Abstract
Stem cell function is thought to be tightly regulated by growth factor concentration in the confines of the microenvironmental niche. Therefore, the response of human mesenchymal stem cells (hMSCs) was studied in culture with mechano-growth factor (MGF), an isoform of IGF-1 known to be expressed in the heart following injury. Chemotactic migration of hMSCs increased in response to a peptide analog corresponding to the E-domain region of the MGF prohormone, which was greater than the IGF-1 polypeptide after 20 hours of culture. Compared to control without growth factor, migration was significantly less with a scrambled peptide (p=0.025) or a peptide harboring a serine to alanine substitution near the carboxy end (p=0.002). The IGF-1 polypeptide increased proliferation of small (5-9 μm) but not large (>13 μm) hMSCs, whereas the E-domain peptide (MGF-E) had no effect on proliferation. Thus, there are complex biological responses of hMSCs to the prehormone of IGF with respect to migration and proliferation. Since neonatal myocytes but not hMSCs express MGF when strained cyclically at 20%, overloading of the heart may trigger immigration of stem cells. It seems possible that regions of the IGF prohormone may act differentially, or in a combinatorial manner, to benefit cardiac tissue recovery after injury.
Keywords: tissue regeneration, insulin growth factor, peptide analogs, cyclic strain
Introduction
Mesenchymal stem cells (MSCs) are advantageous for tissue regeneration because of availability, self-renewal and differentiation into multiple cell types [1]. However, gains are marginal in heart function [2] because of poor homing [3] and lack of myocyte differentiation [4]. Growth factors might enhance clinical outcomes via proliferation and directed migration. Members of the insulin-like growth factor (IGF) family mediate several regenerative processes, including modulation of inflammatory responses, apoptosis and proliferation. However, binding proteins rapidly sequester IGF limiting bioavailability locally and for short duration.
Alternate splicing within the IGF-1 gene produces many preprohormone isoforms that are cleaved by cellular endoproteases leaving the prohormone (mature 70 amino acid peptide + E-domains). Final cleavage yields identical mature peptides but with different E-domains [5], one of which is preferentially expressed in damaged skeletal muscle [6] and found in heart and other tissues [7]. In humans, this isoform is named IGF-1Ec or mechano-growth factor (MGF), Figure 1A.
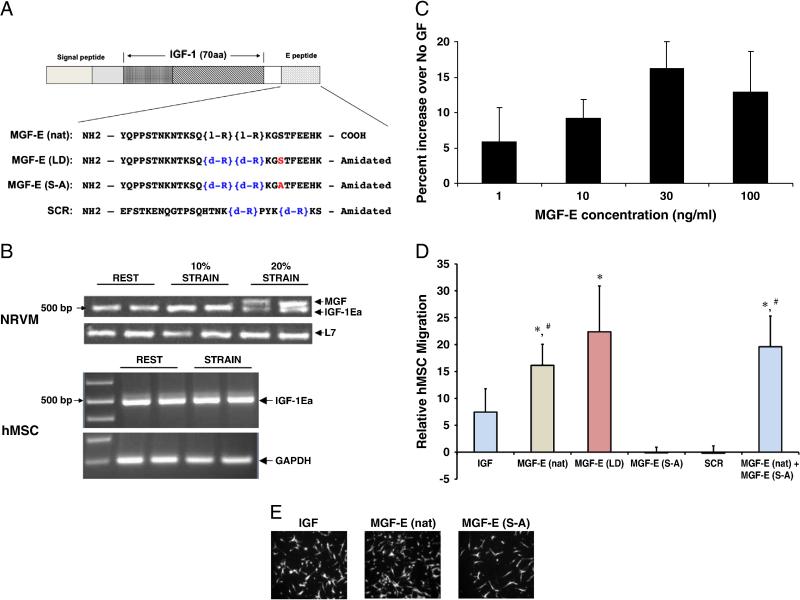
Figure 1. Migration of hMSCs with MGF-E peptide.
Schematic of peptides used for migration and proliferation experiments. A. The prohormone of MGF comprising the 70 amino acid mature IGF-1 and amino acid sequences of E-domain of MGF (MGF-E). The native (MGF-E (nat)), stabilized (MGF-E (LD)) 24 amino acid sequences, and biologically inactive peptides created by substituting the serine at position 18 with alanine (red) (MGF-E (S-A)), and scrambled peptide, containing all 24 amino acids of the E-domain, including the serine (SCR). Note: L-stereoisomer of arginine (R) is denoted by l-R and D-stereoisomer by d-R (blue). B. IGF-1 isoform expression. Neonatal rat ventricular myocytes and hMSC strained at 1Hz for 48hr. Gel images of IGF-1 isoform expression (IGF-1Ea, 500 bp and MGF, 550 bp bands) assessed by RTPCR and normalized to housekeeping genes. MGF expression denoted by higher molecular band is only found at 20% strain in NRVM. C. Migration dose response of hMSCs to MGF-E to determine optimal MGF-E (nat) (n=2, except for 30 ng/mL, n=6). D. Migration of hMSCs increases with MGF-E. hMSC migration to the bottom of a transwell migration insert as determined by increase in fluorescence intensity as percent of no growth factor. 30 ng/mL MGF-E (nat) or MGF-E (LD) in the bottom wells causes a significant increase in migration compared to biologically inactive peptides, MGF-E (S-A) or SCR. Also, MGF-E (nat) and MGF-E (S-A) do not compete. (Values are mean ± SE. *, p<0.05 versus MGF-E (S-A); #, p<0.05 versus SCR, n=3 human donors). E. Representative images of hMSCs which migrated to the bottom of the wells are shown for MGF-E (nat), IGF, MGF-E (S-A).
Biological activity has been demonstrated in both muscle and neuronal tissues of a 24 amino acid peptide analog corresponding to the unique C-terminal E-domain of MGF (MGF-E). MGF-E is a positive regulator of skeletal myoblast proliferation and differentiation leading to muscle hypertrophy [8]. In the mouse heart, MGF-E improves cardiac function after myocardial infarction [9]. MGF-E and IGF-1Ea expression are significantly increased 4 and 8 weeks after myocardial infarction in rat myocardium with MGF-E message and protein levels five-fold higher than IGF-1Ea levels [7]. Additionally, MGF-E causes increased H9C2 cell proliferation independent of the IGF-1 receptor [7]. Thus, further study of MGF-E peptide on proliferation and migration of human MSCs may advance regenerative therapy.
Materials and Methods
Cell Culture
After institutional approval, hMSCs isolated from bone marrow aspirates were cultured in complete culture media (CCM) consisting of MEM-α supplemented with 16.5% FBS, 2 mM L-glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin. hMSCs were obtained from the Tulane University Center for Gene Therapy or AllCells (Berkeley, CA). Passage 2-3 (P2-P3) hMSCs were used for all experiments.
Peptides
The native form of MGF-E (MGF-E (nat)) was synthesized with a C-terminal cysteine cap and purified via high performance liquid chromatography (Genescript Corp, NJ), (Figure 1A). The stabilized form of MGF-E (MGF-E (LD)) is more resistant to degradation due to an L to D stereoisomer switch of two arginines in the 24 amino acid sequence of MGF-E. Biologically inactive peptides were created to test the effects of substituting a serine at a consensus PKA site, 18 amino acids from the N-terminus to alanine (MGF-E (S-A), Genscript Corp, NJ) and scrambling the 24 amino acids of MGF-E (SCR, Genscript Corp, NJ). The stereoisomer switches for MGF-E (LD) are present on both MGF-E (S-A) and SCR peptides (Figure 1A). Peptides were dissolved in 60% acetonitrile (stock 1 mg/mL) and diluted in molecular grade water to 1000 ng/mL yielding a final concentration (30 ng/mL) in media. For no growth factor (No GF) control, equal volumes of molecular grade water were added to media.
Lyophilized recombinant insulin-like growth factor 1 (IGF-1, Sigma) was reconstituted in phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin (stock 50 mg/mL) diluted in molecular grade water to 3333 ng/mL and a final concentration (100 ng/mL) in media; this is an equal molar concentration as for MGF-E.
IGF-1 isoform expression in cyclically strained neonatal rat ventricular myocytes and hMSCs
hMSCs or neonatal rat ventricular myocytes (NRVM) in BioFlex® Culture Plates (Flexcell International, Hillsborough, NC) were strained cyclically with a Flexcell Strain Unit (Model FX-4000, Flexcell International) at 10 or 20% and 1Hz frequency for 48 hours. Total RNA was isolated and used (100 ng) in each RT-PCR reaction to assess changes in IGF-1 isoform expression. Primers were rodent IGF-1 isoforms; forward, 5’-CCTCCAATAAAGATACACATCATGTCG-3’, reverse, 5’-TTTGGCAGGTGTTCCGATGTT-3’. Human IGF-1 isoforms; forward, 5’-CAGCTCTGCCACGGCTGGAC, reverse, 5’-GCAAAGGATCCTGCGGTGGCA. Rodent ribosomal protein (L7); forward, 5’-GAAGCTCATCTATGAGAAGGC-3’, reverse 5’-CAGACGGAGCAGCTGCAGCAC-3’. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH); forward, 5-GTGGACCTGACCTGCCGTCTA-3, reverse 5-GCTTGACAAAGTGGTCGTTGA-3’. Amplification conditions were identical except the annealing temperature for the human IGF-1 isoform primers was 70°C, whereas all others were 55°C.
hMSC migration
Migration of hMSCs was determined with a 10-μm thick polycarbonate porous membrane insert with 8-μm pores (FluoroBlok, BD Biosciences). P2-P3 hMSCs were thawed and cultured to approximately 80% confluence in CCM. Plates were washed with PBS and cells starved with non-serum, low glucose (0.1 mM) DMEM media (LGM) for four hours. Cells were then detached, counted and 50,000 cells in LGM were added to the top of the transwell insert. The bottom of each well contained LGM with or without growth factors. Groups were No GF, IGF, MGF-E (nat), MGF-E (LD), SCR and MGF-E (S-A). Additionally MGF-E (nat) and MGF-E (S-A) were added together in equal concentration to assess any competitive action between them. Cells migrated and attached for 20 hours when the inserts were removed, washed with PBS and stained with Calcein-AM. Plates were then scanned using a FlexStation II (Molecular Devices) bench top scanning fluorometer. Experiments were repeated for three human donors six times (n=6) except where noted.
To confirm hMSC migration, images were collected of Calcein-AM labeled hMSCs, which migrated through the membrane. Fluorescence was observed on a Nikon Microphot-FXA/SA epifluorescent microscope.
hMSC proliferation
P2-P3 hMSCs at approximately 80% confluence in CCM were detached, counted with a hemocytometer and resuspended in reduced culture media (RCM) consisting of MEM-α with 2% FBS and 100 units/mL penicillin and 100 μg/mL streptomycin. 1×104 cells (in 200 μL) were plated into wells of a 96 well plate for each experimental group. Note, cells were restricted to centrally located wells to eliminate parabolic cell growth patterns due to dehydration in the outer perimeter. The colorimetric WST-1 (Roche) assay was used as an index of proliferation of hMSCs. Cell number was estimated at day 0 and four days after growth factor treatment using a plate reader to measure the absorbance at 450 nm. Media was changed once after 48 hours. Mouse MSCs (data not shown) indicated no proliferative effect with the stable version of MGF-E (MGF-E(LD)). Therefore, the groups of interest for proliferation analysis included IGF, MGF-E (nat) and No GF control and experiments were repeated for each human donor (n=3).
To determine differential effects due to cell size and confirm WST-1 proliferation results, hMSC number and size was determined using a Multisizer 3 Coulter counter (Coulter Counter, Fullerton, CA). 6×104 cells in CCM were plated into 10-cm tissue culture dishes and allowed to attach overnight. Some plates were used to obtain day 0 cell counts while RCM with growth factors or control molecular grade water. Cells were cultured for four days with one media change at 48 hours, as above. Cells were then detached and counted and cell counts were made from 5-30 μm diameter to confirm the WST-1 assay. Counts were made of cells with diameters from 5-9 μm or greater than 13 μm to determine effects of growth factors on small or large cells. Experiments were run in triplicate for one human donor.
Results and Discussion
MGF expression in cardiac myocytes following mechanical strain
IGF-1 isoform primers that span exons 4 and 6 detected a higher molecular weight transcript in cardiac myocytes following 20% mechanical strain (Figure 1B). This corresponds to the MGF isoform due to a 49 base pair insert (in rodents) from part of exon 5. In human hMSCs, using the same approach, no apparent MGF expression was noted following mechanical strain. This indicates that the MGF isoform is expressed in cardiac myocytes following mechanical perturbation and may function as a local growth factor in response to injury.
Migration of hMSCs in response to the MGF-E peptide
MGF-E increases hMSC migration as seen in representative images of Calcein-AM labeled hMSCs at the bottom of transwell insert (Figure 1E). Dose response experiments confirm that migration of hMSCs is optimal at 30 ng/mL MGF-E (nat) (Figure 1C), agreeing with other cell types [8,10]. Migration of hMSCs is significantly increased after 20 hours in response to MGF-E (nat) compared to MGF-E (S-A) (p≤0.002) and SCR (p≤0.025) relative to control (No GF) (Figure 1D). Although there was higher variability across 3 human donors, MGF-E (LD) caused significantly more migration compared to MGF-E (S-A) (p≤0.006) with a trend for increase versus SCR (p=0.06) (Figure 1D). Interestingly, the chemotactic effect of MGF-E is blunted with substitution of the serine at position 18 with alanine (MGF-E (S-A)) or scrambling the order of the amino acids of the MGF-E peptide. Additionally, incubation with equal amounts of MGF-E (nat) and MGF-E (S-A) did not blunt hMSC migration, suggesting that lack of phosphorylation at this position does not function in a dominant negative manner.
Migration in hMSCs likely depends on the presence of a serine residue that forms a putative PKA consensus phosphorylation site within the unique region of the E-domain of the MGF prohormone. This would suggest that the E-domain plays a role in mediating intracellular signaling that would be limited to the MGF splice variant, since the serine near the terminus of the E-domain is not present in other IGF-1 isoforms. Indeed, the presence of the E-domain region of the IGF-1 prohormone isoforms (IGF-1Ea and MGF) yields distinct gene expression patterns in skeletal muscle compared to one another and mature IGF-1 [11]. Selective action of MGF-E on skeletal muscle repair is attributed to migration of myoblasts due to increased matrix metalloproteinase expression [10] and increased myoblast stem cell differentiation [12].
Migration is regulated via the SDF-CXCR4 axis. IGF-1 increases murine MSC migratory response via the CXCR4 receptor suggesting that growth factors act in an orchestrated manner with chemokines to reinforce the migratory response [13]. Surprisingly, results here show that IGF-1 does not directly induce hMSC migration whereas MGF-E does. Further, antibody studies have ruled out the action of the MGF-E through the IGF-1 receptor in most cases, suggesting another mechanism must exist for cellular uptake and biologic function [8,10].
Proliferation of hMSCs in response to the MGF-E peptide
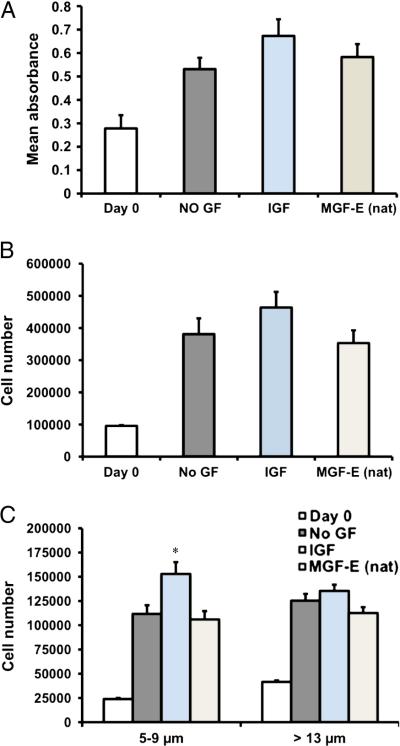
All peptides had minimal effects on proliferation of the whole stem cell population (Figure 2). The WST-1 cell counting assays suggest a trend for increased hMSC proliferation in response to IGF but not MGF-E (nat) treatment (Figures 2A, 2B). Interestingly, IGF treatment causes a significantly greater proportion of small cells (5-9 μm diameter) to proliferate compared to cells without any growth factor or exposed to the natural, MGF-E (Figure 2C). The number of cells greater than 13 μm diameter is not significantly different than No GF after treatment with any of the peptides (Figure 2C).
Figure 2. Proliferation of small and large hMSCs with MGF-E peptides.
A. Cell number was determined at day 0 and for No GF, IGF, and MGF-E (nat) at 4 days for 3 different human donors using the WST-1 assay. Although hMSCs proliferate from day 0 to day 4, there was no significant difference between treatment groups (n=3 human donors). B. Counting of cells 5-30 μm in diameter shows similar trends as those obtained with WST-1 assay. C. Cell number of 5-9 μm is higher (* p<0.05) with IGF treatment. MGF-E (nat) or MGF-E (S-A) does not affect 5-9 μm diameter cells. There is no difference between treatment groups for hMSC with greater than 13 μm diameter (1 human sample, n=3 experiments).
Findings here that IGF-1 does not affect proliferation of the whole hMSC population are consistent with other reports [13]. However, the hMSCs used here are well characterized and have two distinct size sub-populations [14]. It is interesting that the effects of IGF-1 on proliferation are only apparent in the small cells (5-9μm) but not the larger cells (greater than 13 μm). Self-renewal and multipotency are characteristics of the small cells [14], thus suggesting that IGF-1 might increase the proportion of cells capable of differentiation into other cell types useful for tissue regeneration.
Paracrine actions
Beneficial paracrine actions of MSCs include myocyte survival, reduction of fibrotic scar formation [1], and with the marginal functional improvements in the injured heart attributed mainly to enhanced formation of vessels [15]. While little evidence that soluble factors secreted by stem cells act on differentiation and engraftment of myocytes, MGF-E is anti-apoptotic, possibly protecting ischemic myocytes and preserving the injured myocardium [9].
Summary
These new findings suggest that MGF-E can enhance hMSC migration with a mechanism dependent on the serine near the carboxyl terminal region implicating action by phosphorylation that might modify other protein partners or gene expression. MGF-E gene expression is increased in NRVM after 20% cyclic strain, suggesting it is injury-related. Interestingly, although IGF-1 is not thought to affect MSC proliferation [13], small hMSCs do proliferate while larger ones do not. There are complex biological responses of hMSCs to IGF prohormone splice variants with respect to migration and proliferation. Specific members of the IGF family may act differentially and, therefore, a combinatorial approach may be beneficial to the overall tissue recovery after injury.
Acknowledgements
The work was supported by NIH (HL62426, HL090523), the State of Illinois funds for Regenerative Medicine, and AHA predoctoral fellowship 08155359.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Psaltis PJ, Zannettino AC, Worthley SG, Gronthos SG S. Concise review: mesenchymal stromal cells: potential for cardiovascular repair. Stem Cells. 2008;26:2201–10. doi: 10.1634/stemcells.2008-0428. [DOI] [PubMed] [Google Scholar]
- 2.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20–28. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karp JM, Leng Teo GS. Mesenchymal Stem Cell Homing: The Devil Is in the Details. Cell Stem Cell. 2009;4:206–16. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Murry CE, Soonpaa MH, Reinecke H, et al. Haematopoietic Stem Cells Do Not Transdifferentiate into Cardiac Myocytes in Myocardial Infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 5.Foyt HL, LeRoith D, Roberts CT., Jr. Differential Association of Insulin-Like Growth Factor I MRNA Variants with Polysomes in Vivo. J Biol Chem. 1991;266:7300–5. [PubMed] [Google Scholar]
- 6.Hill M, Goldspink G. Expression and Splicing of the Insulin-Like Growth Factor Gene in Rodent Muscle Is Associated with Muscle Satellite (Stem) Cell Activation Following Local Tissue Damage. J Physiol. 2003;549:409–18. doi: 10.1113/jphysiol.2002.035832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stavropoulou A, Halapas A, Sourla A, et al. Igf-1 Expression in Infarcted Myocardium and Mgf E Peptide Actions in Rat Cardiomyocytes in Vitro. Mol Med. 2009;15:127–35. doi: 10.2119/molmed.2009.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang SY, Goldspink G. Different Roles of the Igf-I Ec Peptide (Mgf) and Mature Igf-I in Myoblast Proliferation and Differentiation. FEBS Lett. 2002;522:156–60. doi: 10.1016/s0014-5793(02)02918-6. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter V, Matthews K, Devlin G, et al. Mechano-Growth Factor Reduces Loss of Cardiac Function in Acute Myocardial Infarction. Heart Lung Circ. 2008;17:33–9. doi: 10.1016/j.hlc.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Mills P, Lafreniere JF, Benabdallah BF, et al. A New Pro-Migratory Activity on Human Myogenic Precursor Cells for a Synthetic Peptide within the E Domain of the Mechano Growth Factor. Exp Cell Res. 2007b;313:527–37. doi: 10.1016/j.yexcr.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Barton ER, DeMeo J, Lei H. The insulin-like growth factor (IGF)-I E-peptides are required for isoform-specific gene expression and muscle hypertrophy after local IGF-I production. J Appl Physiol. 2010;108:1069–76. doi: 10.1152/japplphysiol.01308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ates K, Yang SY, Orrell RW, et al. The Igf-I Splice Variant Mgf Increases Progenitor Cells in Als, Dystrophic, and Normal Muscle. FEBS Lett. 2007;581:2727–32. doi: 10.1016/j.febslet.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Yu X, Lin S, et al. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. BBRC. 2007;356:780–84. doi: 10.1016/j.bbrc.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 14.Colter DC, Sekiya I, Prockop DJ. Identification of a Subpopulation of Rapidly Self-Renewing and Multipotential Adult Stem Cells in Colonies of Human Marrow Stromal Cells. Proc Natl Acad Sci U S A. 2001;98:7841–5. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tongers J, Roncalli JG, Losordo DW. Role of Endothelial Progenitor Cells During Ischemia-induced Vasculogenesis and Collateral Formation. Microvasc Res. 2010 doi: 10.1016/j.mvr.2010.01.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]