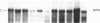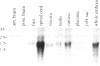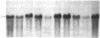Abstract
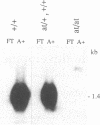
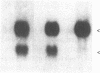
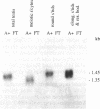
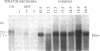
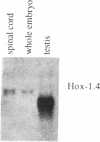
Hox-1.4 is a mouse homeobox-containing gene (initially identified as HBT-1), whose expression appears to be testis-specific in the adult animal. Examination of Hox-1.4 transcripts in RNA from testes of mutant mice deficient in germ cells confirms that Hox-1.4 expression within the testis is germ cell-specific. Enriched populations of spermatogenic cells were used to localize the expression of Hox-1.4 specifically to germ cells that have entered into and progressed beyond the meiotic prophase stage of differentiation and to demonstrate the presence of two different size Hox-1.4 transcripts. Examination of RNA from teratocarcinoma cell cultures and mouse embryos at 10.5-16.5 days of gestation demonstrated the presence of several Hox-1.4 transcripts, which are larger than those present in germ cells. In the midgestation fetus, Hox-1.4 expression is most abundant in the spinal cord.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awgulewitsch A., Utset M. F., Hart C. P., McGinnis W., Ruddle F. H. Spatial restriction in expression of a mouse homoeo box locus within the central nervous system. 1986 Mar 27-Apr 2Nature. 320(6060):328–335. doi: 10.1038/320328a0. [DOI] [PubMed] [Google Scholar]
- Bennett W. I., Gall A. M., Southard J. L., Sidman R. L. Abnormal spermiogenesis in quaking, a myelin-deficient mutant mouse. Biol Reprod. 1971 Aug;5(1):30–58. doi: 10.1093/biolreprod/5.1.30. [DOI] [PubMed] [Google Scholar]
- Bućan M., Yang-Feng T., Colberg-Poley A. M., Wolgemuth D. J., Guenet J. L., Francke U., Lehrach H. Genetic and cytogenetic localisation of the homeo box containing genes on mouse chromosome 6 and human chromosome 7. EMBO J. 1986 Nov;5(11):2899–2905. doi: 10.1002/j.1460-2075.1986.tb04585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Cattanach B. M., Kirk M. Differential activity of maternally and paternally derived chromosome regions in mice. Nature. 1985 Jun 6;315(6019):496–498. doi: 10.1038/315496a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chubb C., Nolan C. Genetic control of steroidogenesis and spermatogenesis in inbred mice. Ann N Y Acad Sci. 1984;438:519–522. doi: 10.1111/j.1749-6632.1984.tb38322.x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Colberg-Poley A. M., Voss S. D., Chowdhury K., Stewart C. L., Wagner E. F., Gruss P. Clustered homeo boxes are differentially expressed during murine development. Cell. 1985 Nov;43(1):39–45. doi: 10.1016/0092-8674(85)90010-8. [DOI] [PubMed] [Google Scholar]
- Duboule D., Baron A., Mähl P., Galliot B. A new homeo-box is present in overlapping cosmid clones which define the mouse Hox-1 locus. EMBO J. 1986 Aug;5(8):1973–1980. doi: 10.1002/j.1460-2075.1986.tb04452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaunt S. J., Miller J. R., Powell D. J., Duboule D. Homoeobox gene expression in mouse embryos varies with position by the primitive streak stage. Nature. 1986 Dec 18;324(6098):662–664. doi: 10.1038/324662a0. [DOI] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Wolgemuth D. J. Expression of the proopiomelanocortin gene is developmentally regulated and affected by germ cells in the male mouse reproductive system. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1600–1604. doi: 10.1073/pnas.84.6.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gizang-Ginsberg E., Wolgemuth D. J. Localization of mRNAs in mouse testes by in situ hybridization: distribution of alpha-tubulin and developmental stage specificity of pro-opiomelanocortin transcripts. Dev Biol. 1985 Oct;111(2):293–305. doi: 10.1016/0012-1606(85)90484-1. [DOI] [PubMed] [Google Scholar]
- Handel M. A., Eppig J. J. Sertoli cell differentiation in the testes of mice genetically deficient in germ cells. Biol Reprod. 1979 Jun;20(5):1031–1038. doi: 10.1095/biolreprod20.5.1031. [DOI] [PubMed] [Google Scholar]
- Hauser C. A., Joyner A. L., Klein R. D., Learned T. K., Martin G. R., Tjian R. Expression of homologous homeo-box-containing genes in differentiated human teratocarcinoma cells and mouse embryos. Cell. 1985 Nov;43(1):19–28. doi: 10.1016/0092-8674(85)90008-x. [DOI] [PubMed] [Google Scholar]
- Hollander W F, Bryan J H, Gowen J W. Pleiotropic Effects of a Mutant at the P Locus from X-Irradiated Mice. Genetics. 1960 Apr;45(4):413–418. doi: 10.1093/genetics/45.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Schofield P., Hogan B. A mouse homoeo box gene is expressed during embryogenesis and in adult kidney. Nature. 1985 Oct 24;317(6039):745–748. doi: 10.1038/317745a0. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Shackleford G. M., Varmus H. E., Martin G. R. Two proto-oncogenes implicated in mammary carcinogenesis, int-1 and int-2, are independently regulated during mouse development. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7806–7810. doi: 10.1073/pnas.83.20.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. R., Hunt D. M. Endocrinological findings in sterile pink-eyed mice. J Reprod Fertil. 1975 Jan;42(1):51–58. doi: 10.1530/jrf.0.0420051. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Kornberg T., Coleman K. G., Cox D. R., Martin G. R. Expression during embryogenesis of a mouse gene with sequence homology to the Drosophila engrailed gene. Cell. 1985 Nov;43(1):29–37. doi: 10.1016/0092-8674(85)90009-1. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Martin G. R. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987 Mar;1(1):29–38. doi: 10.1101/gad.1.1.29. [DOI] [PubMed] [Google Scholar]
- Kilpatrick D. L., Millette C. F. Expression of proenkephalin messenger RNA by mouse spermatogenic cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5015–5018. doi: 10.1073/pnas.83.14.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Evans M. J. Differentiation of clonal lines of teratocarcinoma cells: formation of embryoid bodies in vitro. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1441–1445. doi: 10.1073/pnas.72.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. R. Nomenclature for homoeobox-containing genes. Nature. 1987 Jan 1;325(6099):21–22. doi: 10.1038/325021b0. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Wiley L. M., Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977 Dec;61(2):230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Giampaolo A., Faiella A., Zappavigna V., Acampora D., Poiana G., Russo G., Peschle C., Boncinelli E. Differential and stage-related expression in embryonic tissues of a new human homoeobox gene. Nature. 1986 Dec 18;324(6098):664–668. doi: 10.1038/324664a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- McGrath J., Solter D. Completion of mouse embryogenesis requires both the maternal and paternal genomes. Cell. 1984 May;37(1):179–183. doi: 10.1016/0092-8674(84)90313-1. [DOI] [PubMed] [Google Scholar]
- Mullen R. J., Eicher E. M., Sidman R. L. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976 Jan;73(1):208–212. doi: 10.1073/pnas.73.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimune Y., Haneji T., Maekawa M., Kitamura Y. In vitro demonstration of deleterious effect of steel mutation on spermatogenesis in mice. J Cell Physiol. 1984 Jan;118(1):19–21. doi: 10.1002/jcp.1041180105. [DOI] [PubMed] [Google Scholar]
- Rubin M. R., Toth L. E., Patel M. D., D'Eustachio P., Nguyen-Huu M. C. A mouse homeo box gene is expressed in spermatocytes and embryos. Science. 1986 Aug 8;233(4764):663–667. doi: 10.1126/science.3726554. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone A., Mavilio F., Bottero L., Giampaolo A., Russo G., Faiella A., Boncinelli E., Peschle C. A human homoeo box gene specifically expressed in spinal cord during embryonic development. Nature. 1986 Apr 24;320(6064):763–765. doi: 10.1038/320763a0. [DOI] [PubMed] [Google Scholar]
- Surani M. A., Barton S. C., Norris M. L. Development of reconstituted mouse eggs suggests imprinting of the genome during gametogenesis. Nature. 1984 Apr 5;308(5959):548–550. doi: 10.1038/308548a0. [DOI] [PubMed] [Google Scholar]
- Waters S. H., Distel R. J., Hecht N. B. Mouse testes contain two size classes of actin mRNA that are differentially expressed during spermatogenesis. Mol Cell Biol. 1985 Jul;5(7):1649–1654. doi: 10.1128/mcb.5.7.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Engelmyer E., Duggal R. N., Gizang-Ginsberg E., Mutter G. L., Ponzetto C., Viviano C., Zakeri Z. F. Isolation of a mouse cDNA coding for a developmentally regulated, testis-specific transcript containing homeo box homology. EMBO J. 1986 Jun;5(6):1229–1235. doi: 10.1002/j.1460-2075.1986.tb04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Gizang-Ginsberg E., Engelmyer E., Gavin B. J., Ponzetto C. Separation of mouse testis cells on a Celsep (TM) apparatus and their usefulness as a source of high molecular weight DNA or RNA. Gamete Res. 1985;12:1–10. doi: 10.1002/mrd.1120120102. [DOI] [PubMed] [Google Scholar]