Abstract
Purpose
The urodynamic effects of intravesical PGE2 instillation on bladder function and detrusor overactivity (DO) during the filling phase were investigated in rats by measuring intraabdominal and intravesical pressures simultaneously.
Materials and Methods
Continuous cystometry was performed inconscious, female and male Sprague- Dawley rats. We investigated pressure-, volume-, and DO-related parameters.
Results
Intravesical instillation of PGE2 increased all pressure-related parameters and decreased volume-related ones, compared to the control cystometric ones. However, among the total number of intravesical pressure rises (IVPRs) above 2 cmH2O during the filling phase, only 33% in female rats and 38% in male rats after PGE2 instillation were identified as true DO during the filling phase.
Conclusions
Our findings suggest that the rat model with intravesical PGE2 is inappropriate for observing the effects of some drugs or mechanisms on DO, because only approximately 30% of IVPRs were confirmed as true DO. However, this model of intravesical PGE2 instillation has some advantages for the observation of changes in pressure and volume parameters rather than in DO-related ones.
Keywords: Rat, Detrusor overactivity, Prostaglandin E2, Pressure, Urodynamics
Introduction
Current increasing clinical interest in overactive bladder (OAB) has resulted in considerable progress in basic research in this field. Because the physiology of rat and human bladders has many similarities, many in vivo animal models are available for the study of lower urinary tract function, both under normal conditions and after, for example, inducing the conditions corresponding to OAB in humans. Intravesical administration of PGE2 in rats has been used as an animal model to reproduce the pathophysiologycausing detrusor overactivity (DO) in humans [1]. However, in these studies, DO has been evaluated only by pressure- and volume-related parameters and not by the direct measurement of DO because of a lack of a method for this.
Cystometry is widely used in rats for the evaluation of both voiding and nonvoiding contractions of the bladder. The ultimate goal of cystometry is to detect the detrusor contractions, which requires a methodology for measuring intraabdominal pressure (IAP) as well as intravesical pressure in humans [2]. Previous studies in conscious animals used only the intravesical pressures, so that variations in IAP due to voiding-related abdominal strain or general motor activity might cause misinterpretation of pressure parameters and result in false reports of DO. The methodology for registrations of IAP in awake rat cystometry was first introduced by our group [3]. We also reported direct objective measurements of DO itself, such as pressures or frequency, in conscious male SHRs and found that DO represented approximately 76% of the intravesical pressure rises above 2 cmH2O in SHRs, with the remainder caused by abdominal straining [4]. However, these measurements have been not done in the PGE2 administered rat model, which is widely used in studies of DO.
In this study, we compared the bladder function and characteristics of DO during the filling phase before and after the administration of intravesical PGE2 by measuring intraabdominal and intravesical pressures simultaneously in the DO rat model.
Materials and Methods
Experimental Animals. Sixmale and 6 female age-matched (14 weeks) Sprague-Dawley rats, obtained from Orient BioInc. (GyeongGi, Korea), weighing 250 to 290 g were given PGE2 intravesically to induce DO. They were maintained under standard laboratory conditions with a 12:12 light:dark cycle and free access to food pellets and tap water. All procedures for animal handling and treatment were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and were approved by the author's institution. Simultaneous IAP and IVP were continuously recorded during bladder filling and emptying, as previously described [3].
Surgical procedures
Catheter implantations. Briefly, rats were anesthetized with ketamine (Ketamine, Yuhan Corp, Seoul, Korea; 75 mg kg-1 intraperitoneally) and xylazine (Rompun, Bayer Korea Corp, Korea; 15 mg kg-1 intraperitoneally). Through the lower abdominal incision, a polyethylene catheter (PE-50; Becton Dickinson, Parsippany, NJ, USA) with a cuff was inserted into the dome of the bladder to record the intravesical pressure (IVP). To record IAP, a catheter with an abdominal balloon (Latex, Dawoo Medical, Incheon, Korea) was inserted and attached with a silk tie to the posterior side of the catheter used to record bladder pressure. The catheters were tunneled subcutaneously and anchored to the skin of the back with a silk ligature. The free ends of the catheters were sealed.
Cystometric investigations
Cystometry was performed 3 days after the catheter implantation. The rats were placed in metabolic cages without any restraint and the catheters were connected via a T-tube to a pressure transducer (Research Grade Blood Pressure Transducer; Harvard Apparatus, Holliston, MA, USA) and a microinjection pump (PHD22/2000 pump; Harvard Apparatus). Micturition volumes were recorded with a fluid collector connected to a force displacement transducer (Research Grade Isometric Transducer; Harvard Apparatus). Roomtemperature saline was infused into the bladder continuously at a rate of 10 ml/h. Pressures and micturition volumes were recorded continuously with Acq Knowledge 3.8.1 software and an MP150 data acquisition system (BIOPAC Systems, Goleta, USA) at a sampling rate of 50 Hz.
In normal rats, three reproducible micturition cycles, together corresponding to a 30-min period, were recorded before the intravesical administration of PGE2. The mean values were used as baseline one, to be compared with the mean from three micturition cycles in the first 30-min period after the intravesical administration of PGE2. IAP was defined as the recorded balloon pressure corrected by subtracting the lowest balloon pressure in each voiding cycle. The detrusor pressure (DP) was defined as the IVP minus the abdominal pressure. The intravesical pressure rises (IVPRs) during the filling phase were defined as increments of IVP that exceeded 2 cmH2O from baseline.
The following urodynamic parameters were investigated. Conventional urodynamic pressure- and volume-related parametersincluded the following: maximalpressure (MP=maximum bladder pressure during micturition), basal pressure (BP=the lowest bladder pressure during filling), threshold pressure (TP=bladder pressure immediately before micturition), bladder capacity (BC=residual volume at the most recent previous micturition plus the volume of infused saline at micturition), micturition volume (volume of expelled urine), and residual volume (RV=bladder capacity minus micturition volume) and micturition interval (intercontraction interval).
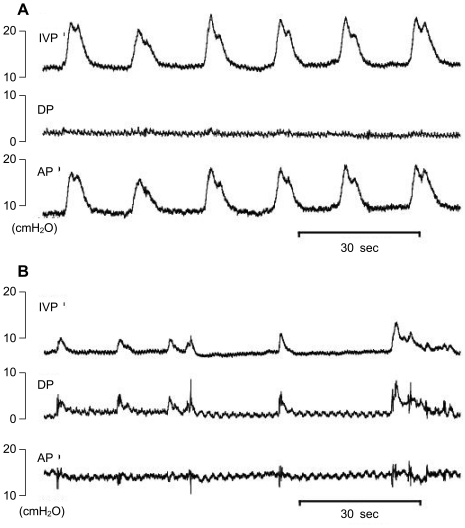
Parameters to investigate hyperactive behavior and DO during the filling phase were as follows: time of filling phase (interval between the end of a micturition and the point immediately before the next micturition), frequency of IVPRs per minute, frequency of abdominal straining (IVPRs with simultaneous changes in IAP Figure 3B), frequency of DO per minute (IVPRs without simultaneous changes in IAP; Figure 3A), and increased amplitude of DO as IVP.
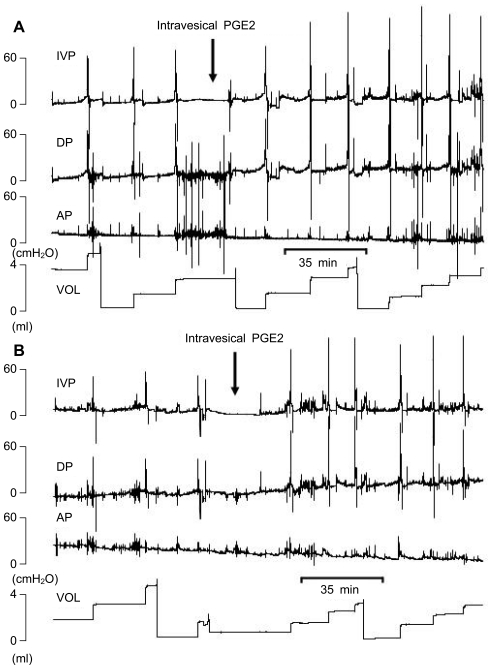
Figure 3.
Representative tracings of rats with intravesical instillation of PGE2. A. Male rats. B. Female Rats.
Administration of drugs. Stock solutions (0.01M) of PGE2 (Sigma Chemical Co, St Louis, MO, USA) were made in absolute ethanol and were then diluted in saline (50 uM) just before use. Prostaglandin E2 was infused intravesically for 28 min by changing the syringe of the micro-injection pump. With the tubing used, PGE2 reached the bladder within 3 min of switching from saline to PGE2-containing saline.
Statistical analysis
The results are given as mean values±standard errors of the mean (SEM). Normal distributions were confirmed by the Shapiro-Wilks' W test. Statistical significance was determined by Student's t test and Bonferroni corrections as appropriate, with statistical significance considered at p<0.05.
Results
Conventional urodynamic pressure- and volume-related parameters
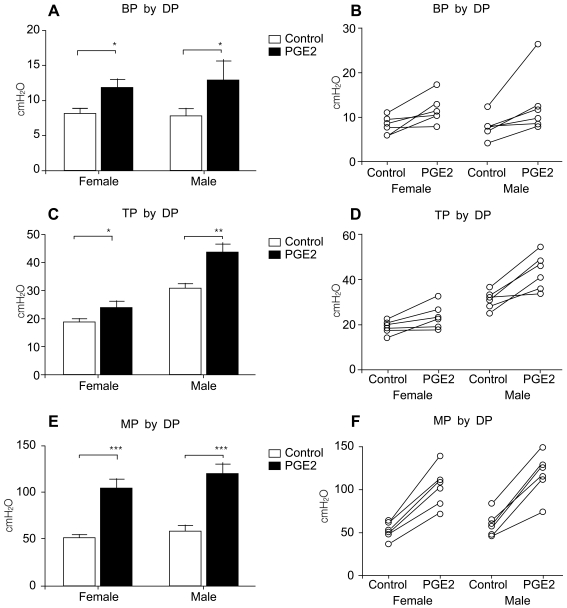
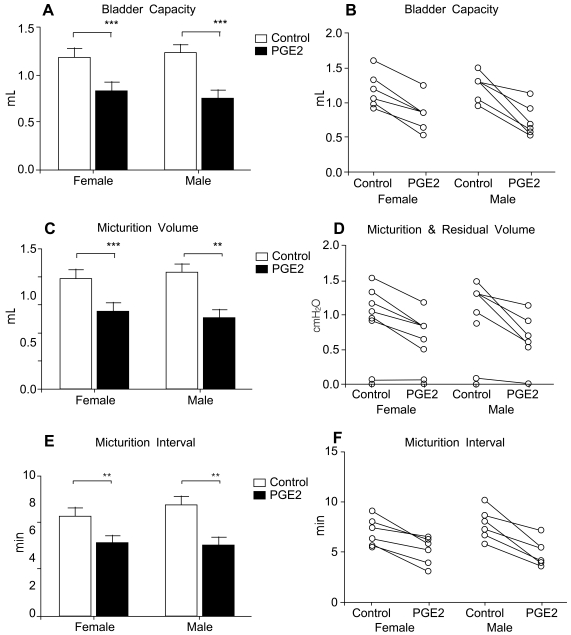
Both male and female rats receiving intravesical PGE2 showed increased pressure parameters and decreased volume parameters compared with thebaseline state (normal rats before PGE2 instillation), which were compatible with typical human OAB in view of pressure and volume changes. The pressure parameters, including basal pressure (BP, P<0.05 Figure 1A), threshold pressure (TP, p<0.01 Figure 1C), and micturition pressure (MP, p<0.01 Figure 1E), increased after PGE2 instillation. All rats showed increased pressure parameters (Figure 1B, D, F). The volume parameters, including bladder capacity (BC, P<0.01 Figure 2A), micturition volume (MV, p<0.01 Figure 2C), and micturition interval (MI, P<0.01 Figure 2E), decreased after PGE2 instillation. All rats showed decreased volume parameters (Figure 2B, D, F, Figure 3).
Figure 1.
Pressure-related parameters in rats with intravesical PGE2 instillation. A, B. Basal pressure. C, D. Threshold pressure. E, F. Maximal pressure.
Figure 2.
Volume-related parameters in rats with intravesical PGE2 instillation. A, B. Bladder capacity. C, D. Micturition volume. E, F. Micturition interval.
Parameters to investigate hyperactive behavior and DO during the filling phase
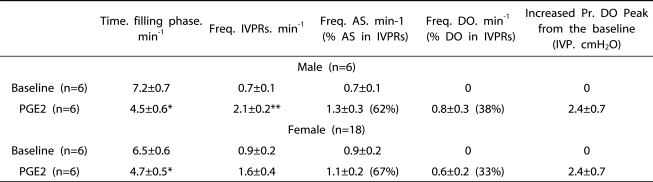
These animals showed typical DO confirmed by the presence of simultaneous changes in the abdominal pressure curve during the filling phase. Rats receiving PGE2 showed a frequency (per minute) of DO of 0.6±0.2 in female rats and 0.8±0.3 in male rats. Rats receiving intravesical PGE2 showed a greater frequency of IVPRs than did the controls. However, among the total frequency of IVPRs events, only 33% in female rats and 38% in male rats after PGE2 instillation were identified as true DO during the filling phase.
The average increased pressures of the DO peak from just before baseline was 2.4±0.7 cmH2O in both male and female rats with intravesical instillation, and there was no statistical significance between these groups (Table 1).
Table 1.
Characteristics of IVPRs During the Filling Phase in Rats During Conscious Cystometry Before and After Intravesical Infusion of PGE2 (50 µM).
IVPR: intravesical pressure rise, IVP: Intravesical pressure, DP: Detrusor pressure, AS: Abdominal straining. Results are expressed as mean±standard error of the mean. *P<0.05. **P<0.01. (paired Student's t-test) versus baseline. Parenthesis shows the percentage of those values among total IVPRs.
Discussion
According to the International Continence Society (ICS), OAB is defined by urgency, with or without urge incontinence, usually with frequency and nocturia in the absence of proven infection or other obvious pathology [5]. This urgency, in which the patient has a sudden compelling desire to pass urine, is difficult to defer. OAB occurs when the patient experiences involuntary detrusor contractions corresponding to DO during the filling phase, as shown by urodynamic study. Many animal models have been used to investigate the mechanisms related to DO, corresponding to urgency in clinical conditions. However, many previous studies used only the conventional pressure or volume parameters of the cystometry [6] or the simple presence of a nonvoiding contraction without objective evidence by IAP [7]. The changes in IAP by inherent actions in rats such as moving, licking, or washing may be blended into the changes in intravesical pressure and make it difficult to differentiate the detrusor action from the intravesical pressure only, because it is the sum of the pressures by the detrusor muscle and the abdominal straining outside the bladder. This can cause misinterpretation of pressure parameters and result in false reports of DO. Therefore, we need to investigate the characteristics of DO in various animal DO models in order to determine which experiments are appropriate for the intended purpose.
According to the socially accepted definition, OAB signifies a patient who has frequency or urgency. However, although the ICS chose urgency rather than frequency as a key symptom, we still use frequency as an important parameter for clinical investigation [5]. Thus, we sometimes need an animal model that has advantages for studying the mechanisms specific to frequency. Our model of intravesical PGE2 instillation showed decreased bladder capacity, micturition volume, and micturition interval in all experimental rats. All rats showed increased pressure parameters, which suggests that the basal tension of the bladder, threshold pressure, and micturition power increased compared with control cystometric ones. As for the DO parameters suggesting urgency in both male and female rats, only approximately 30% in male and female rats were confirmed as DO by IAP among the total IVPRs, which were regarded as nonvoiding contractions if with only the IVP. On the contrary, the rest of the IVPRs signified abdominal straining. There was no difference between male and female rats in DO parameters. In our previous research in other DO rat models, we showed that 70% and 90% of IVPRs in the SHRs and in rats with bladder outlet obstruction, respectively, were confirmed as DO [4,8]. Therefore, we need to use this information to select the DO models appropriate for the specific purpose of the experiment.
There are various theories for the pathogenesis for DO, including the neurogenic [9], myogenic [10], autonomous bladder [11], and urotheliogenic [12] hypotheses. But all of the sub-mechanisms for DO are still unclear, and we need more details. Thus, we need many animal models specific for the sub-mechanisms. To date, there are four representative animal models for DO: the intravesical PGE2 instillation model, the bladder outlet obstruction (BOO) model, the interstitial cystitis (IC) model, and the SHR model. The BOO and IC models are related to specific diseases, such as benign prostatic hyperplasia (BPH) and IC. The SHR model deals with the DO mechanism caused by increased sympathetic tone [13,14]. The intravesical PGE2 model uses prostanoids, which are endogenous modulators of bladder function, in the normal physiologic state and under pathophysiological conditions. PGE2, which is synthesized locally in bladder muscle and mucosa, is known to contract the muscle strip from the human bladder in response to various physiological stimuli, such as detrusor muscle stretch and nerve stimulation as well as injuries and inflammatory mediators [15,16].
Our results showed that the PGE2 model has an advantage in investigating the pressure and volume parameters of OAB rather than the DO parameters, whereas the BOO model is useful for studying the DO parameters rather than the pressure and volume parameters because of the mechanically obstructed outflow.
Conclusions
Our findings suggest that the rat model with intravesical PGE2 administration is inappropriate for observing the effect of drugs or mechanisms on DO, because only approximately 30% of the IVPRs were confirmed as true DO. However, the PGE2 intravesical instillation model has advantages for observing changes in pressure and volume parameters.
Figure 4.
Representative tracings of detrusor overactivity and abdominal straining. A. Detrusor overactivity. B. Abdominal straining.
Acknowledgement
This study was performed by the Inha University Research Fund.
References
- 1.Ishizuka O, Mattiasson A, Andersson KE. Prostaglandin E2-induced bladder hyperactivity in normal, conscious rats: involvement of tachykinins? J Urol. 1995;153:2034–2038. doi: 10.1016/s0022-5347(01)67397-x. [DOI] [PubMed] [Google Scholar]
- 2.Schafer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–274. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 3.Lee T, Andersson KE, Streng T, Hedlund P. Simultaneous registration of intraabdominal and intravesical pressures during cystometry in conscious rats-effects of bladder outlet obstruction and intravesical PGE2. Neurourol Urodyn. 2008;27:88–95. doi: 10.1002/nau.20460. [DOI] [PubMed] [Google Scholar]
- 4.Jin LH, Andersson KE, Kwon YH, Park CS, Yoon SM, Lee T. Substantial detrusor overactivity in conscious spontaneously hypertensive rats with hyperactive behaviour. Scand J Urol Nephrol. 2009;43:3–7. doi: 10.1080/00365590802468750. [DOI] [PubMed] [Google Scholar]
- 5.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 6.Klausner AP, Streng T, Na YG, Raju J, Batts TW, Tuttle JB, et al. The role of corticotropin releasing factor and its antagonist, astressin, on micturition in the rat. Auton Neurosci. 2005;123:26–35. doi: 10.1016/j.autneu.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Christ GJ, Day NS, Day M, Zhao W, Persson K, Pandita RK, et al. Increased connexin43-mediated intercellular communication in a rat model of bladder overactivity in vivo. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1241–R1248. doi: 10.1152/ajpregu.00030.2002. [DOI] [PubMed] [Google Scholar]
- 8.Park SH, Jin LH, Kwon YH, Yoon SM, Lee T. Application and limitations of awake cystometry in Sprague-Dawley rats with partial bladder outlet obstruction as a model of overactive bladder or obstruction. Korean J Urol. 2009;50:486–492. [Google Scholar]
- 9.de Groat WC. A neurologic basis for the overactive bladder. Urology. 1997;50:36–52. doi: 10.1016/s0090-4295(97)00587-6. [DOI] [PubMed] [Google Scholar]
- 10.Brading AF. A myogenic basis for the overactive bladder. Urology. 1997;50:57–67. doi: 10.1016/s0090-4295(97)00591-8. [DOI] [PubMed] [Google Scholar]
- 11.Gillespie JI. The autonomous bladder: a view of the origin of bladder overactivity and sensory urge. BJU Int. 2004;93:478–483. doi: 10.1111/j.1464-410x.2003.04667.x. [DOI] [PubMed] [Google Scholar]
- 12.Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP. Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology. 2004;63:24–31. doi: 10.1016/j.urology.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 13.Persson K, Pandita RK, Spitsbergen JM, Steers WD, Tuttle JB, Andersson KE. Spinal and peripheral mechanisms contributing to hyperactive voiding in spontaneously hypertensive rats. Am J Physiol. 1998;275:R1366–R1373. doi: 10.1152/ajpregu.1998.275.4.R1366. [DOI] [PubMed] [Google Scholar]
- 14.McMurray G, Casey JH, Naylor AM. Animal models in urological disease and sexual dysfunction. Br J Pharmacol. 2006;147:S62–S79. doi: 10.1038/sj.bjp.0706630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson KE, Wein AJ. Pharmacology of the lower urinary tract: basis for current and future treatments of urinary incontinence. Pharmacol Rev. 2004;56:581–631. doi: 10.1124/pr.56.4.4. [DOI] [PubMed] [Google Scholar]
- 16.Andersson KE. Advances in the pharmacological control of the bladder. Exp Physiol. 1999;84:195–213. doi: 10.1111/j.1469-445x.1999.tb00083.x. [DOI] [PubMed] [Google Scholar]