Abstract
Purpose
To evaluate the efficacy and safety of the tension-free placement of a monofilament polypropylene mesh for the repair of an anterior vaginal wall prolapse (AVWP).
Materials and Methods
Women aged ≥ 30 years with an AVWP stage of II or greater were included. Forty-nine women underwent trans-vaginal repair using a Gynemesh™ PS. Forty-six women who had symptomatic stress urinary incontinence received a midurethral sling (MUS). At the 12-month follow-up, evaluations were made for changes in the Pelvic Organ Prolapse Quantification (POP-Q) stage and Pelvic Floor Distress Inventory. Cure was defined as a POP-Q stage of 0 and improvement as a stage of I. Complications were also evaluated.
Results
The cure rate was 71.4%, and the improvement rate was 18.4%. Obstructive/discomfort, irritative, and stress subscale scores of the Urinary Distress Inventory anterior and posterior subscale scores of the POP Distress Inventory and the obstructive subscale score of the Colo-Rectal-Anal Distress Inventory were significantly improved. Thirty-two of the 46 women (69.6%) who received MUS procedures reported no leakage after surgery. Complications were 2 cases of increased intraoperative bleeding and 1 case of vaginal erosion.
Conclusions
Trans-vaginal repair using a Gynemesh™ PS is a feasible and effective procedure for the treatment of AVWP with no significant complications.
Keywords: Pelvic organ prolapse, Lower urinary tract symptoms, Surgery
Introduction
Pelvic organ prolapse (POP) is a common condition that negatively affects the quality of life of a woman. According to research performed in the United States, the lifetime risk of undergoing a single operation for POP or incontinence is 11.1%; up to 30% of those patients will undergo a repeat surgery [1]. In Korea, it is estimated that approximately 32% of the female population has some degree of POP. The anterior vaginal wall is the most common compartment to suffer from prolapse [2]. Anterior vaginal wall prolapse may coexist with lower urinary tract symptoms, pelvic pain or discomfort, and sexual dysfunction [3]. Therefore, restoration of the anatomical defects should be accompanied by improvement of the associated symptoms.
A wide variety of trans-abdominal and transvaginal procedures have been developed for the repair of an anterior wall prolapse. However, the failure or recurrence rate has been reported to be as high as 30% to 50%, regardless of the approach or the technique [4,5]. In a repair of an anterior vaginal wall prolapse, because the pubocervical fascia becomes detached or torn, it is necessary to restore the natural function of the fascia. However, reinforcement of the pubocervical fascia by plication of the damaged fascia or through the placement of an absorbable mesh results in an unacceptably high recurrence rate [4-6]. Accordingly, a nonabsorbable mesh has been used to reinforce or replace the natural structures.
Although the ideal graft has not yet been developed, polypropylene monofilament macrospore mesh appears to be the best at present. It has been shown to have a low risk of infection, long-term stability, and acceptable complication rates [7].
Herein, the purpose of this study was to assess the anatomical and functional outcomes of tension-free placement of a polypropylene monofilament mesh, Gynemesh™ PS (Gynecare, Ethicon Inc., Somerville, NJ, USA), for the treatment of an anterior vaginal wall prolapse.
Materials and methods
This was a prospective, multicenter, observational study. The surgery was performed by six urologists at six university hospitals. Written informed consent was obtained from all participants. The institutional review boards of each study center approved the study protocol.
Participants
A total of 49 consecutive women aged 30 years or older who presented with stage II or greater anterior vaginal wall prolapse underwent tension-free placement of a polypropylene monofilament mesh with a Gynemesh™ PS. The exclusion criteria were severe vaginal atrophy, history of pelvic irradiation therapy, pregnancy or contemplating pregnancy during the study period, and high risk for surgery.
Procedure
All patients were given intravenous antibiotics and iodine antiseptic vaginal suppositories one day before the surgery. The surgeries were performed in the lithotomy position under spinal or general anaesthesia. After the infiltration of mixed normal saline with 1:200,000 epinephrine into the anterior vaginal wall, a vertical midline incision was made from the level of the urethrovesical junction to the uterine cervix. The vaginal epithelium was dissected bilaterally through this incision to the tendineus arc, and the endopelvic fascia was perforated into the retropubic space. A central defect was reinforced by plication of the pubocervical fascia with a 2-0 Vicryl suture. The Gynemesh™ PS was designed and tailored, leaving two tabs on each side. The mesh was approximately 13.7 cm long and 2.4 cm wide (Figure 1) and was positioned by placing the tabs into the retropubic space in a tension-free fashion without sutures. The mesh and pubocervical fascia were sutured by using 3-0 Vicryl at four points. Redundant vaginal mucosa was not excised. The wound was closed by using a 2-0 Vicryl suture in a continuous manner. For women who presented with combined posterior vaginal wall prolapse, posterior colporrhaphy (PC) was performed concomitantly. For women with combined apical vaginal wall prolapse, posterior intravaginal slingplasty (P-IVS) was performed. A concomitant midurethral sling procedure was performed for women with both symptomatic and occult stress urinary incontinence (SUI). The additional procedures were performed after completion of the surgery for anterior vaginal wall prolapse. Vaginal packing and a urethral catheter were left in place for 48 hours. Intravenous antibiotics were given postoperatively for 24 hours, and oral treatment was continued for 14 days.
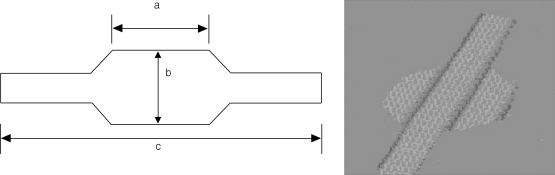
Figure 1.
Prosthesis shape: schematic representation (left panel) and photocopy (right panel).
Mesh size (mean±SD); a: 3.30±1.34 cm, b: 2.37±0.94 cm, c: 13.7±2.59 cm. The tabs of each side of the mesh were inserted into the sides of the retropubic space and were left tension-free.
Assessment
Baseline demographic data were collected. These data included age, body mass index, obstetric and gynecologic history, and medical and surgical history. All subjects underwent a pelvic examination in the seated semi-lithotomy position with the Valsalva maneuver. The Pelvic Organ Prolapse Quantification (POP-Q) standard scoring system and the 3×3 grid system were adopted for staging and recording the nine points of the POP-Q system [8]. During the physical examination, a stress test was performed after reduction of the prolapse to rule out occult SUI. When women presented with lower urinary tract symptoms, including SUI, a multi-channel urodynamic study was performed with reduction of the prolapse. The Pelvic Floor Distress Inventory (PFDI) [9], 3-day frequency volume charts, maximal flow rates (MFRs), and postvoid residuals (PVRs) were also evaluated.
At the 12-month postoperative visit, the POP-Q stage and the 9 points were evaluated to assess the anatomical results. Cure of the anterior vaginal wall prolapse was defined when the postoperative POP-Q system point Ba was found to be stage 0, i.e., the quantitation value for point Ba was ≤-[total vaginal length (TVL)-2] cm. Improvement was defined as stage I i.e., its quantitation value for point Ba was <-1 and >-[TVL-2] cm. Recurrence was assigned to the postoperative POP-Q stage II or greater, i.e., its quantitation value for point Ba was was ≥-1 cm. Cure and improvement were regarded as successful anatomical results. Surgical outcomes of the combined surgery for posterior or apical vaginal prolapses were assessed in the same manner, which defined cure as stage 0, improvement as stage I and recurrence as stage II or greater, as described in the POP-Q system [8]. Cure of SUI was considered when the women reported no leakage with stress or exercise after surgery. Postoperative changes in the PFDI, frequency volume chart, MFR, and PVR were evaluated to assess the functional outcomes. The Patient's Global Impression of Improvement (PGI-I) was evaluated to measure the subjective perception of symptom improvement. Intra- and postoperative complications were also evaluated.
Analysis
Postoperative changes in the POP-Q stage, PFDI, frequency volume chart, MFR, and PVR were evaluated. Statistical significance was determined by using a Wilcoxon signed rank test or a paired t-test according to the normality assumption. All statistical analyses were performed by using SPSS 17.0 software (SPSS, Chicago, IL, USA). A p-value of < 0.05 was considered to be statistically significant.
Results
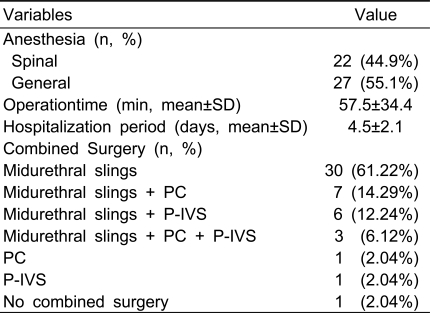
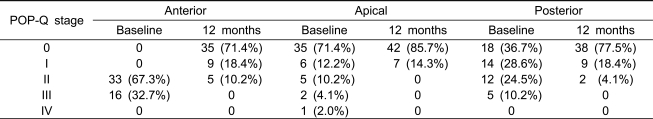
Table 1 shows the baseline characteristics of the 49 women. The average age of the women was 55.1±9.8 years, and their average body mass index was 21.8±4.2kg/m2. No woman had a previous history of surgery for POP. Forty-six (93.9%) women received concomitant midurethral sling procedures for symptomatic or occult SUI. Eleven (22.4%) women underwent PC for posterior vaginal wall prolapse,and 10 (20.4%) women underwent P-IVS for uterine or vaginal vault prolapse. The average duration of surgery was 57.5±34.4 minutes. Table 2 shows the intraoperative data.
Table 1.
Baseline patients' characteristics (n=49)
BMI; body mass index, C/S; caesarean section
Table 2.
Intra-operative findings (n=49)
PC; posterior colporrhaphy, P-IVS; posterior slingplasty
Anatomical outcomes
Thirty-three of the 49 patients (67.3%) had stage II prolapse on the preoperative pelvic examination, and 16 (32.6%) had stage III anterior prolapse. Pre- and postoperative POP-Q stages are shown in Table 3. The cure rate at the 12-month postoperative evaluation was 71.4% (35/49), with an improvement rate of 18.4% (9/49). These results provided a successful anatomical result in 89.8% (44/49) of the women. The recurrence rate was 10.2% (5/49). Three of the patients who suffered from a recurrence had a preoperative POP stage of III, and two had a preoperative POP stage of II. All the patients who suffered a recurrence had a stage II recurrence of the anterior vaginal wall prolapse.
Table 3.
Changes in the POP-Q stage of the anterior, apical, and posterior compartments from baseline to 12-months post-operative (n=49).
POP-Q; pelvic organ prolapse quantification
The cure and improvement rates for the apical prolapse were 85.7% (42/49) and 14.3% (7/49), respectively. The cure and improvement rates for the posterior prolapse were 77.5% (38/49) and 18.4% (9/49), respectively. The two patients who presented with a recurrent posterior vaginal wall prolapse had not undergone surgery for the posterior prolapse, and their preoperative POP-Q stage was II (Table 3).
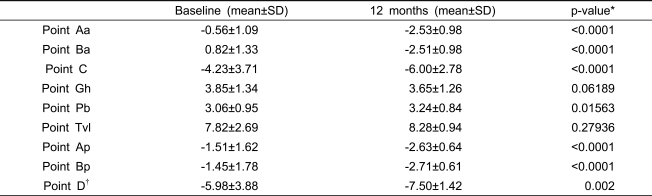
Postoperative changes in the 9 points are summarized in Table 4. The average Ba level changed from 0.82 (±1.33) to -2.51 (±0.98) (p<0.0001), the average C level changed from -4.23 (±3.71) to -6.00 (±2.78) (p<0.0001), and the average Bp level changed from -1.45 (±1.78) to -7.50 (±1.42) (p=0.002).
Table 4.
Changes in the nine points of the POP-Q system from baseline to 12-months post-operative (n=49).
*; Wilcoxon signed rank test.
†; n=43, who had not undergone priorhysterectomy
Functional outcomes
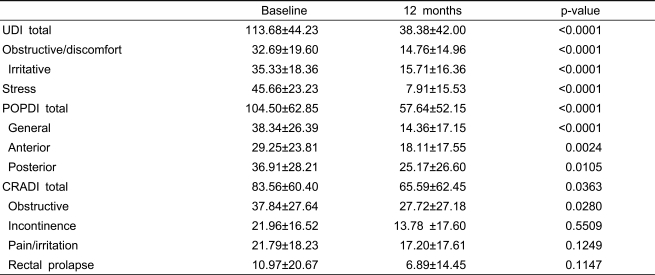
The obstructive/discomfort, irritative, and stress subscale scores of the Urinary Distress Inventory were significantly improved. The anterior and posterior subscale scores of the Pelvic Organ Prolapse Distress Inventory and the obstructive subscale score of the Colo-Rectal-Anal Distress Inventory were also significantly improved (Table 5). Thirty-two of the 46 women (69.6%) who received midurethral sling procedures reported having no leakage with stress or exercise. None of the women in the study reported de novo stress incontinence. The pre- and postoperative MFRs were 22.7 ml/s (±9.4) and 21.3 ml/s (±11.6) (p=0.437), respectively. The pre- and postoperative PVRs were 28.8 ml (±44.9) and 18.8 ml (±25.2) (p=0.176), respectively. Micturition frequency was significantly decreased after surgery from 8.7 per 24 hours (±2.7) to 7.5 per 24 hours (±2.1) (p=0.010), although the number of nocturia episodes did not change significantly [1.2 per 24 hours (±1.0) to 0.7 per 24 hours (±0.8), p=0.070)]. Functional bladder capacity also was not changed significantly [297 ml (±103) to 322 ml (±118), p=0.205]. Overall, 87.7% (43/49) of the patients reported having "much improvement" on the PGI-I questionnaire, 8.2% (4/49) reported "a little improvement," and 4.1% (2/49) reported "no improvement."
Table 5.
Changes in the Pelvic Floor Distress Inventory from baseline to 12-months post-operative (n=49).
UDI; urinary distress inventory
POPDI; pelvic organ prolapse distress inventory
CRADI; colo-rectal-anal distress inventory
Complications
Three patients suffered intraoperative or postoperative complications. Two of these patients received blood transfusions for increased intraoperative bleeding. In both cases, the bleeding began after perforation of the endopelvic fascia and was controlled conservatively with local packing. One of the patients had a concomitant TVT (Ethicon, Somerville, NJ, USA) procedure. Another patient had a TVT-O (Ethicon, Somerville, NJ, USA) procedure and a PC. This woman also had a tape-releasing procedure three days after the surgery as the result of immediate postoperative retention. The voiding symptoms were improved without a recurrence of the urinary stress incontinence.
One woman was diagnosed with asymptomatic minimal vaginalerosion at the 12-month follow-up visit. She had undergone a concomitant TVT and PC. The erosion was treated by partial excision of the mesh and mucosal closure. This procedure was done under local anesthesia in the operating room. The wound was healed without infection or recurrence of the prolapse. There were no other complications, including bladder injury or mesh infection.
Discussion
The anatomical success rate for anterior vaginal wall repair with a tension-free polypropylene mesh (Gynemesh™ PS) was 89.8% after 12 months of follow-up. Improvement in subjective symptoms was reported in 95.9% of the patients. The procedure was found to improve the associated urinary and pelvic floor symptoms. The excellent anatomical results of our study correspond with the 89% objective cure rate reported by de Tayrac et al. [10], whose study had a mean follow-up period of 37 months. Short-term and medium-term cure rates for anterior vaginal wall repair with a Gynemesh™ PS range from 90% to 100% [10-13]. Although there are a variety of forms and sizes, reinforcement of anterior wall defects with a polypropylene mesh provides a superior anatomical result compared with a traditional anterior colporrhaphy or a repair with absorbable mesh [4-6]. The first randomized, controlled study using polypropylene mesh was conducted by Julian in 1996 [14]. That study involved 24 women who had severe recurrent anterior vaginal wall prolapse and used Marlex [14]. Four patients (33.3%) in the control group (who received only an anterior colporrhaphy) and no patients in the treatment group (who received a polypropylene mesh) had recurrent prolapse after 24 months [14]. According to a nonrandomized, retrospective, comparative study, 4% of patients who had a polypropylene mesh experienced a recurrence, compared with 36% of patients who had a porcine dermal graft or 6% who had a traditional anterior colporrhaphy [15].
The high recurrence rate seen with the traditional anterior colporrhaphy could be explained by the fact that it corrects a midline defect only by plication of the weakened and damaged pubocervical fascia. According to Richardson et al. [16], anterior wall prolapse is attributable to four types of defects: transverse, midline, lateral, and pubourethral. Transverse defects happen when the pubocervical fascia is separated from its insertion around the cervix. Midline defects involve an anteroposterior separation of the fascia between the bladder and the vagina. Compared with traditional surgery, our procedure places mesh under the bladder and laterally into the retropubic space. This reinforces and replaces the central and lateral supports of the anterior vaginal wall. However, we had five patients who had persistent or recurrent anterior vaginal wall prolapse. According to DeLancey [17], the arcus tendineus fascia pelvis is detached posteriorly from the ischial spine in most patients who suffer from anterior wall prolapse. Because of this, reinforcement of the supporting tissue between the arcus tendineus fascia pelvis and the ischial spine could provide an additional support for the anterior vaginal wall in our patients with recurrent prolapse.
Severe anterior wall prolapse may coexist with urinary, bowel, and sexual symptoms for which treatment is indicated. The goal of surgery for anterior prolapse includes restoration or maintenance of normal bladder, bowel, and sexual function. Therefore, functional results should be considered to be as important as anatomical outcomes after surgery. Our results show that functional outcomes were correlated with anatomical success. Obstructive, irritative, and urinary stress symptoms, as well as pelvic organ prolapse distress symptoms, were correlated with positive anatomical results. Functional bladder outlet obstruction caused by urethral distortion may contribute to the obstructive and irritative urinary symptoms. In addition, SUI can be masked by the functional obstruction, and de novo incontinence can develop after correction of the prolapse. De novo SUI is one of the outcomes that was evaluated in the POP study. Concomitant anti-incontinence surgeries were performed in most of the patients in our study. None of the patients in our study developed de novo stress incontinence. One patient did suffer from immediate postoperative retention associated with the TVT-O procedure. None of the women had a significant increase in their PVR. The results of this study also showed excellent improvements in the patients' perceptions of symptom improvement, even with a relatively short-term follow-up. According to de Tayrac et al. [10], more than 95% of the women in that study expressed good satisfaction over the 5-year follow-up period.
Mono filament macroporous (pore size > 75 µ m) polypropylene mesh is preferred for transvaginal surgery for POP or stress incontinence because of the low risk of infection and foreign body reactions. The short-and medium-term follow-up results of vaginal repairs for anterior prolapse using polypropylene mesh are promising. However, since this technique was introduced, there have been concerns about vaginal erosion. We had one patient who had mesh erosion at the 1-year follow-up visit. According to previous studies, the rate of vaginal erosion after an anterior vaginal wall repair using a Gynemesh™ PS ranges from 3.8% to 20% [10-13,18], although the definition of erosion in these reports was usually absent or confusing.
Several factors are thought to contribute tomesh erosions. These include a poor healing environment, which is influenced by blood flow, infections, foreign body reactions, patient age, concomitant hysterectomy, and mesh characteristics. de Tayrac et al. [19] reported that vaginal erosion occurred three times more often when a concomitant hysterectomy was performed. The authors explained that this difference was most likely due to vessel injury associated with uterine cervix dissection. Some studies have reported that patient age is another independent risk factor for the development of an erosion [18,20]. Deffieux et al. [18] compared the erosion rate between the use of Gynemesh™ PS and the use of Gynemesh-Soft, which is designed to be more supple and flexible. Gynemesh-Soft did not decrease the incidence of vaginal erosions after 6 months of follow-up. Another study found no significant difference in the incidence of vaginal erosions between the use of Atrium (a polypropylene nonabsorbable, monofilament, macroporous mesh) and the use of Vypro II (a composite polypropylene/polyglactin 910, mono/multifilament, absorbable/nonabsorbable mesh). Vypro II is designed to weigh less than Atrium and also induces less of an inflammatory response [20] than do Mersilene and Marlex mesh [21].
We had two cases of increased bleeding intraoperatively, both of which required a transfusion. Most hemorrhagic complications that occur during a trans-vaginal procedure can be managed conservatively with local packing or a transfusion. This complication is usually associated with concomitant surgeries. Typically, these surgeries involve retropubic or trans-obturator midurethral slings or the repair of a combined posterior or apical prolapse. Major hemorrhagic complications may arise from the Retzius space or the laceration of the external iliac or obturator vessels. There is one case report involving major venous bleeding during a trans-obturator anterior vaginal wall repair [22]. In that case, a terminal anterior branch of the internal hypogastric vein was revealed to be the origin of the bleeding. The bleeding was controlled by embolization of the hypogastric artery and local packing. The authors concluded that pelvic varicose veins were the major risk factor in that case.
One limitation of our study is the small sample size. Also, this study was not a comparative study. It is difficult to determine the outcomes of the combined prolapses and SUI because of the concomitant surgeries. Therefore, a comparative study with a large population is needed to confirm the efficacy of anterior vaginal wall repair with Gynemesh™ PS.
Conclusions
Trans-vaginal repair of an anterior vaginal wall prolapse with the monofilament polypropylene mesh Gynemesh™ PS is an effective and safe procedure. In this study, the anatomical defect was restored and the associated pelvic floor symptoms were improved in women with an anterior vaginal wall prolapse after 1 year of follow-up.
References
- 1.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–506. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Seo JT, Kim JM. Pelvic organ support and prevalence by Pelvic Organ Prolapse-Quantification (POP-Q) in Korean women. J Urol. 2006;175:1769–1772. doi: 10.1016/S0022-5347(05)00993-6. [DOI] [PubMed] [Google Scholar]
- 3.Digesu GA, Chaliha C, Salvatore S, Hutchings A, Khullar V. The relationship of vaginal prolapse severity to symptoms and quality of life. BJOG. 2005;112:971–976. doi: 10.1111/j.1471-0528.2005.00568.x. [DOI] [PubMed] [Google Scholar]
- 4.Weber AM, Walters MD, Piedmonte MR, Ballard LA. Anterior colporrhaphy: a randomized trial of three surgical techniques. Am J Obstet Gynecol. 2001;185:1299–1304. doi: 10.1067/mob.2001.119081. [DOI] [PubMed] [Google Scholar]
- 5.Shull BL. Pelvic organ prolapse: anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol. 1999;181:6–11. doi: 10.1016/s0002-9378(99)70427-8. [DOI] [PubMed] [Google Scholar]
- 6.Sand PK, Koduri S, Lobel RW, Winkler HA, Tomezsko J, Culligan PJ, et al. Prospective randomized trial of polyglactin 910 mesh to prevent recurrence of cystoceles and rectoceles. Am J Obstet Gynecol. 2001;184:1357–1362. doi: 10.1067/mob.2001.115118. [DOI] [PubMed] [Google Scholar]
- 7.Kuuva N, Nilsson CG. A nationwide analysis of complications associated with the tension-free vaginal tape (TVT) procedure. Acta Obstet Gynecol Scand. 2002;81:72–77. doi: 10.1034/j.1600-0412.2002.810113.x. [DOI] [PubMed] [Google Scholar]
- 8.Bump RC, Mattiasson A, Bo K, Brubaker LP, DeLancey JO, Klarskov P, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 9.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 10.de Tayrac R, Deffieux X, Gervaise A, Chauveaud-Lambling A, Fernandez H. Long-term anatomical and functional assessment of trans-vaginal cystocele repair using a tension-free polypropylene mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:483–488. doi: 10.1007/s00192-005-0046-x. [DOI] [PubMed] [Google Scholar]
- 11.Adhoute F, Soyeur L, Pariente JL, Le Guillou M, Ferriere JM. [Use of transvaginal polypropylene mesh (Gynemesh) for the treatment of pelvic floor disorders in women. Prospective study in 52 patients] Prog Urol. 2004;14:192–196. [PubMed] [Google Scholar]
- 12.Hardiman P, Oyawoye S, Browning J. Cystocele repair using polypropylene mesh. Br J Obstet Gynaecol. 2000;107:825–826. [Google Scholar]
- 13.De Tayrac R, Gervaise A, Fernandez H. [Cystocele repair by the vaginal route with a tension-free sub-bladder prosthesis] J Gynecol Obstet Biol Reprod (Paris) 2002;31:597–599. [PubMed] [Google Scholar]
- 14.Julian TM. The efficacy of Marlex mesh in the repair of severe, recurrent vaginal prolapse of the anterior midvaginal wall. Am J Obstet Gynecol. 1996;175:1472–1475. doi: 10.1016/s0002-9378(96)70092-3. [DOI] [PubMed] [Google Scholar]
- 15.Handel LN, Frenkl TL, Kim YH. Results of cystocele repair: a comparison of traditional anterior colporrhaphy, polypropylene mesh and porcine dermis. J Urol. 2007;178:153–156. doi: 10.1016/j.juro.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 16.Richardson AC, Lyon JB, Williams NL. A new look at pelvic relaxation. Am J Obstet Gynecol. 1976;126:568–573. doi: 10.1016/0002-9378(76)90751-1. [DOI] [PubMed] [Google Scholar]
- 17.Delancey JO. Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol. 2002;187:93–98. doi: 10.1067/mob.2002.125733. [DOI] [PubMed] [Google Scholar]
- 18.Deffieux X, de Tayrac R, Huel C, Bottero J, Gervaise A, Bonnet K, et al. Vaginal mesh erosion after transvaginal repairof cystocele using Gynemesh or Gynemesh-Soft in 138 women: a comparative study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:73–79. doi: 10.1007/s0192-005-0041-2. [DOI] [PubMed] [Google Scholar]
- 19.de Tayrac R, Devoldere G, Renaudie J, Villard P, Guilbaud O, Eglin G. Prolapse repair by vaginal route using a new protected low-weight polypropylene mesh: 1-year functional and anatomical outcome in a prospective multicentre study. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:251–256. doi: 10.1007/s00192-006-0135-5. [DOI] [PubMed] [Google Scholar]
- 20.Achtari C, Hiscock R, O'Reilly BA, Schierlitz L, Dwyer PL. Risk factors for mesh erosion after transvaginal surgery using polypropylene (Atrium) or composite polypropylene/polyglactin 910 (Vypro II) mesh. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:389–394. doi: 10.1007/s00192-004-1272-3. [DOI] [PubMed] [Google Scholar]
- 21.Kohli N, Walsh PM, Roat TW, Karram MM. Mesh erosion after abdominal sacrocolpopexy. Obstet Gynecol. 1998;92:999–1004. doi: 10.1016/s0029-7844(98)00330-5. [DOI] [PubMed] [Google Scholar]
- 22.Touboul C, Nizard J, Fauconnier A, Bader G. Major venous hemorrhagic complication during transvaginal cystocele repair using the transobturator approach. Obstet Gynecol. 2008;111:492–495. doi: 10.1097/01.AOG.0000278098.85173.61. [DOI] [PubMed] [Google Scholar]