Abstract
Chronic inflammation has been implicated in the pathology of hypertension; however, the role for specific cytokines remains unclear. We tested whether TNF-α blockade with etanercept (Etan) reduces mean arterial pressure (MAP) in a female mouse model of systemic lupus erythematosus (SLE). SLE is a chronic inflammatory disorder with prevalent hypertension. Thirty week old SLE (NZBWF1) and control mice (NZW/LacJ) received Etan (0.8mg/kg SC weekly) for 4 weeks or vehicle. MAP (mmHg) was increased in SLE mice (150±5 vs. 113±5 in controls, p<0.05) and was lower in Etan treated SLE mice (132±3) but not controls (117±5). Albuminuria (µg/mg creatinine) was elevated in SLE mice (28742±9032 vs. 1075±883 p<0.05) and was lower in Etan treated SLE mice (8154±3899) but not control animals (783±226). Glomerulosclerosis (% of glomeruli) was evident in SLE mice (2.5±1.6 vs. 0.0±0.0 in controls, p<0.05) and was ameliorated in Etan treated SLE mice (0.1±0.1). Renal cortex CD68+ cell staining (% area) was elevated in SLE mice (4.75±0.80 vs. 0.79±0.12 in controls, p<0.05) and was lower in Etan treated SLE mice (2.28±0.32) but not controls (1.43±0.25). Renal cortex NADPH oxidase activity (RLU/mg of protein) was higher in SLE mice compared to controls (10718±1276 vs. 7584±229, p<0.05) and lowered in Etan treated SLE mice (6645±490). Renal cortex NFκB (phosphorylated and non-phosphorylated) was increased in SLE mice compared to controls and lower in Etan treated SLE mice. These data suggest that TNF-α mechanistically contributes to the development of hypertension in a chronic inflammatory disease through increased renal NFκB, oxidative stress and inflammation.
Keywords: Systemic Lupus Erythematosus, Hypertension, Inflammation, TNF-α-Oxidative Stress, cytokine
Introduction
A growing body of literature suggests that chronic inflammation plays an important role in the progression of several forms of hypertension. Plasma levels of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α) and interleukin 6, directly correlate with blood pressure and essential hypertension in humans 1,2. In addition, recent studies demonstrate that immunosuppressive therapy with mycophenolate mofetil (MMF) reduced blood pressure in essential hypertensive patients 3 and in experimental animal models of hypertension 4. The potential mechanistic role of specific inflammatory cytokines, such as TNF-α, in the development of hypertension remains unclear. For example, the effect of TNF-α blockade in experimental models of hypertension varies ranging from having no effect on pressure 5,6 to delaying the progression 7 or even completely ameliorating hypertension 8. Therefore, further studies to understand the contribution of this master immune regulator to the development of hypertension are warranted.
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disorder of unknown etiology that predominantly affects young women. A loss of immunological tolerance during SLE leads to the production of autoantibodies of which anti-double stranded DNA (dsDNA) are the most common and are specific for the disease. Autoantibody production facilitates the formation of immune complexes that deposit in tissues and promote local inflammation and injury with the kidneys being most commonly affected. Numerous inflammatory cytokines are implicated in the pathophysiology of SLE including TNF-α 9,10. TNF-α expression is increased in kidney biopsies 11 and the prevalence of hypertension is elevated in patients with SLE reaching as high as 75% depending upon the cohort 12,13,14,15. These data suggest that SLE may be an important disease model to examine the mechanistic role of renal TNF-α in the development of hypertension.
In the present study, we hypothesize that TNF-α is an important mediator of hypertension during the chronic inflammatory disorder, SLE. This hypothesis will be tested by treating an established mouse model of SLE (female NZBWF1 mice) with etanercept, a clinically available recombinant TNF-α receptor that reduces the biological activity of TNF-α. We previously demonstrated that this model of SLE develops hypertension 16,17,18 and data from others show that renal TNF-α expression is increased in these mice 19. The findings from this study will further advance our understanding of the role for TNF-α in hypertension and have direct clinical relevance to patients with SLE.
Methods
Animals
30 week-old female NZBWF1 (SLE) and NZW/LacJ (control) mice obtained from Jackson Laboratories (Bar Harbor, ME) were randomly assigned to receive a weekly subcutaneous injection of the TNF-α inhibitor etanercept (Enbrel, Wyeth, 0.8 mg/kg) or vehicle (saline) for 4 weeks. Urine was collected weekly and assessed for the presence of albumin as previously described 18. A final dose of etanercept was administered the week of blood pressure recording and tissue harvest. Only mice with no sign of albuminuria at 30 weeks of age were included in the study. Mice were maintained on a 12 hour light/dark cycle in temperature-controlled rooms with access to chow and water ad libitum. Four groups of animals were included as follows: Control untreated mice (Ctrl+Veh), control treated with etanercept (Ctrl+Etan), SLE untreated (SLE+Veh) and SLE treated with etanercept (SLE+Etan). All of the studies were performed with the approval of the University of Mississippi Medical Center Institutional Animal Care and Use Committee and in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Anti-dsDNA
Plasma anti-dsDNA antibodies were measured as previously described 18. Mice were considered anti-dsDNA positive at values ≥ 1 SD from controls. Only NZBWF1 mice with positive anti-dsDNA antibodies were considered to have SLE.
Blood pressure measurements
At the conclusion of the study, mean arterial pressure (MAP) was recorded using indwelling carotid artery catheters in conscious mice as previously described in our laboratory (two consecutive days, 1.5–3 hours/day) 17,18.
Assessment of renal injury
Albuminuria
Urinary albumin was measured using a commercial ELISA (Alpha Diagnostic International Inc) and was normalized to urinary creatinine (CR01 Oxford Biomedical Research, Oxford, MI).
Immunohistochemistry
Immunolocalization of activated macrophages was carried out as previously described 20 using an antibody to CD68 (1:600, Serotec, Oxford, UK). CD68-positive staining was assessed in 15 randomly selected 100µm2 cortical regions and quantified using image analysis software (NIS-Elements, Ver. 2.32; Nikon Instruments, Melville, NY).
Morphology
Glomerulosclerosis was assessed using Periodic Acid Schiff-stained sections as previously described 18.
Assessment of renal cortex oxidative stress
Superoxide production in the renal cortex was assessed by lucigenin-enhanced chemiluminescence as previously described 21 using lucigenin (5 µmol/L) and NADPH (100 µmol/L). The protein concentration was measured using a Bio-Rad (Hercules, CA) protein assay with BCA standards. The data are expressed as RLU per min per mg protein
Renal TNF-α and NFκB protein expression
Renal cortical protein expression of non-phosphorylated (NFκB), phosphorylated (P)-NFκB and TNF-α was determined using standard Western Blot methods as previously described 22. Blots were incubated with either a mouse monoclonal anti-TNF-α antibody (1:250 Santa Cruz, Santa Cruz, CA), rabbit monoclonal anti-P-NF κB p65 (Ser536) (1:1,000; Cell Signaling, Danvers, MA), anti-NFκB p65 (1:2,000; Cell Signaling) and a mouse monoclonal anti-β-actin (1:5,000; Abcam, Cambridge, MA) as a loading control. Secondary antibodies were IR700-conjugated donkey anti-rabbit IgG and IR800-conjugated donkey anti-mouse IgG (1:2,000; Rockland Immunologicals). Antibody labeling was visualized using the Odyssey Infrared Scanner (LI-COR). Data are presented in arbitrary units of protein optical density band normalized to β-actin.
Chemokines and ET-1
Urinary monocyte chemoattractant protein-1 (MCP-1) and urinary endothelin-1 (ET-1) were measured using commercial ELISAs (R&D Systems, MN) as we previously reported 18.
Statistical Analysis
Data are presented as mean±SEM. Statistical analyses were performed using Graph Pad Prism 5 Software. A two factor ANOVA was used to test for drug or group interactions. When a significant interaction was detected, a one way ANOVA with a Student Newman Keuls post-hoc test was used to discern individual differences between groups. Significance was accepted at p<0.05.
Results
Mean arterial pressure and Body Weight
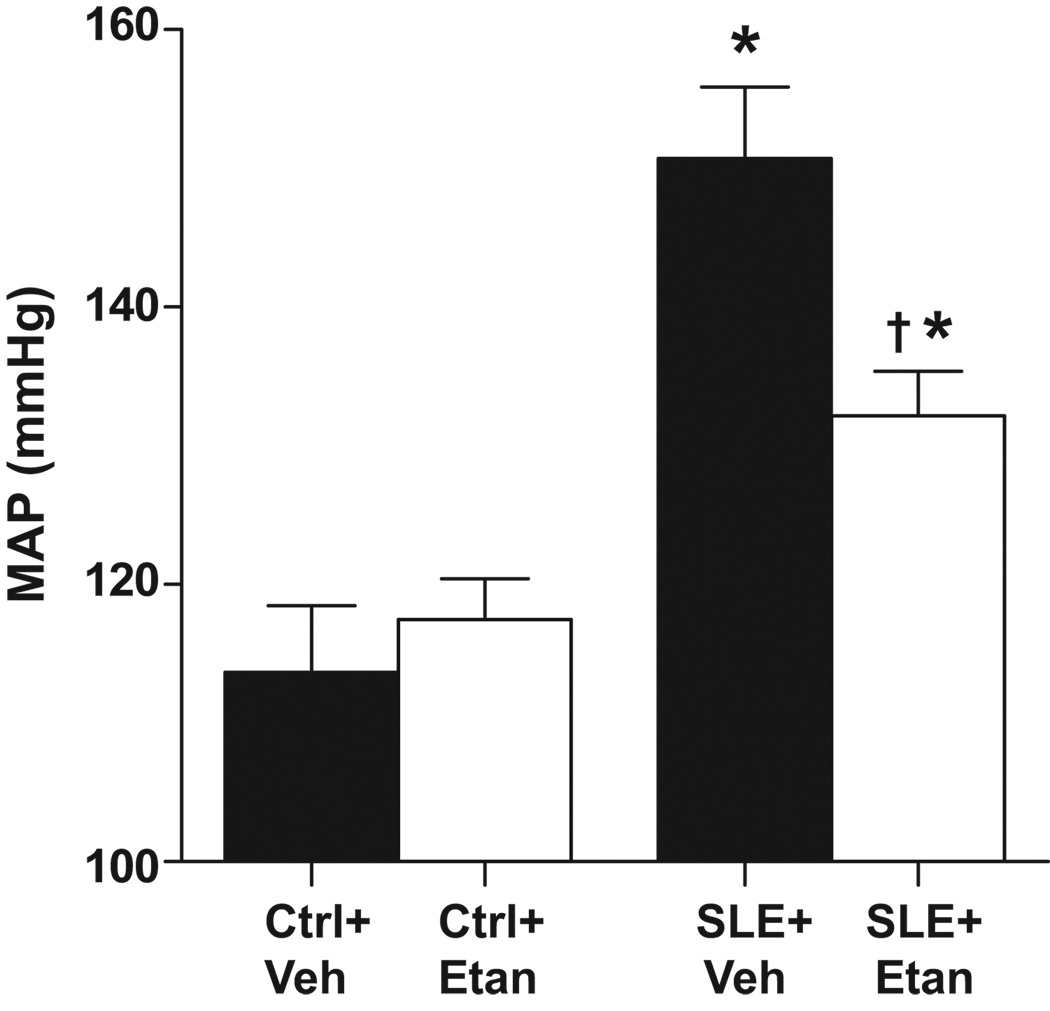
Consistent with our previous studies, MAP was significantly higher in vehicle treated SLE mice compared with vehicle treated control mice (Figure 1). SLE mice treated with etanercept for 4 weeks had significantly lower blood pressure compared to SLE+Veh. Etanercept treatment did not alter blood pressure in control animals. Body weight was greater in SLE mice compared to controls (Ctrl+Veh 34±1 g, Ctrl+Etan 35±1 g, SLE+Veh 43±1 g, SLE+Etan 46±1 g, p<0.0001 SLE vs Ctrl, 2 way ANOVA) as we previously reported 17. Body weight decreased over the course of the experiment in all groups; however, the weight loss was greatest in SLE+Veh treated mice. Because the change in body weight was greater in SLE+Veh than SLE+Etan, it is unlikely that the improved blood pressure can be attributed to weight loss. Please see http://hyper.ahajournals.org. (Figure S1).
Figure 1.
Effect of etanercept on mean arterial pressure (MAP) in SLE and control mice. MAP was significantly higher in SLE mice (n=9) compared to control mice (n=7) (conscious). Etanercept (Etan) significantly reduced MAP in SLE mice (n=10) but had no effect on MAP in control animals (n=9). * p< 0.05 vs. Ctrl + Veh. † p< 0.05 vs. SLE + Veh.
Autoantibodies
Total plasma anti-dsDNA (IgG) antibodies were significantly greater in SLE mice compared to control mice (145±18 vs. 20±14 ng/ml, p<0.05) as previously reported. Treatment with etanercept significantly increased the level of anti-dsDNA in SLE (291±54 ng/ml, p<0.05 vs. SLE+Veh) and control animals (98±21 ng/ml, p<0.05 vs. Ctrl+Veh).
Renal Injury
Albuminuria
Mice with SLE had higher urinary albumin compared to control mice (28742±9032 vs. 1075±883 µg/mg creatinine, p<0.05). SLE mice treated with etanercept for 4 weeks had lower urinary albumin (8154±3899 µg/mg creatinine, p<0.05 vs. SLE+Veh). Urinary albumin was not different in control animals receiving etanercept (Ctrl+Etan: 783±226 µg/mg creatinine).
Renal monocyte/macrophage infiltration
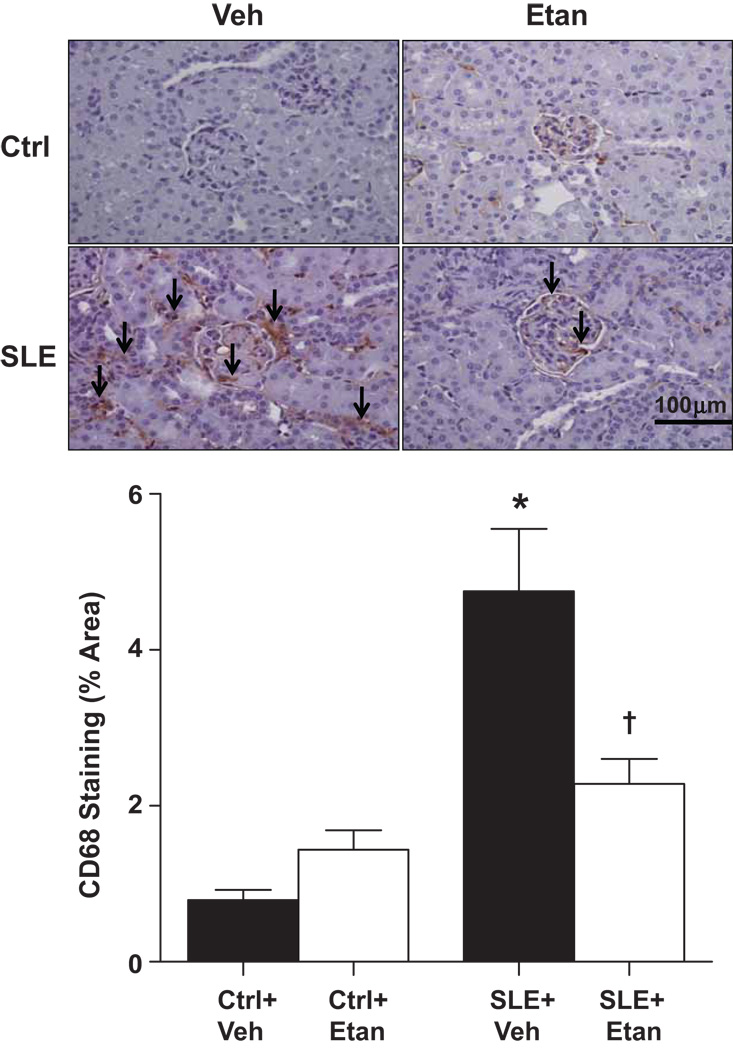
Positive staining for CD68 was significantly higher in kidneys from SLE mice compared to control animals (Figure 2). CD68 staining was significantly reduced in SLE mice treated with etanercept but not in control mice.
Figure 2.
Effect of etanercept on renal monocyte/macrophage infiltration in SLE and control mice. Representative kidney sections of CD68 staining (top panel, 20× magnification, arrows denote cell staining). The area positively stained for CD68 was significantly higher in kidneys from SLE mice (n=6) than control mice (n=10) (bottom panel). Treatment with etanercept for 4 weeks significantly reduced CD68 staining in SLE mice (n=8) but had no effect in control animals (n=9). *p<0.05 vs. Ctrl+Veh , † p<0.05 vs. SLE+Veh.
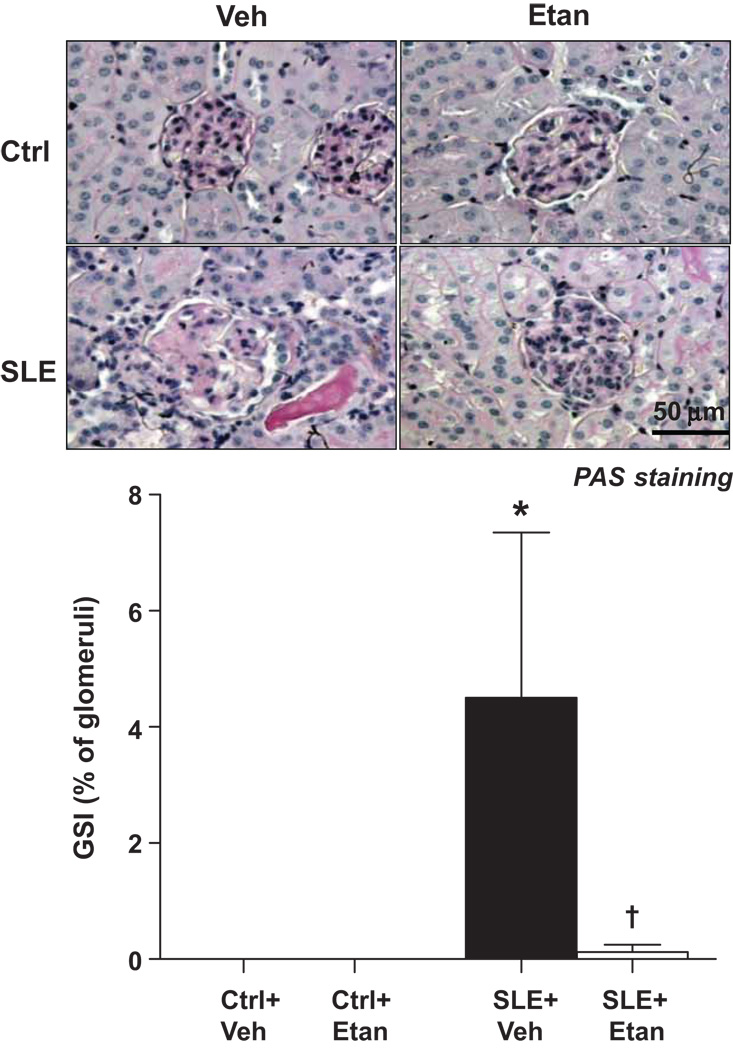
Glomerulosclerosis
The percentage of glomeruli exhibiting a sclerotic area between 51–75% is shown in Figure 3. Glomerulosclerosis was greater in SLE mice compared to control mice. SLE mice treated with etanercept had significantly lower levels of sclerosis. Treatment with etanercept did not affect glomerulosclerosis in control mice. The complete analysis for glomerulosclerosis can be viewed at http://hyper.ahajournals.org. (Figure S2).
Figure 3.
Effect of etanercept on renal glomerulosclerosis in SLE and control mice. Representative kidney sections of PAS staining (top panel, 40× magnification). The percentage of glomeruli exhibiting a sclerotic area between 51–75% was significantly higher in kidneys from SLE mice (n=6) than control mice (n=10) (bottom panel). Treatment with etanercept for 4 weeks significantly reduced glomerulosclerosis in SLE mice (n=8) but had no effect in control animals (n=9). *p<0.05 vs. Ctrl+Veh, † p<0.05 vs. SLE+Veh
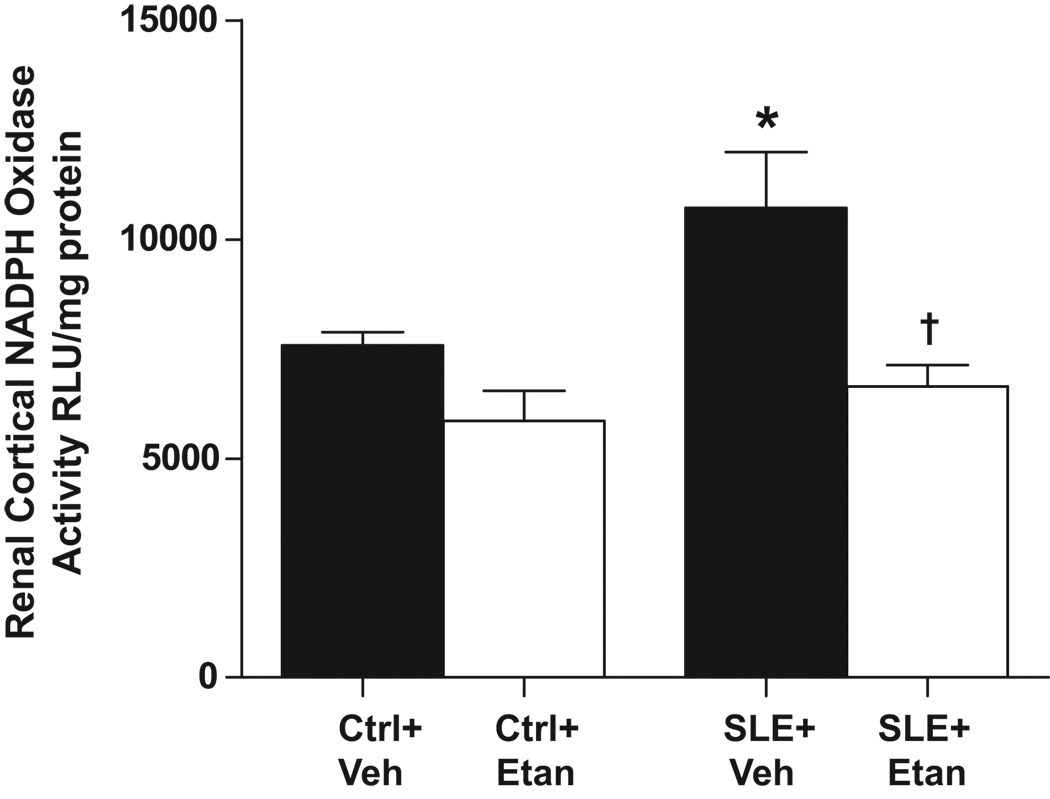
Renal cortical NADPH oxidase activity
NADPH oxidase activity was significantly higher in the cortex of SLE mice than control animals and mice treated with etanercept for 4 weeks had significantly reduced renal cortical NAPDH oxidase activity in SLE. Treatment with etanercept had no effect on control mice (Figure 4). Urinary F2-isoprostanes were measured as previously described 23 in order to assess whole body lipid peroxidation and there was no difference between groups (Please see http://hyper.ahajournals.org. Figure S3).
Figure 4.
Effect of etanercept on renal cortical NADPH oxidase activity in SLE and control mice. NADPH oxidase activity was significantly higher in the cortex of SLE mice (n=12) than control animals (n=11) and treatment with etanercept for 4 weeks significantly reduced NADPH oxidase activity in the renal cortex of SLE mice only (n=10). *p<0.05 vs. Ctrl+Veh, † p<0.05 vs. SLE+Veh
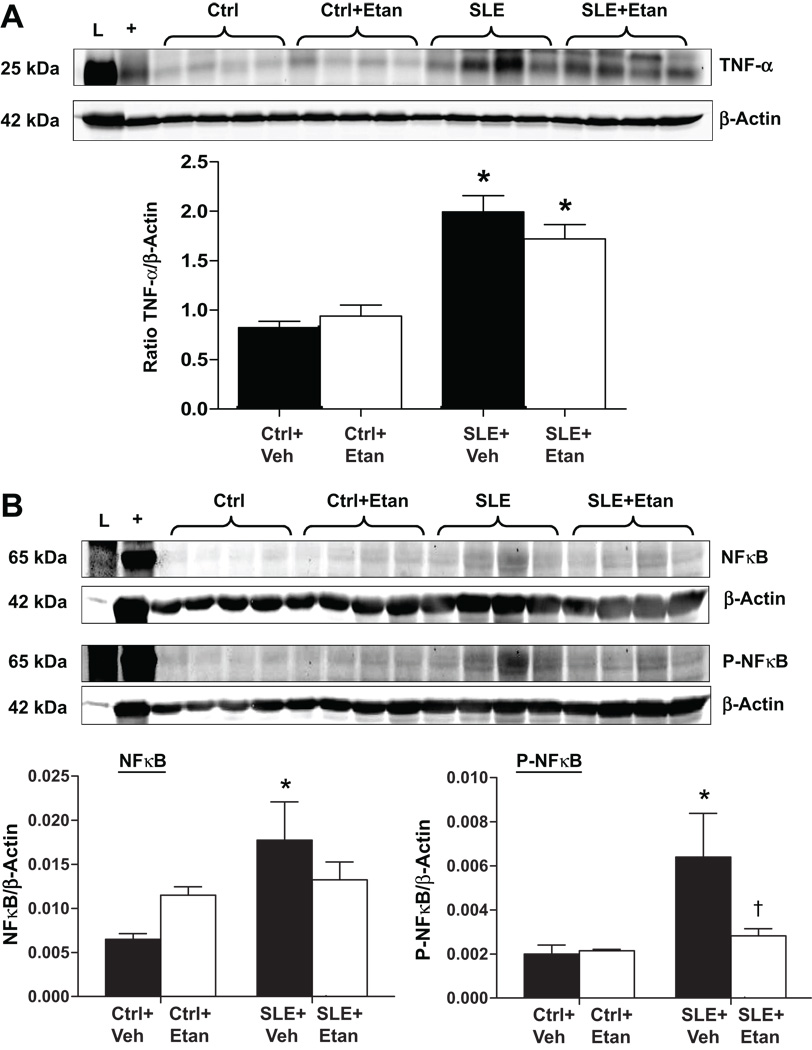
Renal TNF-α and NFκB protein expression
TNF-α protein expression was increased in the renal cortex of SLE mice compared to controls (Figure 5A) and was not significantly reduced in mice treated with etanercept. Total NFκB and phosphorylated NFκB (P-NFκB) were significantly greater in the renal cortex of SLE mice compared to controls (Figure 5B). SLE mice treated with etanercept had a significantly lower level of P-NFκB when compared to vehicle treated SLE mice. The ratio of P-NFκB to non-phosphorylated NFκB protein expression, as an indicator of the relative level of NFκB activity to the total amount, was significantly reduced in the renal cortex of etanercept treated control and SLE mice (Please see http://hyper.ahajournals.org., Figure S4).
Figure 5.
Effect of etanercept on renal TNF-α and NFκB protein expression in SLE and control mice. A. TNF-α protein expression was increased in the renal cortex of SLE mice compared to controls but was not significantly reduced after treatment with etanercept in either group. B. Total phosphorylated (P-NFκB) and non-phosphorylated NFκB are increased in renal cortex homogenates from SLE mice (* p<0.05 vs Ctrl+Veh and Ctrl+Etan). SLE mice treated with etanercept had significantly lower P-NFκB compared to SLE+Veh († p<0.05 vs. SLE+Veh, n=4 per group) "L" denotes the protein ladder and "+ " denotes the positive control.
MCP-1 and ET-1
Urinary levels of monocyte chemoattractant protein-1 (MCP-1) were measured and although the means of each group were not statistically different, 3 out of 5 samples from the vehicle treated SLE mice were markedly higher compared to all other samples (Please see http://hyper.ahajournals.org., Figure S5). Similar to our previous work 18, mice with SLE have increased urinary endothelin-1 (SLE+Veh 1.2 ± 0.2 pg/ml vs. Ctrl+Veh 0.50± 0.2 pg/ml, p<0.05, n=5). ET-1 was not different between SLE and control mice treated with etanercept (SLE+Etan 1.9 ± 0.7 pg/ml vs. Ctrl+Etan 1.8± 0.4 pg/ml, n=5).
Discussion
The major findings of the present study are as follows: 1) A model of chronic inflammatory disease treated with etanercept has lower blood pressure compared to vehicle treated controls; 2) The lower blood pressure in etanercept treated animals occurs despite the persistent elevation in anti-dsDNA autoantibody levels; 3) SLE mice treated with etanercept have less renal injury and inflammatory infiltrates than vehicle treated controls; 4) NADPH oxidase activity is lower in etanercept treated SLE mice compared to vehicle treated controls; and 5) Treatment with etanercept suppresses an important downstream signaling target of TNF-α (NFκB) in the renal cortex of mice with SLE.
Hypertension and chronic inflammation
Several lines of evidence suggest that inflammation may have an important role in the development of hypertension in both humans and experimental animal models. For example, circulating inflammatory cytokines such as TNF-α and interleukin 6 directly correlate with blood pressure 2,1 in humans. In experimental animal models, immunosuppression lowers blood pressure in rat models of salt-sensitive hypertension 24 and mice lacking both B and T lymphocytes (RAG-1 knockout mice) are protected against angiotensin II (AngII) induced hypertension 25. However, the specific role of TNF-α in the development of hypertension is not as clear and varies depending on the experimental animal model studied. AngII hypertension (transgenic model) and DOCA salt hypertension in rats is not attenuated by treatment with etanercept even though the treatment affords renal protection 5,6. In a model of AngII and high salt induced hypertension in rats, treatment with etanercept delayed the development of hypertension 7 whereas hypertension was ameliorated in a fructose fed model of insulin resistance 8 and in a model of placental ischemia that mimics preeclampsia 26. The data from the present study suggest that TNF-α plays an important role in the development of hypertension during SLE, a disease with its origins in immune system dysfunction.
We recently showed that an agonist of peroxisome proliferator activated receptor gamma reduces blood pressure and protects against renal injury in mice with SLE 18. In that study, we proposed that the anti-inflammatory and renal protective effect of rosiglitazone was an important mechanism for the reduced blood pressure. The data in this study further advances the idea that chronic inflammation, and specifically inflammation caused by TNF-α, promotes hypertension during SLE. In addition to these pro-inflammatory effects, TNF-α may also have a role to prevent autoantibody production that occurs during SLE. For example, TNF-α blockade can promote inflammatory cell apoptosis leading to increased autoantibody production 27 (although these antibodies may not be pathogenic 28). In the NZBWF1 model of SLE, IgG2a and IgG2b anti-dsDNA antibodies are closely associated with renal injury whereas IgG1 and IgG3 antibodies are not 29. Because the currently available commercial assays do not distinguish between the IgG subclasses, it is possible that the increased autoantibodies observed here are nonpathogenic. This is indirectly supported by the fact that renal pathology is not exacerbated in either the control or SLE mice treated with etanercept. That etanercept treatment significantly increased autoantibody production in SLE mice, yet reduced blood pressure and protected the kidneys, is consistent with what is observed in humans with SLE and suggests that downstream inflammation is an important mediator of hypertension.
The potential for factors other than TNF-α in the development of hypertension during SLE remains given that the hypertension was not completely normalized in etanercept treated mice. Extra-renal factors such as changes in metabolic parameters, peripheral vascular or central nervous system impairment may also contribute to the hypertension. We chose to focus on the kidneys because of their central role in the long term control of blood pressure and because the NZBWF1 mouse is an established model of lupus nephritis. The concept that TNF-α can directly affect renal function is also supported by a recent study demonstrating that TNF-α infusion in anesthetized mice impairs renal hemodynamics (renal blood flow and glomerular filtration rate) and that this response is prevented by treatment with etanercept 30.
Based on the present data, the possibility of a pressure dependent effect to reduce renal injury cannot be ruled out; however, there are several factors that argue against this. First, the renal injury in SLE is known to be mediated by immune complex deposition and the downstream inflammation. Second, to our knowledge, there is no evidence for a direct blood pressure lowering effect of etanercept as there is with rosiglitazone treatment from our earlier study (rosiglitazone is known to have direct vasodilatory effects through ion channel modulation 31). Finally, several studies in both humans and experimental animal models of SLE, including the NZBWF1 model used here, demonstrate that simply lowering blood pressure is not sufficient to reduce renal injury 32,33,34. For example, blockade of the renin-angiotensin system with captopril and sympathetic blockade with bretylium cause the same drop in blood pressure in NZBWF1 mice, yet only captopril provides renal protection 32. These data strongly suggest that the renal injury during SLE is not simply pressure dependent.
TNF-α and SLE
Because of the varied actions of TNF-α (immunoregulatory vs. proinflammatory), the precise role for TNF-α in the pathogenesis of SLE remains controversial. Some evidence suggests it is protective against SLE disease progression. A recently published retrospective study showed that patients receiving adalimubab or infliximab (monoclonal antibodies against TNF-α) developed lupus-like syndrome with generation of antinuclear and anti-dsDNA antibodies, malar rash, serositis, oral ulcers and hematologic abnormalities, all of which resolved after discontinuation of treatment 35. To the contrary, some patients with SLE respond well to TNF-α inhibition. Treatment with inflixamab has been reported to decrease proteinuria and reduce SLE disease activity score as assessed by the SLE Disease Activity Index (SLEDAI) 36,37. These disparate effects of TNF-α inhibition in humans with SLE may be related to the time course of the treatment. Short-term administration of infliximab (over weeks) appears to have more favorable outcomes for lupus nephritis while long-term treatment (over months) have deleterious renal effects 38. In the present work, we found that mice treated with etanercept have less renal damage as measured by albuminuria, glomerulosclerosis and renal macrophage infiltration. Therefore, the experimental protocol used in the present study mimics the short-term administration of TNF-α inhibition and demonstrates that this type of treatment protocol has implications for blood pressure control in patients with SLE.
TNF-α, Oxidative Stress and Hypertension
Data from humans with SLE show that there is a direct correlation between superoxide generated by isolated polymorphonuclear cells and SLE disease activity 39. The importance of oxidative stress in the development of hypertension is also well known through effects on renal sodium reabsorption and vascular function 40,41,42,43. In this study, the data indicate that mice with SLE have increased renal cortex NADPH oxidase activity that is reduced by the 4-week treatment with etanercept. The multi-subunit enzyme NADPH oxidase is a major cellular source of superoxide. Therefore, one cellular mechanism by which TNF-α inhibition may protect against hypertension during SLE is through decreased renal inflammation and a subsequent reduction in renal oxidative stress. The finding that F2-isoprostanes were not different among the groups is not necessarily surprising given that F2-isprostanes represent a whole body measure of lipid peroxidation and arachadonic acid metabolism in vivo. Moreover, it is well known that the production of reactive oxygen species is highly compartmentalized in tissues and cells. We interpret these data as stronger evidence of a role for renal oxidative stress in SLE and SLE hypertension. Whether antioxidant therapy reduces blood pressure in SLE has yet to be explored, although there is evidence that antioxidants delay mortality and albuminuria in the NZBWF1 model 44.
TNF-α, NFκB, MCP-1 and ET-1
Etanercept is a recombinant receptor with a long half-life (4.35 days 45) that binds to and therefore reduces the biological effectiveness of TNF-α. Consistent with previous studies 19, our data show that renal TNF-α protein expression is increased in mice with SLE compared to controls. Treatment with etanercept did not significantly affect TNF-α expression which is consistent with the concept that etanercept stabilizes the TNF-α protein but decreases its biological activity 46.
Because TNF-α protein expression was not altered after treatment with etanercept, we tested whether an important molecular downstream mediator of TNF-α signaling, NFκB, was altered in the renal cortex of etanercept treated mice. The ratio of P-NFκB to NFκB reported in the online supplement (Figure S4) can be used as an indicator of the relative amount of activated NFκB out of the total. While the ratio was significantly reduced in both control and SLE mice treated with etanercept, it is important to note that the total amount of both NFκB and P-NFκB was significantly greater in SLE mice compared to controls and that SLE mice treated with etanercept had significantly reduced levels of P-NFκB. These data cannot completely rule out the possibility that etanercept modulates the activity of other inflammatory pathways including those activated by the Fc receptor or TNF-β 46; however, they strongly implicate NFκB as an important molecular mediator for the biological actions of TNF-α in the renal cortex during SLE.
We also measured urinary levels of MCP-1 and found that three out of the five samples tested from vehicle treated SLE mice had markedly increased urinary MCP-1 (≈ 8, 24 and 38 fold increased over control); however, the means from the groups were not statistically different. The failure to demonstrate a probability value of less than 0.05 in this experiment is due to the very large variability in the data. This variability is created by the high levels of MCP-1 in the vehicle treated SLE mice whereas none of samples from etanercept treated SLE mice exhibited these levels. This data suggests that at least some mice with SLE have increased urinary MCP-1 that may contribute to increased inflammatory cells in the renal cortex.
Urinary ET-1 was assessed and, consistent with our earlier study, was found be higher in SLE mice when compared to controls 18. Urinary ET-1 levels were comparable between control and SLE mice treated with etanercept suggesting that treatment prevented an increased ET-1 in SLE mice. The specific role of renal ET-1 during SLE hypertension after TNF-α blockade is not yet clear and will require comprehensive studies to evaluate receptor expression and localization as well as hemodynamic effects of ET-1 blockade.
Perspectives
Chronic inflammation is increasingly recognized as an important pathophysiological mechanism leading to hypertension. Plasma TNF-α levels directly correlate with blood pressure in humans; however, the response to TNF-α inhibition in experimental animal models of hypertension has been varied. SLE is a chronic inflammatory disorder with a high prevalence of hypertension and renal disease. In the present study, using a model of disease with its origins in immune system dysfunction, we demonstrate that blockade of TNF-α reduces blood pressure, renal injury, and renal inflammation. Based on the data from this study, our current thinking is that TNF-α is mechanistically an important mediator of hypertension and renal injury likely by activating NFκB mediated pathways leading to increased oxidative stress in the renal cortex. Because etanercept is already clinically available for treatment of autoimmune diseases, these findings may have direct implications for the better treatment of SLE related hypertension as well as for the understanding of the role that TNF-α plays in the development of essential hypertension.
Supplementary Material
Acknowledgements
None
Sources of Funding. Marcia Venegas Pont is the recipient of an American Heart Association Greater Southeast Affiliate Postdoctoral Fellowship (2260874). This work was supported by HL086996 to Heather A. Drummond, DK07583 to Christine Maric, and HL085907, HL085907S3, HL092284 to Michael J. Ryan, and P01HL5197.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest. None
References
- 1.Bautista LE, Vera LM, Arenas IA, Gamarra G. Independent association between inflammatory markers (C-reactive protein, interleukin-6, and TNF-alpha) and essential hypertension. J Hum Hypertens. 2005;19:149–154. doi: 10.1038/sj.jhh.1001785. [DOI] [PubMed] [Google Scholar]
- 2.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 3.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J Am Soc Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 4.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1234–R1239. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 5.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–R83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller DN, Shagdarsuren E, Park JK, Dechend R, Mervaala E, Hampich F, Fiebeler A, Ju X, Finckenberg P, Theuer J, Viedt C, Kreuzer J, Heidecke H, Haller H, Zenke M, Luft FC. Immunosuppressive treatment protects against angiotensin II-induced renal damage. Am J Pathol. 2002;161:1679–1693. doi: 10.1016/S0002-9440(10)64445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–562. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- 8.Tran LT, Macleod KM, McNeill JH. Chronic etanercept treatment prevents the development of hypertension in fructose-fed rats. Mol Cell Biochem. 2009;330:219–228. doi: 10.1007/s11010-009-0136-z. [DOI] [PubMed] [Google Scholar]
- 9.Kelley VR, Wuthrich RP. Cytokines in the pathogenesis of systemic lupus erythematosus. Semin Nephrol. 1999;19:57–66. [PubMed] [Google Scholar]
- 10.Smolen JS, Steiner G, Aringer M. Anti-cytokine therapy in systemic lupus erythematosus. Lupus. 2005;14:189–191. doi: 10.1191/0961203305lu2134oa. [DOI] [PubMed] [Google Scholar]
- 11.Herrera-Esparza R, Barbosa-Cisneros O, Villalobos-Hurtado R, valos-Diaz E. Renal expression of IL-6 and TNFalpha genes in lupus nephritis. Lupus. 1998;7:154–158. doi: 10.1191/096120398678919949. [DOI] [PubMed] [Google Scholar]
- 12.Budman DR, Steinberg AD. Hypertension and renal disease in systemic lupus erythematosus. Arch Intern Med. 1976;136:1003–1007. [PubMed] [Google Scholar]
- 13.Doria A, Shoenfeld Y, Wu R, Gambari PF, Puato M, Ghirardello A, Gilburd B, Corbanese S, Patnaik M, Zampieri S, Peter JB, Favaretto E, Iaccarino L, Sherer Y, Todesco S, Pauletto P. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis. 2003;62:1071–1077. doi: 10.1136/ard.62.11.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petri M. Detection of coronary artery disease and the role of traditional risk factors in the Hopkins Lupus Cohort. Lupus. 2000;9:170–175. doi: 10.1191/096120300678828226. [DOI] [PubMed] [Google Scholar]
- 15.Selzer F, Sutton-Tyrrell K, Fitzgerald S, Tracy R, Kuller L, Manzi S. Vascular stiffness in women with systemic lupus erythematosus. Hypertension. 2001;37:1075–1082. doi: 10.1161/01.hyp.37.4.1075. [DOI] [PubMed] [Google Scholar]
- 16.Ryan MJ, McLemore GR., Jr Hypertension and Impaired Vascular Function in a Female Mouse Model of Systemic Lupus Erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R736–R742. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 17.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin Resistance and Obesity in a Mouse Model of Systemic Lupus Erythematosus. Hypertension. 2006;48:988–993. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 18.Venegas-Pont M, Sartori-Valinotti JC, Maric C, Racusen LC, Glover PH, McLemore GR, Jr, Jones AV, Reckelhoff JF, Ryan MJ. Rosiglitazone decreases blood pressure and renal injury in a female mouse model of systemic lupus erythematosus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1282–R1289. doi: 10.1152/ajpregu.90992.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan DC, Yui MA, Wuthrich RP, Kelley VE. Tumor necrosis factor and IL-1 in New Zealand Black/White mice. Enhanced gene expression and acceleration of renal injury. J Immunol. 1989;143:3470–3475. [PubMed] [Google Scholar]
- 20.Xu Q, Wells CC, Garman JH, Asico L, Escano CS, Maric C. Imbalance in sex hormone levels exacerbates diabetic renal disease. Hypertension. 2008;51:1218–1224. doi: 10.1161/HYPERTENSIONAHA.107.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iliescu R, Cucchiarelli VE, Yanes LL, Iles JW, Reckelhoff JF. Impact of androgen-induced oxidative stress on hypertension in male SHR. Am J Physiol Regul Integr Comp Physiol. 2007;292:R731–R735. doi: 10.1152/ajpregu.00353.2006. [DOI] [PubMed] [Google Scholar]
- 22.Jernigan NL, LaMarca B, Speed J, Galmiche L, Granger JP, Drummond HA. Dietary salt enhances benzamil-sensitive component of myogenic constriction in mesenteric arteries. Am J Physiol Heart Circ Physiol. 2008;294:H409–H420. doi: 10.1152/ajpheart.00571.2007. [DOI] [PubMed] [Google Scholar]
- 23.Venegas-Pont M, Sartori-Valinotti JC, Glover PH, Reckelhoff JF, Ryan MJ. Sexual dimorphism in the blood pressure response to angiotensin II in mice after angiotensin-converting enzyme blockade. Am J Hypertens. 2010;23:92–96. doi: 10.1038/ajh.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 25.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.LaMarca B, Speed J, Fournier L, Babcock SA, Berry H, Cockrell K, Granger JP. Hypertension in response to chronic reductions in uterine perfusion in pregnant rats: effect of tumor necrosis factor-alpha blockade. Hypertension. 2008;52:1161–1167. doi: 10.1161/HYPERTENSIONAHA.108.120881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caramaschi P, Bambara LM, Pieropan S, Tinazzi I, Volpe A, Biasi D. Anti-TNFalpha blockers, autoantibodies and autoimmune diseases. Joint Bone Spine. 2009;76:333–342. doi: 10.1016/j.jbspin.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Ziolkowska M, Maslinski W. Laboratory changes on anti-tumor necrosis factor treatment in rheumatoid arthritis. Curr Opin Rheumatol. 2003;15:267–273. doi: 10.1097/00002281-200305000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Bynote KK, Hackenberg JM, Korach KS, Lubahn DB, Lane PH, Gould KA. Estrogen receptor-alpha deficiency attenuates autoimmune disease in (NZB × NZW)F1 mice. Genes Immun. 2008;9:137–152. doi: 10.1038/sj.gene.6364458. [DOI] [PubMed] [Google Scholar]
- 30.Shahid M, Francis J, Majid DS. Tumor necrosis factor-alpha induces renal vasoconstriction as well as natriuresis in mice. Am J Physiol Renal Physiol. 2008;295:F1836–F1844. doi: 10.1152/ajprenal.90297.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eto K, Ohya Y, Nakamura Y, Abe I, Fujishima M. Comparative actions of insulin sensitizers on ion channels in vascular smooth muscle. Eur J Pharmacol. 2001;423:1–7. doi: 10.1016/s0014-2999(01)01047-0. [DOI] [PubMed] [Google Scholar]
- 32.Herlitz H, Svalander C, Tarkowski A, Westberg G. Effect of captopril on murine systemic lupus erythematosus disease. J Hypertens Suppl. 1988;6:S684–S686. doi: 10.1097/00004872-198812040-00215. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda T, Nakayama D, Gomi T, Sakurai J, Yamazaki T, Yuhara M. Captopril, an angiotensin I-converting enzyme inhibitor, decreases proteinuria in hypertensive patients with renal diseases. Nephron. 1989;52:72–75. doi: 10.1159/000185584. [DOI] [PubMed] [Google Scholar]
- 34.Perez De LG, De WC, Cohen CD, Nieto E, Molina A, Banas B, Luckow B, Vicente AB, Mampaso F, Schlondorff D. Angiotensin inhibition reduces glomerular damage and renal chemokine expression in MRL/lpr mice. J Pharmacol Exp Ther. 2003;307:275–281. doi: 10.1124/jpet.103.053678. [DOI] [PubMed] [Google Scholar]
- 35.Wetter DA, Davis MD. Lupus-like syndrome attributable to anti-tumor necrosis factor alpha therapy in 14 patients during an 8-year period at Mayo Clinic. Mayo Clin Proc. 2009;84:979–984. doi: 10.4065/84.11.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uppal SS, Hayat SJ, Raghupathy R. Efficacy and safety of infliximab in active SLE: a pilot study. Lupus. 2009;18:690–697. doi: 10.1177/0961203309102557. [DOI] [PubMed] [Google Scholar]
- 37.Matsumura R, Umemiya K, Sugiyama T, Sueishi M, Umibe T, Ichikawa K, Yoshimura M. Anti-tumor necrosis factor therapy in patients with difficult-to-treat lupus nephritis: a prospective series of nine patients. Clin Exp Rheumatol. 2009;27:416–421. [PubMed] [Google Scholar]
- 38.Aringer M, Houssiau F, Gordon C, Graninger WB, Voll RE, Rath E, Steiner G, Smolen JS. Adverse events and efficacy of TNF-alpha blockade with infliximab in patients with systemic lupus erythematosus: long-term follow-up of 13 patients. Rheumatology (Oxford) 2009;48:1451–1454. doi: 10.1093/rheumatology/kep270. [DOI] [PubMed] [Google Scholar]
- 39.Waszczykowska E, Robak E, Wozniacka A, Narbutt J, Torzecka JD, Sysa-Jedrzejowska A. Estimation of SLE activity based on the serum level of chosen cytokines and superoxide radical generation. Mediators Inflamm. 1999;8:93–100. doi: 10.1080/09629359990586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garvin JL, Ortiz PA. The role of reactive oxygen species in the regulation of tubular function. Acta Physiol Scand. 2003;179:225–232. doi: 10.1046/j.0001-6772.2003.01203.x. [DOI] [PubMed] [Google Scholar]
- 41.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–R935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 42.Manning RD, Jr, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol. 2005;25:311–317. doi: 10.1159/000086411. [DOI] [PubMed] [Google Scholar]
- 43.Lassegue B, Griendling KK. Reactive oxygen species in hypertension; An update. Am J Hypertens. 2004;17:852–860. doi: 10.1016/j.amjhyper.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 44.Suwannaroj S, Lagoo A, Keisler D, McMurray RW. Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10:258–265. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 45.Galor A, Perez VL, Hammel JP, Lowder CY. Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology. 2006;113:2317–2323. doi: 10.1016/j.ophtha.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Goldenberg MM. Etanercept, a novel drug for the treatment of patients with severe, active rheumatoid arthritis. Clin Ther. 1999;21:75–87. doi: 10.1016/S0149-2918(00)88269-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.