Abstract
Background. Patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI) with the Proxis system (St. Jude Medical, St. Paul, MN, USA) achieved significantly better microvascular flow as measured by ST-segment resolution. However, no differences were observed in left ventricular ejection fraction or infarct size as obtained by cardiovascular magnetic resonance imaging. The goal of the present study was to evaluate the effect of combined proximal embolic protection and thrombus aspiration on core-lab adjudicated angiographic outcomes.
Methods. In the PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST-Elevation (PREPARE) study, patients were randomised to primary PCI with the Proxis system (n=141) or primary PCI alone (n=143). An independent core laboratory re-evaluated all angiograms and adjudicated the angiographic outcomes and computerised quantitative blush evaluation (QuBE) value.
Results. There were no significant differences in Thrombolysis In Myocardial Infarction (TIMI) flow grade, myocardial blush grade, or angiographic signs of distal embolisation among the two arms. QuBE values did not significantly differ between the Proxis-treated patients and control patients (15.1±5.4 vs. 15.8±5.5, respectively, p=0.34).
Conclusion. Primary PCI with combined proximal embolic protection and thrombus aspiration in STEMI patients more frequently resulted in complete immediate ST resolution compared with control patients. However, there were no significant differences in core laboratory adjudicated angiographic outcomes. (Neth Heart J 2010;18:531–6.)
Keywords: ST-Segment Elevation Myocardial Infarction, Primary PCI, Combined Proximal Embolic Protection and Thrombus Aspiration, Computer-Assisted Myocardial Blush
Primary percutaneous coronary intervention (PCI) in patients with ST-segment elevation myocardial infarction (STEMI) results in better coronary reperfusion rates and improved clinical outcomes than fibrinolytic therapy.1 Despite normal epicardial Thrombolysis in Myocardial Infarction (TIMI) flow after primary PCI, myocardial perfusion (as assessed either by myocardial blush or ST-segment resolution) is abnormal in a significant proportion of patients, contributing to increased infarct size and reduced survival.2,3 Several adjunctive mechanical strategies (embolic protection and thrombectomy devices) have been developed to contribute to myocardial perfusion and salvage in patients with STEMI treated with primary PCI.4 Recent data from the TAPAS (Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study) demonstrated that patients with STEMI treated with primary PCI with thrombus aspiration have a significant improvement in clinical outcome one year after the index procedure.5
In the PREPARE (Proximal Embolic Protection in Acute myocardial infarction and Resolution of ST-segment Elevation) study, patients with STEMI treated with primary PCI with combined proximal embolic protection and thrombus aspiration using the Proxis Embolic Protection System (St. Jude Medical, St. Paul, MN, USA) had significantly better immediate microvascular flow measured by ST-segment resolution. Despite improved STsegment resolution in the Proxis-treated patients, infarct size and left ventricular ejection fraction at follow-up were virtually the same in both arms.6,7 Because of these conflicting findings, we additionally performed a re-evaluation of our angiographic outcomes by a blinded independent core lab.
Methods
Participants and study protocol
The PREPARE is a multicentre, randomised open trial. Patients with STEMI were randomly assigned in the catheterisation laboratory to receive primary PCI with combined proximal embolic protection and thrombus aspiration with the Proxis system or primary PCI alone. The Proxis system is a singleoperator full length flexible catheter (6F and 7F guiding catheter compatible) and based on a carbon dioxide gas (CO2) inflation system. It was deployed proximally to the target lesion before crossing. Inflation of the sealing balloon suspends antegrade flow during the period of lesion intervention. Stagnated blood and emboli, liberated during each intervention, were retrieved by aspiration. Crossing of the coronary occlusion with the wire, balloon dilatation, and stent placement were performed through the Proxis system and carried out under full proximal blockade of the vessel. Aspiration and embolic protection by temporary proximal vessel occlusion were repeated during each step of the PCI procedure.8 In all patients, primary PCI was performed according to current guidelines.
Briefly, consecutive patients aged 18 years or older were eligible for enrolment if they experienced onset of symptoms of myocardial infarction less than six hours before presentation and had electrocardiographic evidence of persistent ST-segment elevation of at least 2 mm in two or more contiguous leads and TIMI-graded flow 0 to 1 on diagnostic angiography. Exclusion criteria included any contraindications to the use of glycoprotein IIb/IIIa receptor antagonists, a co-existent condition associated with a limited life expectancy, prior coronary artery bypass grafting or lytics, and recurrence of myocardial infarction in the same myocardial area. The primary endpoint was complete (≥70%) ST-segment resolution at 60 minutes after the last contrast injection by continuous ST Holter monitoring.
In addition, all included patients were asked to participate in an ancillary cardiovascular magnetic resonance (CMR) imaging study. In this study, Proxis-treated patients (n=96) and control patients (n=110) underwent late gadolinium enhancement CMR examination at four to six months after the index procedure.6
Angiographic analysis and quantitative blush evaluation
At the end of PCI, a coronary angiogram was obtained. All coronary angiograms were re-evaluated by the core lab (YLG and FZ) blinded for treatment allocation and clinical data. On the post-procedural angiogram TIMI-graded flow, myocardial blush grade, and angiographic signs of distal embolisation were assessed. Distal embolisation was defined as a filling defect, with an abrupt cut-off in the vessel located distally from the infarct-related coronary lesion.9 The assessment of myocardial blush grade was performed according to van ’t Hof et al.2: 0=no myocardial blush; 1=minimal myocardial blush or contrast density; 2=moderate myocardial blush or contrast density, but less than that obtained during angiography of a contra or ipsilateral non-infarctrelated coronary artery; and 3=normal myocardial blush or contrast density, comparable with that obtained during angiography of a contra or ipsilateral non-infarct-related coronary artery.
Also, analysis of myocardial perfusion was performed by computerised quantitative blush evaluation (QuBE) as previously described.10,11 QuBE was measured blinded to clinical data and treatment assignment by one experienced operator (YLG). The QuBE program loads the coronary angiogram and allows the operator to select the run for quantification of the myocardial perfusion. On the selected run, the operator indicates a region of interest that contains the distal infarct-related area in which the myocardial perfusion should be measured. By quantifying the myocardial contrast density on each frame and by identifying the sum of grey values over time, a continuous value is calculated. High QuBE score was significantly associated with more ST-segment resolution, higher visually assessed myocardial blush grade, and a lower one-year mortality.10
Table 1.
Baseline and procedural characteristics of the patients
| PCI with Proxis (n=141) | Primary PCI alone (n=143) | p value | |||
|---|---|---|---|---|---|
| Age, years | 62 | ± 11 | 59 | ± 11 | 0.03 |
| Men | 112 | (80%) | 114 | (80%) | 0.95 |
| Body mass index | 27 | ± 4 | 27 | ± 4 | 0.17 |
| History | |||||
| Diabetes mellitus | 17 | (12) | 9 | (6) | 0.09 |
| Hypertension | 44 | (31) | 33 | (23) | 0.12 |
| Hypercholesterolaemia | 29 | (21) | 19 | (13) | 0.10 |
| Myocardial infarction | 8 | (6) | 13 | (9) | 0.27 |
| PCI | 9 | (6) | 10 | (7) | 0.84 |
| Cerebrovascular disease | 8 | (6) | 6 | (4) | 0.57 |
| Cardiovascular disease in family | 49 | (35) | 54 | (38) | 0.60 |
| Current smoking | 70 | (50) | 93 | (65) | 0.01 |
| No. of diseased vessels | 0.68 | ||||
| 1 | 95 | (67) | 99 | (69) | |
| 2 | 40 | (28) | 31 | (22) | |
| 3 | 6 | (4) | 13 | (9) | |
| Infarct-related vessel | 0.86 | ||||
| Left anterior descending arter y | 41 | (29) | 42 | (29) | |
| Left circumflex artery | 14 | (10) | 15 | (11) | |
| Right coronary artery | 86 | (61) | 86 | (60) | |
| Baseline TIMI-graded flow | 0.52 | ||||
| 0 | 127 | (90) | 127 | (89) | |
| 1 | 12 | (9) | 11 | (8) | |
| 2 | 2 | (1) | 5 | (4) | |
| Proxis placed | 132 | (94) | - | ||
| GP IIb/IIIa receptor antagonists | 61 | (43) | 50 | (35) | 0.15 |
| Symptom onset to balloon, min | 170 | (132–234) | 153 | (126–212) | 0.07 |
Data are expressed as mean ± SD, median (interquartile range), or number of patients (percent). GP=glycoprotein, TIMI=Thrombolysis in Myocardial Infarction.
Statistical analysis
Analysis was by intention to treat. Categorical outcomes were compared by χ2 and Fisher’s exact test. Continuous normally distributed variables are presented as mean values and standard deviations and were compared with the two-tailed Student’s t test. For non-normally distributed variables, median values with interquartile range are shown, and the variables were compared using the Mann-Whitney U test. A p value <0.05 was considered statistically significant. Statistical analysis was performed using Statistical Package for Social Sciences software (SPSS 16.0 for Windows, SPSS Inc., Chicago, Illinois).
Results
Patient and procedural characteristics
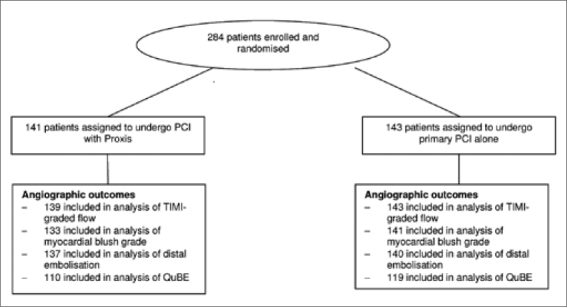
Between 1 March 2006 and 27 June 2008, 284 patients at two centres in the Netherlands and Canada were randomly assigned to receive PCI with combined proximal embolic protection and thrombus aspiration (n=141) or PCI alone (n=143). Figure 1 shows the trial profile. Table 1 shows the baseline and procedural characteristics of the study participants. The proportion of smokers was higher in the control group and patients in the Proxis-treated group were significantly older. Most patients had TIMI grade flow 0 to 1 prior to PCI (98%). The Proxis system could be placed effectively in 94% of the patients.
Angiographic outcomes and QuBE score
Angiographic outcomes are listed in table 2. There were no differences in TIMI-graded flow between primary PCI with combined proximal embolic protection and thrombus aspiration and primary PCI alone (TIMI 3 flow; 92 vs. 88%, p=0.19, respectively). Myocardial blush grade 3 was observed in 29 of 133 patients (22%) randomised to the Proxis arm compared with 41 of 143 patients (29%) randomised to the control arm (p=0.61). Distal embolisation after PCI was noticed in 14 of 137 patients (10%) in the Proxis-treated patients and in 20 of 140 patients (14%) in the control arm (p=0.36).
In 229 of the 284 study participants (81%), the QuBE value could be assessed. Angiograms were excluded for QuBE analysis due to absence of a suitable angiographic run with adequate visualisation of the distal coronary and myocardial territory of the infarct-related artery (n=23), too much diaphragm or panning movement (n=27), or major overlap of a non-infarct-related artery (n=5). The mean (±SD) QuBE value was 15.1±5.4 in patients randomised to PCI with combined proximal embolic protection and thrombus aspiration and 15.8±5.5 in patients randomised to PCI alone. The difference in QuBE score among groups was not significant (p=0.34).
Discussion
This prospective randomised controlled trial demonstrates that combined proximal embolic protection and thrombus aspiration did not improve myocardial perfusion in indices of angiographic outcomes or computer-assisted myocardial blush in patients with STEMI. Despite the fact that Proxistreated patients had significantly more ST-segment resolution immediately after PCI, combined proximal embolic protection and thrombus aspiration did not enhance core-lab adjudicated angiographic outcomes. Consistent with the findings on angiographic outcomes, the area of late gadolinium enhancement on CMR, left ventricular ejection fraction, and segmental myocardial function did not reveal any beneficial effect of the Proxis system.6
There are multiple explanations for why combined proximal embolic protection and thrombus aspiration resulted in conflicting data on continuous ST Holter parameters (ST-segment resolution and ECG injury current over time), angiographic outcomes, infarct size and clinical outcome in this study. First, it appears that the benefit shown from preventing embolisation, procedural myocardial infarction and clinical outcome in saphenous venous graft interventions12 has not translated into a benefit for preventing distal embolisation during primary PCI.3,13 It is more likely that the distal embolisation during primary PCI that can be prevented by protection devices exerts only minor effects compared with the consequences of ischaemic microvascular damage or spontaneous distal embolisation arising from ruptured or eroded plaques during the natural course of an acute coronary syndrome.14 Although the TAPAS study showed a significant reduction in one-year mortality, 5 previous trials of thrombectomy devices have shown conflicting findings,3,13,15 and the method of mechanical thrombectomy may play a significant role in its clinical benefit. Second, patients with a STEMI related to an ostial coronary artery occlusion were not included in the PREPARE study, because of the necessity of a ‘landing zone’ for the Proxis system. For this reason, patients with a very proximal infarct-related left anterior descending artery (LAD) and left circumflex artery (LCx) lesions were not included and more patients with a right coronary artery related myocardial infarction (60%) were prevalent in our study. Subsequently, the smaller myocardial infarctions may have attenuated the effect size that might have been seen in proximal LAD and LCx lesions with larger amounts of myocardium at risk. And third, our trial is a proofof-concept study and was powered to demonstrate benefits in the occurrence of complete ST-segment resolution, and not in terms of angiographic or clinical outcomes.
Table 2.
Assessment of the angiographic outcomes by core lab.
| PCI with Proxis (n=139) | Primary PCI alone (n=143) | p value | |||
|---|---|---|---|---|---|
| TIMI-graded flow | 0.19 | ||||
| 0–1 | 0 | (0%) | 3 | (2%) | |
| 2 | 11 | (8%) | 13 | (9%) | |
| 3 | 128 | (92%) | 127 | (88%) | |
| (n = 133) | (n = 141) | ||||
| Myocardial blush grade | 0.61 | ||||
| 0–1 | 35 | (26%) | 41 | (29%) | |
| 2 | 69 | (52%) | 59 | (42%) | |
| 3 | 29 | (22%) | 41 | (29%) | |
| (n = 137) | (n = 140) | ||||
| Angiographic signs of distal embolisation | 0.36 | ||||
| 14 | (10%) | 20 | (14) | ||
| (n = 110) | (n = 119) | ||||
| QuBE value | 15.1 | ± 5.4 | 15.8 | ± 5.5 | 0.34 |
Data are expressed as number of patients (percent) or mean ± SD. TIMI=Thrombolysis in Myocardial Infarction, QuBE=quantitative blush evaluator.
Our study has several limitations. The study reflects a multi-centre experience in a relatively limited number of patients and represents a mechanical attempt at optimising myocardial reperfusion in patients undergoing primary PCI. Although one of the largest trials of its kind performed to date, the present study was underpowered to detect a difference in clinical outcome with use of the Proxis system. The study was limited to patients with thrombus-containing lesions with TIMI-graded flow 0 to 1 and did not include the entire acute myocardial infarction population.
Conclusion
Primary PCI with combined proximal embolic protection and thrombus aspiration in STEMI patients resulted more frequently in complete immediate ST-resolution compared with control patients. However, there were no significant differences in core laboratory adjudicated angiographic outcomes.
Acknowledgments
We gratefully acknowledge the technical and nursing staff of the Cardiac Catheterisation Laboratory, Coronary Care Unit and Radiology (Heads: M.G. Meesterman, R. Schmeddes and R.D. Snoeks), and M. Klees, E.M. Scheunhage, W.J. Rohling, and M. Shabani for the data acquisition and management.
Trial registration number: ISRCTN71104460
Conflict of interest statement
This study is supported by grants from St. Jude Medical and from the University of Amsterdam. None of the authors has declared any conflict of interest.
References
- 1.Keeley EC, Boura JA, Grines CL. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 2.‘t Hof AW, Liem A, Suryapranata H, Hoorntje JC, de Boer MJ, Zijlstra grade F. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97:2302–2306. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 3.Stone GW, Webb J, Cox DA, Brodie BR, Qureshi M, Kalynych A, et al. Distal microcirculatory protection during percutaneous coronary intervention in acute ST-segment elevation myocardial infarction: a randomized controlled trial. JAMA. 2005;293:1063–1072. doi: 10.1001/jama.293.9.1063. [DOI] [PubMed] [Google Scholar]
- 4.Haeck JD, Verouden NJ, Henriques JP, Koch KT. Current status of distal embolization in percutaneous coronary intervention: mechanical and pharmacological strategies. Future Cardiol. 2009;5:385–402. doi: 10.2217/fca.09.25. [DOI] [PubMed] [Google Scholar]
- 5.Vlaar PJ, Svilaas T, van der Horst I, Diercks GF, Fokkema ML, de Smet BJ, et al. Cardiac death and reinfarction after 1 year in the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS): a 1-year follow-up study. Lancet. 2008;371:1915–1920. doi: 10.1016/S0140-6736(08)60833-8. [DOI] [PubMed] [Google Scholar]
- 6.Haeck JD, Kuijt WJ, Koch KT, Bilodeau L, Henriques JP, Rohling WJ, et al. Infarct size and left ventricular function in the Proximal Embolic Protection in Acute myocardial infarction and Resolution of ST-segment Elevation (PREPARE) trial: ancillary cardiovascular magnetic resonance study. Heart. 2010;96:190–195. doi: 10.1136/hrt.2009.180448. [DOI] [PubMed] [Google Scholar]
- 7.Haeck JD, Koch KT, Bilodeau L, Van der Schaaf RJ, Henriques JP, Vis MM, et al. Randomized comparison of primary embolic protection and thrombus aspiration versus primary percutaneous coronary intervention alone in ST-segment elevation myocardial infarction: the PREPARE (PRoximal Embolic Protection in Acute myocardial infarction and Resolution of ST-Elevation) study. JACC Cardiovasc Interv. 2009;2:934–943. doi: 10.1016/j.jcin.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Koch KT, Haeck JD, Van der Schaaf RJ, Alidjan FM, Henriques JP, Baan J, et al. Proximal embolic protection with aspiration in percutaneous coronary intervention using the Proxis device. Rev Cardiovasc Med. 2007;8:160–166. [PubMed] [Google Scholar]
- 9.Henriques JP, Zijlstra F, Ottervanger JP, de Boer MJ, van ‘t Hof AW, Hoorntje JC, Suryapranata H. Incidence and clinical significance of distal embolization during primary angioplasty for acute myocardial infarction. Eur Heart J. 2002;23:1112–1117. doi: 10.1053/euhj.2001.3035. [DOI] [PubMed] [Google Scholar]
- 10.Vogelzang M, Vlaar PJ, Svilaas T, Amo D, Nijsten MW, Zijlstra F. Computer-assisted myocardial blush quantification after percutaneous coronary angioplasty for acute myocardial infarction: a substudy from the TAPAS trial. Eur Heart J. 2009;30:594–599. doi: 10.1093/eurheartj/ehn542. [DOI] [PubMed] [Google Scholar]
- 11.Fokkema ML, Vlaar PJ, Vogelzang M, Gu YL, Kampinga MA, De Smet BJ, et al. Effect of High-Dose Intracoronary Adenosine Administration During Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction. Circ Cardiovasc Intervent. 2009;2:323–329. doi: 10.1161/CIRCINTERVENTIONS.109.858977. [DOI] [PubMed] [Google Scholar]
- 12.Mauri L, Cox D, Hermiller J, Massaro J, Wahr J, Tay SW, et al. The PROXIMAL trial: proximal protection during saphenous vein graft intervention using the Proxis Embolic Protection System: a randomized, prospective, multicenter clinical trial. J Am Coll Cardiol. 2007;50:1442–1449. doi: 10.1016/j.jacc.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 13.Kaltoft A, Bottcher M, Nielsen SS, Hansen HH, Terkelsen C, Maeng M, et al. Routine thrombectomy in percutaneous coronary intervention for acute ST-segment-elevation myocardial infarction: a randomized, controlled trial. Circulation. 2006;114:40–47. doi: 10.1161/CIRCULATIONAHA.105.595660. [DOI] [PubMed] [Google Scholar]
- 14.Gick M, Jander N, Bestehorn HP, Kienzle RP, Ferenc M, Werner K, et al. Randomized evaluation of the effects of filter-based distal protection on myocardial perfusion and infarct size after primary percutaneous catheter intervention in myocardial infarction with and without ST-segment elevation. Circulation. 2005;112:1462–1469. doi: 10.1161/CIRCULATIONAHA.105.545178. [DOI] [PubMed] [Google Scholar]
- 15.Sardella G, Mancone M, Bucciarelli-Ducci C, Agati L, Scardala R, Carbone I, et al. Thrombus aspiration during primary percutaneous coronary intervention improves myocardial reperfusion and reduces infarct size: the EXPIRA (thrombectomy with export catheter in infarct-related artery during primary percutaneous coronary intervention) prospective, randomized trial. J Am Coll Cardiol. 2009;53:309–315. doi: 10.1016/j.jacc.2008.10.017. [DOI] [PubMed] [Google Scholar]


