Abstract
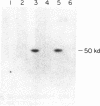
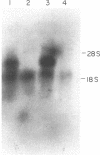
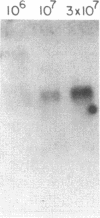
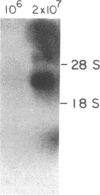
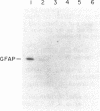
We have found glial fibrillary acidic protein (GFAP), the major component of astrocyte intermediate filaments, to be expressed in cell lines of the RT4 peripheral neurotumor family. The RT4 family is a "stem-cell-like" cell line, RT4-AC, that spontaneously undergoes differentiation in culture to three derivative cell types. This process, termed cell-type conversion, results in a segregation among the derivative cell types of parental cell phenotypes that have been described as neuronal-like or glial-like. We have identified a 50-kDa GFAP-immunoreactive cytoskeletal protein and GFAP mRNA in continuous RT4-AC and RT4-D (glial-type derivative) cell lines, but not in two presumptive neuronal-type cell lines. This result suggests that GFAP gene expression is coordinately coupled with the expression of other glial properties during cell-type conversion. In addition, the RT4-AC and RT4-D sublines were found to significantly express GFAP only at high cell densities and not during logarithmic growth and to express GFAP precociously during morphological differentiation following treatment with 1 mM N6, O2'-dibutyryladenosine 3',5'-cyclic monophosphate. These observations closely reflect reports of glial filament expression in astrocyte cultures, suggesting that a common regulatory mechanism is employed by central and peripheral nervous system glia.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigner D. D., Bigner S. H., Pontén J., Westermark B., Mahaley M. S., Ruoslahti E., Herschman H., Eng L. F., Wikstrand C. J. Heterogeneity of Genotypic and phenotypic characteristics of fifteen permanent cell lines derived from human gliomas. J Neuropathol Exp Neurol. 1981 May;40(3):201–229. doi: 10.1097/00005072-198105000-00001. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25(2):193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- Droms K., Sueoka N. Cell-type-specific responses of RT4 neural cell lines to dibutyryl-cAMP: branch determination versus maturation. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1309–1313. doi: 10.1073/pnas.84.5.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng L. F. Glial fibrillary acidic protein (GFAP): the major protein of glial intermediate filaments in differentiated astrocytes. J Neuroimmunol. 1985 Jun;8(4-6):203–214. doi: 10.1016/s0165-5728(85)80063-1. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Smith M. E., de Vellis J., Skoff R. P. Recent studies of the glial fibrillary acidic protein. Ann N Y Acad Sci. 1985;455:525–537. doi: 10.1111/j.1749-6632.1985.tb50433.x. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Vanderhaeghen J. J., Bignami A., Gerstl B. An acidic protein isolated from fibrous astrocytes. Brain Res. 1971 May 7;28(2):351–354. doi: 10.1016/0006-8993(71)90668-8. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Ferri G. L., Probert L., Cocchia D., Michetti F., Marangos P. J., Polak J. M. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982 Jun 3;297(5865):409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- Frame M. C., Freshney R. I., Vaughan P. F., Graham D. I., Shaw R. Interrelationship between differentiation and malignancy-associated properties in glioma. Br J Cancer. 1984 Mar;49(3):269–280. doi: 10.1038/bjc.1984.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., Rybak S., Kimhi Y., Littauer U. Z. Biphasic regulation by dibutyryl cyclic AMP of tubulin and actin mRNA levels in neuroblastoma cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4243–4247. doi: 10.1073/pnas.80.14.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J. E., Chiu F. C. Dibutyryl cyclic AMP causes intermediate filament accumulation and actin reorganization in astrocytes. Brain Res. 1984 Jul 23;306(1-2):85–95. doi: 10.1016/0006-8993(84)90358-5. [DOI] [PubMed] [Google Scholar]
- Goldman J. E., Chiu F. C. Growth kinetics, cell shape, and the cytoskeleton of primary astrocyte cultures. J Neurochem. 1984 Jan;42(1):175–184. doi: 10.1111/j.1471-4159.1984.tb09714.x. [DOI] [PubMed] [Google Scholar]
- Haag M. M., Soukup S. W., Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. VI. Chromosome analysis. Dev Biol. 1984 Jul;104(1):240–246. doi: 10.1016/0012-1606(84)90051-4. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- Imada M., Sueoka N. A two-dimensional polyacrylamide gel electrophoresis system for the analysis of mammalian cell surface proteins. Biochim Biophys Acta. 1980 Oct 21;625(2):179–192. doi: 10.1016/0005-2795(80)90282-2. [DOI] [PubMed] [Google Scholar]
- Imada M., Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. I. Isolation and characterization of cell lines and cell type conversion. Dev Biol. 1978 Sep;66(1):97–108. doi: 10.1016/0012-1606(78)90276-2. [DOI] [PubMed] [Google Scholar]
- Imada M., Sueoka N. Clonal sublines of rat neurotumor RT4 and cell differentiation. IV. Cell surface proteins. Dev Biol. 1980 Sep;79(1):199–207. doi: 10.1016/0012-1606(80)90083-4. [DOI] [PubMed] [Google Scholar]
- Imada M., Sueoka N., Rifkin D. B. Clonal sublines of rat neurotumor RT4 and cell differentiation. II. A conversion coupling of tumorigenicity and a glial property. Dev Biol. 1978 Sep;66(1):109–116. doi: 10.1016/0012-1606(78)90277-4. [DOI] [PubMed] [Google Scholar]
- Jessen K. R., Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980 Aug 14;286(5774):736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Balcarek J. M., Krek V., Shelanski M., Cowan N. J. Sequence of a cDNA clone encoding mouse glial fibrillary acidic protein: structural conservation of intermediate filaments. Proc Natl Acad Sci U S A. 1984 May;81(9):2743–2746. doi: 10.1073/pnas.81.9.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolait S. J., Harmer J. H., Auteri G., Pedersen J. S., Toh B. H. Expression of glial fibrillary acidic protein, actin, fibronectin and factor VIII antigen in human astrocytomas. Pathology. 1983 Oct;15(4):373–378. doi: 10.3109/00313028309085162. [DOI] [PubMed] [Google Scholar]
- Maurer R. A. Transcriptional regulation of the prolactin gene by ergocryptine and cyclic AMP. Nature. 1981 Nov 5;294(5836):94–97. doi: 10.1038/294094a0. [DOI] [PubMed] [Google Scholar]
- McMorris F. A. Cyclic AMP induction of the myelin enzyme 2',3'-cyclic nucleotide 3'-phosphohydrolase in rat oligodendrocytes. J Neurochem. 1983 Aug;41(2):506–515. doi: 10.1111/j.1471-4159.1983.tb04768.x. [DOI] [PubMed] [Google Scholar]
- Moonen G., Cam Y., Sensenbrenner M., Mandel P. Variability of the effects of serum-free medium, dibutyryl-cyclic AMP or theophylline on the morphology of cultured new-born rat astroblasts. Cell Tissue Res. 1975 Nov 12;163(3):365–372. doi: 10.1007/BF00219470. [DOI] [PubMed] [Google Scholar]
- Nagamine Y., Reich E. Gene expression and cAMP. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4606–4610. doi: 10.1073/pnas.82.14.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamine Y., Sudol M., Reich E. Hormonal regulation of plasminogen activator mRNA production in porcine kidney cells. Cell. 1983 Apr;32(4):1181–1190. doi: 10.1016/0092-8674(83)90301-x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Ludwig-Festl M., Weber K., Bignami A., Dahl D., Bayreuther K. Expression of glial and vimentin type intermediate filaments in cultures derived from human glial material. Differentiation. 1981;19(3):161–167. doi: 10.1111/j.1432-0436.1981.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Quax W., Egberts W. V., Hendriks W., Quax-Jeuken Y., Bloemendal H. The structure of the vimentin gene. Cell. 1983 Nov;35(1):215–223. doi: 10.1016/0092-8674(83)90224-6. [DOI] [PubMed] [Google Scholar]
- Raju T. R., Bignami A., Dahl D. Glial fibrillary acidic protein in monolayer cultures of C-6 glioma cells: effect of aging and dibutyryl cyclic AMP. Brain Res. 1980 Oct 27;200(1):225–230. doi: 10.1016/0006-8993(80)91114-2. [DOI] [PubMed] [Google Scholar]
- Schacterle G. R., Pollack R. L. A simplified method for the quantitative assay of small amounts of protein in biologic material. Anal Biochem. 1973 Feb;51(2):654–655. doi: 10.1016/0003-2697(73)90523-x. [DOI] [PubMed] [Google Scholar]
- Sobue G., Pleasure D. Schwann cell galactocerebroside induced by derivatives of adenosine 3',5'-monophosphate. Science. 1984 Apr 6;224(4644):72–74. doi: 10.1126/science.6322307. [DOI] [PubMed] [Google Scholar]
- Tomozawa Y., Sueoka N. In vitro segregation of different cell lines with neuronal and glial properties from a stem cell line of rat neurotumor RT4. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6305–6309. doi: 10.1073/pnas.75.12.6305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomozawa Y., Sueoka N., Miyake M. Clonal sublines of rat neurotumor RT4 and cell differentiation. V. Comparison of Na+ influx, Rb+ efflux, and action potential among stem-cell, neuronal, and glial cell types. Dev Biol. 1985 Apr;108(2):503–512. doi: 10.1016/0012-1606(85)90053-3. [DOI] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]