Abstract
Purpose
Benign prostatic hyperplasia is often accompanied by age-related comorbidity, such as erectile dysfunction (ED). Recent data suggest an association between ED and lower urinary tract symptoms (LUTS), and increasing evidence indicates that the clinical use of phosphodiesterase type 5 (PDE5) inhibitors provides relief from LUTS. The aim of the present study was to investigate the effects of tadalafil (20 mg once every 3 days for 12 weeks, p.o.) in men with moderate-to-severe ED and LUTS and to investigate the duration of the effects of tadalafil beyond treatment cessation.
Materials and Methods
Men with an International Index of Erectile Function-5 (IIEF-5) score of less than 11 (representing "moderate-to-severe" ED status) and with an International Prostate Symptom Score (IPSS) of more than 8 (representing "moderate-to-severe" LUTS status) were enrolled. IPSS (total score, storage subscore, and voiding subscore) and IIEF-5 scores before treatment (baseline), during treatment (weeks 4 and 12 after treatment commencement), and after treatment (weeks 16 and 20after treatment commencement) were compared.
Results
IPSS and IIEF-5 scores were significantly different between baseline and week 12 after treatment commencement. Furthermore, these scores were significantly different between baseline and week 20 after treatment commencement. However, except for IIEF-5 scores, no significant differences were observed between week 12 and week 20.
Conclusions
Treatment with 20 mg tadalafil (once every 3 days) had beneficial effects on LUTS and ED beyond treatment cessation in patients with moderate-to-severe ED and LUTS.
Keywords: Tadalafil, Erectile dysfunction, Benign
Introduction
Benign prostatic hyperplasia (BPH) is typically characterized by an enlargement of the prostate gland, constriction of the urethra, and the emergence of moderate-to-severe lower urinary tract symptoms (LUTS). LUTS associated with BPH are bothersome and can disrupt normal daily activities and consequently have a substantial impact on the quality of life (QoL) [1,2].
Current medical treatment options for BPH include 5-alpha reductase inhibitors, which block the conversion of testosterone to the more potent dihydrotestosterone, and alpha-adrenergic receptor antagonists, which affect smooth muscle tone by lowering elevated sympathetic adrenergic activity, a common contributor to BPH symptoms [3].
The primary risk factor for BPH is advanced age, and BPH is often accompanied by age-related comorbidities, such as erectile dysfunction (ED), cardiovascular disease, and metabolic syndrome [4,5]. Furthermore, recent reports suggest an association between ED and LUTS, and anecdotally, some patients cite improvements in LUTS when they are taking sildenafil.
Phosphodiesterase (PDE) enzymes are involved in the regulation of the nitric oxide (NO)-cyclic GMP-protein kinase pathway and hence influence smooth muscle tone. Furthermore, the presence of these androgen-regulated enzymes in the urogenital tract is well established [6,7].
Many studies have demonstrated the effects of PDE5 inhibitors on LUTS and ED. However, it was not established in these studies whether chronic administration of PDE5 inhibitors offers sustainable benefits to patients with LUTS and ED after treatment discontinuation.
Thus, the objective of the present study was to investigate the sustainable effects of tadalafil (20 mg once every 3 days for 12 weeks, p.o.) on ED and LUTS beyond the treatment period.
Materials and Methods
This was a prospective, open-label study conducted at a single hospital in accordance with the guidelines of the International Conference on Harmonisation/World Health Organization Good Clinical Practice Standards and the Principles of the Declaration of Helsinki. The study protocol was reviewed and approved by the Gachon University Gil Hospital Institutional Review Board.
Among men aged 45 to 64 with a history of ED for at least 3 months, men with an International Index of Erectile Function-5 (IIEF-5) score of less than 11 (representing an ED status of "moderate-to-severe") and an International Prostate Symptom Score (IPSS) of more than 8 (representing a LUTS status of "moderate-to-severe") were enrolled. All patients were provided written, informed consent before beginning the study.
The exclusion criteria were contraindications to tadalafil, spinal cord injury, prostatitis, a history of prostate or bladder cancer, bladder neck or urethral stricture, urinary retention (postvoid residual volume ≥ 100 ml), pelvic trauma or surgery, a history of any malignancy, and a life expectancy of less than 3 years. Concomitant use of nitrates or NO donors, androgens or anti-androgens, anticoagulants, cytochrome P-450 3A4 inhibitors, or alpha1-adrenoceptor antagonists or any treatment for ED was prohibited. The total treatment period was 12 weeks.
Assessments of IPSS (total score, storage subscore, and voiding subscore) and IIEF-5 scores were carried out at week 0 (baseline), week 12, and week 20 after treatment commencement. Comparisons were made between IPSS and IIEF-5 scores before treatment (week 0), during treatment (week 12), and after treatment (week 20).
Data are expressed as means±SDs. Student's t-test was used to compare IIEF-5 and IPSS scores before, during, and after treatment. Differences with p values of <0.05 were considered significant. All data were analyzed by using SPSS version 12.0 (SPSS Inc., Chicago, IL).
Results
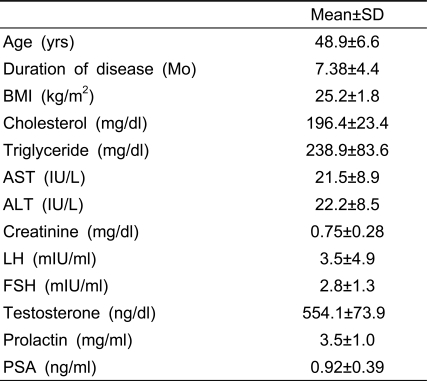
In total, 17 patients were recruited, but only 13 completed the study. Patient characteristics are shown in Table 1. The mean patient age was 48.9±6.6 years, and the mean disease duration was 7.4±4.4 months. There were no abnormal blood test findings.
Table 1.
Characteristics of the patients
BMI: body mass index, LH: luteinizing hormone, FSH: follicle stimulating hormone, PSA: prostatic specific antigen
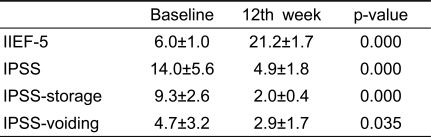
As shown in Table 2, significant differences were found for IIEF-5 and IPSS scores between baseline (before treatment) and week 12 (during treatment), indicating that treatment with 20 mg tadalafil improved LUTS and ED.
Table 2.
Comparisons between baseline and 12th week
IIEF-5: the International Index of Erectile Function, IPSS: International Prostate Symptom Score, IPSS-storage: IPSS storage subscore, IPSS-voiding: IPSS voiding subscore
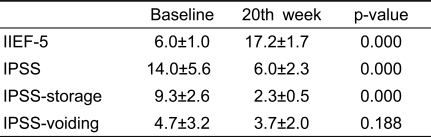
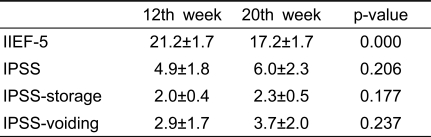
With the exception of IPSS-voiding scores, significant differences were also observed between baseline (before treatment) and week 20 (after treatment) (Table 3). However, except for IIEF-5 scores, no significant differences were observed between week 12 (during treatment) and week 20 (after treatment) (Table 4). These results showed that chronic treatment with PDE5 inhibitors had sustained effects on ED and LUTS beyond treatment completion.
Table 3.
Comparisons between baseline and 20th week
IIEF-5: the International Index of Erectile Function, IPSS: International Prostate Symptom Score, IPSS-storage: IPSS storage subscore, IPSS-voiding: IPSS voiding subscore
Table 4.
Comparisons between 12th and 20th week
IIEF-5: International Index of Erectile Function, IPSS: International Prostate Symptom Score, IPSS-storage: IPSS storage subscore, IPSS-voiding: IPSS voiding subscore
Despite a significant decrease in mean IIEF-5 scores after treatment cessation versus immediately before cessation, IIEF-5 scores at week 20 showed a significant improvement versus baseline. The mean IPSS at week 20 also showed a significant improvement versus baseline. These sustainable effects of PDE5 inhibitors on LUTS after treatment cessation resulted from improved storage symptoms rather than improved voiding symptoms (Tables 2, 3, and 4).
Discussion
PDE5 inhibitors are widely accepted as a first-line treatment for ED [8-10]. PDE5 inhibitor therapy is currently administered "on demand" before sexual activity [11,12]. However, it is known that on-demand therapy does not change the pathophysiology of ED. Christiansen et al [13] reported that, for most patients, sildenafil treatment must be continued for improvements in erectile function to be maintained. Accordingly, it has been suggested that an alternative dosing regimen, such as once-daily dosing, may offer benefits to patients, such as the ability to perform intercourse spontaneously [14].
Furthermore, it has been reported that chronic treatment with PDE5 inhibitors improves spontaneous erectile function. In a prospective open-label trial by Sommer et al [15], 112 men with ED were randomly assigned to receive sildenafil at 50 or 100 mg nightly or as needed for 12 months, followed by a 1-month or a 6-month nonmedicated period. IIEF and peak systolic velocity (PSV) of the penile cavernous arteries were used to measure efficacy. After sildenafil treatment and a 1-month nonmedicated period, IIEF-EF (the erectile function domain of the IIEF) scores were normal in 60.4% of the nightly group vs. in 8.2% of the as-needed group. PSV improved by 11.2 cm/s in the nightly group but by only 3.4 cm/s in the as-needed group. Six months after treatment, IIEF-EF remained normal in 97% of those with a normal IIEF-EF score at 1 month after treatment cessation. Sommer et al [15] suggested that the results were due to an improvement in endothelial function and the oxygenation of penile tissues.
Many clinical studies have reported beneficial effects of once-daily dosing of PDE5 inhibitors [16] thus, it was proposed that once-daily dosing with a PDE5 inhibitor may confer clinical benefits versus on-demand dosing [17]. Furthermore, recent clinical studies have demonstrated that daily administration of vardenafil or tadalafil improved erectile function in men with ED versus baseline levels [18].
However, increasing the dosing frequency of a drug can be burdensome to patients and may reduce compliance. For example, adherence to recommended long-term treatment regimens among patients with a chronic disease was found to be suboptimal [19], and in another study, it was suggested that it may be as low as 50% [20]. In addition, the costs associated with increasing dosing frequencies must be considered. Accordingly, an optimal dosing frequency is required.
In a multicenter, crossover, open-label study [21] conducted in 14 European countries, men with ED (N=4262) were randomly assigned to tadalafil (20 mg) on demand (maximum of one dose per day and before sexual activity) or 3 times per week for 5 to 6 weeks. After a 1-week washout period, patients were crossed over to the other regimen for 5 to 6 weeks. It was found that 57.8% of men preferred the on-demand regimen of tadalafil (20 mg), but that a substantial proportion (42.2%) preferred the 3 times per week treatment. In terms of effectiveness, the two regimens were similar. Thus, in view of patient burden and regimen effectiveness, we administered tadalafil (20 mg) once every 3 days.
Few studies have revealed why chronic administration of a PDE5 inhibitor has a sustained effect after treatment cessation. However, the presence of PDE5 in the urogenital tract is well established [6,7]. PDE5 inhibitors have been shown to inhibit prostate stromal cell proliferation in vitro [22]. Furthermore, these agents have been shown to induce relaxations of bladder, urethral, and prostatic smooth muscle and to relieve the storage symptoms of BPH in vivo [22,23]. Furthermore, increasing evidence supports the clinical use of PDE5 inhibitors to provide relief from LUTS, and randomized, placebo-controlled trials have been performed with sildenafil and with tadalafil in this setting [24,25]. In 2007, McVary et al [25] performed a randomized, double-blind, placebo-controlled study in men with moderate-to-severe LUTS secondary to BPH. The authors introduced daily dosing of tadalafil and concluded that tadalafil (once daily) is well tolerated and induces clinically meaningful and significant improvements in LUTS associated with BPH [25].
Few studies have been conducted using our regimen, and few studies have evaluated the effects of this regimen on LUTS or ED. This is the first study to describe the effects of 20 mg tadalafil on LUTS and ED. On the other hand, many studies have investigated the effects of chronic treatment with PDE5 inhibitors on ED and LUTS. However, few studies have investigated whether chronic administration offers a sustained clinical benefit to patients with LUTS and ED. In the present study, we compared sustained improvements in LUTS and ED through the administration of tadalafil in men with moderate-to-severe LUTS and ED.
The number of patients undergoing medical therapy for ED associated with LUTS is increasing, and this trend is likely to continue as life expectancy increases. A larger, rigorous, comparative clinical trial is needed to assess the efficacy of tadalafil on LUTS and ED.
In the present study, chronic treatment with tadalafil was found to have a sustained effect on ED and LUTS after treatment cessation. In particular, significant improvements in ED and LUTS were observed at week 8 after treatment completion versus baseline. These sustainable effects of PDE5 inhibitors on LUTS after treatment cessation resulted from improved storage symptoms rather than improved voiding symptoms.
Conclusions
Our findings suggest that tadalafil (20 mg once every 3 days for 12 weeks) achieves sustainable effects on LUTS and ED after treatment cessation in patients with moderate-to-severe ED and LUTS.
References
- 1.Calais Da Silva F, Marquis P, Deschaseaux P, Gineste JL, Cauquil J, Patrick DL. Relative importance of sexuality and quality of life in patients with prostatic symptoms. Results of an international study. Eur Urol. 1997;31:272–280. doi: 10.1159/000474467. [DOI] [PubMed] [Google Scholar]
- 2.Kirby RS. The natural history of benign prostatic hyperplasia: what have we learned in the last decade? Urology. 2000;56:3–6. doi: 10.1016/s0090-4295(00)00747-0. [DOI] [PubMed] [Google Scholar]
- 3.Madersbacher S, Marszalek M, Lackner J, Berger P, Schatzl G. The long-term outcome of medical therapy for BPH. Eur Urol. 2007;51:1522–1533. doi: 10.1016/j.eururo.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Kasturi S, Russell S, McVary KT. Metabolic syndrome and lower urinary tract symptoms secondary to benign prostatic hyperplasia. Curr Urol Rep. 2006;7:288–292. doi: 10.1007/s11934-996-0008-y. [DOI] [PubMed] [Google Scholar]
- 5.Costabile RA, Steers WD. How can we best characterize the relationship between erectile dysfunction and benign prostatic hyperplasia? J Sex Med. 2006;3:676–681. doi: 10.1111/j.1743-6109.2006.00274.x. [DOI] [PubMed] [Google Scholar]
- 6.Uckert S, Kuthe A, Jonas U, Stief CG. Characterization and functional relevance of cyclic nucleotide phosphodiesterase isoenzymes of the human prostate. J Urol. 2001;166:2484–2490. [PubMed] [Google Scholar]
- 7.Uckert S, Oelke M, Stief CG, Andersson KE, Jonas U, Hedlund P. Immunohistochemical distribution of cAMP- and cGMP-phosphodiesterase (PDE) isoenzymes in the human prostate. Eur Urol. 2006;49:740–745. doi: 10.1016/j.eururo.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz ER, Kapur V, Rodriguez J, Rastogi S, Rosanio S. The effects of chronic phosphodiesterase-5 inhibitor use on different organ systems. Int J Impot Res. 2007;19:139–148. doi: 10.1038/sj.ijir.3901491. [DOI] [PubMed] [Google Scholar]
- 9.Ziegler D, Merfort F, van Ahlen H, Yassin A, Reblin T, Neureither M. Efficacy and safety of flexible-dose vardenafil in men with type 1 diabetes and erectile dysfunction. J Sex Med. 2006;3:883–891. doi: 10.1111/j.1743-6109.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 10.Rubio-Aurioles E, Porst H, Eardley I, Goldstein I. Comparing vardenafil and sildenafil in the treatment of men with erectile dysfunction and risk factors for cardiovascular disease: a randomized, double-blind, pooled crossoverstudy. J Sex Med. 2006;3:1037–1049. doi: 10.1111/j.1743-6109.2006.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Padma-Nathan H. PDE-5 Inhibitor Therapy for Erectile Dysfunction Secondary to Nerve-Sparing Radical Retropubic Prostatectomy. Rev Urol. 2005;7(Suppl 2):S33–S38. [PMC free article] [PubMed] [Google Scholar]
- 12.Aversa A, Bruzziches R, Pili M, Spera G. Phosphodiesterase 5 inhibitors in the treatment of erectile dysfunction. Curr Pharm Des. 2006;12:3467–3484. doi: 10.2174/138161206778343046. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen E, Guirguis WR, Cox D, Osterloh IH. Long-term efficacy and safety of oral Viagra (sildenafil citrate) in men with erectile dysfunction and the effect of randomised treatment withdrawal. Int J Impot Res. 2000;12:177–182. doi: 10.1038/sj.ijir.3900527. [DOI] [PubMed] [Google Scholar]
- 14.McMahon CG. Treatment of erectile dysfunction with chronic dosing of tadalafil. Eur Urol. 2006;50:215–217. doi: 10.1016/j.eururo.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 15.Sommer F, Klotz T, Engelmann U. Improved spontaneous erectile function in men with mild-to-moderate arteriogenic erectile dysfunction treated with a nightly dose of sildenafil for one year: a randomized trial. Asian J Androl. 2007;9:134–141. doi: 10.1111/j.1745-7262.2007.00233.x. [DOI] [PubMed] [Google Scholar]
- 16.Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47:214–220. doi: 10.1016/j.eururo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Sommer F, Schulze W. Treating erectile dysfunction by endothelial rehabilitation with phosphodiesterase 5 inhibitors. World J Urol. 2005;23:385–392. doi: 10.1007/s00345-005-0021-7. [DOI] [PubMed] [Google Scholar]
- 18.Porst H, Giuliano F, Glina S, Ralph D, Casabe AR, Elion-Mboussa A, et al. Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5mg and 10mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol. 2006;50:351–359. doi: 10.1016/j.eururo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 19.Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38:S2–S6. doi: 10.1016/j.bone.2006.01.150. [DOI] [PubMed] [Google Scholar]
- 20.Burkhart PV, Sabate E. Adherence to long-term therapies: evidence for action. J Nurs Scholarsh. 2003;35:207. [PubMed] [Google Scholar]
- 21.Mirone V, Costa P, Damber JE, Holmes S, Moncada I, Van Ahlen H, et al. An evaluation of an alternative dosing regimen with tadalafil, 3 times/week, for men with erectile dysfunction: SURE study in 14 European countries. Eur Urol. 2005;47:846–854. doi: 10.1016/j.eururo.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Tinel H, Stelte-Ludwig B, Hutter J, Sandner P. Pre-clinical evidence for the use of phosphodiesterase-5 inhibitors for treating benign prostatic hyperplasia and lower urinary tract symptoms. BJU Int. 2006;98:1259–1263. doi: 10.1111/j.1464-410X.2006.06501.x. [DOI] [PubMed] [Google Scholar]
- 23.Filippi S, Morelli A, Sandner P, Fibbi B, Mancina R, Marini M, et al. Characterization and functional role of androgen-dependent PDE5 activity in the bladder. Endocrinology. 2007;148:1019–1029. doi: 10.1210/en.2006-1079. [DOI] [PubMed] [Google Scholar]
- 24.McVary KT, Monnig W, Camps JL, Jr, Young JM, Tseng LJ, van den EG. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol. 2007;177:1071–1077. doi: 10.1016/j.juro.2006.10.055. [DOI] [PubMed] [Google Scholar]
- 25.McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, et al. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol. 2007;177:1401–1407. doi: 10.1016/j.juro.2006.11.037. [DOI] [PubMed] [Google Scholar]