Abstract
Purpose
Ketamine may decrease core-to-peripheral redistribution of heat through direct central sympathetic stimulation and inhibition of norepinephrine uptake into postganglionic sympathetic nerve endings. The purpose of this study was to evaluate the efficacy of epidural ketamine in preventing shivering during transurethral resection of the prostate (TURP) under epidural anesthesia.
Materials and Methods
Ninety-three male patients scheduled for TURP under epidural anesthesia were enrolled in this study. Patients were randomized into one of three groups. Group 1 consisted of 31 patients who received epidural 0.75% ropivacaine, group 2 consisted of 32 patients who received epidural ketamine (0.2 mg/kg) in addition to 0.75% ropivacaine, and group 3 consisted of 30 patients who received epidural ketamine (0.4 mg/kg) in addition to 0.75% ropivacaine. Shivering and side effects such as hypotension, bradycardia, nausea, and hallucination were recorded during the anesthesia and for 2 hours while in the postanesthetic recovery room.
Results
Shivering was statistically more frequent in group 1 than in the other groups. The incidence of sedation was significantly higher in group 3 than in the other groups. The incidences of side effects such as hypotension, bradycardia, and nausea were significantly higher in group 1 than in the other groups.
Conclusions
In this study, epidural ketamine 0.2 mg/kg and 0.4 mg/kg was shown to have a lower incidence of shivering and other side effects except sedation. In patients who undergo TURP under epidural anesthesia, the prophylactic use of low-dose epidural ketamine would be helpful in preventing any adverse effects, including shivering.
Keywords: Epidural anesthesia, Ketamine, Shivering, Transurethral resection of prostate
Introduction
Many published reports support the finding of postoperative hypothermia associated with the use of room-temperature irrigation fluid [1-3]. Winter reported a 63% incidence of hypothermia in patients who underwent TURP with room-temperature irrigation fluid [3].
Unlike general anesthesia, regional anesthesia alters the afferent conduction of the thermal signals as a result of epidural anesthesia; efferent thermoregulatory responses may be terminated as a result of sensory block below the intervention level. The sympathetic blockade, which results in peripheral vasodilation, increased cutaneous blood flow, and subsequent increased heat loss via the skin, may cause heat loss in patients during regional anesthesia [4,5].
Ketamine causes sympathetic stimulation and vasoconstriction in patients at risk of hypothermia. Ketamine probably controls shivering by non-shivering thermogenesis either influencing the hypothalamus or by the beta-adrenergic effect of norepinephrine. Ketamine was also shown to prevent shivering without hemodynamic alteration in patients undergoing regional anesthesia [6]. Ketamine, which is a non-competitive receptor antagonist of N-methyl-D-aspartic acid (NMDA), has a role in thermoregulation on various levels. The NMDA receptors modulate the noradrenergic and serotonergic neurons in the locus ceruleus, and consequently the NMDA receptors in the dorsal horn of the spinal cord provide the transmission of the ascending nociceptive stimuli [7].
The aim of this study was to evaluate the efficacy of epidural ketamine in preventing shivering and unstable hemodynamic changes during TURP under epidural anesthesia.
Materials and methods
After institutional ethics committee approval and written informed consent, 93 patients (ASA physical status I or II) who were scheduled for elective TURP were enrolled in this study. The patients were between 51 and 78 years old (mean age, 70.1±4.8). Patients with hyperthyroidism, cardiopulmonary disease, known history of alcohol or substance abuse, psychological disorder, and an initial body temperature above 38℃ or below 36.5℃ were excluded from this study. Patients were randomly assigned to one of three groups who underwent TURP using room-temperature irrigation fluid. Group 1 consisted of 31 patients who received epidural 0.75% ropivacaine, group 2 consisted of 32 patients who received epidural ketamine (0.2 mg/kg) in addition to 0.75% ropivacaine, and group 3 consisted of 30 patients who received epidural ketamine (0.4 mg/kg) in addition to 0.75% ropivacaine.
On arrival at the operating room, the patient was administered epidural anesthesia at either the L3-4 or L4-5 interspace by use of a 17-gauge Tuhoy needle. Patients did not receive premedication. The temperature of the operating room was maintained at 24℃ The patients in each group received dosages of 0.75% ropivacaine according to height by Bromage (Table 1). Pinprick sensation was examined by using a blunt 21 gauge needle in a cephalic to caudal fashion along the left anterior axillary line. The upper level of sensory block was recorded. If the mean arterial pressure decreased by more than 20% of that recorded before the induction of anesthesia, or heart rate decreased to less than 50 beats/min, 5-10 µg/kg/min of dopamine was given.
Table 1.
Dosage of local anesthetics according to patient height by Bromage
All patients in the study had their body temperature recorded on arrival at the operating room by use of a tympanic thermometer (Thermoscan®, Braun, USA). Operative time, hemodynamic changes, and amount of irrigation fluid (Urosol 3 L®, CJ, Korea) used during the procedure were recorded. Body temperature was checked right after the operation. Side effects such as hypotension, bradycardia, nausea, and hallucination were recorded.
Shivering was graded with a scale similar to that validated by Crossley and Mahajan [8]: 0=no shivering, 1=piloerection or peripheral vasoconstriction but no visible shivering, 2=muscular activity in only one muscle group, 3=muscular activity in more than one muscle group but not generalized shivering, 4=shivering involving the whole body.
Hallucination as a side effect was defined as a false sensory experience in which the patients reported they saw, heard, smelled, tasted, or felt something that was nonexistent. The attending anesthesiologist also assessed the degree of sedation on a 5-point scale where 1=fully awake and oriented, 2=drowsy, 3=eyes closed but rousable to command, 4=eyes closed but rousable to mild physical stimulation, and 5=eyes closed but unrousable to mild physical stimulation.
Shivering and side effects such as hypotension, bradycardia, nausea, and hallucination were recorded during anesthesia and for 2 hours while in the postanesthetic recovery room.
The SPSS 12.0 program was used to analyze the statistical data. The results are presented as means±standard deviation, medians (range), or the number of patients. Comparisons of age, body weight, prostate weight, International Prostate Symptom Score (IPSS), bladder capacity, prostate-specific antigen (PSA), residual urine (RU), resected tissue weight, operation time, and amount of irrigation fluid used during the procedure among groups were conducted by using one-way ANOVA followed by Bonferroni's post-hoc testing. Chi-square test was used to analyze nonparametric data such as frequency of shivering, hypotension, bradycardia, nausea, and hallucination. P<0.05 was considered the minimum level of statistical significance.
Results
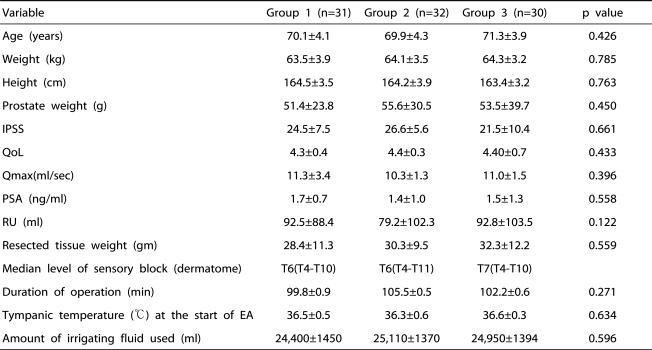
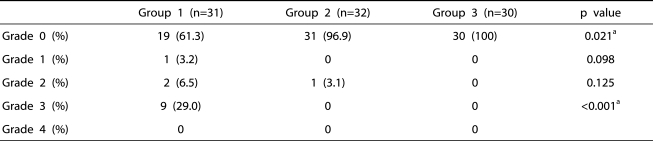
There were no significant differences among the three groups with respect to age, weight, height, prostate weight, IPSS, maximal urinary flow rate, residual urine, serum PSA level, resected tissue weight, median level of sensory block, duration of operation, tympanic temperature at the start of epidural anesthesia, or the amount of irrigation fluid used (Table 2). The number of patients with a shivering grade of 3 was statistically higher in group 1 than in the other groups (p<0.001) (Table 3).
Table 2.
Patients' Characteristics and Intraoperative Data
IPSS: International Prostate Symptom Score, QoL: qaulity of life, PSA: prostate specific antigen, Qmax: maximal urinary flow rate, RU: residual urine, EA: epidural anesthesia. Group 1: patients who received epidural0.75% ropivacaine. Group 2: patients who received epidural ketamine (0.2 mg/kg) in addition to the 0.75% ropivacaine. Group 3: patients who received epidural ketamine (0.4 mg/kg) in addition to the 0.75% ropivacaine.
Table 3.
The Comparison of the Shivering Grade Among Groups
Group 1: patients who received epidural 0.75% ropivacaine, Group 2: patients who received epidural ketamine (0.2 mg/kg) in addition to the 0.75% ropivacaine, Group 3: patients who received epidural ketamine (0.4 mg/kg) in addition to the 0.75% ropivacaine.
a: statistical significant by Chi-square test.
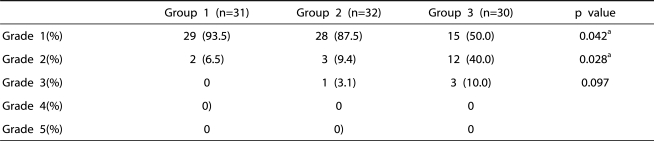
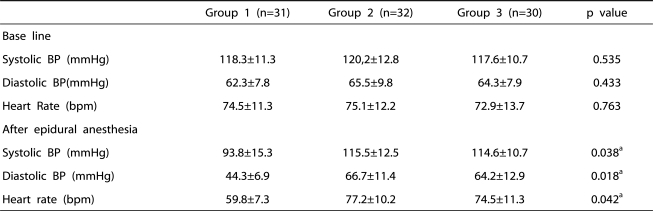
The incidences of grade 1 and 2 sedation were significantly higher in group 3 than in the other groups (p=0.042, 0.028) (Table 4). However, the incidences of over grade 3 sedation were not significantly different among the three groups (Table 4). Systolic and diastolic arterial pressure and heart rate were significantly lower in group 1 than in the other groups, but there was no significant difference in hemodynamic change between group 2 and group 3 (Table 5).
Table 4.
The Comparison of the Sedation Grade Among Groups.
Group 1: patients who received epidural 0.75% ropivacaine, Group 2: patients who received epidural ketamine (0.2 mg/kg) in addition to the 0.75% ropivacaine, Group 3: patients who received epidural ketamine (0.4 mg/kg) in addition to the 0.75% ropivacaine.
a: statistical significant by Chi-square test.
Table 5.
Hemodynamic changes of epidural ketamine anesthesia
Group 1: patients who received epidural 0.75% ropivacaine, Group 2: patients who received epidural ketamine (0.2 mg/kg) in addition to the 0.75% ropivacaine, Group 3: patients who received epidural ketamine (0.4 mg/kg) in addition to the 0.75% ropivacaine.
a: statistical significant by one-way ANOVA test
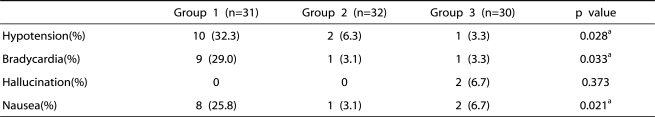
The incidences of side effects such as hypotension, bradycardia, and nausea were significantly higher in group 1 than in the other groups (Table 6). There were no significant differences in side effects between group 2 and group 3 (Table 6).
Table 6.
Incidences of Side Effects
Group 1: patients who received epidural 0.75% ropivacaine, Group 2: patients who epidural ketamine (0.2 mg/kg) in addition to the 0.75% ropivacaine, Group 3: patients who received epidural ketamine (0.4 mg/kg) in addition to the 0.75% ropivacaine.
a: statistical significant by Chi-square test.
Discussion
An approximately 30~40% incidence of shivering during epidural anesthesia has been reported [9-12]. The exact mechanism of shivering under epidural anesthesia has not been fully established. Hypotheses have been formed to explain the phenomenon [13,14]. First, as with general anesthesia, heat is internally redistributed from the core to the peripheral compartment. Second, the loss of thermoregulatory vasoconstriction below the level of the blockade results in increased heat loss from body surfaces in excess of metabolic heat production. Third, there is altered thermoregulation characterized by a small (approximately 0.5℃) decrease in vasoconstriction and a slight increase in the sweating threshold. The incidence of shivering during epidural anesthesia was 38% in our study; this is similar to the results in other previous studies [9-12].
Shivering increases metabolic activity and oxygen consumption. It may also cause arterial hypoxia and lactic acidosis [15]. In patients with compromised cardiac function, this increase in oxygen demand may induce angina pectoris, myocardial infarction, and arrhythmia [4,11]. These all make the prevention of shivering important, especially in elderly patients with a low cardiopulmonary reserve [16]. This is of concern because TURP has been associated with hypothermia and subsequent shivering [1-3].
Pharmacologic agents, skin surface warming, and radiant heat application have been used to prevent shivering [15]. Because the incidence of hypotension is high during regional anesthesia, agents that cause hypotension may not be suitable in preventing shivering. Ketamine was shown to prevent shivering without hemodynamic alteration in patients undergoing regional anesthesia. Ketamine causes sympathetic stimulation and vasoconstriction in patients at risk of hypothermia [17]. Ketamine probably controls shivering by nonshivering thermogenesis either influencing the hypothalamus or by the beta-adrenergic effect of norepinephrine [6].
In our study, it was shown that the incidence of shivering in patients who received epidural ketamine 0.2 mg/kg or 0.4 mg/kg was 3.1% and 0%, respectively. Honarmand et al. reported that the prophylactic use of intravenous ketamine 0.5 mg/kg to prevent shivering during regional anesthesia was effective, and the incidence of shivering was 23.3% [18]. The incidences of hypotension (10.0%) and nausea and vomiting (30.0%) and hallucination (10.0%) as well as shivering were higher than in the group that received epidural ketamine. However, because the protocol of their study differed significantly from ours, a comparison between Honarmand's study and ours would not be beneficial.
In our study, it was shown that the group 2 patients who received epidural ketamine 0.2 mg/kg and the group 3 patients who received epidural ketamine 0.4 mg/kg experienced a lower incidence than the patients in group 1 of the shivering, hypotension, and bradycardia related to epidural anesthesia.
Also, we found no difference in the efficacy of the epidural ketamine dosage regarding hemodynamic values and the antishivering effect between group 2 and group 3.
During regional anesthesia, intravenous sedative drugs are often administered to decrease the patient's discomfort, to maintain cardiorespiratory stability, to improve surgical conditions, and to prevent recall of unpleasant events during surgery [16]. The sedation grades were less than 3 in all groups of patients and were significantly higher in group 3 than in the other groups.
However, sedation in elderly patients during regional anesthesia may cause impaired psychomotor function for several hours after surgery [19]. Therefore, we cannot determine whether the sedative effect of epidural ketamine that was shown in group 3 caused positive effects or not.
There was no graphical evidence of a relationship between the dosage of ketamine and the risk of having hallucinations [20]. Subramaniam et al. reported that hallucinations occurred in 1 of 26 patients who received a single epidural bolus of 1 mg/kg of ketamine [21]. In our study, there was no significant difference in the incidence of hallucination between group 2 and 3 (0% vs 6.7%). However, two patients in group 3 developed hallucination. This is a well-known side effect of ketamine.
The doses of epidural ketamine used in this study were arbitrary, although epidural ketamine 0.2 mg/kg and 0.4 mg/kg was not shown to have a significant difference in shivering or other side effects except sedation. Based on our findings, lower doses of epidural ketamine need to be investigated to determine the optimal dose of ketamine in preventing shivering.
Conclusions
In this study, epidural ketamine 0.2 mg/kg and 0.4 mg/kg was shown to result in a lower incidence of shivering and other side effects except sedation. In patients who underwent TURP under epidural anesthesia, the prophylactic use of low-dose epidural ketamine would be helpful in preventing any adverse effects including shivering. No studies have described the efficacy of epidural ketamine for prophylaxis of postanesthetic shivering. Therefore, the optimal dosage regimen of epidural ketamine in preventing shivering is currently undetermined. We think that additional study is necessary.
Acknowledgement
This work was supported by Wonkwang University in 2010
References
- 1.Ogura K, Fukuyama T, Nakagawa K. The effects of warm irrigating fluid during and after transurethral prostatectomy. Clin Ther. 1988;10:20–21. [PubMed] [Google Scholar]
- 2.Heathcote PS, Dyer PM. The effect of warm irrigation on blood loss during transurethral prostatectomy under spinal anaesthesia. Br J Urol. 1986;58:669–671. doi: 10.1111/j.1464-410x.1986.tb05909.x. [DOI] [PubMed] [Google Scholar]
- 3.Winter M. Effects of irrigation fluid warming on hypothermia during urologic surgery. Urol Nurs. 1994;14:6–8. [PubMed] [Google Scholar]
- 4.Chan AM, Ng KF, Tong EW, Jan GS. Control of shivering under regional anesthesia in obstetric patients with tramadol. Can J Anaesth. 1999;46:253–258. doi: 10.1007/BF03012605. [DOI] [PubMed] [Google Scholar]
- 5.Buggy DJ, Crossley AW. Thermoregulation, mild perioperative hypothermia and postanaesthetic shivering. Br J Anaesth. 2000;84:615–628. doi: 10.1093/bja/84.5.615. [DOI] [PubMed] [Google Scholar]
- 6.Sharma DR, Thakur JR. Ketamine and shivering. Anaesthesia. 1990;45:252–253. doi: 10.1111/j.1365-2044.1990.tb14709.x. [DOI] [PubMed] [Google Scholar]
- 7.Dal D, Kose A, Honca M, Akinci SB, Basgul E, Aypar U. Efficacy of prophylactic ketamine in preventing postoperative shivering. Br J Anaesth. 2005;95:189–192. doi: 10.1093/bja/aei148. [DOI] [PubMed] [Google Scholar]
- 8.Crossley AW, Mahajan RP. The intensity of postoperative shivering is unrelated to axillary temperature. Anaesthesia. 1994;49:205–207. doi: 10.1111/j.1365-2044.1994.tb03422.x. [DOI] [PubMed] [Google Scholar]
- 9.Webb PJ, James FM, 3rd, Wheeler AS. Shivering during epidural analgesia in women in labor. Anesthesiology. 1981;55:706–707. doi: 10.1097/00000542-198155060-00024. [DOI] [PubMed] [Google Scholar]
- 10.Chan VW, Morley-Forster PK, Vosu HA. Temperature changes and shivering after epidural anesthesia for cesarean section. Reg Anesth. 1989;14:48–52. [PubMed] [Google Scholar]
- 11.Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93:1288–1292. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 12.Sessler DI, Ponte J. Shivering during epidural anesthesia. Anesthesiology. 1990;72:816–821. doi: 10.1097/00000542-199005000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Matsukawa T, Sessler DI, Christensen R, Ozaki M, Schroeder M. Heat flow and distribution during epidural anesthesia. Anesthesiology. 1995;83:961–967. doi: 10.1097/00000542-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki M, Kurz A, Sessler DI, Lenhardt R, Schroeder M, Moayeri A, et al. Thermoregulatory thresholds during epidural and spinal anesthesia. Anesthesiology. 1994;81:282–288. doi: 10.1097/00000542-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Bilotta F, Pietropaoli P, La Rosa I, Spinelli F, Rosa G. Effects of shivering prevention on haemodynamic and metabolic demands in hypothermic postoperative neurosurgical patients. Anaesthesia. 2001;56:514–519. doi: 10.1046/j.1365-2044.2001.02057.x. [DOI] [PubMed] [Google Scholar]
- 16.Piper SN, Suttner SW, Schmidt CC, Maleck WH, Kumle B, Boldt J. Nefopam and clonidine in the prevention of postanaesthetic shivering. Anaesthesia. 1999;54:695–699. doi: 10.1046/j.1365-2044.1999.00849.x. [DOI] [PubMed] [Google Scholar]
- 17.Sagir O, Gulhas N, Toprak H, Yucel A, Begec Z, Ersoy O. Control of shivering during regional anaesthesia: prophylactic ketamine and granisetron. Acta Anaesthesiol Scand. 2007;51:44–49. doi: 10.1111/j.1399-6576.2006.01196.x. [DOI] [PubMed] [Google Scholar]
- 18.Honarmand A, Safavi MR. Comparison of prophylactic use of midazolam, ketamine, and ketamine plus midazolam for prevention of shivering during regional anaesthesia: a randomized double-blind placebo controlled trial. Br J Anaesth. 2008;101:557–562. doi: 10.1093/bja/aen205. [DOI] [PubMed] [Google Scholar]
- 19.Nishiyama T, Yokoyama T, Hanaoka K. Sedation guidelines for midazolam infusion during spinal and epidural anesthesia. J Clin Anesth. 2004;16:568–572. doi: 10.1016/j.jclinane.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Shinozaki M, Usui Y, Yamaguchi S, Okuda Y, Kitajima T. Recovery of psychomotor function after propofol sedation is prolonged in the elderly. Can J Anaesth. 2002;49:927–931. doi: 10.1007/BF03016876. [DOI] [PubMed] [Google Scholar]
- 21.Subramaniam B, Subramaniam K, Pawar DK, Sennaraj B. Preoperative epidural ketamine in combination with morphine does not have a clinically relevant intra- and postoperative opioid-sparing effect. Anesth Analg. 2001;93:1321–1326. doi: 10.1097/00000539-200111000-00059. [DOI] [PubMed] [Google Scholar]