Abstract
Purpose
Aquaporins (AQPs) have been reported to be expressed in rat and human urothelium. Nitric oxide (NO) is thought to play an important role in the bladder overactivity related to menopause. The purpose of this study was to investigate the effect of hormonal alteration on the expression of AQP1 and eNOS in menopausal rat urinary bladder.
Materials and Methods
Female Sprague-Dawley rats (230-240 g, N=30) were divided into three groups: control (N=10), bilateral ovariectomy (Ovx, N=10), and bilateral ovariectomy followed by subcutaneous injections of 17β-estradiol (50 mg/kg/day, Ovx+Est, N=10). After 4 weeks, urodynamic studies measuring the contraction interval and contraction pressure were done. The expression and cellular localization of AQP1 and eNOS were determined by performing Western blotting and immunohistochemistry on the rat urinary bladder.
Results
The approximate contraction interval (min) was significantly decreased in the Ovx group (3.9±0.25) compared to the control group (6.7±0.15), and was increased after estrogen treatment (9.7±0.22) (p<0.05). The AQP1 and eNOS immunoreactivities were localized in the same areas: capillaries, arterioles, and venules of the lamina propria. The protein expression of AQP1 was not changed significantly, whereas eNOS expression was significantly decreased in the Ovx group and restored to the control value in the Ovx+Est group.
Conclusions
This study showed that ovariectomy causes a significant change in e-NOS expression without a change in AQP1 in menopausal rat urinary bladder. This may imply that e-NOS has a functional role in the bladder overactivity that occurs in association with menopause.
Keywords: AQP1, eNOS, Urinary bladder, Menopause
Introduction
The female urinary tracts arise from the urogenital sinus, which is sensitive to sex steroid hormones. Female sex steroid hormone is essential for the physiological maintenance of the female genitourinary tracts. Postmenopausal women are subject to several urologic dysfunctions, including urinary incontinence, urinary tract infection, and detrusor overactivity [1]. The presence of estrogen receptors throughout the urinary tract and pelvic floor muscle suggests that female sex steroid hormone plays an important role in mediating bladder dysfunction [2]. In an animal study, ovariectomy resulted in bladder mucosal and muscular atrophy, decreased bladder compliance, and decreased detrusor contractility [3]. In clinical studies, estrogen replacement therapy increased blood flow around the bladder neck in menopausal women and significantly reduced urgency symptoms [4].
Detrusor overactivity is a major cause of urinary tract symptoms and is commonly seen in older menopausal women. A better understanding of the underlying pathophysiology of bladder dysfunction in menopausal women is essential because many patients with menopause suffer from the symptoms itself and related harmful effects on their daily lives and quality of life. The mechanisms for bladder dysfunction in menopausal patients have not yet been fully revealed, and the mechanism involved in the release of neurotransmitters or sensory nerve activation for sensing bladder fullness remains to be evaluated.
Aquaporins (AQPs) are a family of water channel proteins that facilitate bidirectional water movement across cell membranes, and in some cases, the movement of glycerol or some solute [5]. Until now, data about AQPs in the mammalian urinary bladder have been limited. Spector et al. [6] investigated the regulation of AQPs in the urothelium of rats following 24 hours of dehydration and water loading tests and reported the localization of AQP1, 2, and 3 in rat urothelium. They suggested that the AQPs in urothelium may play a role in regulating epithelial cell volume and osmolarity. They introduced the possibility of bulk water movement across the urothelium. Also, Rubenwolf et al. [7] demonstrated that AQPs are expressed in cultured human urothelium, suggesting a potential role in transurothelial water and solute transport. However, until now, there have been no studies investigating the expression of AQP family members in the urothelium in an ovariectomy animal model or the functional activity of these proteins in response to hormonal alteration.
Combet et al. [8] tried to explain the role of AQP1 and nitric oxide synthase (NOS) in the peritoneal membrane in experimental ultrafiltration failure in peritoneal dialysis. They suggested that nitric oxide (NO) could modify microvascular permeability by an effective increase in the peritoneal surface area and could regulate plasma membrane proteins such as AQP1 in the peritoneal endothelial cells. NO is thought to play a role in bladder overactivity, which may be associated with impairment in the NO signaling pathway [9]. On the basis of these findings, we hypothesized that AQPs and endothelial NOS (eNOS) might be involved in the physiology of bladder function and dysfunction induced by ovariectomy. The purposes of this study were therefore to investigate the impact of hormonal alteration on AQPs and eNOS isoforms in rat urinary bladder.
Materials and methods
Experimental model
Female Sprague-Dawley rats (230~240 g, N=30) were divided into three groups: control (N=10), bilateral ovariectomy (Ovx, N=10), and bilateral ovariectomy followed by subcutaneous injections of 17β-estradiol (50 mg/kg/day, Ovx+Est, Sigma Chemical Co., St. Louis, MO, USA, N=10). Animals were premedicated with xylazine (2.2 mg/kg, IM) and anesthetized with a zolazepam/tiletamine cocktail (4.4 mg/kg, IM). The control group underwent a sham operation. The Ovx group underwent a bilateral ovariectomy and was treated with an oil vehicle. The Ovx+Est group underwent a bilateral ovariectomy, followed by treatment with subcutaneous estradiol daily (50 mg/kg/day) for 7 days after ovariectomy. All experimental animals were kept on a standard diet up until the day before the experiment. The day before the experiment, food was withheld from the animals. Four weeks after ovariectomy and after 3 weeks of vehicle or hormonal replacement, animals confirmed to have an estrous cycle through a vaginal smear were premedicated with xylazine (2.2 mg/kg, IM) and anesthetized with a zolazepam/tiletamine cocktail (4.4 mg/kg, IM). The protocol for animal surgery was approved by the Ethics Committee of the author's institution.
Cystometrogram
Four weeks after the operation, rats (N=10 in each group) were anesthetized with 1.2 g/kg urethane injected subcutaneously. A suprapubic midline incision was performed to expose the bladder, a transvesical catheter with a fire-flared tip (polyethylene catheter-50) was inserted into the dome of the bladder and secured with a ligature, and the abdomen was closed. The catheter was connected to a pressure transducer and syringe pump via a 3-way stopcock to record intravesical pressure and to infuse saline into the bladder. After the bladder was emptied, cystometry was performed with saline infused at 0.04 ml/min. The contraction pressure and contraction interval were recorded.
Immunohistochemistry for AQP1 and eNOS
The bladder tissue (N=10 in each group) was dissected from both lateral walls of the bladder. The tissue was placed in 4% paraformaldehyde fixative for 16 hours and was then processed for washing and dehydration. The tissue was routinely embedded in paraffin, and 6-µm sections were prepared. Tissues were stained with H&E. Immunohistochemistry was performed by using an immunoperoxidase procedure (Vector ABC Kit; Vector Laboratories, Burlingame, CA, USA). The tissue sections were deparaffinized in xylene, dehydrated in a graded series of ethanol, rinsed twice in phosphate-buffered saline (PBS), and then treated with 3% H2O2 in 60% methanol for 30 minutes to quench endogenous peroxidase activity. After washing twice (5 minutes) in PBS, the sections were incubated for 12 to 14 hours with purified rabbit antibodies for AQP1 (Chemicon, Temecula, CA, USA) and monoclonal rabbit antibodies against eNOS (Santa Cruz, CA, USA) in PBS with 0.3% bovine serum albumin. In the negative control, the sections were incubated in PBS containing 5% normal goat serum only. The 10sections in each tissue sample were then rinsed 3 times in PBS and incubated sequentially for 30 minutes, each with the biotinylated secondary antibody and the ABC reagent. The sections were then incubated for 5 minutes with the peroxidase substrate solution contained in the kit. Finally, the tissue sections were examined and photographed under a light microscope.
Western blot
The tissue homogenates (N=10 in each group 30 µg of protein) were separated by 12% SDS-polyacrylamide gel electrophoresis and transferred to PVDF membranes (Amersham Pharmacia Biotech, England, UK). The blots were then washed with Tris-Buffered Saline Tween-20 (TBST) (10 mM Tris-HCl, pH 7.6, 150 mMNaCl, 0.05% Tween-20). The membrane was blocked with 5% skimmed milk for 1 hour and incubated with the appropriate primary antibody. Monoclonal rabbit antibodies for AQP1 (Chemicon, Temecula, CA, USA) and eNOS (Santa Cruz, CA, USA) and monoclonal rabbit antibody against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Sigma, St. Louis, MO, USA) were used. The membrane was then washed and detected with goat anti-rabbit-IgG conjugated to horseradish peroxidase. Antibody incubations were performed in a 4℃ incubator. The bands were visualized by using enhanced chemiluminescence (Amersham Pharmacia Biotech, England, UK). GAPDH was used as an internal control. Densitometry analysis was performed with a studio Star Scanner using the NIH image V1-57 software.
Statistical analysis
The results were expressed as the mean±the standard error of mean. Analysis of variance (ANOVA) was performed for the statistical analysis. Differences were considered significant at p<0.05.
Results
All animals survived for 4 weeks after the operation. Body weights (g) were significantly increased in the Ovx group (333.3±11.8) compared with the control group (277.5±16.4). Treatment of ovariectomized animals with 17β-estradiol reduced the body weight to the control level (266.2±12.4) (p<0.05). There was no significant difference in bladder weight between groups.
Cystometric change
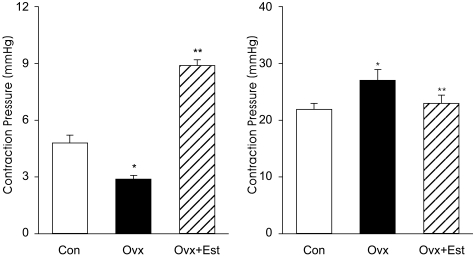
In cystometrograms performed 4 weeks after the operation, the contraction interval (min) was significantly decreased in the Ovx group (2.9±0.34) compared to the control group (4.9±0.65) and was increased after estrogen treatment (8.8±0.49) (p<0.05). Conversely, the average contraction pressure (mmHg) was increased in the Ovx group (27.2±3.2) compared to the control group (21.8±1.79) and decreased after estrogen treatment (22.1±2.53) (p<0.05) (Figure 1).
Figure 1.
Representative urodynamic profiles of control (Con), ovariectomy (Ovx) and estrogen treatment bladders (Ovx+Est). A: The contraction interval of the Ovx group is significantly less than that of the control (*p<0.05) and restored to the control value after estrogen treatment (**p<0.05). B: Note the increased peak pressures on each voiding contraction in the Ovx group and recovery to the control value in Ovx+Est group. The lower panels denote the means±standard deviation of 10 experiments for each condition determined by cystometrogram. *p<0.05 vs. control. **p<0.05 vs. Ovx.
Histologic change
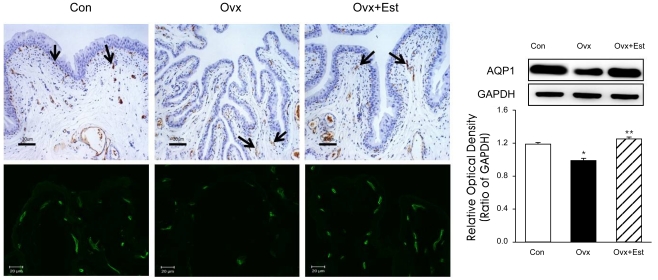
In control rats, the urothelium consisted of 4 to 5 layers of transitional cells and lamina propria. Numerous microvasculatures were observed throughout the lamina propria and muscular layers. Each microvascular structure was surrounded by scattered smooth muscle bundles and connective tissues. In the Ovx group compared to the control, the histology of the bladder tissue showed relative thinning of the mucosal epithelium and atrophic change (Figure 2). In the Ovx+Est group, the above histologic changes were restored to the control level.
Figure 2.
Immunohistochemistry of AQP1 in urinary bladder tissue from animals of the control (Con), ovariectomy (Ovx), and ovariectomy plus 17β-estradiol treatment (Ovx+Est) groups. Immunolabeling of AQP appears in brown (arrows). AQP1 was mainly expressed in the capillaries and venules. The panels below are displayed at immunofluorescence stain in each group. The anti-AQP antibodies recognized 27-29 kDa bands, corresponding to glycosylated AQPs. Anti-actin antibody recognized the 42 kDa band. AQP-1 protein was present in all groups, and there were no significant changes among the groups. The right panels denote the means±standard deviation of 10 experiments for each condition determined by densitometry relative to GAPDH. *p<0.05 vs. control. **p<0.05 vs. Ovx. AQP=aquaporin; Con=control; Ovx=ovariectomy; Ovx+Est=ovariectomy plus 17β-estradiol treatment. Horizontal scale bar at the bottom of each figure indicates the magnification power.
Effect of hormonal alteration on the expression of AQP1 and eNOS
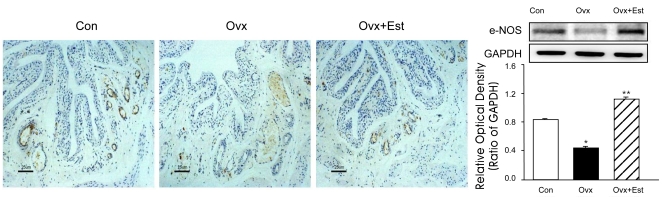
AQP1 was mainly expressed in the subepithelial capillaries and venules of the urinary bladder (Figure 2). Western blot analysis showed that the AQP1 protein was recognized in all groups, but there were no significant differences among the groups (Figure 2). Immunoreactivity for eNOS was also located in the capillaries and venules, the same areas of AQP1 (Figure 3). The protein expression of e-NOS was significantly lower in the ovariectomy group than in the control group. However, expression was restored to the control value after 17β-estradiol treatment (p<0.05) (Figure 3). Western blot analysis revealed bands at 28 kDa and 140 kDa for AQP1 and eNOS protein, respectively.
Figure 3.
Immunohistochemistry of eNOS in animals of the control (Con), ovariectomy (Ovx), and ovariectomy followed by 17β-estradiol treatment (Ovx+Est) groups. Immunoreactivity of endothelial nitric oxide synthase (eNOS) was detected in the capillaries and venules. The eNOS protein expression was significantly reduced after ovariectomy, but was restored to the control value after 17β-estradiol treatment. The right panels denote the means±standard deviation of 10 experiments for each condition determined by densitometry relative to GAPDH. *p<0.05 vs. control. **p<0.05 vs. Ovx. Con=control; Ovx=ovariectomy; Ovx+Est=ovariectomy plus 17β-estradiol treatment. Horizontal scale bar at the bottom of each figure indicates the magnification power.
Discussion
This study has demonstrated the altered expression of eNOS in the bladder following ovariectomy without a significant change in AQP1, which is located in the same location as eNOS in female rats. Four weeks after surgery, cystometric results showed that ovariectomy bladders exhibited a decreased voiding interval and increased voiding pressure compared with control bladders, and these indexes returned to the normal control value after estrogen treatment. In the ovariectomy group, the histological findings showed a decreased thickness of the urothelial layer. The immunohistochemical study showed that the localization of AQP1 and eNOS in the urinary bladder shared the same areas of capillaries, venules, and arterioles in subepithelial layers. However, AQP1 was not changed significantly between groups, whereas eNOS expression was significantly decreased after ovariectomy and restored to the control value after estrogen treatment. These results provide evidence that estrogen deprivation induces bladder instability; eNOS may play a role in the bladder dysfunction induced by female sex hormonal change. To our knowledge, this study is the first to shed some light on the possible occurrence of water and solute movement via AQPs in bladder dysfunction induced in an ovariectomy rat model.
Historically, the urinary bladder is a simple organ for the storage and emptying of urine. Urothelial impermeability is considered essential for maintaining a blood-urine barrier that allows urine to be stored for up to several hours without recirculation [10]. However, recent studies demonstrated that the urothelium is a responsive organ and it is now understood that the urothelium plays an active role in the regulation of bladder activity. Questions on whether the bladder epithelium has a role in water and solute movement from the urine to the systemic circulation have been raised. It has been noted that the urothelium has very low permeability to several urinary solutes and substrates. Mammalian urothelium modifies urine concentration and composition by reabsorbing potassium (K+), urea, and creatine and secreting sodium (Na+) [11,12], and the composition of urine changes during its transport through urine passages from the renal pelvis to the urinary bladder, a finding showing a modifying function of the urothelium [11]. A dramatic example of net water or solute movement is in hibernating bears, which are capable of reabsorbing their entire daily urine production during the months of winter [13]. Furthermore, Araki et al. [14] tried to investigate the role of the epithelial sodium channel (ENaC) in bladder overactivity in patients who were diagnosed clinically with bladder outlet obstruction. They showed that ENaC expression correlated significantly with the patients' storage symptom score, and the expression levels of ENaC in patients with bladder outlet obstruction were significantly greater than those of controls.
AQPs are family of hydrophobic intrinsic membrane proteins that function as water channels in many fluid-containing tissues, and they are involved in many physiological mechanisms including the transportation of transepithelial fluid, the concentration of urine, and gland fluid secretion [5]. Until now, there has been little research concerning AQPs in the urinary bladder. The water channel AQP1 is expressed strongly throughout the microvasculature of endothelial cells outside of the central nervous system in various organs [5]. Therefore, in this study we investigated the expression of AQPs with a focus on AQP1 expression in the urinary bladder to support the hypothesis of water movement in the bladder from the subepithelial capillaries to the urothelium.
Reports about the hormonal regulation of AQPs have implicated these proteins in the water movement in various organs. In reproductive organs, the expression profile of AQPs in the peri-implantation mouse uterus was down-regulated in ovariectomized mice and upregulated after hormonal replacement [15]. Jablonski et al. [16] explored the estrogen effect of water movement in the mouse uterine endometrium and explained the water movement across the endometrium by the mechanism of estrogen-stimulated water transport of AQPs in the uterus.
NO is thought to play a role in bladder overactivity and it has been strongly suggested that bladder instability may be associated with impairment in the NO signaling pathway [9]. NO induces vasodilatation and modulates microvascular permeability [17], and Hayashi et al. [18] reported that the vasoprotective effect of estrogen maybe mediated by increased expression and activity of e-NOS. The current study also showed an apparent downregulation of eNOS expression after ovariectomy and the restoration of expression in the rat urinary bladder by 17β-estradiol treatment.
The water transport mechanism of AQPs may be influenced by the NO-cGMP pathway. NOS has been implicated in modulating renal blood flow and in the regulation of sodium handling [19]. Several studies about the relationship between AQPs and NOS isoforms in water permeability have been reported. Bouley et al. investigated the functional role of NOS and AQPs in water movement in the kidney and showed that NO stimulates cGMP-dependent movement of AQP molecules from the epithelial cells to the cell membrane. In another report, Combet et al. used a rat peritonitis model to explain the role of AQP1 and NOS isoforms in the peritoneal membrane to explain the mechanism of ultrafiltration failure in dialysis [20]. The authors of the aforementioned study showed that the loss of water permeability and ultrafiltration failure in acute peritonitis is mainly due to increased peritoneal transport of small solutes, which might be primarily mediated by the increased NOS isoforms without a change in AQP1 expression. However, they explained that increased NO levels, secondary to eNOS upregulation, modify AQP1 and interfere with its function in endothelial cells of the peritoneal microvasculature.
These results support the findings of the current study, which show a significant change in eNOS isoforms without an apparent change in AQP1 expression in the urinary bladder after ovariectomy. In the current study, we found that ovariectomy induced epithelial thinning of the bladder mucosa and a significant decrease in eNOS protein expression, which were restored to the control value after estrogen treatment. It is possible that estrogen may interfere not with AQP1 but only with eNOS in subepithelial capillaries, which regulates blood flow and may induce water or solute movement from the capillaries to the subepithelial layer via increases of fluid pressure possibly through the modification of AQP1 in response to estrogen stimulation. The limitation of this study is that the precise functional relationship between AQP1 and eNOS isoforms has not been fully elucidated, even though we presented the possible relationship of AQPs and NOS isoforms in bladder dysfunction in a menopausal rat model. The potential role of AQPs in water transport in the urinary bladder might differ according to the types of AQPs. Further studies are needed to investigate the underlying mechanism of NOS isoforms and other types of AQPs to elucidate their functional role in bladder function.
Conclusions
Our studies have shown that detrusor overactivity induced by estrogen deprivation causes a significant decrease of eNOS expression without a significant change in AQP1 expression in rat urinary bladder. This may imply that estrogen deprivation-induced bladder dysfunction may be primarily due to the functional changes of eNOS isoforms and not directly to AQP1 in the urinary bladder.
References
- 1.Davila GW, Guerette N. Current treatment options for female urinary incontinence-a review. Int J Fertil Womens Med. 2004;49:102–112. [PubMed] [Google Scholar]
- 2.Batra SC, Iosif CS. Progesterone receptors in the female lower urinary tract. J Urol. 1987;138:1301–1304. doi: 10.1016/s0022-5347(17)43588-9. [DOI] [PubMed] [Google Scholar]
- 3.Lin AD, Levin R, Kogan B, Whitbeck C, Chichester P, Sokol R, et al. Estrogen induced functional hypertrophy and increased force generation of the female rabbit bladder. Neurourol Urodyn. 2006;25:473–479. doi: 10.1002/nau.20258. [DOI] [PubMed] [Google Scholar]
- 4.Zullo MA, Plotti F, Calcagno M, Palaia I, Muzii L, Manci N, et al. Vaginal estrogen therapy and overactive bladder symptoms in postmenopausal patients after a tension free vaginal tape procedure: A randomized clinical trial. Menopause. 2005;12:421–427. doi: 10.1097/01.GME.0000148645.93603.62. [DOI] [PubMed] [Google Scholar]
- 5.Verkman AS. More than just water channels: unexpected cellular roles of aquaporins. J Cell Sci. 2005;118:3225–3235. doi: 10.1242/jcs.02519. [DOI] [PubMed] [Google Scholar]
- 6.Spector DA, Wade JB, Dillow R, Steplock DA, Weinman EJ. Expression, localization, and regulation of aquaporin-1 to -3 in rat urothelia. Am J Physiol Renal Physiol. 2002;282:F1034–F1042. doi: 10.1152/ajprenal.00136.2001. [DOI] [PubMed] [Google Scholar]
- 7.Rubenwolf PC, Georgopoulos NT, Clements LA, Feather S, Holland P, Thomas DF, et al. Expression and localization of aquaporin water channels in human urothelium in situ and in vitro. Eur Urol. 2009;56:1013–1024. doi: 10.1016/j.eururo.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Combet S, Van Landschoot M, Moulin P, Piech A, Verbavatz JM, Goffin E, et al. Regulation of aquaporin-1 and nitric oxide synthase isoforms in a rat model of acute peritonitis. J Am Soc Nephrol. 1999;10:2185–2196. doi: 10.1681/ASN.V10102185. [DOI] [PubMed] [Google Scholar]
- 9.Mamas MA, Reynard JM, Brading AF. Nitric oxide and the lower urinary tract: current concept, future prospects. Urology. 2003;61:1079–1085. doi: 10.1016/s0090-4295(03)00131-6. [DOI] [PubMed] [Google Scholar]
- 10.Hohlbrugger G. The vesical blood-urine barrier: a relevant and dynamic interface between renal function and nervous bladder control. J Urol. 1995;154:6–15. doi: 10.1016/s0022-5347(01)67208-2. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Nielsen B. Excretion in mammals: role of the renal pelvis in the modification of the urinary concentration and composition. Fed Proc. 1977;36:2493–2503. [PubMed] [Google Scholar]
- 12.Levinsky NG, Berliner RW. Changes in composition of the urine in ureter and bladder at low urine flow. Am J Physiol. 1959;196:549–553. doi: 10.1152/ajplegacy.1959.196.3.549. [DOI] [PubMed] [Google Scholar]
- 13.Nelson RA, Jones JD, Wahner HW, McGill DB, Code CF. Nitrogen metabolism in bears: urea metabolism in summer starvation and in winter sleep and role of urinary bladder in water and nitrogen conservation. Mayo Clin Proc. 1975;50:141–146. [PubMed] [Google Scholar]
- 14.Araki I, Du S, Kamiyama M, Mikami Y, Matsushita K, Komuro M, et al. Overexpression of epithelial sodium channels in epithelium of human urinary bladder with outlet obstruction. Urology. 2004;64:1255–1260. doi: 10.1016/j.urology.2004.06.064. [DOI] [PubMed] [Google Scholar]
- 15.Richard C, Gao J, LaFleur B, Christman BW, Anderson J, Brown N, et al. Patency of the preterm fetal ductus arteriosus is regulated by endothelial nitric oxide synthase and is independent of vasa vasorum in the mouse. Am J Physiol Regul Integr Comp Physiol. 2004;287:R652–R660. doi: 10.1152/ajpregu.00049.2004. [DOI] [PubMed] [Google Scholar]
- 16.Jablonski EM, McConnell NA, Hughes FM, Jr, Huet-Hudson YM. Estrogen regulation of aquaporins in the mouse uterus: Potential roles in uterine water movement. Biol Reprod. 2003;69:1481–1487. doi: 10.1095/biolreprod.103.019927. [DOI] [PubMed] [Google Scholar]
- 17.Kone BC. Nitric oxide synthesis in the kidney: Isoforms, biosynthesis, and functions in health. Semin Nephrol. 2004;24:299–315. doi: 10.1016/j.semnephrol.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Yamada K, Esaki T, Mutoh E, Iguchi A. Effect of estrogen on isoforms of nitric oxide synthase: Possible mechanism of anti-atherosclerotic effect of estrogen. Gerontology. 1997;43:24–34. doi: 10.1159/000213883. [DOI] [PubMed] [Google Scholar]
- 19.Braam B. Renal endothelial and macula densa NOS: integrated response to changes in extracellular fluid volume. Am J Physiol. 1999;276:R1551–R1561. doi: 10.1152/ajpregu.1999.276.6.R1551. [DOI] [PubMed] [Google Scholar]
- 20.Combet S, Van Landschoot M, Moulin P, Piech A, Verbavatz JM, Goffin E, et al. Regulation of aquaporin-1 and nitric oxide synthase isoforms in a rat model of acute peritonitis. J Am Soc Nephrol. 1999;10:2185–2196. doi: 10.1681/ASN.V10102185. [DOI] [PubMed] [Google Scholar]