Abstract
High-intensity focused ultrasound (HIFU) is a novel therapeutic modality that permits noninvasive treatment of various benign and malignant solid tumors, including prostatic cancer, uterine fibroids, hepatic tumors, renal tumors, breast cancers, and pancreatic cancers. Several preclinical and clinical studies have investigated the safety and efficacy of HIFU for treating solid tumors, including pancreatic cancer. The results of nonrandomized studies of HIFU therapy in patients with pancreatic cancer have suggested that HIFU treatment can effectively alleviate cancer-related pain without any significant complications. This noninvasive method of delivering ultrasound energy into the body has recently been evolving from a method for purely thermal ablation to harnessing the mechanical effects of HIFU to induce a systemic immune response and to enhance targeted drug delivery. This review provides a brief overview of HIFU, describes current clinical applications of HIFU for pancreatic cancer, and discusses future applications and challenges.
Keywords: Pancreatic cancer, High-intensity focused ultrasound, Therapy, Review
INTRODUCTION
Ultrasound is one of the most useful diagnostic tools in current clinical practice. Most clinicians are familiar with diagnostic ultrasound; however, the use of high intensity focused ultrasound (HIFU) for therapeutic purposes is an emerging medical technology that many physicians are unfamiliar with. HIFU is a completely non-invasive method to achieve ablation in solid tissue using focused ultrasound energy from an extracorporeal source to a target within the body.
HIFU is an emerging therapeutic technique but has a long history. The initial investigation of ultrasound in biologic systems was not for imaging but for its biologic effects.1 In the 1950's Fry et al.2,3 applied HIFU to the human brain with the intent to create discrete lesions to treat hyperkinetic disorders such as Parkinson's disease. With recent technical advances, clinical devices have been developed leading to several clinical studies of HIFU for treatment of benign and malignant solid tumors of the liver, prostate, bladder, kidney, uterus, breast and pancreas.
Pancreatic cancer is the fourth leading cause of cancer death in the United States and more than 42,470 people were newly diagnosed with pancreatic cancer in the United States last year and most patients dying within one year.4 Approximately 80% of patients have unresectable disease at the time of diagnosis with an overall 5-year survival rate of less than 1%. The median survival of the patients with pancreatic cancer is less than 3 months without therapy and less than 6 to 12 months with therapy. Therefore, the primary goals of treatment for advanced pancreatic cancer patients are to improve overall survival and palliation. Preliminary studies suggest that HIFU may be useful for the palliative therapy of cancer-related pain in patients with unresectable pancreatic cancer.4-7 This review provides an overview of HIFU, discusses current clinical applications of HIFU for pancreatic cancers, and addresses future applications and challenges.
OVERVIEW OF HIFU
Ultrasound is a form of mechanical energy that can propagate through most of the human body. HIFU can be described in an analogous fashion to light. Light is a form of electromagnetic energy that can be used to see objects (imaging). Similarly, ultrasound is a form of mechanical energy that can be used to perform imaging. However, if the energy is focused, e.g., using a magnifying glass to focus sunlight, the concentrated energy is sufficient to burn an object at the focus. Similarly, when high intensity ultrasound energy is generated by an ultrasound transducer that is focused either mechanically (spherically curved or using a focusing lens) or electronically by phasing an array of transducers, ultrasound energy can heat and ablate tissue. The focal characteristics of most clinically available transducers are similar in size to a grain of rice, typically 2-3 mm in diameter and 8-10 mm in length. The acoustic intensities used in HIFU differ from diagnostic ultrasound in that the time averaged acoustic intensity at the focus is several orders of magnitude greater for HIFU. Diagnostic ultrasound typically produces time averaged acoustic intensities of around 100 mW/cm2 whereas HIFU can deliver intensities at the focus that is over 10 kW/cm2.
1. Biologic effects of HIFU
HIFU is able to produce both thermal and mechanical effects in tissue. Thermal effects from HIFU result from heat generation at the focus due to absorption of acoustic energy in tissue. The absorption of ultrasound energy in tissue can elevate the temperature rapidly up to 100℃. Focusing of the ultrasound energy minimizes the potential for thermal damage to intervening tissue between the transducer and the focal point because the intensities are substantially lower outside the focal region. If the temperature is elevated to 100℃ then boiling occurs at the focus and coagulative necrosis occurs immediately. However, if the temperature is not elevated to over 100℃ then a phenomenon termed thermal fixation can occur where the cells do not undergo lysis and the tissue architecture remains relatively intact but the cells are no longer viable. This has been seen in patients treated with HIFU followed by surgical resection.8 As the lesion evolves the cells degenerate resulting in coagulative necrosis; however, this effect is significant for the treatment of the pancreas where cell lysis has potential to release autodigestive enzymes and lead to pancreatitis. With HIFU treatments that result in thermal fixation, pancreatic cells do not undergo lysis until the intracellular enzymes have been completely denatured and inactivated, theoretically reducing the risk of pancreatitis with HIFU therapy.
HIFU is also able to produce mechanical bioeffects that are not seen with low intensity US. Mechanical effects include acoustic cavitation, radiation force, shear stress and acoustic streaming/microstreaming. Acoustic cavitation is the most significant mechanical phenomenon that occurs when a gas-filled bubble interacts with an ultrasound field. Gas-filled bubbles can form in tissue in response to ultrasound fields with high peak negative pressures that extract gas from the tissue resulting in bubble formation or they can be introduced intravascularly in the form of ultrasound contrast agents. Bubbles can undergo oscillations in size in response to positive and negative pressures from the ultrasound field. Given the appropriate ultrasound parameters (frequency, pulse length, pulse repetition frequency, and pressure amplitudes) and bubble conditions (concentration, size, and composition) a bubble can undergo expansion followed by violent collapse of the bubble which is termed inertial cavitaion.9 If a bubble undergoes inertial cavitation, the force produced from the collapse of the bubble can be sufficient to cause cell lysis.10 Radiation force is another mechanical phenomenon that can occur from HIFU exposure which results when a wave is either absorbed or reflected. If the reflecting or absorbing medium is tissue or other solid material, the acoustic force presses against the medium, producing a radiation force. If the medium is liquid and can move under pressure, then acoustic streaming results. Acoustic streaming caused by an oscillating bubble in an acoustic field immediately surrounding the bubbles is specifically referred to as acoustic microstreaming.11
2. Monitoring of HIFU treatment
Currently available commercial devices employ two different methods of imaging, ultrasound and MR, for guiding and monitoring HIFU therapy. Ultrasound is relatively inexpensive and provides real-time imaging using the same energy modality as HIFU. Adequate ultrasound imaging of the target suggests that there is no obstruction (e.g., bowel gas or bone) to ultrasound energy reaching the target. However, ultrasound imaging used in current clinical devices does not have the capability of performing thermometry or other monitoring of the focal region for lesion formation with the exception of identifying hyperechoic changes. The hyperechoic changes on ultasound imaging represent gas bubble formation due to tissue boiling, and do not precisely correlate with the actual lesion that is created.12
MR-guided HIFU provides excellent cross-sectional imaging of the target. Furthermore, MR imaging allows for near-real-time monitoring of HIFU treatments by providing temperature information within seconds after HIFU treatment. However, motion artifact and spatial resolution can limit its accuracy in the clinical setting.
HIFU OF PANCREATIC TUMORS
As previously mentioned, most patients with pancreatic cancer are deemed inoperable at the time of diagnosis and the efficacy of systemic chemotherapy is limited. Palliative therapy for pain relief is an important aspect of managing these patients. Furthermore, development of effective local therapies will likely be essential as systemic therapies improve. Currently, HIFU treatment of pancreatic cancer is widely available in China, with limited availability in South Korea and Europe. A preclinical in vivo study of HIFU ablation of the pancreas in swine resulted in effective ablation of the pancreas without any significant adverse effects such as skin burns or evidence of pancreatitis on biochemical analysis suggesting that HIFU treatment for pancreatic cancer may be feasible and safe.13
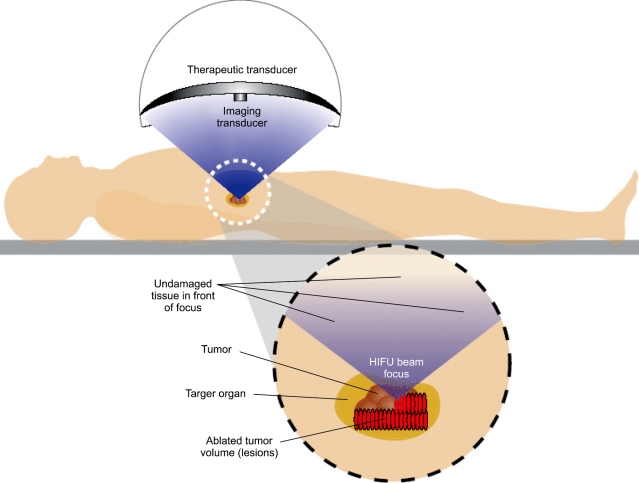
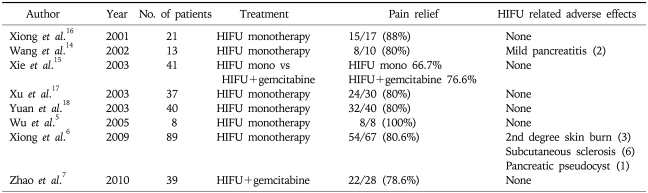
HIFU treatment is non-invasive with ultrasound energy delivered from an extracorporeal source (Fig. 1). There have been several case series reporting on the use of HIFU to treat patients with pancreatic cancer (Table 1).5,6,13-19 These reports, all from China, investigated the use of HIFU as monotherapy or as combination therapy with chemotherapy. In general, these reports suggest that HIFU is an effective therapy for palliation of pain related to pancreatic cancer. Furthermore, no significant adverse effects were reported in any of these studies. The mechanism of pain relief in these patients is still unclear but may be due to damage of nerve fibers innervating the tumor. In these reports objective tumor response rates ranged from 14.6% to 74% with some cases showing significant reduction of tumor volume. However, there have been no comparative studies performed to demonstrate that treatment with HIFU confers a survival benefit.
Fig. 1.
Illustration of high-intensity focused ultrasound (HIFU) treatment of a pancreatic tumor from an extracorporeal HIFU system (Reproduced with permission from Dubinsky et al., AJR Am J Roentgenol 2008;190:191-199.).
Table 1.
Previous Studies of HIFU Therapy for Pancreatic Cancer
HIFU, high-intensity focused ultrasound.
1. Enhancement of treatment efficacy
Several factors impact the efficacy of HIFU treatment of pancreatic cancers. Most importantly, an adequate acoustic window is essential for effective and predictable ablation of pancreatic tumors. Obstruction of the acoustic beam by bowel gas will prevent effective transmission of the acoustic energy to the target for effective ablation. Furthermore, the presence of bowel gas in the path of the acoustic beam increases the risk of complications such as thermal injury to the bowel. Therefore, it is important to minimize bowel gas prior to the procedure. Another factor that is important in achieving a consistent and effective therapy is to have a treatment protocol that delivers a consistent 'dose' to the target. To date, a standardized HIFU dose has not been established. If MR guidance is used for monitoring the HIFU therapy then a thermal dose can be determined. However, ultrasound-guided systems currently do not have the capability of thermometry. Therefore, in order to deliver a consistent therapy, a method for determining the amount of acoustic energy delivered to the tumor is needed in this situation. It is important to account for attenuation of the acoustic energy between the transducer and the target by the intervening tissue (primarily abdominal wall and viscera) when determining the amount of energy delivered to the tumor.13 Lastly, respiratory movement of the tumor during the treatment must be considered and minimized. Respiratory motion tracking techniques to achieve more safe and effective therapy are currently in development.
FUTURE CLINICAL APPLICATIONS OF HIFU
1. Enhancement of cancer-specific immunity
Reports have suggested that local ablative therapies, such as RF ablation and cryosurgery, may lead to activation of the host immune system, which is sensitized to the ablated tumor lysates, to stimulate a systemic host tumor-specific immune response.20,21 Similar findings have also been reported after HIFU ablation.22,23 Preliminary clinical studies have suggested that the immune response can be altered after HIFU treatment. Increased NK cell activity has been observed in tumors and increases in the population of CD4+ lymphocytes and the ratio of CD4+/CD8+ in the blood circulation of cancer patients have been observed after HIFU treatments.14 In addition to an enhanced systemic immune response, HIFU may lead to enhance local antitumor immunity as well. Some clinical studies have shown greater concentrations of dendritic cells, macrophages, and B lymphocytes in the HIFU treatment group than control groups.23,24 These results suggest that local HIFU treatment may have systemic effects and could potentially be combined with systemic chemotherapy for local therapy as well as control of local tumor recurrence and metastasis. Further studies are needed to understand the mechanisms involved in HIFU-enhanced systemic immune response and to identify the optimal HIFU treatment parameters to elicit this response.
2. Enhancement of drug delivery
Ultrasound-enhanced drug delivery has become a highly active area of research area. The goal for ultrasound-enhanced drug delivery is to improve the efficacy of drugs by increasing local concentrations of drug while reducing systemic toxicity.25,26 Preliminary animal studies showed that pulsed HIFU increased delivery of chemotherapeutic agents to targeted tumors.27,28 The mechanisms of HIFU-enhanced drug delivery likely include: 1) increasing the permeability of the vascular endothelial cells (most likely due to intravascular cavitation) allowing the chemotherapeutic agent to escape the vascular space into the interstitial space of the tumor; and 2) aiding the distribution of the chemotherapeutic agent into the tumor due to radiation force from the ultrasound field.
CONCLUSION
Pancreatic cancer is a logical target for HIFU therapy given the poor treatment options and the non-invasive nature of the therapy. Although further research is needed in determining the optimal methods for using HIFU to treat the pancreas, initial results to date are promising. Furthermore, the potential for new applications of HIFU, such as stimulation of the host immune system against the tumor (both local and metastatic) and targeted enhancement of drug delivery to the tumor, provide hope for improving outcomes in pancreatic cancer therapy.
References
- 1.Harvey EN, Loomis AL. High frequency sound waves of small intensity and thier biological effects. Nature. 1928;121:622–624. [Google Scholar]
- 2.Fry WJ, Barnard JW, Fry FJ, Brennan JF. Ultrasonically produced localized selective lesions in the central nervous system. Am J Phys Med. 1955;34:413–423. [PubMed] [Google Scholar]
- 3.Fry WJ, Fry FJ. Fundamental neurological research and human neurosurgery using intense ultrasound. IRE Trans Med Electron. 1960;ME-7:166–181. doi: 10.1109/iret-me.1960.5008041. [DOI] [PubMed] [Google Scholar]
- 4.Horner MJ, Ries LA, Krapcho M, et al. SEER cancer statistics review, 1975-2006. Bethesda: National Cancer Institute; 2009. [Google Scholar]
- 5.Wu F, Wang ZB, Zhu H, et al. Feasibility of US-guided high-intensity focused ultrasound treatment in patients with advanced pancreatic cancer: initial experience. Radiology. 2005;236:1034–1040. doi: 10.1148/radiol.2362041105. [DOI] [PubMed] [Google Scholar]
- 6.Xiong LL, Hwang JH, Huang XB, et al. Early clinical experience using high intensity focused ultrasound for palliation of inoperable pancreatic cancer. JOP. 2009;10:123–129. [PubMed] [Google Scholar]
- 7.Zhao H, Yang G, Wang D, et al. Concurrent gemcitabine and high-intensity focused ultrasound therapy in patients with locally advanced pancreatic cancer. Anticancer Drugs. 2010;21:447–452. doi: 10.1097/CAD.0b013e32833641a7. [DOI] [PubMed] [Google Scholar]
- 8.Wu F, Wang ZB, Cao YD, et al. Heat fixation of cancer cells ablated with high-intensity-focused ultrasound in patients with breast cancer. Am J Surg. 2006;192:179–184. doi: 10.1016/j.amjsurg.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 9.Coleman AJ, Saunders JE, Crum LA, Dyson M. Acoustic cavitation generated by an extracorporeal shockwave lithotripter. Ultrasound Med Biol. 1987;13:69–76. doi: 10.1016/0301-5629(87)90076-7. [DOI] [PubMed] [Google Scholar]
- 10.Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. Correlation between inertial cavitation dose and endothelial cell damage in vivo. Ultrasound Med Biol. 2006;32:1611–1619. doi: 10.1016/j.ultrasmedbio.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Holland CK, Apfel RE. Thresholds for transient cavitation produced by pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am. 1990;88:2059–2069. doi: 10.1121/1.400102. [DOI] [PubMed] [Google Scholar]
- 12.Khokhlova VA, Bailey MR, Reed JA, Cunitz BW, Kaczkowski PJ, Crum LA. Effects of nonlinear propagation, cavitation, and boiling in lesion formation by high intensity focused ultrasound in a gel phantom. J Acoust Soc Am. 2006;119:1834–1848. doi: 10.1121/1.2161440. [DOI] [PubMed] [Google Scholar]
- 13.Hwang JH, Wang YN, Warren C, et al. Preclinical in vivo evaluation of an extracorporeal HIFU device for ablation of pancreatic tumors. Ultrasound Med Biol. 2009;35:967–975. doi: 10.1016/j.ultrasmedbio.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Sun JZ. Preliminary study of high intensity focused ultrasound in treating patients with advanced pancreatic carcinoma. Chin J Gen Surg. 2002;17:654–655. [Google Scholar]
- 15.Xie DR, Chen D, Teng H. A multicenter non-randomized clinical study of high intensity focused ultrasound in treating patients with local advanced pancreatic carcinoma. Chin J Clin Oncol. 2003;30:630–634. [Google Scholar]
- 16.Xiong LL, He CJ, Yao SS, et al. The preliminary clinical results of the treatment for advanced pancreatic carcinoma by high intensity focused ultrasound. Chin J Gen Surg. 2005;16:345–347. [Google Scholar]
- 17.Xu YQ, Wang GM, Gu YZ, Zhang HF. The acesodyne effect of high intensity focused ultrasound on the treatment of advanced pancreatic carcinoma. Clin Med J China. 2003;10:322–323. [Google Scholar]
- 18.Yuan C, Yang L, Yao C. Observation of high intensity focused ultrasound treating 40 cases of pancreatic cancer. Chin J Clin Hep. 2003;19:145. [Google Scholar]
- 19.Zhou Q, Zhu XQ, Zhang J, Xu ZL, Lu P, Wu F. Changes in circulating immunosuppressive cytokine levels of cancer patients after high intensity focused ultrasound treatment. Ultrasound Med Biol. 2008;34:81–87. doi: 10.1016/j.ultrasmedbio.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Dromi SA, Walsh MP, Herby S, et al. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58–66. doi: 10.1148/radiol.2511072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sabel M. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology. 2009;58:1–11. doi: 10.1016/j.cryobiol.2008.10.126. [DOI] [PubMed] [Google Scholar]
- 22.Kramer G, Steiner GE, Grobl M, et al. Response to sublethal heat treatment of prostatic tumor cells and of prostatic tumor infiltrating T-cells. Prostate. 2004;58:109–120. doi: 10.1002/pros.10314. [DOI] [PubMed] [Google Scholar]
- 23.Wu F, Zhou L, Chen WR. Host antitumour immune responses to HIFU ablation. Int J Hyperthermia. 2007;23:165–171. doi: 10.1080/02656730701206638. [DOI] [PubMed] [Google Scholar]
- 24.Deng J, Zhang Y, Feng J, Wu F. Dendritic cells loaded with ultrasound-ablated tumour induce in vivo specific antitumour immune responses. Ultrasound Med Biol. 2010;36:441–448. doi: 10.1016/j.ultrasmedbio.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Jung JT, Lee DH, Hwang JH. Enhanced chemotherapeutic drug delivery to tumor tissue by high intensity focused ultrasound. Korean J Gastroenterol. 2009;53:216–220. [PubMed] [Google Scholar]
- 26.Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF. The distribution of the anticancer drug Doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res. 2005;11:8782–8788. doi: 10.1158/1078-0432.CCR-05-1664. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill BE, Vo H, Angstadt M, Li KP, Quinn T, Frenkel V. Pulsed high intensity focused ultrasound mediated nanoparticle delivery: mechanisms and efficacy in murine muscle. Ultrasound Med Biol. 2009;35:416–424. doi: 10.1016/j.ultrasmedbio.2008.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuh EL, Shulman SG, Mehta SA, et al. Delivery of systemic chemotherapeutic agent to tumors by using focused ultrasound: study in a murine model. Radiology. 2005;234:431–437. doi: 10.1148/radiol.2342030889. [DOI] [PubMed] [Google Scholar]