Abstract
Recurrent tumour after radical pancreaticoduodenectomy may cause obstruction of the small bowel loop draining the liver. Roux-loop obstruction presents a particular therapeutic challenge, since the postsurgical anatomy usually prevents endoscopic access. Careful multidisciplinary discussion and multimodality preprocedure imaging are essential to accurately demonstrate the cause and anatomical location of the obstruction. Transhepatic or direct percutaneous stent placement should be possible in most cases, thereby avoiding long-term external biliary drainage. Gastropexy T-fasteners will secure the percutaneous access and reduce the risk of bile leakage. The static bile is invariably contaminated by gut bacteria, and systemic sepsis is to be expected. Enteral stents are preferable to biliary stents, and compound covered stents in a sandwich construction are likely to give the best long-term results. Transhepatic and direct percutaneous enteral stent insertion after jejunopexy is illustrated and the literature reviewed.
Keywords: Anastomosis, Roux-en-Y; Cholestasis; Gastropexy; Jejunostomy; Palliation
INTRODUCTION
Radical pancreatico-duodenectomy (Whipple's procedure) includes resection of the lower bile duct and requires anastomosis of the remaining biliary tree with the bowel. An interposition of a loop of small bowel, which drains into the side of the lower jejunum is currently regarded as the standard procedure. The resulting Y-configuration, in conjunction with the name of the French surgeon, who described it in 1893 gave this type of reconstruction it's name: Roux-en-Y.1
Subsequent biliary obstruction may occur either at the anastomosis from fibrotic strictures or tumour recurrence or less frequently from tumour involvement of the afferent Roux loop of small bowel.
Endoscopic biliary access to the Roux loop may be gained by complex methods,2-4 but is usually impossible. Further surgical bypass is rarely option and in these cases further management requires a percutaneous approach involving cross-sectional imaging as well as fluoroscopy.
If the obstruction is at the level of the bilioenteric anastomosis, access is by standard percutaneous transhepatic cholangiography. Benign anastomotic strictures are a particular challenge, as their recurrence rate is high. Multiple strategies have described including balloon dilatation, long-term internal/external drainage or maintaining the track with an indwelling catheter.5 First case reports on the use of biodegradable stents in benign biliary strictures suggest that symptom free intervals of 1-2 years can be achieved.6 However it remains to be seen whether the temporary stenting period of 3-4 months results in stricture remodelling and long-term patency.
Tumour recurrence at the bilioenteric anastomosis is in most cases readily dealt with by percutaneous insertion of self-expanding metal stents (SEMS).
However if the Roux-loop is obstructed by tumour further downstream, transhepatic access to the stenosis becomes increasingly difficult with having to negotiate the afferent small bowel loop.7
This article illustrates transhepatic as well as direct percutaneous insertion of a self-expanding duodenal stent into an obstructed Roux-loop.
CASE REPORT
1. Case 1
A 71-year-old man had a Whipple's procedure for a T4 N1 M0 ampullary carcinoma. 4/24 resected lymph nodes were involved but an R0 resection margin was achieved. Surgery was followed with adjuvant chemotherapy.
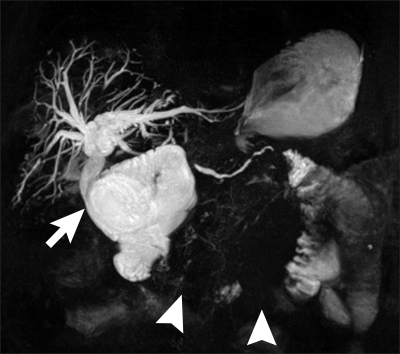
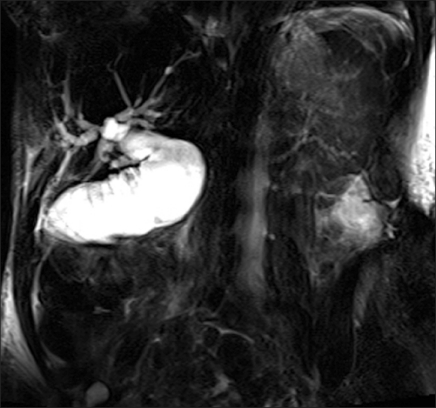
Six months after the original surgery a rise in Ca199 was observed. Ultrasound demonstrated mild biliary dilatation and a computed tomography (CT) scan confirmed local tumour recurrence. Over the next fortnight the patient became jaundiced and magnetic resonance cholangiopancreatography (MRCP) was performed for planning of percutaneous biliary drainage and possible stent insertion. This confirmed a patent choledocho-jejunostomy and a short segment of dilated bowel above the stricture (Fig. 1).
Fig. 1.
Patient 1. Magnetic resonance cholangiopancreatography showing a patent choledochojejunostomy (arrow) with obstruction of the afferent loop (arrowheads).
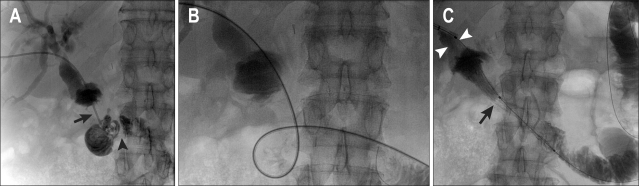
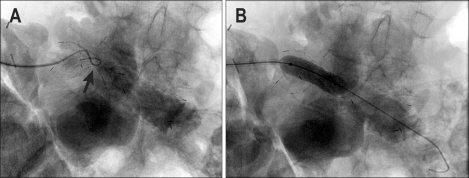
Percutaneous transhepatic cholangiography was performed through a right-sided approach. The markedly dilated biliary tree was punctured under real time ultrasound guidance using a Skater biliary access set (Manatech, Burton-on-Trent, UK). Cholangiography confirmed the choledocho-jejunostomy to be widely patent, with a short segment of dilated duodenum above a tortuous stricture (Fig. 2). This loop was difficult to negotiate, but straightened out after insertion of a stiff "Amplatz" guidewire, (Boston Scientific, St Albans, UK).
Fig. 2.
(A) Transhepatic cholangiogram confirms a tight stricture (arrow) with further narrowing distally (arrowhead). (B) Tortuosity of the loop made catheterization difficult. (C) A 22-mm ComVi stent after deployment with limited expansion across the stricture (arrow). The proximal end is positioned just below the anastomosis (arrowheads).
At this point the patient became septic with marked rigors, hypotension and hypoxia of 95% on nasal oxygen. The patient was resuscitated by the interventional nursing team, while awaiting support from the critical care outreach team.
While the patient was being stabilised, a 22×100 mm compound ComVi enteral stent (TaeWoong Medical, Seoul, Korea) was placed with its upper end below the choledocho-jejunostomy without difficulty. Immediate stent expansion of approximately 40% resulted in a sufficient lumen for biliary drainage, but in view of the inability to fully assess the result in the septic patient, a 6 Fr. locked external drain (NeoHydro; UK Medical, Sheffield, UK) was placed and the procedure terminated.
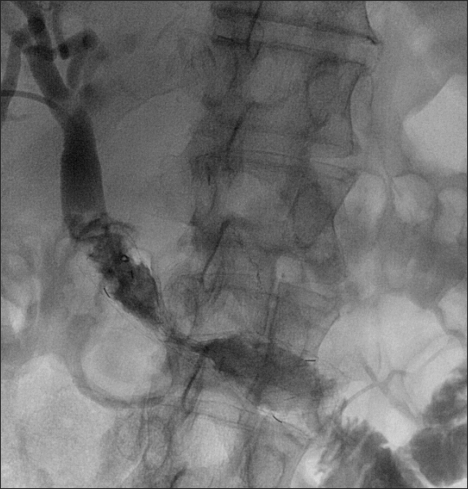
The patient made an uneventful recovery, check cholangiography after two days confirmed free biliary drainage (Fig. 3) and the external drain was removed.
Fig. 3.
Check cholangiogram showing decompression of the biliary tree and the free flow of contrast agent through the stent.
The pre-procedure bilirubin of three times the upper normal limits (68µmol/L [<20]) normalised within four days, the markedly raised alkaline phosphatise (1,457 U/l [25-110]) remained elevated for several weeks.
The patient died of disease progression two months later without evidence of recurrent biliary obstruction, not requiring any further intervention.
2. Case 2
One year after a Whipple's procedure for adenocarcinoma of the pancreas, a 77-year-old woman was diagnosed with tumour recurrence in the pancreatic bed and development of liver metastases. She was fit for first line chemotherapy and was enrolled in a trial (AVITA). The combination chemotherapy was tolerated well over the following two years except for intermittent anaemia, thrombocytopenia and pleural effusions. Her disease remained stable over a further year but she presented with jaundice 4½ years following her original pancreaticoduodenectomy.
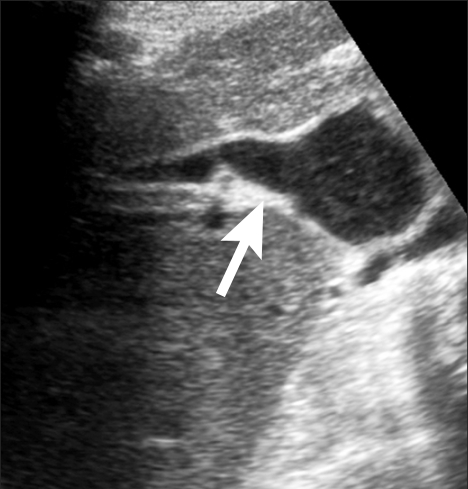
Ultrasound showed dilatation of the whole of the biliary tree with a patent anastomosis (Fig. 4). This was confirmed on MRCP, which demonstrated marked dilatation of the upper Roux loop (Fig. 5).
Fig. 4.
Patient 2. US shows a dilated hepaticojejunostomy (arrow).
Fig. 5.
Magnetic resonance cholangiopancreatography demonstrating the dilated Roux loop.
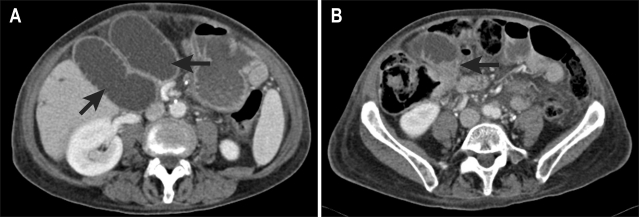
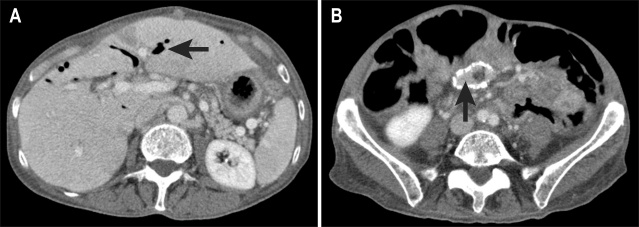
A CT scan was performed for further assessment, which showed that recurrent tumour had obstructed the draining loop centrally in the upper pelvis. The Roux loop above this was long and tortuous, but immediately underlying the anterior abdominal wall (Fig. 6). A transhepatic approach was deemed inappropriate, as the convoluted approach through the liver and upper Roux loop was unlikely to be successful, potentially resulting in permanent external drainage.
Fig. 6.
(A) CT shows two limbs of the fluid-filled afferent loop (arrows) directly beneath the anterior abdominal wall. (B) The Roux loop is obstructed in the upper pelvis by a tumor nodule (arrow).
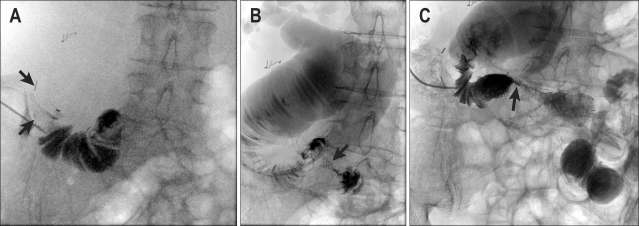
Under ultrasound guidance the subcutaneous part of the Roux loop was fixed with two "Harpon" T-fasteners (Pyramed, Basingstoke, UK) designed for gastropexy during radiologic gastrostomy (Fig. 7). The small bowel was then punctured with an 18 gauge needle (Fig. 8) and a stiff wire inserted (Amplatz; Boston Scientific). A peel-away sheath was placed and the stricture crossed with a 6 Fr biliary manipulation catheter (William Cook, Bjaeverskov, Denmark) and a hydrophilic guidewire (Terumo, Tokyo, Japan).
Fig. 7.
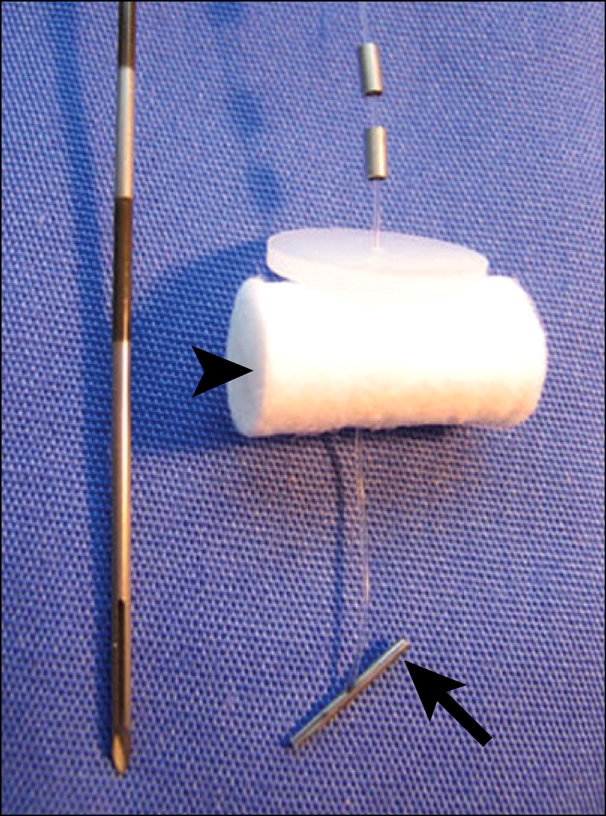
"Harpon" T-fastener and insertion needle. The metal bar (arrow) retracts the stomach, and the cotton wool bud (arrowhead) is fixed against the skin after insertion.
Fig. 8.
(A) Percutaneous puncture of the Roux loop. The T-fasteners (arrows) are evident above the needle. (B) Injection of contrast agent shows a massively dilated proximal loop with a tight stricture (arrow). (C) Deployed 22 mm Niti-S D-stent with limited initial expansion (arrow) but still allowing the free flow of contrast agent across the stricture.
A 22×80 uncovered Niti-S D-stent (TaeWoong) was placed into good position with initial 15-20% expansion.
A locked 6 Fr. pigtail catheter was inserted to ensure adequate biliary decompression and reduce the risk of a peritoneal bile leak.
The patient also had an episode of rigors and transient hypotension in the radiology recovery room as well as some transient abdominal pain, presumably from a small bile leak. She recovered quickly with conservative management by the interventional nurses. A check cholangiogram the following day showed slight further expansion of the stent, adequate for biliary drainage (Fig. 9). However, in view of the possibility of continued bile leak after drain removal, dilatation with a 12 mm angioplasty balloon (Smash; Bard Ltd., Crawley, UK) was performed. Some recoil of the stent occurred, but after aspiration of bile and drain removal, the patient made an uneventful recovery.
Fig. 9.
(A) After 24 hours there is further expansion (arrow). (B) Dilatation with a 12 mm balloon.
Minor infection occurred at the puncture site (Fig. 10), the gastropexy sutures were cut after 14 days; no further leakage occurred.
Fig. 10.
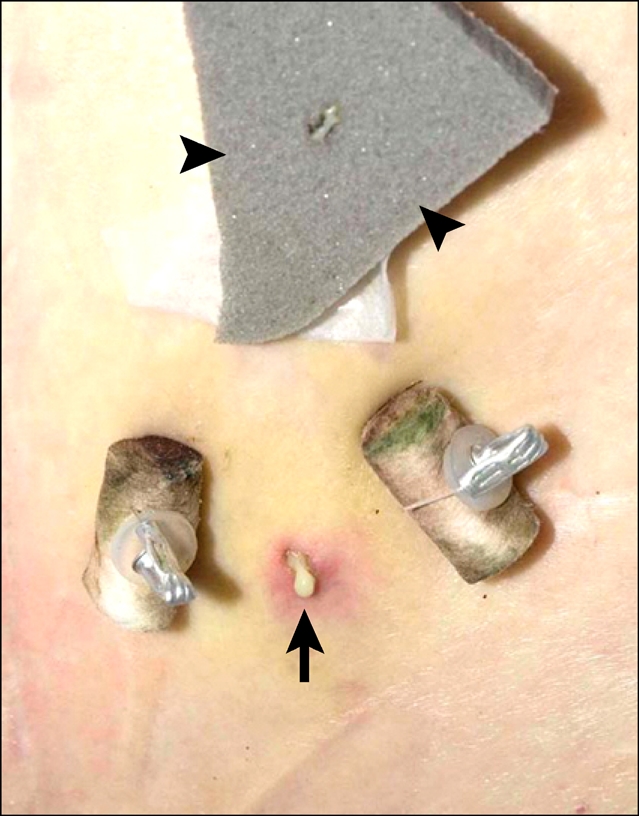
3 days after drain removal, pus is seen draining from the puncture site (arrow) between the gastropexy sutures. This was successfully treated by applying a silver dressing (arrowheads).
Fifteen months after the percutaneous stent insertion the patient is still alive, but with evidence of further disease progression. The CT showed tumour in-growth into the stent, but there was no clinical or biochemical evidence of biliary obstruction and extensive pneumobilia was present (Fig. 11).
Fig. 11.
(A) CT performed 15 months after stent insertion shows air in both lobes of the liver (arrow), indicating stent patency. (B) However, some tumour ingrowth (arrow) into the uncovered stent is evident.
DISCUSSION
Median survival after radical pancreatico-duodenectomy is 18-14 months depending on resection margins.8
With advances in chemotherapy, this is likely to increase, with the potential for slow disease progression and intra-abdominal dissemination causing obstruction of the bilioenteric anastomosis. Endoscopic access is usually impossible and percutaneous techniques need to be applied.
Occasionally the Roux loop may have been fixed surgically to the abdominal wall, in order to provide retrograde percutaneous access to the biliary tree.9 Some authors have advocated this as a routine procedure,10 but this is not accepted as a standard. Direct puncture of the unfixed draining loop for retrograde biliary access has been described11 and there are a number of reports of insertion of jejunostomy drainage catheters.12,13 However permanent external drainage is a very unsatisfactory outcome, leaving the patient disfigured by a drainage bag full of bile and the carers with the challenge of maintaining it. Reports of transhepatic placement of enteral stents are rare14-18 and we have only found one previous case of direct percutaneous enteral stent insertion for Roux loop obstruction.7
With adoption of techniques used for other interventions it should be possible to achieve internal drainage in most cases, but a number of careful considerations are required.
A clear understanding of the level of obstruction and the anatomy of the bypass is essential. This may well be demonstrated by one imaging modality alone,19,20 however as illustrated the techniques are complementary and a combination gives the most complete assessment.
If the stricture is well below the bilioenteric anastomosis it may not be accessible through a transhepatic approach. Once the Roux loop has been decompressed by insertion of a transhepatic drain, percutaneous puncture into it may no longer be possible. At that point the patient may be condemned to permanent external drainage.
Direct percutaneous stenting is possible if the distended afferent loop is underlying the abdominal wall. However after puncture the loop may decompress and collapse with a high risk of bile leaking into the peritoneum.
T-bar gastropexy is well established for reducing peritoneal leaks during and after gastrostomy insertion.21-23 It offers a simple way of fixing bowel as well as stomach against the anterior abdominal wall. This secures access, reduces the risk of biliary peritonitis and allows for a staged procedure if necessary.7
After successful stenting the sutures should be left in place for several days to allow the puncture site to heal. If they are permanently left in situ, infection of the site will occur. Resorbable sutures (Safe-T-pexy; Kimberly-Clark, Roswell, GA, USA) are available if follow-up is a problem.
In contrast to cholestasis within the biliary tree itself, static bile in the afferent loop is invariably contaminated with small bowel bacteria. These patients are of very high risk of developing bacteraemia and sepsis during, or immediately after the procedure and antibiotics should be given routinely.24 The team needs to be prepared for dealing with the acute onset of septic shock. If this occurs it may be better to only establish drainage and perform stenting as a staged procedure.
Although stenting is performed to relieve jaundice and no food needs to pass through the stent, the Roux-loop occlusion should be regarded as a conventional small bowel obstruction requiring enteral stent insertion.
Technically a 10 mm biliary stent would be sufficient to establish biliary drainage. However uncovered enteral stents have a high risk of tumour ingrowth and occlusion; this is increased if a small biliary stent is used. Equally a biliary stent is more likely to displace within the larger lumen of the small bowel, particularly if a covered stent is used to reduce the risk of re-occlusion.
Enteral stents may only available on an endoscopic ("through the scope", TTS) delivery system and the radiologist needs to ensure that wires of sufficient length (260-450 cm) are available in the department. TTS delivery systems are mostly between 10 and 11 Fr (3.2-3.5 mm). These can usually be advanced without too much difficulty through the liver, but should be inserted over a stiff guidewire and/or through a peel-away sheath. The creation of such a large track has an increased risk of haemorrhage and biliary leak and insertion of an external drain at the end of the procedure should be considered.
A number of agents can be used to plug the track. Powdered collagen (Avitene flour, Bard Ltd., Crawley, UK) - designed to achieve haemostasis during surgery - can be mixed with 5-7 mL of contrast resulting in radiopaque paste which is easily applied on withdrawing of the transhepatic sheath. However it is not licensed for this application.
Uncovered gastroduodenal stents occlude in 20-30% from tumour ingrowth, with patency being less than one year even in long-term survivors with further chemotherapy.25 Fully covered stents have a reduced risk of ingrowth, but migrate in 20-25%,26-28 although this may be less in a loop of bowel excluded from the passage of food.
Newer stent designs using a layer of covered membrane, sandwiched between two layers of Nitinol are now available (ComVi, TaeWoong, Seoul; Egis, S&G Biotech, Seoul, Korea). These should combine the benefits of adequate stent fixation with reduced in-growth, although so far only the reduced migration has been confirmed.29 In our centre they are currently the preferred stent construction for malignant enteral strictures in the small and large bowel.
Even patients with metastatic disease may survive many months or even years as illustrated by our second case. Survival is likely to increase even further with improvements in palliative chemotherapy regimens. It therefore becomes ever more important to consider the need for future interventions when planning the initial procedure.
The use of enteral instead of biliary stents and newer covered stent constructions will delay re-occlusion by tumour growth and should be considered as devices of choice.
References
- 1.Roux C. De la gastroenterostomie. Rev Chir. 1893;13:402–403. [Google Scholar]
- 2.Hankins BC, Johnson MS, Lehman GA. Combined percutaneous and endoscopic stent placement for an obstructed Roux limb after pancreaticojejunostomy for chronic pancreatitis. J Vasc Interv Radiol. 1997;8:465–468. doi: 10.1016/s1051-0443(97)70590-6. [DOI] [PubMed] [Google Scholar]
- 3.Moreels TG, Roth B, Vandervliet EJ, Parizel PM, Dutre J, Pelckmans PA. The use of the double-balloon enteroscope for endoscopic retrograde cholangiopancreatography and biliary stent placement after Roux-en-Y hepaticojejunostomy. Endoscopy. 2007;39(Suppl 1):E196–E197. doi: 10.1055/s-2007-966410. [DOI] [PubMed] [Google Scholar]
- 4.Koornstra JJ, Alkefaji H. Self-expandable metal stent placement combining double balloon endoscopy with a percutaneous approach in a Roux-en-Y hepaticojejunostomy. J Gastrointestin Liver Dis. 2009;18:375–377. [PubMed] [Google Scholar]
- 5.Ho CS, Voss MD. Self-expandable metallic biliary stents with permanent access. AJR Am J Roentgenol. 2005;184:410–414. doi: 10.2214/ajr.184.2.01840410. [DOI] [PubMed] [Google Scholar]
- 6.Petrtyl J, Bruha R, Horak L, Zadorova Z, Dosedel J, Laasch HU. Management of benign intrahepatic bile duct strictures: initial experience with polydioxanone biodegradable stents. Endoscopy. 2010;42(Suppl 2):E89–E90. doi: 10.1055/s-0029-1243880. [DOI] [PubMed] [Google Scholar]
- 7.Chevallier P, Novellas S, Motamedi JP, Gugenheim J, Brunner P, Bruneton JN. Percutaneous jejunostomy and stent placement for treatment of malignant Roux-en-Y obstruction: a case report. Clin Imaging. 2006;30:283–286. doi: 10.1016/j.clinimag.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Fusai G, Warnaar N, Sabin CA, Archibong S, Davidson BR. Outcome of R1 resection in patients undergoing pancreatico-duodenectomy for pancreatic cancer. Eur J Surg Oncol. 2008;34:1309–1315. doi: 10.1016/j.ejso.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Krige JE, Beningfield SJ, Beckingham IJ. Technical factors in the construction and use of a biliary access loop. Radiology. 1998;209:883–884. doi: 10.1148/radiology.209.3.9844694. [DOI] [PubMed] [Google Scholar]
- 10.McPherson SJ, Gibson RN, Collier NA, Speer TG, Sherson ND. Percutaneous transjejunal biliary intervention: 10-year experience with access via Roux-en-Y loops. Radiology. 1998;206:665–672. doi: 10.1148/radiology.206.3.9494484. [DOI] [PubMed] [Google Scholar]
- 11.Perry LJ, Stokes KR, Lewis WD, Jenkins RL, Clouse ME. Biliary intervention by means of percutaneous puncture of the antecolic jejunal loop. Radiology. 1995;195:163–167. doi: 10.1148/radiology.195.1.7892460. [DOI] [PubMed] [Google Scholar]
- 12.Maile CW, Hanna PD. Direct percutaneous drainage of an obstructed afferent loop. AJR Am J Roentgenol. 1989;152:521–522. doi: 10.2214/ajr.152.3.521. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Han JK, Lee KH, Kim TK, Kim KW, Choi BI. Palliative percutaneous tube enterostomy in afferent-loop syndrome presenting as jaundice: clinical effectiveness. J Vasc Interv Radiol. 2002;13:845–849. doi: 10.1016/s1051-0443(07)61995-2. [DOI] [PubMed] [Google Scholar]
- 14.Milson A, Abdellaoui A, Fraser C, Watkinson AF. Transhepatic assisted transoral placement of a duodenal stent in malignant gastric outlet obstruction. J Vasc Interv Radiol. 2010;21:590–592. doi: 10.1016/j.jvir.2009.12.386. [DOI] [PubMed] [Google Scholar]
- 15.Keymling M, Wagner HJ, Vakil N, Knyrim K. Relief of malignant duodenal obstruction by percutaneous insertion of a metal stent. Gastrointest Endosc. 1993;39:439–441. doi: 10.1016/s0016-5107(93)70125-x. [DOI] [PubMed] [Google Scholar]
- 16.Caldicott DG, Ziprin P, Morgan R. Transhepatic insertion of a metallic stent for the relief of malignant afferent loop obstruction. Cardiovasc Intervent Radiol. 2000;23:138–140. doi: 10.1007/s002709910027. [DOI] [PubMed] [Google Scholar]
- 17.Johnsson E, Delle M, Lundell L, Liedman B. Transhepatic placement of an enteral stent to treat jaundice in a tumor recurrence obstructed afferent loop after a whipple procedure. Dig Surg. 2003;20:329–331. doi: 10.1159/000071760. [DOI] [PubMed] [Google Scholar]
- 18.Gwon DI. Percutaneous transhepatic placement of covered, self-expandable nitinol stent for the relief of afferent loop syndrome: report of two cases. J Vasc Interv Radiol. 2007;18:157–163. doi: 10.1016/j.jvir.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Lim JH, Ko YT. Afferent loop syndrome: sonographic findings in seven cases. AJR Am J Roentgenol. 1991;157:41–43. doi: 10.2214/ajr.157.1.2048535. [DOI] [PubMed] [Google Scholar]
- 20.Kim HC, Han JK, Kim KW, et al. Afferent loop obstruction after gastric cancer surgery: helical CT findings. Abdom Imaging. 2003;28:624–630. doi: 10.1007/s00261-002-0070-y. [DOI] [PubMed] [Google Scholar]
- 21.Ryan JM, Hahn PF, Boland GW, McDowell RK, Saini S, Mueller PR. Percutaneous gastrostomy with T-fastener gastropexy: results of 316 consecutive procedures. Radiology. 1997;203:496–500. doi: 10.1148/radiology.203.2.9114111. [DOI] [PubMed] [Google Scholar]
- 22.Thornton FJ, Fotheringham T, Haslam PJ, McGrath FP, Keeling F, Lee MJ. Percutaneous radiologic gastrostomy with and without T-fastener gastropexy: a randomized comparison study. Cardiovasc Intervent Radiol. 2002;25:467–471. doi: 10.1007/s00270-001-0089-4. [DOI] [PubMed] [Google Scholar]
- 23.Laasch HU, Martin DF. Radiologic gastrostomy. Endoscopy. 2007;39:247–255. doi: 10.1055/s-2006-945119. [DOI] [PubMed] [Google Scholar]
- 24.Morita S, Takemura T, Matsumoto S, Odani R. Septic shock after percutaneous transhepatic drainage of obstructed afferent loop: case report. Cardiovasc Intervent Radiol. 1989;12:66–68. doi: 10.1007/BF02577389. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Song HY, Shin JH, et al. Metallic stent placement in the palliative treatment of malignant gastroduodenal obstructions: prospective evaluation of results and factors influencing outcome in 213 patients. Gastrointest Endosc. 2007;66:256–264. doi: 10.1016/j.gie.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 26.Jung GS, Song HY, Kang SG, et al. Malignant gastroduodenal obstructions: treatment by means of a covered expandable metallic stent-initial experience. Radiology. 2000;216:758–763. doi: 10.1148/radiology.216.3.r00au05758. [DOI] [PubMed] [Google Scholar]
- 27.Lopera JE, Alvarez O, Castano R, Castaneda-Zuniga W. Initial experience with Song's covered duodenal stent in the treatment of malignant gastroduodenal obstruction. J Vasc Interv Radiol. 2001;12:1297–1303. doi: 10.1016/s1051-0443(07)61555-3. [DOI] [PubMed] [Google Scholar]
- 28.Laasch HU, Martin DF, Maetani I. Enteral stents in the gastric outlet and duodenum. Endoscopy. 2005;37:74–81. doi: 10.1055/s-2004-826103. [DOI] [PubMed] [Google Scholar]
- 29.Isayama H, Kawabe T, Nakai Y, et al. Management of distal malignant biliary obstruction with the ComVi stent, a new covered metallic stent. Surg Endosc. 2010;24:131–137. doi: 10.1007/s00464-009-0537-9. [DOI] [PubMed] [Google Scholar]