische Untersuchungen in der Veronica-Sektion Chamaedrys Griseb. Rep. Spec. Nov. Regni Veg. Beih. 1935;79:1–68. [Google Scholar]
- Rogers A.R., Harpending H. Population growth makes waves in the distribution of pairwise genetic differences. Mol. Biol. Evol. 1992;9:552–569. doi: 10.1093/oxfordjournals.molbev.a040727. [DOI] [PubMed] [Google Scholar]
- Rohlf, F.J., 1998. NTSYS-pc. Numerical Taxonomy and Multivariate Analysis System, Version 2.0. Exeter Software, New York.
- Santucci F., Emerson B.C., Hewitt G.M. Mitochondrial DNA phylogeography of European hedgehogs. Mol. Ecol. 1998;7:1163–1172. doi: 10.1046/j.1365-294x.1998.00436.x. [DOI] [PubMed] [Google Scholar]
- Schmitt T., Habel J.C., Zimmermann M., Müller P. Genetic differentiation of the marbled white butterfly, Melanargia galathea, accounts for glacial distribution patterns and postglacial range expansion in southeastern Europe. Mol. Ecol. 2006;15:1889–1901. doi: 10.1111/j.1365-294X.2006.02900.x. [DOI] [PubMed] [Google Scholar]
- Schneider S., Excoffier L. Estimating of past demographic parameters from the distribution of pairwise differences when the mutation rates vary among sites: application to human mitochondrial DNA. Genetics. 1999;152:1079–1089. doi: 10.1093/genetics/152.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönswetter P., Solstad H., Escobar García P., Elven R. A combined molecular and morphological approach to the taxonomically intricate European mountain plant Papaver alpinum s.l. (Papaveraceae)—taxa or informal phylogeographical groups? Taxon. 2009;58:1326–1343. [Google Scholar]
- Shaw J., Lickey E.B., Schilling E.E., Small R.L. Comparison of whole chloroplast genome sequences to choose noncoding regions for phylogenetic studies in angiosperms: the tortoise and the hare III. Am. J. Bot. 2007;94:275–288. doi: 10.3732/ajb.94.3.275. [DOI] [PubMed] [Google Scholar]
- Smith C.I., Pellmyr O., Althoff D.M., Balcázar-Lara M., Leebens-Mack J., Segraves K.A. Pattern and timing of diversification in Yucca (Agavaceae): specialized pollination does not escalate rates of diversification. Proc. R. Soc. Lond. Ser. B. 2008;275:249–258. doi: 10.1098/rspb.2007.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis D.E., Soltis P.S., Tate J.A. Advances in the study of polyploidy since Plant speciation. New Phytol. 2003;161:173–191. [Google Scholar]
- Sotiropoulos K., Eleftherakos K., Džukić G., Kalezić M., Legakis A., Polymeni R. Phylogeny and biogeography of the alpine newt Mesotriton alpestris (Salamandridae, Caudata), inferred from mtDNA sequences. Mol. Phylogenet. Evol. 2007;45:211–226. doi: 10.1016/j.ympev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Stefanović S., Lakušić D., Kuzmina M., MeđEdović S., Tan K., Stevanović V. Molecular phylogeny of Edraianthus (Grassy Bells; Campanulaceae) based on non-coding plastid DNA sequences. Taxon. 2008;57:452–475. [Google Scholar]
- Stewart J.R., Lister A.M. Cryptic northern refugia and the origins of the modern biota. Trends Ecol. Evol. 2001;16:608–613. [Google Scholar]
- Strid A., Franzén R. Chromosome numbers in flowering plants from Greece (materials for the mountain Flora of Greece, 22) Willdenowia. 1984;13:329–333. [Google Scholar]
- Suda J., Malcova R., Abazid D., Banas M., Prochazka F., Sida O., Stech M. Cytotype distribution in Empetrum (Ericaceae) at various spatial scales in the Czech Republic. Folia Geobot. 2004;39:161–171. [Google Scholar]
- Suda J., Krahulcová A., Trávníček P., Krahulec F. Ploidy level versus DNA ploidy level: an appeal for consistent terminology. Taxon. 2006;55:447–450. [Google Scholar]
- Swofford, D.L., 2001. PAUP∗: Phylogenetic Analysis Using Parsimony (∗and Other Methods), Version 4.0b.10 for 32-Bit Microsoft Windows. Sinauer Associates, Sunderland, MA, USA.
- Taberlet P. Mitochondrial DNA polymorphism, phylogeography and conservation genetics of the brown bear Ursus arctos in Europe. Proc. R. Soc. Lond. Ser. B. 1994;255:195–200. doi: 10.1098/rspb.1994.0028. [DOI] [PubMed] [Google Scholar]
- Taberlet P., Fumagalli L., Wust-Saucy A.-G., Cosson J.-F. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 1998;7:453–464. doi: 10.1046/j.1365-294x.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- Tajima F. Evolutionary relationship of DNA sequences in finite populations. Genetics. 1983;105:437–460. doi: 10.1093/genetics/105.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F. Measurement of DNA polymorphism. In: Takahata N., Clark A.G., editors. Mechanisms of Molecular Evolution: Introduction to Molecular Paleopopulation Biology. Japan Scientific Societies Press/Sinauer Associates Inc.; Tokyo/Sunderland, MA: 1993. pp. 37–59. [Google Scholar]
- Tan K., Iatrou G., Bent J. Gad Publisher; Copenhagen: 2001. The Peloponnese. [Google Scholar]
- Tate J.A., Simpson B.B. Paraphyly of Tarasa (Malvaceae) and diverse origins of polyploid species. Syst. Bot. 2003;28:723–737. [Google Scholar]
- Trewick S.A., Morgan-Richards M., Russell S.J., Herderson S., Rumsey F.J., Pintér I., Barrett J.A., Gibby M., Vogel J.C. Polyploidy, phylogeography and Pleistocene refugia of the rockfern Asplenium ceterach: evidence from chloroplast DNA. Mol. Ecol. 2002;11:2003–2012. doi: 10.1046/j.1365-294x.2002.01583.x. [DOI] [PubMed] [Google Scholar]
- Turrill W.B. Oxford University Press; Oxford: 1929. The Plant Life of the Balkan Peninsula. [Google Scholar]
- Tzedakis P.C., Lawson I.T., Frogley M.R., Hewitt G.M., Preece R.C. Buffered tree population changes in a Quaternary refugium: evolutionary implications. Science. 2002;297:2044–2047. doi: 10.1126/science.1073083. [DOI] [PubMed] [Google Scholar]
- Ursenbacher S., Schweiger S., Tomovi L., Crnobrnja-Isailovi J., Fumagalli L., Mayer W. Molecular phylogeography of the nose-horned viper (Vipera ammodytes, Linnaeus (1758)): evidence for high genetic diversity and multiple refugia in the Balkan Peninsula. Mol. Phylogenet. Evol. 2008;46:1116–1128. doi: 10.1016/j.ympev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y., De Wachter R. Construction of evolutionary distance trees with TREECON for Windows: accounting for variation in nucleotide substitution rate among sites. Comput. Appl. Biosci. 1997;13:227–230. doi: 10.1093/bioinformatics/13.3.227. [DOI] [PubMed] [Google Scholar]
- Van Dijk P., Bakx-Schotman T. Chloroplast DNA phylogeography and cytotype geography in autopolyploid Plantago media. Mol. Ecol. 1997;6:345–352. [Google Scholar]
- Vos P., Hogers R., Bleeker M., Reijans M., van de Lee T., Hornes M., Frijeters A., Pot J., Peleman J., Kuiper M., Zabeau M. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters S.M., Webb D.A. Veronica L. In: Tutin T.G., Heywood V.H., Burges N.A., Moore D.M., Valentine D.H., Walters S.M., Webb D.A., editors. vol. 3. Cambridge University Press; Cambridge: 1972. pp. 242–251. (Flora Europaea). [Google Scholar]
- Wendel J.F. Genome evolution in polyploids. Plant Mol. Biol. 2000;42:225–249. [PubMed] [Google Scholar]
- Willis K.J. The vegetational history of the Balkans. Quat. Sci. Rev. 1994;13:769–788. [Google Scholar]
- Willis K.J., van Andel T.H. Trees or no trees? The environments of central and eastern Europe during the Last Glaciation. Quat. Sci. Rev. 2004;23:2369–2387. [Google Scholar]
- Willner W., Di Pietro R., Bergmeier E. Phytogeographical evidence for post-glacial dispersal limitation of European beech forest species. Ecography. 2009;32:1011–1018. [Google Scholar]
- Yamane K., Yasui Y., Ohnishi O. Intraspecific cpDNA variations of diploid and tetraploid perennial buckwheat, Fagopyrum cymosum (Polygonaceae) Am. J. Bot. 2003;90:339–346. doi: 10.3732/ajb.90.3.339. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Liu Y., Sodmergen Examination of the cytoplasmic DNA in male reproductive cells to determine the potential for cytoplasmic inheritance in 295 angiosperm species. Plant Cell Physiol. 2003;44:941–951. doi: 10.1093/pcp/pcg121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.