Abstract
TGF-β is one of the key cytokines controlling immune responses. Measuring TGF-β from culture supernatants in vitro is an important index of immune function. However, fetal bovine serum (FBS) contains a high level of latent TGF-β that often hampers measuring T cell-derived TGF-β in culture using FBS-supplemented medium. In this report, we generated anti-latency associated peptide (LAP) monoclonal antibodies which cross-react with bovine LAP, and developed a protocol to deplete TGF-β from FBS. This provides the ability to reliably quantify TGF-β in vitro without relying on serum-free media which do not support growth of murine T cells.
Keywords: TGF-β, LAP, fetal bovine serum, depletion, monoclonal antibody
1. Introduction
TGF-β is an important cytokine controlling immune responses. TGF-β suppresses Th1 cells, cytotoxic T cells, and other inflammatory reactions. TGF-β also affects naive T cell differentiation either into Foxp3+ Tregs or Th17 cells (Rubtsov and Rudensky, 2007). Thus, measuring production of TGF-β in T cell culture is a crucial method of describing immune status in vitro.
TGF-β is synthesized as a pro-TGF-β from. Pro-TGF-β is intracellularly processed by furin and forms latent TGF-β which is a non-covalently associated complex consisting of latency-associated peptide (LAP) and mature TGF-β (Miyazono, 1993). Latent TGF-β cannot bind TGF-β receptors, and an activation step is required for TGF-β to have biological activity. How T cell-produced TGF-β is activated is as yet unknown.
In general, murine T cell culture supernatants do not contain active TGF-β, which is measured without the acidification step by ELISA. Investigators usually measure “total” TGF-β after the acidification and neutralization steps on the assumption that total TGF-β reflects TGF-β activity, though this may not be true. Fetal bovine serum-supplemented medium is standardly used for murine T cell culture. 10% FBS-supplemented media contain 1,000 – 2,000 pg/ml of latent TGF-β (Danielpour, 1989, and our observation). T cell-derived TGF-β in culture supernatants is usually much less than FBS-derived TGF-β. Because of this, some investigators only use serum-free medium for TGF-β measurements to avoid contribution of FBS-derived TGF-β in the culture medium. Commercially available serum-free media, however, often do not support murine T cell growth in vitro. Thus, either subtraction of the background TGF-β level of FBS-supplemented medium or using a poorly growth-supporting serum-free medium, may make TGF-β measurements unreliable. If one would be able to deplete TGF-β from FBS, it would obviate this problem. As part of our investigations of TGF-β, we raised anti-human latency-associated peptide (LAP) mAbs, and selected clones which cross-reacted with bovine LAP. We found that by using these anti-LAP antibodies, the latent TGF-β in FBS was successfully reduced by 90%. We found that the FBS depleted of TGF-β by anti-LAP antibody supported the T cell response as well as normal FBS, and thus enabled the reliable measurement of TGF-β from T cells.
2. Materials and Methods
2.1 Preparation of anti-LAP monoclonal antibodies
Anti-LAP mAbs were raised as described (Oida and Weiner, in press). In brief, BALB/c mice were immunized with recombinant human LAP (R&D Systems, Minneapolis, MN) and boosted with TGFB1-transduced P3U1 cells. Lymph node cells were fused with P3U1 myeloma cells and anti-LAP hybridoma clones were selected. Among these anti-human LAP mAbs, clones cross-reacting with bovine LAP were selected by FACS staining of bovine TGFB1-transduced P3U1 cells. The Selected hybridoma (TW4-5A8, mouse IgG1,κ) cells were grown in CELLine culture flask (Integra Biosciences, Zizers Switzerland) in ADCF-Mab protein-free medium (Hyclone/Thermo, Rockford, IL) and the antibody was purified T-Gel Absorbent (Pierce/Thermo, Rockford, IL).
2.2 Immunological depletion of latent TGF-β from FBS
20 μg anti-LAP mAb TW4-5A8 was coupled to 200 μl of 1 mg/ml goat anti-mouse IgG BioMag Plus beads (Polysciences, Warrington, PA) in a 1.5 ml microcentrifuge tube according the manufacturer's protocol and the beads were washed with PBS for three times. 1 ml of heat-inactivated FBS (Hyclone) was added to the bead pellet and incubated with rotation at 4°C for 8 hrs. The beads were removed by magnetic separation (DynaMag-Spin, Invitrogen, Carlsbad, CA) and the depletion was repeated for two more times. The depletion volume can be scale-up to 10 times by using a 15 ml tube and a corresponding magnet separator. The final TGF-β-depleted FBS was filtered through 0.22 μm to completely remove the magnetic particles.
2.3 TGF-β ELISA
FBS and culture supernatants were used for TGF-β ELISA with or without acidification. FBS was first diluted tenfold with PBS. Acidification was done by adding 1/10 volume of 1N HCl, incubating at room temperature for 10 min, and neutralizing with 1/10 volume of 1N NaOH/0.1M Tris. The samples were then diluted twofold by adding 25 mM Tris buffered saline. TGF-β ELISA was performed using anti-TGF-β mAb 1D11 (ATCC, Manassas, VA) as a coating antibody and biotinylated chicken anti-TGF-β IgY (BAF240, R&D Systems) as a detection antibody. Recombinant human TGF-β (R&D Systems) was used as a standard (0 – 2,000 pg/ml).
2.4 T cell stimulation
Mouse CD4 T cells were separated from BALB/c spleen and lymph nodes using MACS CD4 Isolation Kit (Miltenyi, Bergisch Gladbach, Germany). CD4 T cells were stimulated with plate-bound anti-CD3 (145-2C11, BD Biosciences, San Diego, CA, 4 μg/ml) and soluble anti-CD28 (37.51, Biolegend, San Diego, CA, 2 μg/ml) in 10% normal FBS-IMDM, 10% TGF-β-depleted FBS-IMDM, or X-vivo15 serum-free medium (Lonza, Basel, Switzerland) at 5 × 105 cells/ml, and culture supernatants were collected at 48 hrs and 72 hrs.
3. Results and Discussion
3.1 Selection of anti-LAP monoclonal antibodies cross-reacting to bovine LAP
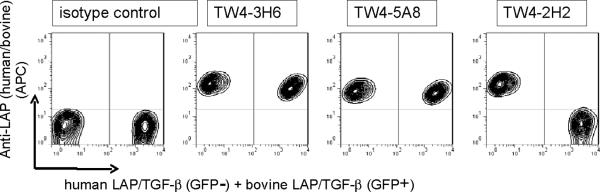
We have recently reported that TGFB1-transduced P3U1 cells express LAP on the cell surface (Oida and Weiner, 2010). We made anti-human LAP mAbs by immunizing mice with recombinant human LAP. To select clones cross-reacting bovine LAP, bovine TGFB1-transduced P3U1 containing IRES-GFP cells were made, and stained with our in-house anti-LAP mAbs. TW4-5A8 and TW4-3H6 stained bovine TGFB1-transduced P3U1 cells (Fig.1, GFP+ cells) as well as human TGFB1-transduced P3U1 cells (Fig1, GFP− cells), while other clones, such as TW4-2H2, stained only human TGFB1-transduced cells. Thus, we found anti-LAP mAbs which cross-react with bovine LAP. TW4-5A8 or TW4-3H6 do not stain mouse Tgfb1-transduced P3U1 cells (data not shown), indicating that these anti-LAP mAbs do not cross-react with mouse LAP.
Figure 1. Selection of anti-LAP clones that cross-react with bovine LAP.
P3U1-bovine TGF-β #4 cells (with IRES-GFP) mixed with P3U1-human TGF-β #32 cells (lacking IRES-GFP) were surface stained anti-human LAP mAbs. TW4-3H6 and TW4-5A8 stained both GFP+ cells and GFP− cells, while TW4-2H2 stained only GFP− cells.
3.2 Immunological depletion of latent TGF-beta from FBS
Fetal bovine serum contains approximately 10 – 20 ng/ml of total TGF-β by ELISA (Danielpour, 1989, and our observation) although it may vary depending on lots. Thus, a 10% FBS-medium usually contains 1,000 – 2,000 pg/ml of total TGF-β. Active TGF-β, which is measured without sample acidification, is virtually not detectable in FBS. Thus, TGF-β exists only as the latent form in FBS, and hence does not have biological activity. However, the amount of FBS-derived TGF-β is often high enough to interfere with measuring T cell-derived TGF-β.
To deplete latent TGF-β from FBS, anti-LAP mAb TW4-5A8 was coupled to goat anti-mouse IgG BioMag beads, and the beads were mixed with FBS. After 8 hr incubation at 4°C, the beads were magnetically removed, and TGF-β depleted FBS was recovered. The depletion process was repeated for a total of three times. We found that the initial total TGF-β amount in our FBS lot was 14.9 ng/ml, and it became 3.2 ng/ml after the first depletion, 2.1 ng/ml after the second depletion, and 1.5 ng/ml after the third depletion (Table 1). As shown in Table 1 the variability of TGF-β measurements was between 5–8%. Clone TW4-3H6 also depleted TGF-β from FBS as well as TW4-5A8 (data not shown). Remaining magnetic particles, if any, were completely removed by filtration through a 0.22 μm filter unit. Free anti-LAP mAb detached from the magnetic beads is expected to be minimal, and it should be noted that the anti-LAP mAb does not cross-react with mouse LAP and hence it does not interfere murine T cell culture even if it is released from the beads.
Table 1. Depletion of TGF-β from FBS.
TGF-β was immunologically depleted with anti-LAP TW4-5A8 for three rounds. Remaining TGF-β in FBS was measured by ELISA after acidification. S.D. from three independent depletion procedures is also shown.
| No depletion | 1st dep. | 2nd dep. | 3rd dep. | |
|---|---|---|---|---|
| TGF-β (pg/ml) | 14,920 +/− 1,150 | 3,170 +/− 150 | 2,100 +/− 80 | 1,520 +/− 80 |
3.3 Measuring T cell-derived TGF-β
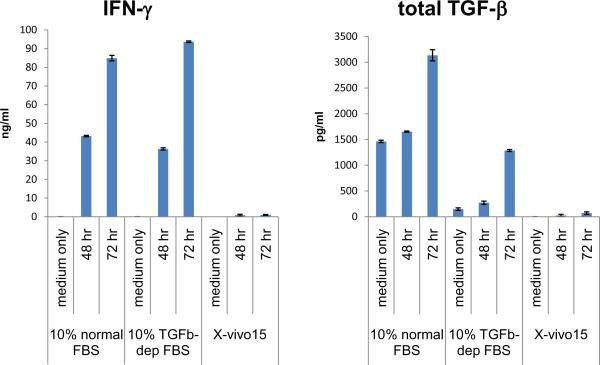
Mouse CD4+ T cells were purified and stimulated with plate-bound anti-CD3 and soluble anti-CD28 at 5 × 105 cells/ml for 48 and 72 hrs in the following media: 1) 10% normal FBS-IMDM, 2) 10% TGF-β-depleted FBS-IMDM, and 3) X-vivo15 serum-free medium. The culture supernatants were measured for TGF-β with/without acidification. IFN-γ was also measured as an indicator of cell activity. T cells cultured in 10% TGF-β-depleted FBS-IMDM showed a similar amount of IFN-γ production as in 10% normal FBS IMDM, indicating that the TGF-β depletion process does not affect overall T cell-supporting ability of FBS. T cells cultured in X-vivo15 produced much less IFN-γ than cultured in 10% FBS medium, suggesting that the serum-free medium does not support T cell growth well.
Active TGF-β was not detected (below 15 pg/ml) without acidification in any T cell culture supernatants or in 10% FBS-IMDM, and hence total TGF-β was measured after acidification.
A representative experiment is shown in Fig 2. 10% normal FBS-IMDM contained 1,462 pg/ml TGF-β. The T cell culture supernatant at 48 hrs grown in 10% normal FBS-IMDM showed 1,653 pg/ml TGF-β. Although the subtraction of the background indicates an increase of 191 pg/ml of TGF-β, one cannot rely on this number since the change is only a 13% increase from the base line. On the other hand, by using TGF-β-depleted FBS, The T cell culture supernatant at 48 hr contained 271 pg/ml TGF-β while 10% TGF-β-depleted FBS IMDM only showed 148 pg/ml TGF-β. The subtracted amount (123 pg/ml) is more reliable as the T cell-derived TGF-β amount.
Figure 2. TGF-β measurement from T cell cultures in different media.
Murine CD4+ T cells were stimulated with plate-bound anti-CD3 and soluble anti-CD28 for 48 and 72 hrs in 10% normal FBS-IMDM, in 10% TGF-β-depleted FBS-IMDM, or in X-vivo15 serum-free medium. Culture supernatants or the original media were measured for TGF-β and IFN-γ by ELISA.
At 72 hrs, The T cell culture supernatant grown in 10% normal FBS-IMDM showed 3,134 pg/ml and the background (1,462 pg/ml) subtraction calculated 1,672 pg/ml. T cell culture sup in 10% TGF-β-depleted FBS IMDM contained 1,286 pg/ml, and the background (148 pg/ml) subtraction became 1,138 pg/ml. Although T cell-derived TGF-β was observed even in 10% normal FBS-IMDM at this time point, the increase in 10% TGF-β-depleted FBS IMDM was more apparent and reliable than the amount in 10% normal FBS.
4. Conclusion
FBS-derived TGF-β makes it difficult to measure in vitro TGF-β production from murine T cells. Here we selected anti-LAP mAbs cross-reacting with bovine LAP from our in-house anti-human LAP mAbs, and we successfully depleted bovine latent TGF-β from FBS by the simple protocol. We did not find that commercially available anti-human LAP antibodies cross-reacted with bovine LAP (data not shown). Our use of anti-LAP antibodies to deplete TGF from FBS enables us to obtain more reliable TGF-β measurements from T cell cultures, especially at low cell number conditions and/or at early time points, without requiring serum-free media which does not support T cell growth well and thus does not provide sensitive biologic assessment of TGF-β.
Acknowledgments
This work is supported by grants from the National Institutes of Health Grants AI435801 and NS38037 (H. L. W.).
Abbreviations
- mAb
monoclonal antibody
- TGF-β
transforming growth factor-β1
- LAP
latency-associated peptide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors declare that they have no competing financial interests.
References
- 1.Danielpour D, Kim KY, Dart LL, Watanabe S, Roberts AB, Sporn MB. Sandwich enzyme-linked immunosorbent assays (SELISAs) quantitate and distinguish two forms of transforming growth factor-beta (TGF-β1 and TGF-β2) in complex biological fluids. Growth Factors. 1989;2:61–71. doi: 10.3109/08977198909069082. [DOI] [PubMed] [Google Scholar]
- 2.Miyazono K, Ichijo H, Heldin CH. Transforming growth factor-β: latent forms, binding proteins and receptors. Growth Factors. 1993;8:11–22. doi: 10.3109/08977199309029130. [DOI] [PubMed] [Google Scholar]
- 3.Oida T, Weiner HL. Overexpression of TGF-β1 gene induces cell surface localized, GRP78-associated LAP/TGF-β. J. Immunol. 2010 doi: 10.4049/jimmunol.0904121. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubtsov YP, Rudensky AY. TGFβ signalling in control of T-cell-mediated self-reactivity. Nat. Rev. Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]