Abstract
Lysophosphatidic acid (LPA, 1-radyl-2-hydroxy-sn-glycero-3-phosphate) is the prototype member of a family of lipid mediators and second messengers. LPA and its naturally occurring analogues interact with G protein-coupled receptors on the cell surface and a nuclear hormone receptor within the cell. In addition, there are several enzymes that utilize LPA as a substrate or generate it as a product and are under its regulatory control. LPA is present in biological fluids, and attempts have been made to link changes in its concentration and molecular composition to specific disease conditions. Through their many targets, members of the LPA family regulate cell survival, apoptosis, motility, shape, differentiation, gene transcription, malignant transformation and more. The present review depicts arbitrary aspects of the physiological and pathophysiological actions of LPA and attempts to link them with select targets. Many of us are now convinced that therapies targeting LPA biosynthesis and signalling are feasible for the treatment of devastating human diseases such as cancer, fibrosis and degenerative conditions. However, successful targeting of the pathways associated with this pleiotropic lipid will depend on the future development of as yet undeveloped pharmacons.
Keywords: lysophospholipid, cyclic phosphatidic acid, alkyl glycerophosphate, endothelial differentiation gene, GPR23, GPR92, GPR87, P2Y5, P2Y10, PPARγ
The lysophosphatidic acid-like lipid mediator family
Lysophosphatidic acid (1-acyl-2-hydroxy-sn-glycero-3-phosphate; LPA) represents the minimal glycerophospholipid based on the radyl-glycerol-phosphate scaffold. However, LPA is only one member of a family of endogenous lipid-like substances endowed with a host of biological actions that are often mediated through high-affinity interactions with specific cell surface and intracellular proteins (Figure 1). The specific recognition of LPA-like molecules by several receptors and enzymes provides a diversity of biological actions that have fascinated many investigators. What biomolecules constitute the LPA family? First, for the sake of brevity, we will ignore lysophospholipids built up on the sphingoid backbone. Although this creates an artificial oversimplification, it nevertheless allows for a single focus on a distinct subset of lipids, which in many instances interact with the same molecular targets. In most cases, sphingolipids, best represented by sphingosine-1-phosphate (S1P), have their own set of distinct targets that rarely overlap (but in some instances do; discussed below) with those of LPA.
Figure 1.
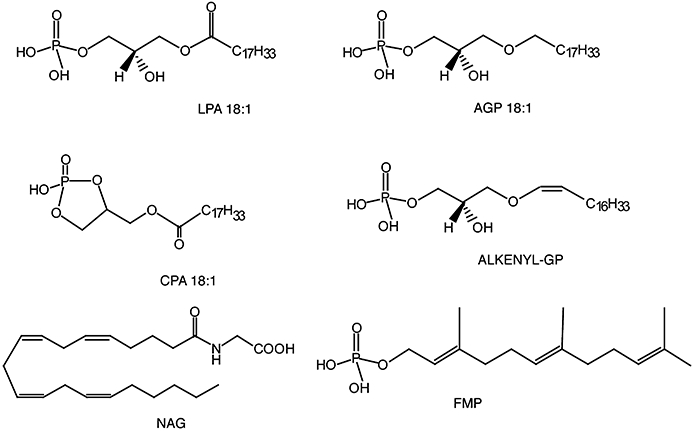
Naturally occurring ligands of LPA targets. LPA 18:1, oleoyl-lysophosphatidic acid; AGP 18:1, 1-O-octadecyl glycerophosphate; CPA 18:1, oleoyl-cyclic phosphatidic acid; ALKENYL-GP, alkenyl glycerophosphate; NAG, N-arachidonoyl glycine; FMP, farnesyl monophosphate.
Different molecular species of LPA, dominated by 16-, 18- and 20-carbon long acyl chains, have been detected in biological fluids as saturated, and mono- and poly-unsaturated variants of either the sn1 or the sn2 regioisomers. This natural variety within the LPA class leads to important biological and pharmacological consequences. First, most LPA G protein-coupled receptors (GPCR) show lower EC50 values (higher potency) for unsaturated species (Bandoh et al., 2000; Fujiwara et al., 2005; Williams et al., 2009). An extreme case of target selectivity is found for the peroxisome proliferator-activated receptor gamma (PPARγ), an intracellular target of LPA that is exclusively activated by unsaturated acyl species of LPA (Tsukahara et al., 2006). The LPA concentration in plasma is in the low nanomolar range, whereas in serum it increases to several micromoles (Baker et al., 2001; Watanabe et al., 2007b; Hosogaya et al., 2008). The poly-unsaturated 18:2 (38%) and 20:4 (39%) and the mono-unsaturated 18:1 (9%) LPA species constitute the overwhelming majority (86%) of LPA in serum (Sano et al., 2002). This enrichment in the unsaturated species increases the likelihood of the activation of those LPA targets that show preference for these fatty acyl species. In addition, sn1 and sn2 regioisomers of acyl LPA have been detected, and the LPA3, the purinoreceptor-5 (P2Y5) and GPR35 receptors have been suggested to show a slight preference for sn2 over sn1 LPA containing identical fatty acid chains (Bandoh et al., 2000; Yanagida et al., 2009; Oka et al., 2010). One unresolved concern is the stability of these regioisomers in vivo due to the relatively fast rate of acyl migration towards a 9:1 (sn1: sn2) equilibrium ratio of the isomers (Wang et al., 1997; Croset et al., 2000).
There are two mouse phospholipase A1 enzymes that have been linked to production of unsaturated sn-2 LPA. A phosphatidic acid-specific PLA1α and β (Sonoda et al., 2002; Hiramatsu et al., 2003) from mice have been cloned, which are the equivalents of the human enzymes LIPH and LIPI respectively (Kazantseva et al., 2006; Aoki et al., 2007; Aoki et al., 2008; Pasternack et al., 2009; Shimomura et al., 2009a,b). Mutations in the LIPH/mPA-PLA1α gene have been identified as the cause of the hereditary disorder woolly hair, as it's gene product supplies LPA to the P2Y5 receptor expressed in the hair bulb (Kazantseva et al., 2006; Pasternack et al., 2008; 2009; Shimomura et al., 2008; 2009a,b). Yet, another mouse PLA1 enzyme, designated PS-PLA1, secreted from rat platelets and specific to phosphatidylserine, has been identified by the Aoki group (Horigome et al., 1987; Sato et al., 1997). However, this enzyme is not found in human platelets and its role in human pathophysiology is unclear.
Alkyl-ether (Sugiura et al., 1999; Nakane et al., 2001) and alkenyl-ether analogues (Liliom et al., 1998) of LPA have been described. These lower-abundance mediators have been shown to be weaker agonists of LPA-specific GPCR (Bandoh et al., 2000; Fujiwara et al., 2005; Williams et al., 2009) with one exception. The LPA5/GPR92 receptor shows a preference for 1-O-alkyl glycerophosphate over the acyl analogues with the same chain length (Williams et al., 2009). Human platelets show preferential activation by 1-O-alkyl glycerophosphate over LPA; and the structure-activity relationship of the platelet response is similar, although not identical, to that of LPA5 (Simon et al., 1982; Williams et al., 2009). Interestingly, 1-O-alkyl glycerophosphate shows a higher potency than LPA with respect to PPARγ activation (Zhang et al., 2004; Tsukahara et al., 2006).
Cyclic phosphatidic acid (1-acyl-sn-glycerol-2,3-cyclic phosphate; CPA) is an additional class of acyl LPA analogues in which the sn-2 hydroxyl group has formed a 5-membered ring with the sn-3 phosphate via the elimination of a water molecule. CPA is also a naturally occurring analogue of LPA present in mammalian blood and brain, as well as in slime mold, the organism in which CPA was originally identified (Murakami-Murofushi et al., 1993; Kobayashi et al., 1999). CPA can be generated by autotaxin (ATX) under non-physiological conditions using a two-phase water/ether reaction system (Tsuda et al., 2006), but whether this enzyme contributes to the formation of CPA in blood remains an open question. Bacterial phospholipase D (PLD) can also yield CPA production (Friedman et al., 1996). In mammalian cells, CPA is generated intracellularly by PLD2 and acts as a second messenger which inhibits PPARγ (Tsukahara et al., 2010). CPA is a weak agonist of many LPA GPCR (Fujiwara et al., 2005; Williams et al., 2009). However, its synthetic hydrolysis-resistant 3-carba analogue (3CCPA 18:1) is a selective weak agonist of LPA5/GPR92 that fails to activate LPA1/2/3/4 and also inhibits ATX (Baker et al., 2006; Fujiwara, 2008). CPA and its synthetic 3CCPA analogues inhibit cancer metastasis through an as yet unidentified mechanism, which includes the inhibition of ATX (Baker et al., 2006; Uchiyama et al., 2007; Fujiwara, 2008).
In search of the minimal pharmacophore that activates LPA GPCR, we identified fatty alcohol phosphates that, depending on the length of the hydrocarbon chain and the headgroup (phosphate or thiophosphate), function as either antagonists or agonists (Durgam et al., 2005; Deng et al., 2007; Zhang et al., 2009a). Naturally occurring farnesyl phosphates are structurally similar to fatty alcohol phosphates. This similarity prompted us to confirm that farnesyl mono- and diphosphate activate various LPA GPCR targets (Liliom et al., 2006). Oh et al. (Oh da et al., 2008) reported that farnesyl monophosphate and arachidonoyl glycerol were more potent activators of LPA5 than LPA. However, other groups have reported that LPA and 1-O-alkyl glycerophosphate are the most potent agonists of LPA5 identified so far (Kotarsky et al., 2006; Lee et al., 2006; Williams et al., 2009). Despite this controversy, farnesyl mono- and diphosphate are important pharmacological tools that allow for the selective activation of LPA5 (∼50 nM EC50) without activation of LPA1,2,3,4 (in fact these reagents are weak inhibitors of LPA2,3,4 with IC50 values in the mid-micromolar range) (Williams et al., 2009).
S1P is currently classified as a specific ligand for five cognate receptors, S1P1,2,3,4,5, which are not activated by physiological concentrations of LPA. It is important to recognize that LPA1,2,3 receptors, at least when expressed heterologously, do show activation to S1P at concentrations in excess of 1 µM (Valentine et al., 2008a). Conversely, LPA binds to (Kd = 2.3 µM) and activates the S1P1 receptor as a low-affinity agonist (Lee et al., 1998). Because LPA concentrations can reach several micromoles in serum and tumour cell effusates (Baker et al., 2001; 2002; Sutphen et al., 2004), promiscuous activation of S1P1 by LPA in pathophysiological conditions may occur. Murakami et al. (Murakami et al., 2008) recently reported that P2Y10 is a receptor for LPA that is also activated by S1P, making P2Y10, if confirmed, a dual-specificity receptor. To make things even more interesting, heterologously expressed P2Y10 is also activated by lysophosphatidyl serine, although it is much less potent than LPA on this target.
LPA GPCRs
The LPA GPCR are the most studied and best understood targets of LPA. In mammals, LPA GPCRs have been identified in two distinct gene families (Figure 2). Chronologically, the endothelial gene (EDG) family was the first shown to encode GPCR specific for S1P and LPA. Within the EDG family, three receptors – LPA1, LPA2 and LPA3– are activated by LPA and its analogues. These three receptors share 45–56% overall amino acid identity. The overall homology of the transmembrane domains of these three GPCR is 86%, whereas only 6% of the amino acids are conserved in their C-terminal tails. In addition, five S1P-specific receptors, S1P1, S1P2, S1P3, S1P4 and S1P5, belong to this gene cluster. There are many excellent recent reviews on the biological function and signal transduction associated with the EDG family receptors; we refer the reader to these for a more in-depth description (Tigyi and Parrill, 2003; Choi et al., 2008; Mutoh and Chun, 2008; Rivera and Chun, 2008; Ishii et al., 2009; Noguchi et al., 2009; Peyruchaud, 2009).
Figure 2.
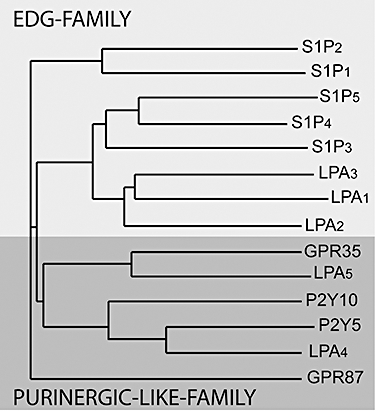
Phylogenetic tree of established and putative LPA GPCR generated by the NGBW program (http://www.ngbw.org). GPCR, G protein-coupled receptor.
A second cluster of LPA GPCR is found within the P2Y gene cluster (Ishii et al., 2009). The better-characterized LPA-sensitive members of this cluster are P2Y9/GPR23/LPA4 (this will be abbreviated as LPA4) (Noguchi et al., 2003; Yanagida et al., 2007) and GPR92/LPA5 (this will be abbreviated as LPA5) (Kotarsky et al., 2006; Lee et al., 2006; Williams et al., 2009; Yin et al., 2009). There are additional related GPCR that appear to be activated by LPA. In chronological order based on the first publication, these include the GPR87 (Tabata et al., 2007; Wetter et al., 2009), P2Y5 (Pasternack et al., 2008; Shimomura et al., 2008), P2Y10 (Murakami et al., 2008) and GPR35 (Oka et al., 2010) gene products. The LPA GPCR nomenclature was developed by the International Union of Basic and Clinical Pharmacology (IUPHAR) nomenclature committee (Chun et al., 2002), which has not met since 2002; but efforts to update this nomenclature are underway. Hence, the assignment of numbers to LPA GPCR beyond LPA5 has been arbitrary and confusing. According to the IUPHAR committee, numbering assignments should follow the chronological order of publication, yet some authors tend to ignore the report on GPR87 in 2007 (Tabata et al., 2007) that preceded publications on P2Y5 in 2008 (Pasternack et al., 2008; Shimomura et al., 2008). Now that GPR87 has been shown independently to be activated by LPA (Wetter et al., 2009), it might qualify for the designation LPA6; consequently, P2Y5 would be LPA7, P2Y10 and GPR35, if confirmed, would be LPA8 and LPA9, respectively. However, these designations will have to be officially assigned by the IUPHAR pending the publication of additional confirmatory evidence concerning the identity of their most potent natural ligand. A summary of the different established and putative LPA receptors and their ligands is shown in Table 1 and Figure 3.
Table 1.
Confirmed and putative LPA receptor
| Receptor | Location | Agonist preference# | KD (LPA 18 : 1) | High expression | Antagonist | Signalling | References |
|---|---|---|---|---|---|---|---|
| LPA1 | 9q31.3 | 18 : 2 > 18 : 3 > 20 : 4 > 18 : 1 > 16 : 0 > 18 : 0 | N/A | Brain, heart, gut, kidney, stomach | AP29660*,Ki16425, DGPP, VPC12449, H2L5105099, H2L-5765834, tetradecyl-phosphonate, LPA α-bromophosphonate | Gi, Gq, G12/13, PDZ | (Ishii et al., 2004; Lee-Kwon et al., 2003; Fischer et al., 2001; Fujiwara et al., 2005; Heise et al., 2001; Ohta et al., 2003; Williams et al., 2009; Fells et al., 2009; Zhang et al., 2009a) |
| LPA2 | 19p12 | 18 : 3 > 20 : 4 > 18 : 2 = 18 : 1 > 18 : 0 > 16 : 0 ≫ 20 : 0 | N/A | Heart, lung, gut, stomach, brain | Amgen25, tetradecyl-phosphonate, H2L-5105099, NSC47091, LPA α-bromophosphonate | Gi, Gq, G12/13, PDZ, LIM | (Ishii et al., 2004; Beck et al., 2008; Durgam et al., 2005; E et al., 2009; Fells et al., 2009; Lin et al., 2007) |
| LPA3 | 1p22.3 | 18 : 3 > 18 : 2 > OMPT > 18 : 1 > 20 : 4 ≫ 18 : 0 ≫ 16 : 0 | N/A | Testis, kidney, lung, brain | NSC161613, DGPP, VPC12449, H2L-5765834, tetradecyl-phosphonate, LPA α-bromophosphonate | Gi, Gq | (Ishii et al., 2004; Durgam et al., 2005; Fells et al., 2008; Fischer et al., 2001; Heise et al., 2001; Zhang et al., 2009a) |
| LPA4/PR23/P2Y9 | Xq13-q21.1 | 18 : 1 > 18 : 0 > 16 > 0 > 14 : 0 > AGP18 : 0 > AKGP18 : 1 | KD 77 nM | Uterus, ovary, placenta, platelets, mesenchymal cells | H2L-5987411, LPA α-bromophosphonate | Gq, G12/13, cAMP↑ | (Noguchi et al., 2003; Lee et al., 2006; 2007; Williams et al., 2009; Zhang et al., 2009a) |
| LPA5/GPR92 | 12p13.31 | AGP18 : 1 > AGP 16 : 0 > 18 : 3 > 18 : 2> 18 : 1 ≃ 16 : 0 ≃ 20 : 4 > FDP > FMP > AGP 18 : 0 > 18 : 0 > CPA 18 : 1 > CPA 16 : 0 > NAG | KD 6.4 nM | CD8+ lymphocytes, B cells, platelets, dorsal root ganglion cells | H2L-5987411, H2L-5765834 | Gq, cAMP↑ | (Kotarsky et al., 2006; Lee et al., 2006; Williams et al., 2009) |
| GPR87 | 3q25 | 18 : 1 > CPA18 : 1 > 2CCPA18 : 1 | N/A | Squamos cell carcinomas | N/A | Gq, G16 | (Tabata et al., 2007; Wetter et al., 2009; Gugger et al., 2008; Zhang et al., 2009b) |
| P2Y5 | 13q14.2 | 18 : 2 > OMPT > sn2-18 : 1 > sn1-18 : 1 > 20 : 4 > 18 : 0 > 16 : 0 > 14 > 0 | N/A | Henle and Huxley layers of scalp hair follicles | N/A | Gq, Gs, cAMP↑ G12/13 | (Pasternack et al., 2008; Shimomura et al., 2008) |
| P2Y10 | Xq21.1 | 18 : 1 > S1P > LPS18 : 1 | N/A | Uterus, brain, prostate, lung, placenta, skeletal muscle, B cells | N/A | Gq, G16, RhoA-ROCK | (Murakami et al., 2008) |
| GPR35 | 2q37.3 | sn2-18 : 2 > sn2-18 : 1 > sn2-20 : 4 > sn1-18 : 1 ≫ Zaprinast | N/A | Gastrointestinal, lymphoid and the central and peripheral nervous tissues | N/A | ERK1/2, RhoA, Ca2+ | (Oka et al. 2010) |
| PPARγ | 3p25 | Unsaturated AGP > Unsaturated LPA | KD 60 nM (AGP18 : 1) | Adipocytes, muscle, macrophage, vascular smooth muscle | GW9662 | Genes with peroxisome proliferator response elements | (McIntyre et al., 2003; Tsukahara et al., 2006; Zhang et al., 2004) |
Compounds in italics show at least a 10-fold selectivity for the receptor subtype.
Numbers alone always refer to LPA, AKGP 18:1 denotes alkenyl glycerophosphate 18:1.
LPA, lysophosphatidic acid; N/A, not available.
Figure 3.
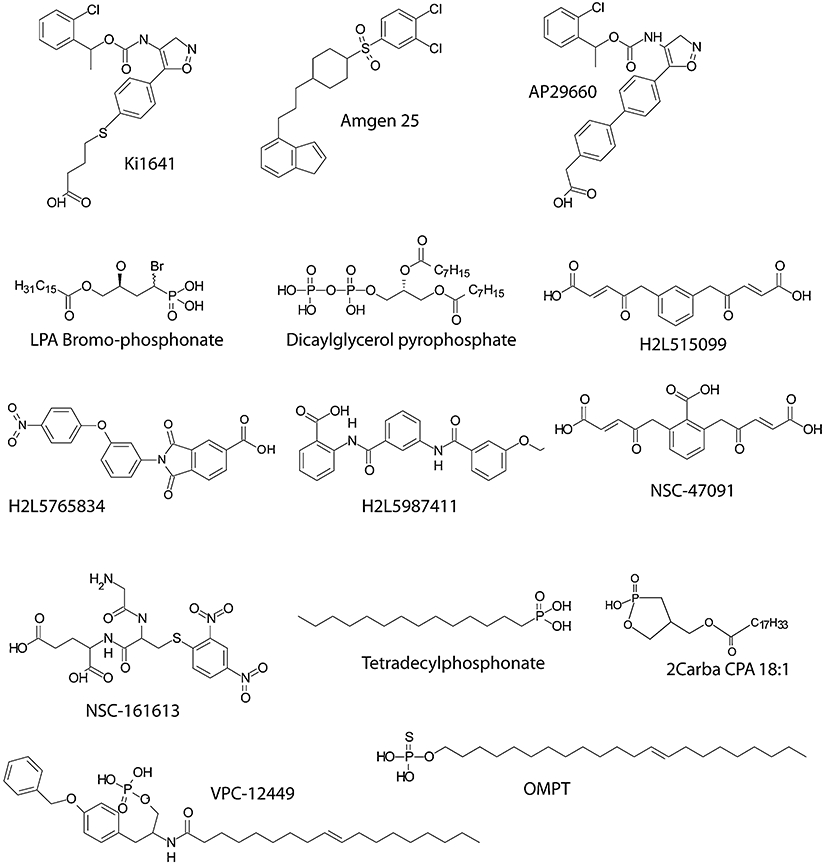
Synthetic ligands of LPA GPCR (see Table 1 for details). GPCR, G protein-coupled receptor; LPA, lysophosphatidic acid.
LPA4 shows a wide tissue distribution with the highest density of transcripts in the ovary, uterus and placenta (Ishii et al., 2009). Interestingly, this receptor was not detectable by Northern blotting in the liver, spleen and testis. LPA4 has high transcript abundance at sites of implantation within the uterus and increased expression in the ovary during pregnancy. The ligand preference of LPA4 is LPA 18:1 > 18:0 > 16:0 > 14:0 > 1-O-alkyl glycerophosphate >1-O-alkenyl glycerophosphate, and the Kd for LPA 18:1 binding to the human orthologue is 77 nM (Noguchi et al., 2003). The brain expresses relatively high levels of LPA4; and it has been shown, using heterologous expression in B103 neuroblastoma cells, to cause LPA-dependent growth cone collapse and neurite retraction through G12/13-RhoA-Rho-associated kinase (ROCK) activation (Lee et al., 2007). Although a practical issue, it is important to note that heterologous expression of the non-EDG family receptors is often difficult and requires special plasmids and/or receptor-G protein fusion constructs (Noguchi et al., 2003; Tabata et al., 2007; Murakami et al., 2008). Knockout mice for LPA4 do not display a noticeable phenotype, although mouse embryonic fibroblasts (MEF) isolated from the knockouts show increased migration in response to LPA, supporting the hypothesis that this receptor inhibits cell motility (Lee et al., 2008). Along with LPA5, LPA4 has been implicated in the inhibition of neurosphere formation and neuronal differentiation of human embryonic stem cells (Dottori et al., 2008). LPA4 shows high expression in platelets (Khandoga et al., 2008), and Smyth et al. proposed that it might mediate LPA-induced inhibition of platelet activation (Pamuklar et al., 2008). In patients whose platelets do not respond to LPA, LPA4 mRNA is increased sixfold over platelets from patients that are LPA-responsive. Thus, LPA4 might be a cardioprotective receptor as patients with LPA-non-responsive platelets are less likely to suffer from coronary heart disease.
LPA5 is present in the heart, placenta, brain, dorsal root ganglia, small intestine and spleen. Intestinal CD8+ lymphocytes, as well as B cells, show abundant expression of LPA5 (Kotarsky et al., 2006), suggesting an immunoregulatory role. This receptor subtype is also highly expressed in human platelets, and pharmacological evidence of its preference for 1-O-alkyl glycerophosphate suggests that it mediates platelet activation (Williams et al., 2009). The ligand preference of LPA5 is 1-O-alkyl glycerophosphate 18:1 ≥ 1-O-alkyl glycerophosphate 18:0 > LPA 18:1 > LPA 20:4 = LPA 16:0 = LPA 18:3 > farnesyl monophosphate > farnesyl diphosphate > LPA 18:0 > LPA 20:4 > CPA 18:1 > CPA 16:0 > N-arachidonyl glycine (Williams et al., 2009), and the Kd for LPA 18:1 binding is 6.4 nM (Kotarsky et al., 2006). The physiological/pathophysiological classification of this receptor subtype is rudimentary at the present time. Analysis of LPA5 is currently limited to heterologous expression studies, and experiments using farnesyl phosphate as a selective, although not a specific, agonist. LPA5 knockout mice have deficits in the response to neuropathic pain (Sheardown et al., 2004; Kinloch and Cox, 2005). LPA5-lacZ reporter gene-expressing mice showed the strongest expression in cell bodies of dorsal root ganglion cells that extend C-fibres (sensory nerves conveying pain signals) but not in those neurons with Aβ fibres (sensory nerves conveying non-pain signals). In these animals, LPA5 staining was noted in the trigeminal ganglia but not the spinal cord or brain. Behavioural testing showed that the mutant mice had no abnormalities on a wide range of standard tests, including von Frey responses. There was, however, a significant delay in the tail flick test, indicating impaired nociceptive function. In vivo electrophysiological recordings (Kinloch and Cox, 2005) from the dorsal horn of mutant animals revealed significantly lower numbers of action potentials in response to noxious mechanical, thermal and cold stimuli applied to the hind foot than in wild-type mice. However, there was no difference in the number of action potentials recorded in response to non-noxious brush stimulation or to temperature in the non-noxious range. Knockout mice failed to develop allodynia using the Chung model of neuropathic pain for 13 days as measured by von Frey thresholds. In contrast wild-type mice developed allodynia in this model (Sheardown et al., 2004; Kinloch and Cox, 2005). In another study, LPA5 protein was detected using immunohistology in dorsal root ganglion cells of mice and humans (Oh da et al., 2008). In cultured small dorsal root ganglion (DRG) cell neurons, farnesyl diphosphate and N-arachidonoyl glycine both elicited Ca2+ transients that were abolished by LPA5 small interfering RNA (siRNA) transfection, suggesting a potential role in nociception (Oh da et al., 2008). These in vitro findings are consistent with the role of LPA5 in pain processing. Hence, antagonists of LPA5 might offer a therapeutic approach to the treatment of neuropathic pain.
Chronologically GPR87 was the next de-orphaned GPCR showing activation by LPA (Tabata et al., 2007; Wetter et al., 2009). GPR87 has a modest 27% and 25% homology with LPA4 and LPA5 respectively. Its sequence shows 41–48% identity with the P2Y12-13-14 receptors and is nestled into this cluster of genes on human chromosome 3q25. Fujita et al. developed an in silico screening method to discover surrogate ligands for the P2Y receptor family (Hiramoto et al., 2002; 2004; Nonaka et al., 2005). The data supporting LPA as a ligand for GPR87 come from heterologous expression studies conducted in stably transfected Chinese hamster ovary (CHO) cells with a GPR87-G16 fusion protein (Tabata et al., 2007) and from using the Tango™β-arrestin recruitment-based assay in U2OS cells (Wetter et al., 2009). Expression of GPR87 alone is difficult, suggesting that the posttranslational processing of this receptor might be complex and that attaching G16 to the putative receptor increases plasma membrane expression of the fusion construct (Dr Norihisa Fujita – personal communication). GPR87-G16-transfected CHO cells mobilize Ca2+ in response to LPA with an EC50 of 36 nM. This response is completely abolished with a GPR87 siRNA. These cells fail to respond to uridine diphosphate (UDP) and UDP-glucose; however, uridine triphosphate (UTP) treatment of the cells elicits a desensitization-like phenomenon of the LPA response. GPR87 transcripts were detected in the brain, skeletal muscle and the reproductive organs. The initial report on GPR87 also showed that the response to LPA (100 nM) was blocked by Ki16425 (1 µM) and diacylglycerol pyrophosphate (50 µM), which have been previously identified as antagonists of the LPA1-3 receptors (Tabata et al., 2007). Clearly, more information is necessary to fully characterize this receptor's ligand selectivity and signal transduction properties, both of which should be derived from LPA-hyporesponsive cells. GPR87 is under the transcriptional regulation of p53; shows a very high expression level in squamous cell carcinomas, lung adenocarcinomas and transitional carcinomas of the urinary bladder and is involved in promoting cell survival and proliferation (Glatt et al., 2008; Gugger et al., 2008; Zhang et al., 2009b).
An unbiased genetic search for the gene causing familial Hypotrichosis simplex and autosomal recessive woolly hair has identified mutations in the P2Y5 receptor gene on chromosome 13q14.2–14.3 (Z = 17.97) (Pasternack et al., 2008; Shimomura et al., 2008) underlying the disease. Woolly hair is characterized by the presence of fine and tightly curled hair and is also linked to another locus on chromosome 3q27. This region contains the lipase H (LIPH) gene, which has been recently shown to underlie an autosomal-recessive form of hypotrichosis (Pasternack et al., 2009; Shimomura et al., 2009a,b). Lipase H is the human orthologue of the PA-PLA1 enzyme that produces sn2-LPA from PA. The phenotypically indistinguishable autosomal-recessive woolly hair syndrome caused either by mutations in the P2Y5 or PA-PLA1/LIPH genes points to the signalling axis that is colocalized to the Henle's and Huxley's layers of the inner root of the hair follicle. There are differences in LPA receptor expression between hair follicles in the eyebrow and those in the scalp. The former express LPA5 in addition to P2Y5, whereas the latter express P2Y5 only; hence, hypotrichosis manifests only in the scalp (Pasternack et al., 2008). LIPH was originally identified by Kazantseva et al. (Kazantseva et al., 2006) as a gene linked to hair growth deficiency in Finno-Ugric families in current-day Russia. LIPH is homologous to mPA-PLA1α, the enzyme that cleaves the sn1 fatty acid in phosphatidate to generate sn2-LPA (Sonoda et al., 2002). LIPH/mPA-PLA1α is expressed in the skin concentrated near the hair bulb and follicle, lung, kidney and pancreas. Currently, the tissue expression of P2Y5 is not known outside the skin and the hair. P2Y5 shows a Kd of 88.6 nM for sn1 LPA 18:1 binding and a rank order of activation of LPA 18:2 > LPA 18:1 > LPA 20:4 > LPA 18:0 > LPA 16:0 > LPA 14:0 (Yanagida et al., 2009). Yanagida et al. also compared the activation properties of P2Y5 by sn1 and sn2-LPA species and found that the sn2-substituted regioisomers were more potent and, in the case of arachidonoyl LPA, more efficacious than the corresponding sn1 regioisomer (Yanagida et al., 2009). d-sn-1-O-oleyl-2-O-methyl-glyceryl-3-phosphothionate (OMPT), a previously proposed LPA3-selective agonist, was more potent at the P2Y5 receptor than LPA 18:1 (Yanagida et al., 2009). P2Y5 in heterologous expression systems has been found to couple to G12/13 and Gs, whereas its ability to elicit Ca2+ transients is disputed [(Yanagida et al., 2009) vs. (Pasternack et al., 2008)]. Interestingly, human umbilical vein endothelial cells (HUVEC) express LPA1 and P2Y5; and knock-down of the latter partially inhibited LPA-induced contraction, suggesting a role for this receptor in the barrier properties of vascular endothelia (Yanagida et al., 2009). Of note is the observation that transgenic mice overexpressing lipid phosphate phosphatase-1 (LPP1/PAP2a), an ectoenzyme thought to degrade LPA, show hair growth defects that is consistent with LPP1-mediated degradation of LPA in the skin (Yue et al., 2004). The physiological and pathophysiological functions of P2Y5 other than in hair growth will have to be addressed in future studies.
P2Y10 was de-orphaned as an LPA-activated receptor by the Fujita group, using the same in silico process that led to their discovery of GPR87's responsiveness to LPA (Murakami et al., 2008). The P2Y10 gene is localized to region 21.1 of the X chromosome near the gene for the LPA4 receptor. LPA4, LPA5 and GPR87/LPA6 show high 56%, 52% and 45% homology to P2Y10, which is similar to the 45% amino acid sequence identity among LPA1, LPA2 and LPA3. Transcripts for this putative LPA receptor were detected in the uterus, prostate, brain, lung, placenta and skeletal muscle. P2Y10 is transcriptionally regulated by PU.1 and Spi-B of the Ets transcription factor family in B cells (Rao et al., 1999) and also expressed in platelets (Amisten et al., 2008). P2Y10 have been expressed functionally in Flp-In™ CHO cells either alone or as a G16 fusion construct. In the stable transfectants, LPA and S1P evoke Ca2+ transients with EC50 values of 53 nM and 130 nM respectively. Empty vector-transfected or G16-transfected CHO cells do not show Ca2+ transients in response to S1P, and their endogenous LPA response is augmented in its size with a dose–response shifted to the left. These authors found no activation by dihydro-S1P or sphingosylphosphorylcholine or other lysophospholipids. Kitamura et al. (Kitamura et al., 2009) expressed P2Y10 in NIH3T3 and HEK293 cells and found that transfectants show morphological changes and TNFα secretion in response to lysophosphatidyl serine (LPS); the latter was inhibited by Y-27632, an inhibitor of the RhoA-ROCK signalling axis. These cells were highly responsive to LPA and S1P, and these lipids elicited similar responses in P2Y- and mock-transfected cells. In our hands, P2Y10-CHO cells respond to LPA > S1P > LPS (Figure 4), which is consistent with the findings of the Fujita and Aoki groups. GPR34, another P2Y family GPCR, has been recently de-orphaned as a receptor for LPS (Sugo et al., 2006). As for GPR87, also for P2Y10: additional studies will be needed to establish whether LPA is their most potent endogenous ligand. During the redaction of this article, Oka et al. (2010) reported that GPR35 responds to LPA with a unique preference for sn2-substituted LPAs. The rank order of ligand efficacy at GPR35 was sn2-18:2 > sn2-18:1 > sn2-20:4 > sn1-18:1. GPR35 activates the Rho GTPase and the ERK1/2 mitogen activated kinase. Kynurenic acid at concentrations ≥ 100 µM activated GPR35.
Figure 4.
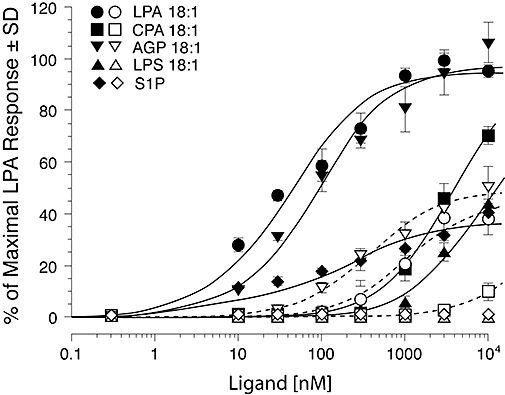
Activation of Ca2+ transients in P2Y10-transfected (filled symbols) and empty vector-transfected Gα16-expressing CHO cells (open symbols) by LPA, AGP, CPA, S1P and LPS. Note that P2Y10-transfected cells dose-dependently respond to low micromolar CPA, S1P and LPS. These ligands either elicit no response (S1P and LPS) or greatly diminished response (CPA) in the vector transfected Gα16-expressing CHO cells. All ligands display lower EC50 and higher Emax values in P2Y10-transfected cells compared with controls. The dose–response curves were generated using Fura2 in a FLEXStation.
Intracellular actions of LPA
Identification of its many GPCR shifted the interest to the mediator role of LPA while many early reports on its intracellular actions became ignored (Tsai et al., 1989; Sando and Chertihin, 1996; Yang et al., 2000; Chemin et al., 2005). LPA is generated intracellularly through several pathways. Glycerol-3-phosphate acyl transferase (GPAT) links fatty acyl-CoA with glycerol-3-phosphate to yield LPA. The metabolic fate of fatty acids taken up into cells goes through this important lipid biosynthetic pathway. In cells with the ability to take up fatty acids, this LPA production pathway might have an important regulatory role through activation of the nuclear hormone receptor PPARγ. PPARγ plays an essential role in regulating lipid and glucose homeostasis (Evans, 2005), as well as cell proliferation (Mueller et al., 1998), apoptosis (Elstner et al., 1998), and inflammation (Ricote and Glass, 2007). These responses have a direct impact on human diseases, particularly diabetes (Lehmann et al., 1995), atherosclerosis (Li et al., 2000) and cancer (Sarraf et al., 1998). Synthetic agonists of PPARγ include the thiazolidinedione (TZD) class, widely used to treat type 2 diabetes. Physiological agonists of PPARγ include 15d-PGJ2 (Forman et al., 1995), modified fatty acids (Baker et al., 2005) (Nagy et al., 1998), oxidized phospholipids (Davies et al., 2001) and select forms of LPA (McIntyre et al., 2003). The structure-activity relationship of PPARγ's activation by LPA differs from that of the GPCR (Tsukahara et al., 2006). Whereas LPA GPCR are activated by both saturated and unsaturated fatty acid-substituted LPAs, PPARγ is only activated by unsaturated LPA. LPA GPCR do not show stereoselective responses to the S- and R-isomers of LPA; in contrast, PPARγ is only activated by the S-stereoisomer. Furthermore, with the exception of LPA5, the alkyl analogues of LPA are less potent agonists of the remaining LPA GPCR; however, 1-O-alkyl glycerophosphate is considerably more potent than LPA at PPARγ (Zhang et al., 2004; Tsukahara et al., 2006). The apparent Kd of 1-O-octadecenyl glycerophosphate binding was 60 nM in the PPARγ ligand binding domain, which is similar to that of rosiglitazone at 40 nM (Tsukahara et al., 2006). Some of the residues required for activation of PPARγ by 1-O-octadecenyl glycerophosphate differ from those required by rosiglitazone. When mutated to alanine, histidines 323 and 449 within the ligand-binding domain of PPARγ failed to abolish binding to and activation by 1-O-octadecenyl glycerophosphate but completely abolished the binding of and activation induced by rosiglitazone. Alanine mutation of arginine 288 significantly reduced binding and activation induced by 1-O-octadecenyl glycerophosphate but not those induced by rosiglitazone. Mutation of tyrosine 273 to phenylalanine abolished binding to and activation by both agonists (Tsukahara et al., 2006).
What is the physiological role of PPARγ activation by LPA? LPA is a key intermediate of fatty acid metabolism. Fatty acids taken up by cells are activated to form acyl-CoAs. Acyl-CoAs can be targeted for β-oxidation by conversion to acyl-carnitine or converted to LPA by different isoforms of GPAT. The resulting LPA can feed either phospholipid synthesis or fuel triacylglycerol synthesis and lipid storage. The LPA formed by GPAT can up-regulate PPARγ target genes involved in lipogenesis, lipid storage and adipocytic differentiation. In a pathophysiological context, LPA might be a key factor in non-alcoholic fatty liver disease (Nagle et al., 2009; Wendel et al., 2009). Thus, LPA might be a molecular link between fatty acid uptake and lipid storage. This hypothesis, although plausible, awaits experimental proof.
Another mechanism regulated by LPA-mediated activation of PPARγ is arterial wall remodelling, which can lead to plaque formation. All major cell types of the arterial wall respond to LPA. In endothelial cells, LPA has been shown to regulate the expression of adhesion molecules and stimulate proliferation, apoptosis, permeability, motility and cell-to-cell contacts responsible for transendothelial permeability (Rizza et al., 1999; Lin et al., 2006). LPA induces vascular smooth muscle cell (VSMC) contraction, proliferation (Tokumura et al., 1994) and phenotypic transdifferentiation in vitro (Hayashi et al., 2001; Zhang et al., 2004). LPA and oxidized low-density lipoprotein (LDL) inhibit macrophage/dendritic cell egress across endothelial cell monolayers (Angeli et al., 2004; Llodra et al., 2004). LPA also stimulates the formation of platelet-monocyte aggregates, which are considered an early marker of acute myocardial infarction (Fueller et al., 2003; Siess, 2006).
Yoshida et al. were the first to test the effects of lumenally applied LPA on non-injured arterial wall in vivo (Yoshida et al., 2003). These authors infused LPA through the external carotid artery of rats into a ligated section of the common/internal carotid artery that was previously rinsed free of blood and maintained near the mean arterial perfusion pressure. In this model, which involves no mechanical injury or removal of endothelial cells within the common carotid artery, a 1-hour exposure to unsaturated but not to saturated species of LPA at low micromolar concentrations leads to neointima development. The structure-activity relationship of neointima induction does not match that of the known LPA GPCR but matches that for PPARγ activation by LPA. PPARγ has long been implicated in atherogenesis (Diep and Schiffrin, 2001; Li and Glass, 2002). GW9662, a specific irreversible antagonist of PPARγ (Leesnitzer et al., 2002), completely abolished 1-O-octadecenyl glycerophosphate-, LPA- and rosiglitazone-induced neointima formation in the rat model (Zhang et al., 2004). In addition to this pharmacological evidence, studies conducted with LPA1&2 and PPARγ conditional knockout mice confirmed the requirement of PPARγ and not LPA1&2 GPCR for arterial wall remodelling in the non-injury model (Cheng et al., 2009). Several investigators have reported that systemic and chronic administration of rosiglitazone attenuates neointima in models with mechanical injury of the arterial wall, which differs from findings reported using the non-injury model (Lim et al., 2006; Lee et al., 2009). When 1-O-octadecenyl glycerophosphate was applied to an injured carotid artery, neointima formation was augmented, whereas rosiglitazone attenuated it. These observations underscore the differences between the non-injury and injury-induced models. It is important to note that circulating LPA bound to carrier proteins such as albumin is unable to activate PPARγ due to limited internalization of the lipid-protein complex (Zhang et al., 2004). Thus, the source of LPA is ether intracellular or although the uptake of lipid particles such as oxidized LDL (Siess et al., 1999; Zhang et al., 2004). The role of LPA in regulating vascular wall physiology is a very promising area for future research.
The LPA-PPARγ axis is also involved in mast cell and dendritic cell differentiation (Bagga et al., 2004; Leslie et al., 2008). Bagga et al. have shown that LPA caused mast cell hyperplasia by inducing the proliferation and maturation of these cells. These cellular responses are under dual control of LPA1/3 GPCR and PPARγ as they were partially inhibited by GW9662, pertussis toxin or VPC-32179. Dendritic cells play an important role in antigen presentation. Serum lipids promote the expression of CD1d antigen-presenting molecules and enhance the activation of immune responses mediated by CD1d-restricted T cells. LPA and cardiolipin were identified as the two active ingredients in serum (Leslie et al., 2008). CD1 expression in immature dendritic cells was under the transcriptional control of PPARγ. This finding raises the hypothesis that LPA can regulate antigen presentation by dendritic cells through the expression of CD1 molecules. Although not mediated by PPARγ, another important immunoregulatory role of LPA has been described by Kanda et al. (Kanda et al., 2008), who found high expression of ATX in the high endothelial venules of lymph nodes. LPA induces chemokinesis in T lymphocytes and could regulate their homing into the lymph node. These observations combined with the role of S1P in lymphocyte egress underscore the importance of lysophospholipids in lymphocyte trafficking and assign LPA and S1P to distinct steps, homing and egress respectively.
Lysophosphatidic acid is also produced in a spatially regulated fashion by the Ca2+-independent phospholipase A2 (iPLA2) at the leading edge of the migrating monocyte (Carnevale and Cathcart, 2001; Mishra et al., 2008). Cathcart et al. have shown that whereas cPLA2α generates arachidonic acid in the cytoplasm and localizes to the trailing edge in monocytes migrating towards MCP-1 chemokine, iPLA2 produces LPA and is localized to the pseudopods where actin polymerization is actively regulated. LPA GPCR have long been known to signal through the Rho-Rac-Cdc42 small GTPase-coupled pathways regulating the actin cytoskeleton (Ridley and Hall, 1992; 1994; Ridley et al., 1992; Ridley et al., 2004). However, there is compelling evidence for the interaction of LPA with actin-binding proteins, including gelsolin, formin, adseverin (Meerschaert et al., 1998; Goetzl et al., 2000b; Mintzer et al., 2006) and villin, the later of which is restricted to epithelial cells (Khurana et al., 2008; Tomar et al., 2009). LPA generation via iPLA2 in migrating cells could regulate actin capping, severing and polymerization through direct high-affinity interactions with these actin-binding proteins. The high-affinity interaction of acting binding proteins with phosphatidylinositol 4,5-bisphosphate (PIP2) has long been known among cell biologists. In this context, findings of the Khurana group are of importance (Khurana et al., 2008; Tomar et al., 2009). These authors showed higher affinity binding of LPA to villin (Kd 22 µM) than that of PIP2 (Kd 39.5 µM) and demonstrated the LPA displaced villin-bound PIP2 in vivo. They also provided evidence that LPA can regulate actin nucleation, depolymerization and capping, the latter of which is not affected by PIP2. LPA has been found to enhance Src-mediated phosphorylation of villin (Khurana et al., 2008; Tomar et al., 2009) and gelsolin (Meerschaert et al., 1998). This polarized production coupled with the direct interaction of LPA with members of the gelsolin-villin family proteins implicates it in the cellular compass mechanism underlying directional cell migration. Extracellularly applied LPA induces cell polarization, directional motility and metabolic burst in human neutrophils (Chettibi et al., 1994). It is intriguing to speculate how iPLA2 could be redistributed to the leading edge of the cell exposed to a chemokinetic gradient and whether extracellular LPA signals originating from LPA GPCR through an inside-out mechanism could feed back into the regulation of iPLA2 and the cellular compass (Figure 5).
Figure 5.
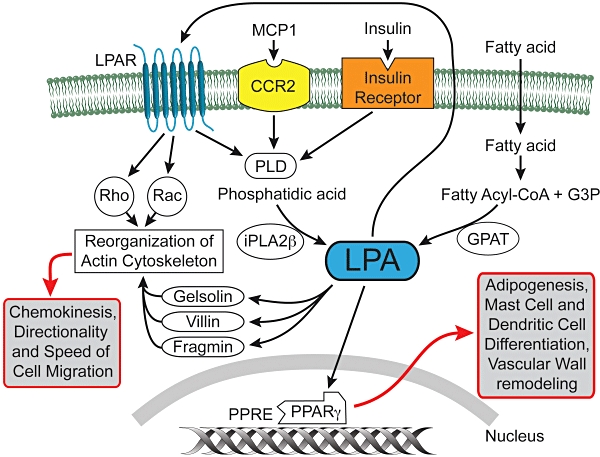
Intracellular sources, targets and actions of LPA. LPA can be generated by iPLA2β in response to MCP1 or insulin stimulation at the leading-edge of migrating macrophages and regulate actin-binding/severing proteins. LPA is also generated by GPAT from fatty acids and can interact with PPARγ, which in turn up-regulates genes involved in adipogenesis and lipid storage. LPA can potentially excreted from cells and stimulate cell surface LPA GPCR setting up an inside-out signalling paradigm. LPA GPCR can synergize with the activation of intracellular targets as it may happen in the case of regulation of the actin cytoskeleton via Rho and Rac.
Lysophosphatidic acid can also be produced intracellularly by PLD from lysophosphatidyl choline and potentially be a source of ligand for intracellular and/or cell surface LPA receptors (Xie et al., 2002).
Autotaxin – how to target it in vivo?
In extracellular biological fluids, LPA production is linked to ATX, which is a lysophospholipase D that cleaves the headgroup from lysophospholipids to generate LPA. ATX is a member of the nucleotide pyrophosphatase/phosphodiesterase (NPP) family and is also known as NPP2. ATX was originally identified as a secreted phosphatase in melanoma culture supernatant that promoted the motility of cancer cells (Stracke et al., 1992). Ten years after its original discovery, ATX was identified as a lysophospholipase D responsible for the production of LPA in serum (Umezu-Goto et al., 2002; Tokumura et al., 2002a). Knocking out ATX leads to embryonic lethality due to a defect in blood vessel formation (van Meeteren et al., 2006; Tanaka et al., 2006) and a lack of large lysosomes in the yolk sac visceral endoderm cells (Koike et al., 2009). This latter defect is due to a lack of LPA-mediated constitutive activation of the Rho-ROCK and LIM kinase pathways. Conditional knockout of ATX in the nervous system causes a neural tube defect that can be corrected in explants by the addition of LPA in the culture medium (Fotopoulou et al., 2010). Neural tube closure involves LPA-mediated activation of the hypoxia-inducible factor HIF-1α. In the developing nervous system, ATX also regulates the differentiation of oligodendrocytes (Fuss et al., 1997; Fox et al., 2003; 2004; Dennis et al., 2005; 2008; Yuelling and Fuss, 2008; Nogaroli et al., 2009). In adult mice, the level of ATX affects haemostasis and thrombosis (Pamuklar et al., 2009).
Many cancers secrete ATX, which contributes to their invasive properties. Gene copy numbers increase in ovarian cancer in chromosomal region 8q24, which also contains the genes encoding the Myc oncogene (Dimova et al., 2006). This raises the possibility that the ATX gene might be amplified in ovarian cancer. Ectopic expression of ATX in mice has recently been shown to lead to the development of chronic mastitis, hyperplasia, mammary intraepithelial neoplasia, and invasive and metastatic tumours (Liu et al., 2009). Ovarian cancer cells produce high levels of LPA in tumour ascites (Baker et al., 2002; Sutphen et al., 2004). ATX inhibits paclitaxel-induced apoptosis in breast cancer cells (Samadi et al., 2009), and LPA renders ovarian cancer cells chemoresistant to cisplatin and adriamycin (E et al., 2009). ATX is overexpressed in patients with recurrent disease after prior treatment with chemotherapy (Jazaeri et al., 2005). An siRNA screen has identified ATX as a candidate drug-resistance gene in ovarian cancer (Vidot et al., 2010).
Autotaxin shows product feedback inhibition, meaning that LPA and S1P inhibit its activity (Durgam et al., 2005; van Meeteren et al., 2005). This feedback inhibition of ATX by LPA has been proposed to be a factor in maintaining plasma levels of LPA in the low nanomolar range (van Meeteren et al., 2005). However, the rate of ATX production seems to be an overriding factor because ATX knockout heterozygous mice have half the normal plasma level of LPA (van Meeteren et al., 2006; Tanaka et al., 2006). Nevertheless, the search for ATX inhibitors is intensifying due to the potential therapeutic applicability of such compounds in cancer therapy (Clair et al., 2005; Baker et al., 2006; Ferry et al., 2008; van Meeteren et al., 2008; Parrill and Baker, 2008; Parrill et al., 2008; North et al., 2009; Zhang et al., 2009a). As proof of this concept, we and others have shown that CCPA analogues inhibit ATX-mediated melanoma invasion in vitro and metastasis in vivo (Baker et al., 2006; Uchiyama et al., 2007). However, inhibition of ATX does not lead to an anti-tumour effect, as cancer cells continue to proliferate although their metastasis is reduced. Zhang et al. (Zhang et al., 2009a) have developed alpha-bromophosphonate analogues of LPA that inhibit ATX activity and also are antagonists of LPA1,2,3,4,5 GPCR and showed that these dual-action compounds not only reduce breast cancer metastasis but also inhibit tumour growth. Hence, the challenge now is to combine receptor antagonism with the inhibition of ATX to obtain anti-tumour and anti-metastatic drug candidates. Pure ATX inhibitors also face another challenge; intravenously injected recombinant ATX is very rapidly >10-min cleared within the liver sinusoids (Jansen et al., 2009). This rapid clearance might also apply to endogenous ATX. Another new direction is to explore agonists of LPA4 and LPA5 that also inhibit ATX. LPA4 inhibits invasion and migration elicited by the EDG-family LPA receptors LPA1 and LPA2 (Lee et al., 2008). Hence, selective agonists of this receptor subtype that inhibit ATX might prove to be useful in limiting cancer invasion and metastasis (Baker et al., 2006; Williams et al., 2009). LPA receptors of the EDG family are dysregulated in ovarian cancer. In a study with 30 ovarian cancer patients, LPA1 expression was decreased, whereas LPA2 and LPA3 were increased (Murph et al., 2008) in the tumour tissue. siRNA-mediated knock-down of LPA2 and LPA3 in SKOV and OVCAR-3 ovarian cancer cell lines reduced aggressiveness and increased survival after xenografting (Yu et al., 2008).
LPA in malignant transformation, cancer metastasis, and radiation- and chemo-resistance
Lysophosphatidic acid is a mitogen, motogen and anti-apoptotic agent, all of which combined provide survival advantages to cells that generate LPA and utilize it in an autocrine or paracrine fashion. In the last decade, many reports have confirmed that several types of cancer cells secrete ATX, which in turn generates LPA in the immediate vicinity of the cell, so long as lysophosphatidylcholine is available. Biological fluids are rich in lysophosphatidylcholine. Perhaps the best-established example for the role of LPA in cancer pathobiology is ovarian cancer. Ovarian cancer ascites has highly elevated levels of LPA (Xu et al., 1995; Baker et al., 2002; Sutphen et al., 2004) due to increased ATX expression (Dimova et al., 2006). Ovarian cancers predominantly express LPA2 receptors, whereas this receptor is barely detected in ovarian epithelial cells (Goetzl et al., 1999a). In addition to LPA2, LPA4 is highly expressed in normal ovarian tissues (Noguchi et al., 2003). LPA2 might play an important role in the aggressive behaviour of ovarian cancer in at least two ways. First, LPA2 promotes the production of vascular endothelial growth factor (VEGF), urokinase (uPA) and matrix metalloproteinases (MMP) (Zebrowski et al., 1999; Hu et al., 2001; Huang et al., 2004; So et al., 2004; 2005). LPA increases VEGF production, and VEGF in turn up-regulates ATX production, which increases LPA levels (Ptaszynska et al., 2008) – a potential feed-forward loop. LPA-induced uPA production promotes invasion by several ovarian cancer cell lines (Pustilnik et al., 1999; Gil et al., 2008). uPA levels carry a prognostic value in ovarian cancer and correlate with poor prognosis (Schmalfeldt et al., 2001; Murthi et al., 2004). LPA conveys resistance to chemo- and radiation-therapy through its anti-apoptotic action (Fang et al., 2000; Deng et al., 2007; E et al., 2009). The LPA2 receptor plays a unique role in chemoresistance, mediated through its C-terminal interaction with the thyroid receptor-interacting protein 6 (TRIP6), the pro-apoptotic transcription factor Siva-1 and the PSD-95/Disc-large/ZO-1 domain (PDZ domain)-binding proteins NHERF2 and MAGI-3 (Yamada et al., 2005; Lin et al., 2007; Zhang et al., 2007a,b; E et al., 2009). LPA2 binds Siva-1 through a–C 311XXC motif and the complex becomes polyubiquitinated and degraded by the proteasome, which leads to the attenuation of the DNA-damage-induced apoptosis. Siva-1 has also been shown to bind and sequester the anti-apoptotic BCL-XL protein and promote the progression of apoptosis via the mithochondrial pathway. LPA-induced molecular complex between LPA2 and Siva-1 plays a major role in the anti-apoptotic effect of LPA. LPA2 can form a ternary complex with TRIP6 and NHERF2 in such a way that TRIP6 and NHERF2 also attach to each other. This ternary complex is formed via the –347DSTL PDZ motif of LPA2 and the PDZ2 domain of NHERF2. NHERF2 homodimerizes with an other NHERF2 via its PDZ1 binding domain, leaving the second PDZ2 domain available to bind the –TTDC PDZ motif of TRIP6. TRIP6 also binds to the –CXXC motif in LPA2 via its LIM3 domain completing the ternary complex (Figure 6). The ternary complex is required for the full activation of ERK1/2 and AKT kinases and through them essential for LPA-induced protection against radiation- and DNA-damage-induced apoptosis. LPA2-associated macromolecular signalosomes have been detected in ovarian cancer cells and for this reason also represent novel targets for prevention of therapeutic resistance of cancer (Figure 6). MMPs represent yet another group of important effectors of LPA in cancer cells (Fishman et al., 2001; Meng et al., 2004; So et al., 2004; 2005; Hope et al., 2009). LPA up-regulates MMP production, which in turn promotes the proliferation of cancer cells and invasion. Increased MMP production correlates with poor prognosis of ovarian cancer (Davidson et al., 1999a,b; Schmalfeldt et al., 2001). In view of the mitogenic, motogenic, pro-invasive and anti-apoptotic effects of LPA, receptor antagonists and ATX inhibitors offer potential for anti-cancer therapeutics.
Figure 6.
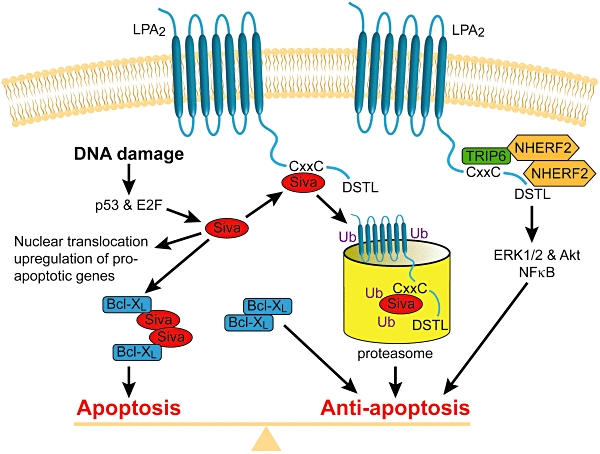
Macromolecular complex-mediated anti-apoptotic signalling by the LPA2 receptor. The C-terminal tail of LPA2 contains docking sites for PDZ-binding proteins (NHERF2) and LIM family proteins (Siva-1 and TRIP6). LPA2 activation captures the pro-apoptotic Siva-1 and targets it to proteasomal degradation. LPA2 receptor activation recruits a ternary complex formed with NHERF2 and TRIP6, which augments anti-apoptotic signals mediated via ERK1/2 and Akt kinase pathways.
LPA in the nervous system
The LPA1 receptor was originally identified from neuronal progenitor cells in the ventricular zone of the developing brain (Hecht et al., 1996). LPA is important in myelination and Schwann cell survival (Weiner et al., 1998; Moller et al., 1999; Li et al., 2003; Nogaroli et al., 2009). Perhaps the most profound morphological effect of LPA is on the development of cortical folds that lead to the development of gyri in the cerebral cortex (Kingsbury et al., 2003; Estivill-Torrus et al., 2008; Matas-Rico et al., 2008). There are several reviews dedicated to the topic of LPA in the brain, and the reader is referred to those for a more in-depth coverage of this exciting aspect of LPA developmental neurobiology (Birgbauer and Chun, 2006; Herr and Chun, 2007; Choi et al., 2008; Rivera and Chun, 2008; Noguchi et al., 2009).
One significant therapeutic effect of LPA appears to be its mediator role in neuropathic pain. Intrathecally injected lysophosphatidylcholine (LPC) has been shown to elicit neuropathic pain and cause demyelination (Wallace et al., 2003; Inoue et al., 2008b). These effects of LPC require ATX; and the bioactive compound is LPA, the product of ATX-mediated LPC hydrolysis (Inoue et al., 2008b; Ahn et al., 2009). LPA initiates neuropathic pain and regulates the production of pain-related molecules through the LPA1 receptor (Inoue et al., 2004; 2008a,b; Ueda, 2006; Fujita et al., 2007; Xie et al., 2008). Recently, Ueda et al. proposed a feed-forward loop that involves LPA3 receptor-mediated production of lysophosphatidylcholine, which supplies the substrate to ATX, rich in the cerebrospinal fluid. These authors showed that LPA produced by ATX in turn can lead to LPA1-mediated neuropathic pain (Ma et al., 2009).
LPA1 up-regulates the voltage-gated Ca2+ channel in the dorsal root ganglion cell, through a Rho-Rho kinase-coupled mechanism, causes demyelination of the fibres originating from the ganglion cells and leads to the reorganization of Aβ-fibres (Inoue et al., 2004; Fujita et al., 2007; Xie et al., 2008). In addition to the LPA1 receptor, LPA5 has also been implicated in neuropathic pain, although the mechanism responsible remains to be elucidated (Sheardown et al., 2004; Kinloch and Cox, 2005). Antagonists targeting LPA1 and LPA5 receptors might provide new ways to treat this currently difficult-to-manage neurological condition. Perhaps more than a curiosity is the fact that B lymphocytes from patients with bipolar disorders are hyper-responsive to LPA (Wasserman et al., 2004; Perova et al., 2008; 2009); and in patients with schizophrenia, LPA1 is among 18 genes that show close association with the disease (Bowden et al., 2006).
LPA in bone metabolism
LPA1 knockout mice show decreased bone density, shorter bones (J.P. Salles – personal communication) and a craniofacial dysmorphic phenotype due to abnormal development of the frontal bones of the skull (Contos et al., 2000). This abnormality might represent only one manifestation of defects in osteoclast and osteoblast function regulated by the LPA1 receptor. LPA has been identified as a mitogen for osteoblasts (Caverzasio et al., 2000; Grey et al., 2001; Lyons and Karin, 2001); it prevents osteoblast apoptosis (Grey et al., 2002) and stimulates alkaline phosphatase expression (Dziak et al., 2003) and cell migration. Osteocytes develop a mechanosensory network within the bone matrix and communicate through gap junctions established at their dendritic termini. LPA is a potent stimulator of dendrite outgrowth, which is blocked by the LPA1/LPA3 antagonist Ki16425 and pertussis toxin (Karagiosis and Karin, 2007). Resting zone chondrocytes generate LPA, which in turn promotes osteoblast differentiation, proliferation and survival (Hurst-Kennedy et al., 2009). Osteolytic metastasis of breast cancers also utilizes LPA as a mediator. LPA1 regulates the secretion of IL-6 and IL-8, which are potent bone resorption stimulators. Silencing expression or utilizing pharmacological inhibition of LPA1 in cancer cells reduces bone metastasis progression. Future pharmacological manipulation of LPA receptors might provide opportunities to prevent bone loss and reduce the bone metastasis of cancers.
LPA in reproductive disorders
The effects of LPA on cells from the female reproductive system have been known since the late 1980s. We previously identified and purified LPA as a serum factor based on its activation of oscillatory Cl- currents in Xenopus oocytes (Tigyi et al., 1990; Tigyi and Miledi, 1992). LPA enhances embryonic development from the pronuclear stage to the blastocyst stage in mice (Kobayashi et al., 1994). In luteal cells, LPA stimulates type V adenylyl cyclase, which is important for the maintenance of pregnancy (Budnik and Mukhopadhyay, 1997). This response is likely to be mediated through LPA4, which, in contrast to the EDG family receptors, elevates cAMP and is highly expressed in the ovary (Noguchi et al., 2003). LPA stimulates contraction in uterine smooth muscle cells (Tokumura et al., 1980) and increases embryo transport in the oviduct of mice (Kunikata et al., 1999; Tokumura et al., 1999). In the follicular fluid, LPA concentration is as high as 25 nmol·mL−1, which is nearly double its serum concentration of c. 15 nmol·mL−1 (Tokumura et al., 1999). The LPA3 receptor, which is up-regulated by progesterone and down-regulated by oestrogen, is required for embryo implantation and affects embryo spacing in the womb (Ye et al., 2005; Hama et al., 2007). In view of its effects on implantation, it is plausible that LPA is involved in placental pathologies, including non-receptive endometrium, decidualization, embryo crowding, placenta previa and placenta acreta, any of which could lead to sterility or loss of pregnancy (Ye, 2008). There are some selective LPA3 antagonists available that include diacylglycerol pyrophosphate (Fischer et al., 2001), VPC12449 (Heise et al., 2001) and Ki16425 (Ohta et al., 2003). However, these antagonists also inhibit LPA1. Recently, a specific non-lipid antagonist, NSC161613, has been identified showing an IC50 of 24 nM (Fells et al., 2008).
LPA1/2/3 are highly expressed in the male reproductive system. Mice deficient in all three receptors show a testosterone-independent reduction of mating activity and sperm production, with an increased prevalence of azoospermia in aging animals. These triple knockouts show a significant increase in germ cell apoptosis, leading to a reduction in germ cell proliferation (Ye et al., 2008). Similar azoospermia and reduced germ cell numbers were found in transgenic animals that overexpress lipid phosphate phosphatase, an enzyme that degrades LPA (Yue et al., 2004). There is a wealth of evidence that links LPA, LPA3 and LPA1 to the regulation of prostate cancer cells (Qi et al., 1998; Prenzel et al., 1999; Im et al., 2000; Daaka, 2002; Guo et al., 2006; Hwang et al., 2006). In contrast, little is known about the physiological role of LPA in the normal prostate. Selective ligands of the LPA3 receptor would likely offer novel drug candidates to modulate both male and female reproduction.
LPA in vascular pathologies
In 1978, Tokumura et al. reported that soy lipid extract contains a vasopressor lipid that they identified as LPA (Tokumura et al., 1978a; Tokumura et al., 1978b). LPA had hypertensive effects in rats and guinea pigs and hypotensive effects in cats and rabbits. Schumacher et al. discovered that factors developed during ‘aging’ of heparinized plasma kept at 36°C for 18–24 h and that these factors activated platelet aggregation and caused constriction of pulmonary vessels (Schumacher et al., 1979). These authors identified LPA as being one of the active factors and noted that it caused desensitization of the platelet response, a hallmark of GPCR action. Simon et al. discovered that the alkyl-ether analogue of LPA is a potent activator of human platelets at concentrations as low as 10−10 M, making this analogue nearly as potent as platelet-activating factor (Simon et al., 1982; 1984). The barrier function of vascular endothelial cells is altered by LPA (Schulze et al., 1997; Alexander et al., 1998; English et al., 1999), and LPA induces the expression of VCAM-1 and E-selectin on the surface of cultured human endothelial cells (Rizza et al., 1999). LPA induces endothelin-1 production in vitro and in vivo (Chua et al., 1998; Yakubu and Leffler, 1999). LPA is a mitogen for VSMC (Tokumura et al., 1994) and also promotes their phenotypic dedifferentiation from a contractile phenotype to a secretory phenotype (Hayashi et al., 2001; Yoshida et al., 2003). Topical application of unsaturated LPA species into the non-injured carotid artery of rodents induces arterial wall remodelling, and this response requires PPARγ but not LPA1 or LPA2 (Hayashi et al., 2001; Yoshida et al., 2003; Zhang et al., 2004; Cheng et al., 2009).
Lysophosphatidic acid is required for normal vascular development, as indicated by the embryonic lethality of ATX knockout mice at a stage when vascular stabilization begins (van Meeteren et al., 2006; Tanaka et al., 2006). In adult mice, ATX overexpression leads to haemorrhages due to an inhibitory effect of LPA on platelets, whereas ATX ± heterozygotes, which have a reduced plasma LPA concentration, are more prone to thrombosis (Pamuklar et al., 2009). LPA and ATX have been implicated in wet-type macular degeneration by promoting angiogenesis in the retina.
Nanomolar concentrations of LPA and minimally oxidized LDL induce shape change of washed platelets through LPA receptor-linked signal transduction pathways that presumably involve the activation of the heterotrimeric G12/G13 protein, the small GTPase Rho and Rho kinase, the Rho kinase-mediated inhibition of myosin light-chain phosphatase, and stimulation of myosin light-chain phosphorylation (Bauer et al., 1999; Retzer and Essler, 2000; Retzer et al., 2000). It seems that there are two types of LPA receptors that regulate LPA responsiveness in human platelets. One subtype inhibits platelet responses by elevating cAMP levels, which in turn attenuates activation, as proposed by Pamuklar et al. (Pamuklar et al., 2008). These authors ascribed this effect to LPA4. Khandoga et al. could not detect elevation in cAMP in LPA-treated human platelets (Khandoga et al., 2008). However, these authors found that the structure-activity relationship, characterized by the preference for alkyl over acyl LPA analogues, is similar to that of LPA5, which makes this receptor a candidate for the activating LPA receptor subtype in platelets (Williams et al., 2009). Williams et al. identified two non-lipid antagonists of LPA5, H2L 5987411 and H2L 5765834, that also blocked platelet activation by LPA (Williams et al., 2009). The former compound but not the latter is also a weak antagonist of LPA4 (Ki 741 nM), yet both inhibited platelet responses to LPA. LPA4 and LPA5 transcripts are both abundant in human platelets mixed from multiple donors (Amisten et al., 2008). Tokumura et al. described human donors whose platelets failed to respond to alkyl glycerophosphate while maintaining normal responsiveness to acyl analogues of LPA (Tokumura et al., 2002b). This observation lends support to the hypothesis that multiple receptors are involved in platelet responses to LPA.
Lysophosphatidic acid also activates haptotactic migration in monocytes/macrophages (Zhou et al., 1995), inhibits the egress of dendritic cells from the vessel wall (Llodra et al., 2004), and promotes the formation of monocyte/platelet aggregates that are an early marker of acute myocardial infarction (Fueller et al., 2003). Acyl and alkyl LPA accumulate in human atherosclerotic plaques and likely activate platelets and initiate thrombus formation upon plaque rupture (Siess et al., 1999; Rother et al., 2003). LPA production and the LPA receptor subtypes expressed by different subsets of blood cells and cells of the vessel wall are undoubtedly important potential drug targets for manipulating pathological angiogenesis and thrombosis.
LPA as a mediator of organ fibrosis
Lysophosphatidic acid has long been known for its mitogenic effect on fibroblasts (van Corven et al., 1989; 1992). In addition to being a mitogen, LPA increases connective tissue growth factor production, which promotes tissue fibrosis (Hahn et al., 2000; Muehlich et al., 2004; Vial et al., 2008). LPA induces the expression and activation of the CIC-3 chloride channel that is required for myofibroblast dedifferentiation during wound healing (Wang et al., 2002; Yin and Watsky, 2005; Yin et al., 2008). In light of these findings, it is perhaps not surprising that LPA has been implicated in renal (Pradere et al., 2007), hepatic (Watanabe et al., 2007a,b) and pulmonary fibrosis (Tager et al., 2008; Xu et al., 2009). In several models, inhibition of the LPA1 receptor inhibits the progression of fibrosis (Pradere et al., 2007; Tager et al., 2008), but LPA-induced αVβ6 integrin-mediated TGF-β activity is mediated via the LPA2 receptor (Xu et al., 2009). Tager et al. showed that LPA levels increase in bronchoalveolar lavage fluid following lung injury in the bleomycin model of pulmonary fibrosis and that mice lacking LPA1 are protected from fibrosis in this model. These investigators showed that patients with idiopathic pulmonary fibrosis, had increased LPA levels in bronchoalveolar lavage fluid and that inhibition of LPA1 reduced fibroblast responses to the chemotactic activity of the lavage fluid. A recent study using plasma and serum samples from systemic scleroderma patients found elevated LPA and S1P levels. Furthermore, serum LPA : LPC ratios of the 18:2 and 20:4 molecular species, and also the ratio of all species combined, were significantly higher in systemic scleroderma patients versus controls (Tokumura et al., 2009). Currently, a diagnosis of organ fibrosis leaves the physician with very limited therapeutic options that often come with severe side effects. Exploration of the role of LPA- and LPA receptor antagonist-based therapies might offer an entirely new therapeutic avenue of treatment.
LPA in infection and immunity
Activation of the RhoA GTPase and phospholipase d-dependent acidification of phagolysosomes can have profound effects on viral and bacterial entry into cells and also on the intracellular killing of bacteria. The cysteine-rich protein 61 (CYR61) and connective tissue growth factor (CTGF) regulate adhesion, migration, extracellular matrix deposition and cell differentiation and play a role in wound healing. Bacterial lipid extracts derived from Yersinia, E. coli, Pseudomonas aeruginosa, Enterococcus faecalis or Staphylococcus aureus induce expression of CYR61 and CTGF in epithelial cells. Wiedmaier et al. have shown that Ki16425, a selective antagonist of LPA1/3 receptors (Ohta et al., 2003), inhibits Cyr61 and CTGF expression (Wiedmaier et al., 2008). LPA-induced CYR61 and CTGF mRNA expression requires Rho GTPases because it is abolished by Clostridium difficile toxin B, which inhibits RhoA and Rac-1. These authors raised the hypothesis that LPA GPCR could be involved in sensing bacterial lipid products. thereby regulating the host's response to infection via CYR61 and CTGF expression.
Tsurudome et al. reported that LPA promotes cell-cell fusion in parainfluenza virus-infected Vero cells (Tsurudome et al., 2008). This process was sensitive to the Rho kinase inhibitor Y-27632. Thus, LPA, at least theoretically, could promote the cytopathic effect of parainfluenza virus infection. This hypothesis awaits experimental testing. Inhibition of RhoA activation elicited by respiratory syncytial virus infection is implicated in the antiviral mechanism of the macrolide antibiotics bafilomycin and clarithromycin (Asada et al., 2009). RhoA activation is associated with entry and exocytosis of viruses, and LPA might synergize with these events and also attenuate the antiviral efficacy of macrolide antibiotics.
Recent reports assign anti-microbial activity to LPA in Mycobacterium tuberculosis infection (Garg et al., 2006; Greco et al., 2010). LPA and S1P exert a cytoprotective effect in Mycobacterium tuberculosis-infected type II alveolar cells and activate PLD, which in turn leads to phagolysosome maturation, leading to mycobacterial killing and inhibition of bacterial dissemination in vitro (Greco et al., 2010). Garg et al. have shown that LPA enhances anti-mycobacterial activity in vitro and ex vivo in cells derived from the bronchoalveolar lavage of patients with tuberculosis. LPA activated PLD-dependent acidification of the phagolysosomes in these cells that had been chronically infected with endogenous mycobacteria in the lungs of the patients. The molecular target(s) of LPA underlying its anti-mycobacterial effect remain to be identified. Nonetheless, LPA is known to activate PLD (Qi et al., 1998; Zhao et al., 2005) through a Ca2+-dependent mechanism. Furthermore, ATX is important for normal lysosomal morphology and function (Koike et al., 2009). These exciting studies on the role of LPA in infection are at a very early stage; hence, it is difficult to predict whether LPA or LPA-based pharmacons might have therapeutic value in infection control.
The role of LPA in immune cells cannot be underestimated, and research in this field will likely lead to therapeutically important new discoveries. T lymphocytes (Goetzl et al., 2000a), B cells (Rosskopf et al., 1998; Satoh et al., 2007; Perova et al., 2008), eosinophils (Idzko et al., 2004), neutrophils (Chettibi et al., 1994), macrophages (Hornuss et al., 2001), mast cells (Bagga et al., 2004) and dendritic cells (Panther et al., 2002; Llodra et al., 2004; Chan et al., 2007) express functional LPA receptors. Natural killer cells respond to LPA with increased IFNγ production and chemotaxis (Jin et al., 2003; Maghazachi, 2003; Jo et al., 2008), although it was also found to inhibit their cytotoxic response through a cAMP-PKA-dependent mechanism. Lipopolysaccharide treatment of TH-1 lymphocytes increases ATX expression that can supply LPA upon contact of the T cell with other immune cells (Li and Zhang, 2009). These findings lend support to the role of LPA in innate as well as acquired immunity. In the preceding sections, we have already described some effects of LPA on mast cell and dendritic cell differentiation and the regulation of T cell homing regulated by ATX-LPA GPCR and PPARγ (Bagga et al., 2004; Llodra et al., 2004; Kanda et al., 2008; Leslie et al., 2008; Nakasaki et al., 2008). LPA has been shown to stimulate proliferation, migration and survival of T cells and macrophages (Koh et al., 1998; Goetzl et al., 1999b; Zheng et al., 2000; 2001). LPA regulates chemokine and cytokine production by immune cells that include IL-1β, IL-2, IL-8, IL-13, TNFα, MCP-1 and MIP-1β (Zheng et al., 2000; Lee et al., 2002; Panther et al., 2002; Gobeil et al., 2003; Lin and Boyce, 2005; Rubenfeld et al., 2006). Perhaps the most compelling evidence for the immunomodulatory role of LPA comes from Leslie et al. (Leslie et al., 2008), who showed that it differentially regulates the development of group 1 (CD1a, CD1b and CD1c positive) and group 2 (CD1d) dendritic cells. LPA, in a completely reversible PPARγ-dependent mechanism, inhibited the transcription and cell surface expression of group 1-specific CD1 markers, and conversely increased CD1d, involved in lipid antigen presentation by dendritic cells to T cells. Group 1-reactive T cells display a cytotoxic phenotype and secrete T helper type 1 cytokines, suggesting their role in microbial infection (Brigl and Brenner, 2004). CD1 molecules are also involved in T cell recognition of self-lipids (Leslie et al., 2002; Vincent et al., 2002; 2003). Thus, CD1-restricted T cells mediate not only host defense mechanisms but also pro-inflammatory responses in human autoimmune diseases. including systemic lupus erythematodes (Sieling et al., 2000), multiple sclerosis (De Libero et al., 2002) and autoimmune thyroiditis (Roura-Mir et al., 2005). Based on these reports, LPA through its effect on dendritic cell maturation might promote the expansion of T helper 2 type cells relative to T helper 1 cells (Panther et al., 2002; Leslie et al., 2008), which has far-reaching implications for the physiological regulation of autoimmunity and host defense.
Conclusion
In recent years, there has been an explosion in the number of reports describing the numerous actions of LPA, making it impossible to write a completely up-to-date and comprehensive review of this rapidly changing field. The objective of the present article was not to be comprehensive but to highlight only certain aspects of the LPA story. The reader is referred to the many excellent and more in-depth reviews on specific aspects of LPA biology. Our aim in this article was to generate additional interest for this exciting and novel field of lipid research.
Lysophosphatidic acid, with its pleiotropic effects and simple structure, provides an enticing opportunity for therapeutic exploration. LPA engages targets both as a mediator and a second messenger in almost every cell type in the body. The many LPA receptors represent an unparalleled redundancy of cellular signalling. The multiplicity of cell surface and intracellular LPA targets/receptors, often co-expressed in the same cell type and coupling to overlapping signal transduction pathways, poses an unmet challenge to designing LPA-based therapeutics.
A fundamental problem often neglected is the endogenous expression of LPA receptor transcripts in every mammalian cell line. Although there are a few cell lines that do not respond to LPA with Ca2+ transients (RH7777 and B103) and although heterologous overexpression of LPA1,2,3,4,5 in these cell lines conveys LPA-elicited Ca2+ responses, these cells still express low levels of LPA GPCR transcripts and show endogenous signalling responses to LPA (Valentine et al., 2008b). Functional expression of LPAR of the purinergic subcluster often requires plasmids with strong promoters such as pCXN2.1 (Niwa et al., 1991) and/or co-expression of promiscuous G protein α-subunits (G16) due to low coupling efficiency of some LPAR. However, overexpression of Gα16 can increase the coupling efficiency of the endogenous LPAR and introduce Ca2+ transients into RH7777 and B103 cells even in the absence of heterologous LPAR (Valentine and Tigyi, unpublished). This complicates the characterization of the novel LPAR and emphasizes the need for ‘cleaner’ assay platforms (Wetter et al., 2009).
The S1P field has surpassed the LPA field in therapeutic exploration because the S1P receptor agonist FTY720 is now near completion of phase III clinical trials for the treatment of multiple sclerosis, whereas no LPA-based therapeutic has advanced this far. What might be the reason for such disparity between the two lysophospholipid fields? Perhaps one key difference lies in the obligatory role of S1P1 in lymphocyte egress. Thus far, no matching (patho-)physiologically essential cellular response has been linked to a single LPAR, although the role of LPA3 in embryo implantation offers some degree of similarity. Thus, the greater redundancy and overlapping signalling pathways that prevail among the LPAR families might be the reason that no LPA-based drug has emerged thus far. Perhaps, the redundancy of LPA signalling is an indicator of the fundamental necessity of this mediator/second messenger for normal cell function. Indeed the S1P1 receptor knockout mouse is the only embryonic lethal lysophospholipid receptor knockout, whereas none of the LPAR knockouts cause embryonic lethality. This contrasts with the ATX knockout, which also causes embryonic lethality and indicates the essential requirement for LPA during development. More thorough characterization of LPAR knockouts under different pathophysiological challenges will likely pinpoint distinct and unique functions associated with a single or a select few LPAR.
The field still lacks drug-like compounds with the desired specificity or ubiquity for LPA targets. Currently, identified lipid-like LPA analogues lack the necessary potency and selectivity, and they have suboptimal pharmacokinetic profiles. With the identification of the novel LPAR, the selectivity and specificity of the previously developed and presumed ‘selective’ pharmacons begs further characterization. From now on, any new compound will have to be tested on the nine established and putative LPAR and the five S1P receptors. While the opportunity to control LPA-mediated pathophysiologies is apparent, harnessing the LPA system through pharmacological tools remains a stimulating task for the academic community and a lucrative opportunity for the pharmaceutical industry.
Acknowledgments
This publication was made possible by grants CA92160, HL79004 and AI080405 from NIH. Its contents are solely the responsibility of the author and do not necessarily represent the official views of the NIH. The author thanks Prof Norihisa Fujita (Ritsumeikan University, Japan) for sharing his GPR87- and P2Y10-transfected cell lines, Drs Yuko Fujiwara and Mari Gotoh (University of Tennessee Health Science Center Memphis) for providing the SAR of the P2Y10 receptor, Drs Abby L. Parrill and Daniel L. Baker (University of Memphis) for their helpful comments on this manuscript and Dr James Fells for his help with molecular structures.
Glossary
Abbreviations
- 3CCPA
18:1 oleoyl-3-carba cyclic phosphatidic acid
- ATX
autotaxin
- CHO
Chinese hamster ovary
- CPA
cyclic phosphatidic acid (1-acyl-sn-glycerol-2,3-cyclic phosphate)
- CTGF
connective tissue growth factor
- CYR61
cysteine-rich protein 61
- DRG
dorsal root ganglion
- EDG
endothelial gene
- GPAT
glycerol-3-phosphate acyl transferase
- GPCR
G protein-coupled receptor
- HUVEC
human umbilical vein endothelial cells
- iPLA2
Ca2+-independent phospholipase A2
- IUPHAR
International Union of Basic and Clinical Pharmacology
- LDL
low-density lipoprotein
- LPA
1-acyl-2-hydroxy-sn-glycero-3-phosphate
- LPC
lysophosphatidylcholine
- LPS
lysophosphatidyl serine
- MEF
mouse embryonic fibroblast
- MMP
matrix metalloproteinases
- NPP
nucleotide pyrophosphatase/phosphodiesterase
- OMPT
D-sn-1-O-oleyl-2-O-methyl-glyceryl-3-phosphothionate
- P2Y
purinoreceptor
- PDZ domain
PSD-95/Disc-large/ZO-1 domain
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLD
phospholipase D
- PPARγ
peroxisome proliferator-activated receptor gamma
- ROCK
Rho-associated kinase
- S1P
sphingosine-1-phosphate
- siRNA
small interfering RNA
- TRIP6
thyroid receptor-interacting protein 6
- TZD
thiazolidinedione
- UDP
uridine diphosphate
- UTP
uridine triphosphate
- uPA
urokinase
- VEGF
vascular endothelial growth factor
- VSMC
vascular smooth muscle cells
Conflicts of interest
The author is a shareholder in RxBio Inc.
Supplemental material
Supporting Information: Teaching Materials; Figs 1–6 as PowerPoint slide.
References
- Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, et al. Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain. 2009;146:114–120. doi: 10.1016/j.pain.2009.07.012. [DOI] [PubMed] [Google Scholar]
- Alexander JS, Patton WF, Christman BW, Cuiper LL, Haselton FR. Platelet-derived lysophosphatidic acid decreases endothelial permeability in vitro. Am J Physiol. 1998;274(1 Pt 2):H115–H122. doi: 10.1152/ajpheart.1998.274.1.H115. [DOI] [PubMed] [Google Scholar]
- Amisten S, Braun OO, Bengtsson A, Erlinge D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res. 2008;122:47–57. doi: 10.1016/j.thromres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, et al. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Aoki J, Inoue A, Makide K, Saiki N, Arai H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 2007;89:197–204. doi: 10.1016/j.biochi.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta. 2008;1781:513–518. doi: 10.1016/j.bbalip.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Asada M, Yoshida M, Suzuki T, Hatachi Y, Sasaki T, Yasuda H, et al. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antiviral Res. 2009;83:191–200. doi: 10.1016/j.antiviral.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Bagga S, Price KS, Lin DA, Friend DS, Austen KF, Boyce JA. Lysophosphatidic acid accelerates the development of human mast cells. Blood. 2004;104:4080–4087. doi: 10.1182/blood-2004-03-1166. [DOI] [PubMed] [Google Scholar]
- Baker DL, Desiderio DM, Miller DD, Tolley B, Tigyi GJ. Direct quantitative analysis of lysophosphatidic acid molecular species by stable isotope dilution electrospray ionization liquid chromatography-mass spectrometry. Anal Biochem. 2001;292:287–295. doi: 10.1006/abio.2001.5063. [DOI] [PubMed] [Google Scholar]
- Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, et al. Plasma lysophosphatidic Acid concentration and ovarian cancer. JAMA. 2002;287:3081–3082. doi: 10.1001/jama.287.23.3081. [DOI] [PubMed] [Google Scholar]
- Baker PR, Lin Y, Schopfer FJ, Woodcock SR, Groeger AL, Batthyany C, et al. Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands. J Biol Chem. 2005;280:42464–42475. doi: 10.1074/jbc.M504212200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006;281:22786–22793. doi: 10.1074/jbc.M512486200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000;478:159–165. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- Bauer M, Retzer M, Wilde JI, Maschberger P, Essler M, Aepfelbacher M, et al. Dichotomous regulation of myosin phosphorylation and shape change by Rho-kinase and calcium in intact human platelets. Blood. 1999;94:1665–1672. [PubMed] [Google Scholar]
- Beck HP, Kohn T, Rubenstein S, Hedberg C, Schwandner R, Hasslinger K, et al. Discovery of potent LPA2 (EDG4) antagonists as potential anticancer agents. Bioorg Med Chem Lett. 2008;18:1037–1041. doi: 10.1016/j.bmcl.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Birgbauer E, Chun J. New developments in the biological functions of lysophospholipids. Cell Mol Life Sci. 2006;63:2695–2701. doi: 10.1007/s00018-006-6155-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden NA, Weidenhofer J, Scott RJ, Schall U, Todd J, Michie PT, et al. Preliminary investigation of gene expression profiles in peripheral blood lymphocytes in schizophrenia. Schizophr Res. 2006;82:175–183. doi: 10.1016/j.schres.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Brigl M, Brenner MB. CD1: antigen presentation and T cell function. Annu Rev Immunol. 2004;22:817–890. doi: 10.1146/annurev.immunol.22.012703.104608. [DOI] [PubMed] [Google Scholar]
- Budnik LT, Mukhopadhyay AK. Functional lysophosphatidic acid receptor in bovine luteal cells. FEBS Lett. 1997;419:4–8. doi: 10.1016/s0014-5793(97)01408-7. [DOI] [PubMed] [Google Scholar]
- Carnevale KA, Cathcart MK. Calcium-independent phospholipase A(2) is required for human monocyte chemotaxis to monocyte chemoattractant protein 1. J Immunol. 2001;167:3414–3421. doi: 10.4049/jimmunol.167.6.3414. [DOI] [PubMed] [Google Scholar]
- Caverzasio J, Palmer G, Suzuki A, Bonjour JP. Evidence for the involvement of two pathways in activation of extracellular signal-regulated kinase (Erk) and cell proliferation by Gi and Gq protein-coupled receptors in osteoblast-like cells. J Bone Miner Res. 2000;15:1697–1706. doi: 10.1359/jbmr.2000.15.9.1697. [DOI] [PubMed] [Google Scholar]
- Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82:1193–1200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- Chemin J, Patel A, Duprat F, Zanzouri M, Lazdunski M, Honore E. Lysophosphatidic acid-operated K+ channels. J Biol Chem. 2005;280:4415–4421. doi: 10.1074/jbc.M408246200. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Makarova N, Tsukahara R, Guo H, E S, Farrar P, et al. Lysophosphatidic acid-induced arterial wall remodeling: requirement of PPARγ but Not LPA1 or LPA2 GPCR. Cell Signal. 2009;12:1874–1884. doi: 10.1016/j.cellsig.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettibi S, Lawrence AJ, Stevenson RD, Young JD. Effect of lysophosphatidic acid on motility, polarisation and metabolic burst of human neutrophils. FEMS Immunol Med Microbiol. 1994;8:271–281. doi: 10.1111/j.1574-695X.1994.tb00452.x. [DOI] [PubMed] [Google Scholar]
- Choi JW, Lee CW, Chun J. Biological roles of lysophospholipid receptors revealed by genetic null mice: an update. Biochim Biophys Acta. 2008;1781:531–539. doi: 10.1016/j.bbalip.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CC, Hamdy RC, Chua BH. Upregulation of endothelin-1 production by lysophosphatidic acid in rat aortic endothelial cells. Biochim Biophys Acta. 1998;1405:29–34. doi: 10.1016/s0167-4889(98)90093-3. [DOI] [PubMed] [Google Scholar]
- Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, et al. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- Clair T, Koh E, Ptaszynska M, Bandle RW, Liotta LA, Schiffmann E, et al. L-histidine inhibits production of lysophosphatidic acid by the tumor-associated cytokine, autotaxin. Lipids Health Dis. 2005;4:5. doi: 10.1186/1476-511X-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contos JJ, Fukushima N, Weiner JA, Kaushal D, Chun J. Requirement for the lpA1 lysophosphatidic acid receptor gene in normal suckling behavior. Proc Natl Acad Sci USA. 2000;97:13384–13389. doi: 10.1073/pnas.97.24.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH. Lysophosphatidate-induced cell proliferation: Identification and dissection of signaling pathways mediated by G proteins. Cell. 1989;59:45–54. doi: 10.1016/0092-8674(89)90868-4. [DOI] [PubMed] [Google Scholar]
- van Corven EJ, van Rijswijk A, Jalink K, van der Bend RL, van Blitterswijk WJ, Moolenaar WH. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset M, Brossard N, Polette A, Lagarde M. Characterization of plasma unsaturated lysophosphatidylcholines in human and rat. Biochem J. 2000;345(Pt 1):61–67. [PMC free article] [PubMed] [Google Scholar]
- Daaka Y. Mitogenic action of LPA in prostate. Biochim Biophys Acta. 2002;1582:265–269. doi: 10.1016/s1388-1981(02)00180-4. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999a;17:799–808. doi: 10.1023/a:1006723011835. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Kopolovic J, Lerner-Geva L, Gotlieb WH, Ben-Baruch G, et al. MMP-2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma – a clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol Oncol. 1999b;73:372–382. doi: 10.1006/gyno.1999.5381. [DOI] [PubMed] [Google Scholar]
- Davies SS, Pontsler AV, Marathe GK, Harrison KA, Murphy RC, Hinshaw JC, et al. Oxidized alkyl phospholipids are specific, high affinity peroxisome proliferator-activated receptor gamma ligands and agonists. J Biol Chem. 2001;276:16015–16023. doi: 10.1074/jbc.M100878200. [DOI] [PubMed] [Google Scholar]
- De Libero G, Donda A, Gober HJ, Manolova V, Mazorra Z, Shamshiev A, et al. A new aspect in glycolipid biology: glycosphingolipids as antigens recognized by T lymphocytes. Neurochem Res. 2002;27:675–685. doi: 10.1023/a:1020280201809. [DOI] [PubMed] [Google Scholar]
- Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, et al. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis J, Nogaroli L, Fuss B. Phosphodiesterase-Ialpha/autotaxin (PD-Ialpha/ATX): a multifunctional protein involved in central nervous system development and disease. J Neurosci Res. 2005;82:737–742. doi: 10.1002/jnr.20686. [DOI] [PubMed] [Google Scholar]
- Dennis J, White MA, Forrest AD, Yuelling LM, Nogaroli L, Afshari FS, et al. Phosphodiesterase-Ialpha/autotaxin's MORFO domain regulates oligodendroglial process network formation and focal adhesion organization. Mol Cell Neurosci. 2008;37:412–424. doi: 10.1016/j.mcn.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep QN, Schiffrin EL. Increased expression of peroxisome proliferator-activated receptor-alpha and -gamma in blood vessels of spontaneously hypertensive rats. Hypertension. 2001;38:249–254. doi: 10.1161/01.hyp.38.2.249. [DOI] [PubMed] [Google Scholar]
- Dimova I, Raitcheva S, Dimitrov R, Doganov N, Toncheva D. Correlations between c-myc gene copy-number and clinicopathological parameters of ovarian tumours. Eur J Cancer. 2006;42:674–679. doi: 10.1016/j.ejca.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Dottori M, Leung J, Turnley AM, Pebay A. Lysophosphatidic acid inhibits neuronal differentiation of neural stem/progenitor cells derived from human embryonic stem cells. Stem Cells. 2008;26:1146–1154. doi: 10.1634/stemcells.2007-1118. [DOI] [PubMed] [Google Scholar]
- Durgam GG, Virag T, Walker MD, Tsukahara R, Yasuda S, Liliom K, et al. Synthesis, structure-activity relationships, and biological evaluation of fatty alcohol phosphates as lysophosphatidic acid receptor ligands, activators of PPARgamma, and inhibitors of autotaxin. J Med Chem. 2005;48:4919–4930. doi: 10.1021/jm049609r. [DOI] [PubMed] [Google Scholar]
- Dziak R, Yang BM, Leung BW, Li S, Marzec N, Margarone J, et al. Effects of sphingosine-1-phosphate and lysophosphatidic acid on human osteoblastic cells. Prostaglandins Leukot Essent Fatty Acids. 2003;68:239–249. doi: 10.1016/s0952-3278(02)00277-6. [DOI] [PubMed] [Google Scholar]
- E S, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, et al. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elstner E, Muller C, Koshizuka K, Williamson EA, Park D, Asou H, et al. Ligands for peroxisome proliferator-activated receptorgamma and retinoic acid receptor inhibit growth and induce apoptosis of human breast cancer cells in vitro and in BNX mice. Proc Natl Acad Sci USA. 1998;95:8806–8811. doi: 10.1073/pnas.95.15.8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D, Kovala AT, Welch Z, Harvey KA, Siddiqui RA, Brindley DN, et al. Induction of endothelial cell chemotaxis by sphingosine 1-phosphate and stabilization of endothelial monolayer barrier function by lysophosphatidic acid, potential mediators of hematopoietic angiogenesis. J Hematother Stem Cell Res. 1999;8:627–634. doi: 10.1089/152581699319795. [DOI] [PubMed] [Google Scholar]
- Estivill-Torrus G, Llebrez-Zayas P, Matas-Rico E, Santin L, Pedraza C, De Diego I, et al. Absence of LPA1 signaling results in defective cortical development. Cereb Cortex. 2008;18:938–950. doi: 10.1093/cercor/bhm132. [DOI] [PubMed] [Google Scholar]
- Evans RM. The nuclear receptor superfamily: a rosetta stone for physiology. Mol Endocrinol. 2005;19:1429–1438. doi: 10.1210/me.2005-0046. [DOI] [PubMed] [Google Scholar]
- Fang X, Yu S, LaPushin R, Lu Y, Furui T, Penn LZ, et al. Lysophosphatidic acid prevents apoptosis in fibroblasts via G(i)-protein-mediated activation of mitogen-activated protein kinase. Biochem J. 2000;352:135–143. [PMC free article] [PubMed] [Google Scholar]
- Fells JI, Tsukahara R, Fujiwara Y, Liu J, Perygin DH, Osborne DA, et al. Identification of non-lipid LPA3 antagonists by virtual screening. Bioorg Med Chem. 2008;16:6207–6217. doi: 10.1016/j.bmc.2008.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fells JI, Tsukahara R, Liu J, Tigyi G, Parrill AL. Structure-based drug design identifies novel LPA3 antagonists. Bioorg Med Chem. 2009;17:7457–7464. doi: 10.1016/j.bmc.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferry G, Moulharat N, Pradere JP, Desos P, Try A, Genton A, et al. S32826, a nanomolar inhibitor of autotaxin: discovery, synthesis and applications as a pharmacological tool. J Pharmacol Exp Ther. 2008;327:809–819. doi: 10.1124/jpet.108.141911. [DOI] [PubMed] [Google Scholar]
- Fischer DJ, Nusser N, Virag T, Yokoyama K, Wang D, Baker DL, et al. Short-chain phosphatidates are subtype-selective antagonists of lysophosphatidic acid receptors. Mol Pharmacol. 2001;60:776–784. [PubMed] [Google Scholar]
- Fishman DA, Liu Y, Ellerbroek SM, Stack MS. Lysophosphatidic acid promotes matrix metalloproteinase (MMP) activation and MMP-dependent invasion in ovarian cancer cells. Cancer Res. 2001;61:3194–3199. [PubMed] [Google Scholar]
- Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- Fotopoulou S, Oikonomou N, Grigrorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, et al. ATX expression and LPA signaling are vital for the development of the nervous system. Dev Biol. 2010;339:451–464. doi: 10.1016/j.ydbio.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Fox MA, Colello RJ, Macklin WB, Fuss B. Phosphodiesterase-Ialpha/autotaxin: a counteradhesive protein expressed by oligodendrocytes during onset of myelination. Mol Cell Neurosci. 2003;23:507–519. doi: 10.1016/s1044-7431(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Fox MA, Alexander JK, Afshari FS, Colello RJ, Fuss B. Phosphodiesterase-I alpha/autotaxin controls cytoskeletal organization and FAK phosphorylation during myelination. Mol Cell Neurosci. 2004;27:140–150. doi: 10.1016/j.mcn.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Friedman P, Haimovitz R, Markman O, Roberts MF, Shinitzky M. Conversion of lysophospholipids to cyclic lysophosphatidic acid by phospholipase D. J Biol Chem. 1996;271:953–957. doi: 10.1074/jbc.271.2.953. [DOI] [PubMed] [Google Scholar]
- Fueller M, Wang DA, Tigyi G, Siess W. Activation of human monocytic cells by lysophosphatidic acid and sphingosine-1-phosphate. Cell Signal. 2003;15:367–375. doi: 10.1016/s0898-6568(02)00117-1. [DOI] [PubMed] [Google Scholar]
- Fujita R, Kiguchi N, Ueda H. LPA-mediated demyelination in ex vivo culture of dorsal root. Neurochem Int. 2007;50:351–355. doi: 10.1016/j.neuint.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y. Cyclic phosphatidic acid – a unique bioactive phospholipid. Biochim Biophys Acta. 2008;1781:519–524. doi: 10.1016/j.bbalip.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, et al. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- Fuss B, Baba H, Phan T, Tuohy VK, Macklin WB. Phosphodiesterase I, a novel adhesion molecule and/or cytokine involved in oligodendrocyte function. J Neurosci. 1997;17:9095–9103. doi: 10.1523/JNEUROSCI.17-23-09095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg SK, Valente E, Greco E, Santucci MB, De Spirito M, Papi M, et al. Lysophosphatidic acid enhances antimycobacterial activity both in vitro and ex vivo. Clin Immunol. 2006;121:23–28. doi: 10.1016/j.clim.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Gil OD, Lee C, Ariztia EV, Wang FQ, Smith PJ, Hope JM, et al. Lysophosphatidic acid (LPA) promotes E-cadherin ectodomain shedding and OVCA429 cell invasion in an uPA-dependent manner. Gynecol Oncol. 2008;108:361–369. doi: 10.1016/j.ygyno.2007.10.027. [DOI] [PubMed] [Google Scholar]
- Glatt S, Halbauer D, Heindl S, Wernitznig A, Kozina D, Su KC, et al. hGPR87 contributes to viability of human tumor cells. Int J Cancer. 2008;122:2008–2016. doi: 10.1002/ijc.23349. [DOI] [PubMed] [Google Scholar]
- Gobeil F, Jr, Bernier SG, Vazquez-Tello A, Brault S, Beauchamp MH, Quiniou C, et al. Modulation of pro-inflammatory gene expression by nuclear lysophosphatidic acid receptor type-1. J Biol Chem. 2003;278:38875–38883. doi: 10.1074/jbc.M212481200. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Dolezalova H, Kong Y, Hu YL, Jaffe RB, Kalli KR, et al. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 1999a;59:5370–5375. [PubMed] [Google Scholar]
- Goetzl EJ, Kong Y, Mei B. Lysophosphatidic acid and sphingosine 1-phosphate protection of T cells from apoptosis in association with suppression of Bax. J Immunol. 1999b;162:2049–2056. [PubMed] [Google Scholar]
- Goetzl EJ, Kong Y, Voice JK. Cutting edge: differential constitutive expression of functional receptors for lysophosphatidic acid by human blood lymphocytes. J Immunol. 2000a;164:4996–4999. doi: 10.4049/jimmunol.164.10.4996. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ, Lee H, Azuma T, Stossel TP, Turck CW, Karliner JS. Gelsolin binding and cellular presentation of lysophosphatidic acid. J Biol Chem. 2000b;275:14573–14578. doi: 10.1074/jbc.275.19.14573. [DOI] [PubMed] [Google Scholar]
- Greco E, Santucci MB, Sali M, De Angelis FR, Papi M, De Spirito M, et al. Natural lysophospholipids reduce Mycobacterium tuberculosis-induced cytotoxicity and induce anti-mycobacterial activity by a phagolysosome maturation-dependent mechanism in A549 type II alveolar epithelial cells. Immunology. 2010;129:125–132. doi: 10.1111/j.1365-2567.2009.03145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey A, Banovic T, Naot D, Hill B, Callon K, Reid I, et al. Lysophosphatidic acid is an osteoblast mitogen whose proliferative actions involve G(i) proteins and protein kinase C, but not P42/44 mitogen-activated protein kinases. Endocrinology. 2001;142:1098–1106. doi: 10.1210/endo.142.3.8011. [DOI] [PubMed] [Google Scholar]
- Grey A, Chen Q, Callon K, Xu X, Reid IR, Cornish J. The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving G(i) proteins and phosphatidylinositol-3 kinase. Endocrinology. 2002;143:4755–4763. doi: 10.1210/en.2002-220347. [DOI] [PubMed] [Google Scholar]
- Gugger M, White R, Song S, Waser B, Cescato R, Riviere P, et al. GPR87 is an overexpressed G-protein coupled receptor in squamous cell carcinoma of the lung. Dis Markers. 2008;24:41–50. doi: 10.1155/2008/857474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R, Kasbohm EA, Arora P, Sample CJ, Baban B, Sud N, et al. Expression and function of lysophosphatidic acid LPA1 receptor in prostate cancer cells. Endocrinology. 2006;147:4883–4892. doi: 10.1210/en.2005-1635. [DOI] [PubMed] [Google Scholar]
- Hahn A, Heusinger-Ribeiro J, Lanz T, Zenkel S, Goppelt-Struebe M. Induction of connective tissue growth factor by activation of heptahelical receptors. Modulation by Rho proteins and the actin cytoskeleton. J Biol Chem. 2000;275:37429–37435. doi: 10.1074/jbc.M000976200. [DOI] [PubMed] [Google Scholar]
- Hama K, Aoki J, Inoue A, Endo T, Amano T, Motoki R, et al. Embryo spacing and implantation timing are differentially regulated by LPA3-mediated lysophosphatidic acid signaling in mice. Biol Reprod. 2007;77:954–959. doi: 10.1095/biolreprod.107.060293. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Takahashi M, Nishida W, Yoshida K, Ohkawa Y, Kitabatake A, et al. Phenotypic modulation of vascular smooth muscle cells induced by unsaturated lysophosphatidic acids. Circ Res. 2001;89:251–258. doi: 10.1161/hh1501.094265. [DOI] [PubMed] [Google Scholar]
- Hecht JH, Weiner JA, Post SR, Chun J. Ventricular Zone Gene-1 (vzg-1) Encodes a Lysophosphatidic Acid Receptor Expressed in Neurogenic Regions of the Developing Cerebral Cortex. J Cell Biol. 1996;135:1071–1083. doi: 10.1083/jcb.135.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise CE, Santos WL, Schreihofer AM, Heasley BH, Mukhin YV, Macdonald TL, et al. Activity of 2-substituted lysophosphatidic acid (LPA) analogs at LPA receptors: discovery of a LPA1/LPA3 receptor antagonist. Mol Pharmacol. 2001;60:1173–1180. doi: 10.1124/mol.60.6.1173. [DOI] [PubMed] [Google Scholar]
- Herr DR, Chun J. Effects of LPA and S1P on the nervous system and implications for their involvement in disease. Curr Drug Targets. 2007;8:155–167. doi: 10.2174/138945007779315669. [DOI] [PubMed] [Google Scholar]
- Hiramatsu T, Sonoda H, Takanezawa Y, Morikawa R, Ishida M, Kasahara K, et al. Biochemical and molecular characterization of two phosphatidic acid-selective phospholipase A1s, mPA-PLA1alpha and mPA-PLA1beta. J Biol Chem. 2003;278:49438–49447. doi: 10.1074/jbc.M213018200. [DOI] [PubMed] [Google Scholar]
- Hiramoto T, Nemoto W, Kikuchi T, Fujita N. Construction of hypothetical three-dimensional structure of P2Y1 receptor based on Fourier transform analysis. J Protein Chem. 2002;21:537–545. doi: 10.1023/a:1022429722651. [DOI] [PubMed] [Google Scholar]
- Hiramoto T, Nonaka Y, Inoue K, Yamamoto T, Omatsu-Kanbe M, Matsuura H, et al. Identification of endogenous surrogate ligands for human P2Y receptors through an in silico search. J Pharmacol Sci. 2004;95:81–93. doi: 10.1254/jphs.95.81. [DOI] [PubMed] [Google Scholar]
- Hope JM, Wang FQ, Whyte JS, Ariztia EV, Abdalla W, Long K, et al. LPA receptor 2 mediates LPA-induced endometrial cancer invasion. Gynecol Oncol. 2009;112:215–223. doi: 10.1016/j.ygyno.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Horigome K, Hayakawa M, Inoue K, Nojima S. Selective release of phospholipase A2 and lysophosphatidylserine-specific lysophospholipase from rat platelets. J Biochem. 1987;101:53–61. doi: 10.1093/oxfordjournals.jbchem.a121907. [DOI] [PubMed] [Google Scholar]
- Hornuss C, Hammermann R, Fuhrmann M, Juergens UR, Racke K. Human and rat alveolar macrophages express multiple EDG receptors. Eur J Pharmacol. 2001;429:303–308. doi: 10.1016/s0014-2999(01)01329-2. [DOI] [PubMed] [Google Scholar]
- Hosogaya S, Yatomi Y, Nakamura K, Ohkawa R, Okubo S, Yokota H, et al. Measurement of plasma lysophosphatidic acid concentration in healthy subjects: strong correlation with lysophospholipase D activity. Ann Clin Biochem. 2008;45(Pt 4):364–368. doi: 10.1258/acb.2008.007242. [DOI] [PubMed] [Google Scholar]
- Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001;93:762–768. doi: 10.1093/jnci/93.10.762. [DOI] [PubMed] [Google Scholar]
- Huang MC, Lee HY, Yeh CC, Kong Y, Zaloudek CJ, Goetzl EJ. Induction of protein growth factor systems in the ovaries of transgenic mice overexpressing human type 2 lysophosphatidic acid G protein-coupled receptor (LPA2) Oncogene. 2004;23:122–129. doi: 10.1038/sj.onc.1206986. [DOI] [PubMed] [Google Scholar]
- Hurst-Kennedy J, Boyan BD, Schwartz Z. Lysophosphatidic acid signaling promotes proliferation, differentiation, and cell survival in rat growth plate chondrocytes. Biochim Biophys Acta. 2009;1793:836–846. doi: 10.1016/j.bbamcr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Hwang YS, Hodge JC, Sivapurapu N, Lindholm PF. Lysophosphatidic acid stimulates PC-3 prostate cancer cell Matrigel invasion through activation of RhoA and NF-kappaB activity. Mol Carcinog. 2006;45:518–529. doi: 10.1002/mc.20183. [DOI] [PubMed] [Google Scholar]
- Idzko M, Laut M, Panther E, Sorichter S, Durk T, Fluhr JW, et al. Lysophosphatidic acid induces chemotaxis, oxygen radical production, CD11b up-regulation, Ca2+ mobilization, and actin reorganization in human eosinophils via pertussis toxin-sensitive G proteins. J Immunol. 2004;172:4480–4485. doi: 10.4049/jimmunol.172.7.4480. [DOI] [PubMed] [Google Scholar]
- Im DS, Heise CE, Harding MA, George SR, O'Dowd BF, Theodorescu D, et al. Molecular cloning and characterization of a lysophosphatidic acid receptor, Edg-7, expressed in prostate. Mol Pharmacol. 2000;57:753–759. [PubMed] [Google Scholar]
- Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H. Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med. 2004;10:712–718. doi: 10.1038/nm1060. [DOI] [PubMed] [Google Scholar]
- Inoue M, Ma L, Aoki J, Chun J, Ueda H. Autotaxin, a synthetic enzyme of lysophosphatidic acid (LPA), mediates the induction of nerve-injured neuropathic pain. Mol Pain. 2008a;4:6. doi: 10.1186/1744-8069-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M, Xie W, Matsushita Y, Chun J, Aoki J, Ueda H. Lysophosphatidylcholine induces neuropathic pain through an action of autotaxin to generate lysophosphatidic acid. Neuroscience. 2008b;152:296–298. doi: 10.1016/j.neuroscience.2007.12.041. [DOI] [PubMed] [Google Scholar]
- Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: signaling and biology. Annu Rev Biochem. 2004;73:321–354. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- Ishii S, Noguchi K, Yanagida K. Non-Edg family lysophosphatidic acid (LPA) receptors. Prostaglandins Other Lipid Mediat. 2009;89:57–65. doi: 10.1016/j.prostaglandins.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Jansen S, Andries M, Vekemans K, Vanbilloen H, Verbruggen A, Bollen M. Rapid clearance of the circulating metastatic factor autotaxin by the scavenger receptors of liver sinusoidal endothelial cells. Cancer Lett. 2009;284:216–221. doi: 10.1016/j.canlet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, et al. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005;11:6300–6310. doi: 10.1158/1078-0432.CCR-04-2682. [DOI] [PubMed] [Google Scholar]
- Jin Y, Knudsen E, Wang L, Maghazachi AA. Lysophosphatidic acid induces human natural killer cell chemotaxis and intracellular calcium mobilization. Eur J Immunol. 2003;33:2083–2089. doi: 10.1002/eji.200323711. [DOI] [PubMed] [Google Scholar]
- Jo SH, Kim SD, Kim JM, Lee HY, Lee SY, Shim JW, et al. Lysophosphatidylglycerol stimulates chemotactic migration in human natural killer cells. Biochem Biophys Res Commun. 2008;372:147–151. doi: 10.1016/j.bbrc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Kanda H, Newton R, Klein R, Morita Y, Gunn MD, Rosen SD. Autotaxin, an ectoenzyme that produces lysophosphatidic acid, promotes the entry of lymphocytes into secondary lymphoid organs. Nat Immunol. 2008;9:415–423. doi: 10.1038/ni1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiosis SA, Karin NJ. Lysophosphatidic acid induces osteocyte dendrite outgrowth. Biochem Biophys Res Commun. 2007;357:194–199. doi: 10.1016/j.bbrc.2007.03.121. [DOI] [PubMed] [Google Scholar]
- Kazantseva A, Goltsov A, Zinchenko R, Grigorenko AP, Abrukova AV, Moliaka YK, et al. Human hair growth deficiency is linked to a genetic defect in the phospholipase gene LIPH. Science. 2006;314:982–985. doi: 10.1126/science.1133276. [DOI] [PubMed] [Google Scholar]
- Khandoga AL, Fujiwara Y, Goyal P, Pandey D, Tsukahara R, Bolen A, et al. Lysophosphatidic acid-induced platelet shape change revealed through LPA(1-5) receptor-selective probes and albumin. Platelets. 2008;19:415–427. doi: 10.1080/09537100802220468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S, Tomar A, George SP, Wang Y, Siddiqui MR, Guo H, et al. Autotaxin and lysophosphatidic acid stimulate intestinal cell motility by redistribution of the actin modifying protein villin to the developing lamellipodia. Exp Cell Res. 2008;314:530–542. doi: 10.1016/j.yexcr.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- Kinloch RA, Cox PJ. New targets for neuropathic pain therapeutics. Expert Opin Ther Targets. 2005;9:685–698. doi: 10.1517/14728222.9.4.685. [DOI] [PubMed] [Google Scholar]
- Kitamura H, Makide K, Okutani M, Arima N, Inoue A, Aoki J. Identification of a novel lysophosphatidylserine receptor. In: Arai H, editor. 4th International Conference on Phospholipase A2 and Lipid Mediators. Tokyo: Japan Science and Technology Agency; 2009. p. 132. [Google Scholar]
- Kobayashi T, Yamano S, Murayama S, Ishikawa H, Tokumura A, Aono T. Effect of lysophosphatidic acid on the preimplantation development of mouse embryos. FEBS Lett. 1994;351:38–40. doi: 10.1016/0014-5793(94)00815-9. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Tanaka-Ishii R, Taguchi R, Ikezawa H, Murakami-Murofushi K. Existence of a Bioactive Lipid, Cyclic Phosphatidic Acid in Human Serum. Life Sci. 1999;56:245–253. doi: 10.1016/s0024-3205(99)00483-x. [DOI] [PubMed] [Google Scholar]
- Koh JS, Lieberthal W, Heydrick S, Levine JS. Lysophosphatidic acid is a major serum noncytokine survival factor for murine macrophages which acts via the phosphatidylinositol 3-kinase signaling pathway. J Clin Invest. 1998;102:716–727. doi: 10.1172/JCI1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Keino-Masu K, Ohto T, Sugiyama F, Takahashi S, Masu M. Autotaxin/lysophospholipase d-mediated lysophosphatidic acid signaling is required to form distinctive large lysosomes in the visceral endoderm cells of the mouse yolk sac. J Biol Chem. 2009;284:33561–33570. doi: 10.1074/jbc.M109.012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, et al. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- Kunikata K, Yamano S, Tokumura A, Aono T. Effect of lysophosphatidic acid on the ovum transport in mouse oviducts. Life Sci. 1999;65:833–840. doi: 10.1016/s0024-3205(99)00310-0. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Thangada S, Liu CH, Thompson BD, Hla T. Lysophosphatidic acid stimulates the G-protein-coupled receptor EDG-1 as a low affinity agonist. J Biol Chem. 1998;273:22105–22112. doi: 10.1074/jbc.273.34.22105. [DOI] [PubMed] [Google Scholar]
- Lee H, Liao JJ, Graeler M, Huang MC, Goetzl EJ. Lysophospholipid regulation of mononuclear phagocytes. Biochim Biophys Acta. 2002;1582:175–177. doi: 10.1016/s1388-1981(02)00153-1. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282:4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- Lee Z, Cheng CT, Zhang H, Subler MA, Wu J, Mukherjee A, et al. Role of LPA4/p2y9/GPR23 in negative regulation of cell motility. Mol Biol Cell. 2008;19:5435–5445. doi: 10.1091/mbc.E08-03-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CS, Kwon YW, Yang HM, Kim SH, Kim TY, Hur J, et al. New mechanism of rosiglitazone to reduce neointimal hyperplasia: activation of glycogen synthase kinase-3beta followed by inhibition of MMP-9. Arterioscler Thromb Vasc Biol. 2009;29:472–479. doi: 10.1161/ATVBAHA.108.176230. [DOI] [PubMed] [Google Scholar]
- Lee-Kwon W, Kawano K, Choi JW, Kim JH, Donowitz M. Lysophosphatidic acid stimulates brush border Na+/H+ exchanger 3 (NHE3) activity by increasing its exocytosis by an NHE3 kinase A regulatory protein-dependent mechanism. J Biol Chem. 2003;278:16494–16501. doi: 10.1074/jbc.M300580200. [DOI] [PubMed] [Google Scholar]
- Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, et al. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie DS, Dascher CC, Cembrola K, Townes MA, Hava DL, Hugendubler LC, et al. Serum lipids regulate dendritic cell CD1 expression and function. Immunology. 2008;125:289–301. doi: 10.1111/j.1365-2567.2008.02842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AC, Glass CK. The macrophage foam cell as a target for therapeutic intervention. Nat Med. 2002;8:1235–1242. doi: 10.1038/nm1102-1235. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang J. Lipopolysaccharide induces autotaxin expression in human monocytic THP-1 cells. Biochem Biophys Res Commun. 2009;378:264–268. doi: 10.1016/j.bbrc.2008.11.047. [DOI] [PubMed] [Google Scholar]
- Li AC, Brown KK, Silvestre MJ, Willson TM, Palinski W, Glass CK. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gonzalez MI, Meinkoth JL, Field J, Kazanietz MG, Tennekoon GI. Lysophosphatidic acid promotes survival and differentiation of rat Schwann cells. J Biol Chem. 2003;278:9585–9591. doi: 10.1074/jbc.M213244200. [DOI] [PubMed] [Google Scholar]
- Liliom K, Fischer DJ, Virag T, Sun G, Miller DD, Tseng JL, et al. Identification of a novel growth factor-like lipid, 1-O-cis-alk-1′-enyl-2-lyso-sn-glycero-3-phosphate (alkenyl-GP) that is present in commercial sphingolipid preparations. J Biol Chem. 1998;273:13461–13468. doi: 10.1074/jbc.273.22.13461. [DOI] [PubMed] [Google Scholar]
- Liliom K, Tsukahara T, Tsukahara R, Zelman-Femiak M, Swiezewska E, Tigyi G. Farnesyl phosphates are endogenous ligands of lysophosphatidic acid receptors: inhibition of LPA GPCR and activation of PPARs. Biochim Biophys Acta. 2006;1761:1506–1514. doi: 10.1016/j.bbalip.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S, Jin CJ, Kim M, Chung SS, Park HS, Lee IK, et al. PPARgamma gene transfer sustains apoptosis, inhibits vascular smooth muscle cell proliferation, and reduces neointima formation after balloon injury in rats. Arterioscler Thromb Vasc Biol. 2006;26:808–813. doi: 10.1161/01.ATV.0000204634.26163.a7. [DOI] [PubMed] [Google Scholar]
- Lin DA, Boyce JA. IL-4 regulates MEK expression required for lysophosphatidic acid-mediated chemokine generation by human mast cells. J Immunol. 2005;175:5430–5438. doi: 10.4049/jimmunol.175.8.5430. [DOI] [PubMed] [Google Scholar]
- Lin CI, Chen CN, Chen JH, Lee H. Lysophospholipids increase IL-8 and MCP-1 expressions in human umbilical cord vein endothelial cells through an IL-1-dependent mechanism. J Cell Biochem. 2006;99:1216–1232. doi: 10.1002/jcb.20963. [DOI] [PubMed] [Google Scholar]
- Lin FT, Lai YJ, Makarova N, Tigyi G, Lin WC. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J Biol Chem. 2007;282:37759–37769. doi: 10.1074/jbc.M705025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Umezu-Goto M, Murph M, Lu Y, Liu W, Zhang F, et al. Expression of autotaxin and lysophosphatidic acid receptors increases mammary tumorigenesis, invasion, and metastases. Cancer Cell. 2009;15:539–550. doi: 10.1016/j.ccr.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llodra J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons JM, Karin NJ. A role for G protein-coupled lysophospholipid receptors in sphingolipid-induced Ca2+ signaling in MC3T3-E1 osteoblastic cells. J Bone Miner Res. 2001;16:2035–2042. doi: 10.1359/jbmr.2001.16.11.2035. [DOI] [PubMed] [Google Scholar]
- Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, et al. Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol Pain. 2009;5:64. doi: 10.1186/1744-8069-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre TM, Pontsler AV, Silva AR, St Hilaire A, Xu Y, Hinshaw JC, et al. Identification of an intracellular receptor for lysophosphatidic acid (LPA): LPA is a transcellular PPARgamma agonist. Proc Natl Acad Sci USA. 2003;100:131–136. doi: 10.1073/pnas.0135855100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghazachi AA. G protein-coupled receptors in natural killer cells. J Leukoc Biol. 2003;74:16–24. doi: 10.1189/jlb.0103019. [DOI] [PubMed] [Google Scholar]
- Matas-Rico E, Garcia-Diaz B, Llebrez-Zayas P, Lopez-Barroso D, Santin L, Pedraza C, et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol Cell Neurosci. 2008;39:342–355. doi: 10.1016/j.mcn.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerschaert K, De Corte V, De Ville Y, Vandekerckhove J, Gettemans J. Gelsolin and functionally similar actin-binding proteins are regulated by lysophosphatidic acid. EMBO J. 1998;17:5923–5932. doi: 10.1093/emboj/17.20.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Christodoulou E, Goding JW, Takakusa H, Kikuchi K, et al. Inhibition of autotaxin by lysophosphatidic acid and sphingosine 1-phosphate. J Biol Chem. 2005;280:21155–21161. doi: 10.1074/jbc.M413183200. [DOI] [PubMed] [Google Scholar]
- van Meeteren LA, Ruurs P, Stortelers C, Bouwman P, van Rooijen MA, Pradere JP, et al. Autotaxin, a secreted lysophospholipase D, is essential for blood vessel formation during development. Mol Cell Biol. 2006;26:5015–5022. doi: 10.1128/MCB.02419-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Brinkmann V, Saulnier-Blache JS, Lynch KR, Moolenaar WH. Anticancer activity of FTY720: phosphorylated FTY720 inhibits autotaxin, a metastasis-enhancing and angiogenic lysophospholipase D. Cancer Lett. 2008;266:203–208. doi: 10.1016/j.canlet.2008.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Y, Graves L, Do TV, So J, Fishman DA. Upregulation of FasL by LPA on ovarian cancer cell surface leads to apoptosis of activated lymphocytes. Gynecol Oncol. 2004;95:488–495. doi: 10.1016/j.ygyno.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Mintzer E, Sargsyan H, Bittman R. Lysophosphatidic acid and lipopolysaccharide bind to the PIP2-binding domain of gelsolin. Biochim Biophys Acta. 2006;1758:85–89. doi: 10.1016/j.bbamem.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Mishra RS, Carnevale KA, Cathcart MK. iPLA2beta: front and center in human monocyte chemotaxis to MCP-1. J Exp Med. 2008;205:347–359. doi: 10.1084/jem.20071243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller T, Musante DB, Ransom BR. Lysophosphatidic acid-induced calcium signals in cultured rat oligodendrocytes. Neuroreport. 1999;10:2929–2932. doi: 10.1097/00001756-199909290-00010. [DOI] [PubMed] [Google Scholar]
- Muehlich S, Schneider N, Hinkmann F, Garlichs CD, Goppelt-Struebe M. Induction of connective tissue growth factor (CTGF) in human endothelial cells by lysophosphatidic acid, sphingosine-1-phosphate, and platelets. Atherosclerosis. 2004;175:261–268. doi: 10.1016/j.atherosclerosis.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- Murakami M, Shiraishi A, Tabata K, Fujita N. Identification of the orphan GPCR, P2Y(10) receptor as the sphingosine-1-phosphate and lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2008;371:707–712. doi: 10.1016/j.bbrc.2008.04.145. [DOI] [PubMed] [Google Scholar]
- Murakami-Murofushi K, Kaji K, Kano K, Fukuda M, Shioda M, Murofushi H. Inhibition of cell proliferation by a unique lysophosphatidic acid, PHYLPA, isolated from Physarum polycephalum: signaling events of antiproliferative action by PHYLPA. Cell Struct Funct. 1993;18:363–370. doi: 10.1247/csf.18.363. [DOI] [PubMed] [Google Scholar]
- Murph MM, Nguyen GH, Radhakrishna H, Mills GB. Sharpening the edges of understanding the structure/function of the LPA1 receptor: expression in cancer and mechanisms of regulation. Biochim Biophys Acta. 2008;1781:547–557. doi: 10.1016/j.bbalip.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthi P, Barker G, Nowell CJ, Rice GE, Baker MS, Kalionis B, et al. Plasminogen fragmentation and increased production of extracellular matrix-degrading proteinases are associated with serous epithelial ovarian cancer progression. Gynecol Oncol. 2004;92:80–88. doi: 10.1016/j.ygyno.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Mutoh T, Chun J. Lysophospholipid activation of G protein-coupled receptors. Subcell Biochem. 2008;49:269–297. doi: 10.1007/978-1-4020-8831-5_10. [DOI] [PubMed] [Google Scholar]
- Nagle CA, Klett EL, Coleman RA. Hepatic triacylglycerol accumulation and insulin resistance. J Lipid Res. 2009;50(Suppl):S74–S79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- Nakane S, Tokumura A, Waku K, Sugiura T. Hen egg yolk and white contain high amounts of lysophosphatidic acids, growth factor-like lipids: distinct molecular species compositions. Lipids. 2001;36:413–419. doi: 10.1007/s11745-001-0737-1. [DOI] [PubMed] [Google Scholar]
- Nakasaki T, Tanaka T, Okudaira S, Hirosawa M, Umemoto E, Otani K, et al. Involvement of the lysophosphatidic acid-generating enzyme autotaxin in lymphocyte-endothelial cell interactions. Am J Pathol. 2008;173:1566–1576. doi: 10.2353/ajpath.2008.071153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Nogaroli L, Yuelling LM, Dennis J, Gorse K, Payne SG, Fuss B. Lysophosphatidic acid can support the formation of membranous structures and an increase in MBP mRNA levels in differentiating oligodendrocytes. Neurochem Res. 2009;34:182–193. doi: 10.1007/s11064-008-9772-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Herr D, Mutoh T, Chun J. Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol. 2009;9:15–23. doi: 10.1016/j.coph.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Hiramoto T, Fujita N. Identification of endogenous surrogate ligands for human P2Y12 receptors by in silico and in vitro methods. Biochem Biophys Res Commun. 2005;337:281–288. doi: 10.1016/j.bbrc.2005.09.052. [DOI] [PubMed] [Google Scholar]
- North EJ, Osborne DA, Bridson PK, Baker DL, Parrill AL. Autotaxin structure-activity relationships revealed through lysophosphatidylcholine analogs. Bioorg Med Chem. 2009;17:3433–3442. doi: 10.1016/j.bmc.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh da Y, Yoon JM, Moon MJ, Hwang JI, Choe H, Lee JY, et al. Identification of farnesyl pyrophosphate and N-arachidonylglycine as endogenous ligands for GPR92. J Biol Chem. 2008;283:21054–21064. doi: 10.1074/jbc.M708908200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta H, Sato K, Murata N, Damirin A, Malchinkhuu E, Kon J, et al. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol Pharmacol. 2003;64:994–1005. doi: 10.1124/mol.64.4.994. [DOI] [PubMed] [Google Scholar]
- Oka S, Ota R, Shima M, Yamashita A, Sugiura T. GPR35 is a novel lysophosphatidic acid receptor. BBBRC. 2010;395:232–237. doi: 10.1016/j.bbrc.2010.03.169. [DOI] [PubMed] [Google Scholar]
- Pamuklar Z, Lee JS, Cheng HY, Panchatcharam M, Steinhubl S, Morris AJ, et al. Individual heterogeneity in platelet response to lysophosphatidic acid: evidence for a novel inhibitory pathway. Arterioscler Thromb Vasc Biol. 2008;28:555–561. doi: 10.1161/ATVBAHA.107.151837. [DOI] [PubMed] [Google Scholar]
- Pamuklar Z, Federico L, Liu S, Umezu-Goto M, Dong A, Panchatcharam M, et al. Autotaxin/lysopholipase D and lysophosphatidic acid regulate murine hemostasis and thrombosis. J Biol Chem. 2009;284:7385–7394. doi: 10.1074/jbc.M807820200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panther E, Idzko M, Corinti S, Ferrari D, Herouy Y, Mockenhaupt M, et al. The influence of lysophosphatidic acid on the functions of human dendritic cells. J Immunol. 2002;169:4129–4135. doi: 10.4049/jimmunol.169.8.4129. [DOI] [PubMed] [Google Scholar]
- Parrill AL, Baker DL. Autotaxin inhibition: challenges and progress toward novel anti-cancer agents. Anticancer Agents Med Chem. 2008;8:917–923. doi: 10.2174/187152008786847765. [DOI] [PubMed] [Google Scholar]
- Parrill AL, Echols U, Nguyen T, Pham TC, Hoeglund A, Baker DL. Virtual screening approaches for the identification of non-lipid autotaxin inhibitors. Bioorg Med Chem. 2008;16:1784–1795. doi: 10.1016/j.bmc.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, et al. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- Pasternack SM, von Kugelgen I, Muller M, Oji V, Traupe H, Sprecher E, et al. In Vitro Analysis of LIPH Mutations Causing Hypotrichosis Simplex: Evidence Confirming the Role of Lipase H and Lysophosphatidic Acid in Hair Growth. J Invest Dermatol. 2009;129:2772–2776. doi: 10.1038/jid.2009.154. [DOI] [PubMed] [Google Scholar]
- Perova T, Wasserman MJ, Li PP, Warsh JJ. Hyperactive intracellular calcium dynamics in B lymphoblasts from patients with bipolar I disorder. Int J Neuropsychopharmacol. 2008;11:185–196. doi: 10.1017/S1461145707007973. [DOI] [PubMed] [Google Scholar]
- Perova T, Kwan M, Li PP, Warsh JJ. Differential modulation of intracellular Ca2+ responses in B lymphoblasts by mood stabilizers. Int J Neuropsychopharmacol. 2009;29:1–10. doi: 10.1017/S1461145709000261. [DOI] [PubMed] [Google Scholar]
- Peyruchaud O. Novel implications for lysophospholipids, lysophosphatidic acid and sphingosine 1-phosphate, as drug targets in cancer. Anticancer Agents Med Chem. 2009;9:381–391. doi: 10.2174/1871520610909040381. [DOI] [PubMed] [Google Scholar]
- Pradere JP, Klein J, Gres S, Guigne C, Neau E, Valet P, et al. LPA1 receptor activation promotes renal interstitial fibrosis. J Am Soc Nephrol. 2007;18:3110–3118. doi: 10.1681/ASN.2007020196. [DOI] [PubMed] [Google Scholar]
- Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature. 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- Ptaszynska MM, Pendrak ML, Bandle RW, Stracke ML, Roberts DD. Positive feedback between vascular endothelial growth factor-A and autotaxin in ovarian cancer cells. Mol Cancer Res. 2008;6:352–363. doi: 10.1158/1541-7786.MCR-07-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999;5:3704–3710. [PubMed] [Google Scholar]
- Qi C, Park JH, Gibbs TC, Shirley DW, Bradshaw CD, Ella KM, et al. Lysophosphatidic acid stimulates phospholipase D activity and cell proliferation in PC-3 human prostate cancer cells. J Cell Physiol. 1998;174:261–272. doi: 10.1002/(SICI)1097-4652(199802)174:2<261::AID-JCP13>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Rao S, Garrett-Sinha LA, Yoon J, Simon MC. The Ets factors PU.1 and Spi-B regulate the transcription in vivo of P2Y10, a lymphoid restricted heptahelical receptor. J Biol Chem. 1999;274:34245–34252. doi: 10.1074/jbc.274.48.34245. [DOI] [PubMed] [Google Scholar]
- Retzer M, Essler M. Lysophosphatidic acid-induced platelet shape change proceeds via Rho/Rho kinase-mediated myosin light-chain and moesin phosphorylation. Cell Signal. 2000;12:645–648. doi: 10.1016/s0898-6568(00)00108-x. [DOI] [PubMed] [Google Scholar]
- Retzer M, Siess W, Essler M. Mildly oxidised low density lipoprotein induces platelet shape change via Rho-kinase-dependent phosphorylation of myosin light chain and moesin. FEBS Lett. 2000;466:70–74. doi: 10.1016/s0014-5793(99)01762-7. [DOI] [PubMed] [Google Scholar]
- Ricote M, Glass CK. PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta. 2007;1771:926–935. doi: 10.1016/j.bbalip.2007.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J. 1994;13:2600–2610. doi: 10.1002/j.1460-2075.1994.tb06550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- Rizza C, Leitinger N, Yue J, Fischer DJ, Wang DA, Shih PT, et al. Lysophosphatidic acid as a regulator of endothelial/leukocyte interaction. Lab Invest. 1999;79:1227–1235. [PubMed] [Google Scholar]
- Rosskopf D, Daelman W, Busch S, Schurks M, Hartung K, Kribben A, et al. Growth factor-like action of lysophosphatidic acid on human B lymphoblasts. Am J Physiol. 1998;274(6 Pt 1):C1573–C1582. doi: 10.1152/ajpcell.1998.274.6.C1573. [DOI] [PubMed] [Google Scholar]
- Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, et al. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- Roura-Mir C, Catalfamo M, Cheng TY, Marqusee E, Besra GS, Jaraquemada D, et al. CD1a and CD1c activate intrathyroidal T cells during Graves' disease and Hashimoto's thyroiditis. J Immunol. 2005;174:3773–3780. doi: 10.4049/jimmunol.174.6.3773. [DOI] [PubMed] [Google Scholar]
- Rubenfeld J, Guo J, Sookrung N, Chen R, Chaicumpa W, Casolaro V, et al. Lysophosphatidic acid enhances interleukin-13 gene expression and promoter activity in T cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L66–L74. doi: 10.1152/ajplung.00473.2004. [DOI] [PubMed] [Google Scholar]
- Samadi N, Gaetano C, Goping IS, Brindley DN. Autotaxin protects MCF-7 breast cancer and MDA-MB-435 melanoma cells against Taxol-induced apoptosis. Oncogene. 2009;28:1028–1039. doi: 10.1038/onc.2008.442. [DOI] [PubMed] [Google Scholar]
- Sando JJ, Chertihin OI. Activation of protein kinase C by lysophosphatidic acid: dependence on composition of phospholipid vesicles. Biochem J. 1996;317(Pt 2):583–588. doi: 10.1042/bj3170583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, et al. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, et al. Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nat Med. 1998;4:1046–1052. doi: 10.1038/2030. [DOI] [PubMed] [Google Scholar]
- Sato T, Aoki J, Nagai Y, Dohmae N, Takio K, Doi T, et al. Serine phospholipid-specific phospholipase A that is secreted from activated platelets. A new member of the lipase family. J Biol Chem. 1997;272:2192–2198. doi: 10.1074/jbc.272.4.2192. [DOI] [PubMed] [Google Scholar]
- Satoh Y, Ohkawa R, Nakamura K, Higashi K, Kaneko M, Yokota H, et al. Lysophosphatidic acid protection against apoptosis in the human pre-B-cell line Nalm-6. Eur J Haematol. 2007;78:510–517. doi: 10.1111/j.1600-0609.2007.00849.x. [DOI] [PubMed] [Google Scholar]
- Schmalfeldt B, Prechtel D, Harting K, Spathe K, Rutke S, Konik E, et al. Increased expression of matrix metalloproteinases (MMP)-2, MMP-9, and the urokinase-type plasminogen activator is associated with progression from benign to advanced ovarian cancer. Clin Cancer Res. 2001;7:2396–2404. [PubMed] [Google Scholar]
- Schulze C, Smales C, Rubin LL, Staddon JM. Lysophosphatidic acid increases tight junction permeability in cultured brain endothelial cells. J Neurochem. 1997;68:991–1000. doi: 10.1046/j.1471-4159.1997.68030991.x. [DOI] [PubMed] [Google Scholar]
- Schumacher KA, Classen HG, Spath M. Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: identity with substances in aged serum (DAS) Thromb Haemost. 1979;42:631–640. [PubMed] [Google Scholar]
- Sheardown M, Messager S, Mathews E, Dickenson AH, Aparicio S, Brice NL. Society of Neuroscience Abstracts. Washington, DC: Society for Neuroscience; 2004. Knockout of GPRr92 reveals key role in neuropathic and acute pain; p. 64.16. [Google Scholar]
- Shimomura Y, Wajid M, Ishii Y, Shapiro L, Petukhova L, Gordon D, et al. Disruption of P2RY5, an orphan G protein-coupled receptor, underlies autosomal recessive woolly hair. Nat Genet. 2008;40:335–339. doi: 10.1038/ng.100. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Wajid M, Petukhova L, Shapiro L, Christiano AM. Mutations in the lipase H gene underlie autosomal recessive woolly hair/hypotrichosis. J Invest Dermatol. 2009a;129:622–628. doi: 10.1038/jid.2008.290. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Wajid M, Zlotogorski A, Lee YJ, Rice RH, Christiano AM. Founder mutations in the lipase h gene in families with autosomal recessive woolly hair/hypotrichosis. J Invest Dermatol. 2009b;129:1927–1934. doi: 10.1038/jid.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieling PA, Porcelli SA, Duong BT, Spada F, Bloom BR, Diamond B, et al. Human double-negative T cells in systemic lupus erythematosus provide help for IgG and are restricted by CD1c. J Immunol. 2000;165:5338–5344. doi: 10.4049/jimmunol.165.9.5338. [DOI] [PubMed] [Google Scholar]
- Siess W. Platelet interaction with bioactive lipids formed by mild oxidation of low-density lipoprotein. Pathophysiol Haemost Thromb. 2006;35:292–304. doi: 10.1159/000093222. [DOI] [PubMed] [Google Scholar]
- Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, et al. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci USA. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MF, Chap H, Douste-Blazy L. Human platelet aggregation induced by 1-alkyl-lysophosphatidic acid and its analogs: a new group of phospholipid mediators? Biochem Biophys Res Commun. 1982;108:1743–1750. doi: 10.1016/s0006-291x(82)80113-7. [DOI] [PubMed] [Google Scholar]
- Simon MF, Chap H, Douste-Blazy L. Platelet aggregating activity of lysophosphatidic acids is not related to their calcium ionophore properties. FEBS Lett. 1984;166:115–119. doi: 10.1016/0014-5793(84)80055-1. [DOI] [PubMed] [Google Scholar]
- So J, Navari J, Wang FQ, Fishman DA. Lysophosphatidic acid enhances epithelial ovarian carcinoma invasion through the increased expression of interleukin-8. Gynecol Oncol. 2004;95:314–322. doi: 10.1016/j.ygyno.2004.08.001. [DOI] [PubMed] [Google Scholar]
- So J, Wang FQ, Navari J, Schreher J, Fishman DA. LPA-induced epithelial ovarian cancer (EOC) in vitro invasion and migration are mediated by VEGF receptor-2 (VEGF-R2) Gynecol Oncol. 2005;97:870–878. doi: 10.1016/j.ygyno.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, et al. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem. 2002;277:34254–34263. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- Stracke ML, Krutzsch HC, Unsworth EJ, Arestad A, Cioce V, Schiffmann E, et al. Identification, purification, and partial sequence analysis of autotaxin, a novel motility-stimulating protein. J Biol Chem. 1992;267:2524–2529. [PubMed] [Google Scholar]
- Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A, et al. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim Biophys Acta. 1999;1440:194–204. doi: 10.1016/s1388-1981(99)00127-4. [DOI] [PubMed] [Google Scholar]
- Sugo T, Tachimoto H, Chikatsu T, Murakami Y, Kikukawa Y, Sato S, et al. Identification of a lysophosphatidylserine receptor on mast cells. Biochem Biophys Res Commun. 2006;341:1078–1087. doi: 10.1016/j.bbrc.2006.01.069. [DOI] [PubMed] [Google Scholar]
- Sutphen R, Xu Y, Wilbanks GD, Fiorica J, Grendys EC, Jr, LaPolla JP, et al. Lysophospholipids are potential biomarkers of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1185–1191. [PubMed] [Google Scholar]
- Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Okudaira S, Kishi Y, Ohkawa R, Iseki S, Ota M, et al. Autotaxin stabilizes blood vessels and is required for embryonic vasculature by producing lysophosphatidic acid. J Biol Chem. 2006;281:25822–25830. doi: 10.1074/jbc.M605142200. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- Tigyi G, Parrill AL. Molecular mechanisms of lysophosphatidic acid action. Prog Lipid Res. 2003;42:498–526. doi: 10.1016/s0163-7827(03)00035-3. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Dyer D, Matute C, Miledi R. A serum factor that activates the phosphatidylinositol phosphate signaling system in Xenopus oocytes. Proc Natl Acad Sci USA. 1990;87:1521–1525. doi: 10.1073/pnas.87.4.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumura A, Fukuzawa K, Akamatsu Y, Yamada S, Suzuki T, Tsukatani H. Identification of vasopressor phospholipid in crude soybean lecithin. Lipids. 1978a;13:468–472. doi: 10.1007/BF02533615. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Fukuzawa K, Tsukatani H. Effects of synthetic and natural lysophosphatidic acids on the arterial blood pressure of different animal species. Lipids. 1978b;13:572–574. doi: 10.1007/BF02533598. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Fukuzawa K, Yamada S, Tsukatani H. Stimulatory effect of lysophosphatidic acids on uterine smooth muscles of non-pregant rats. Arch Inter Pharmacodyn Ther. 1980;245:74–83. [PubMed] [Google Scholar]
- Tokumura A, Iimori M, Nishioka Y, Kitahara M, Sakashita M, Tanaka S. Lysophosphatidic acids induce proliferation of cultured vascular smooth muscle cells from rat aorta. Am J Physiol. 1994;267(1 Pt 1):C204–C210. doi: 10.1152/ajpcell.1994.267.1.C204. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Miyake M, Nishioka Y, Yamano S, Aono T, Fukuzawa K. Production of lysophosphatidic acids by lysophospholipase D in human follicular fluids of in vitro fertilization patients. Biol Reprod. 1999;61:159–199. doi: 10.1095/biolreprod61.1.195. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, et al. Identification of Human Plasma Lysophospholipase D, a Lysophosphatidic acid-producing Enzyme, as Autotaxin, a Multifunctional Phosphodiesterase. J Biol Chem. 2002a;277:39436–39442. doi: 10.1074/jbc.M205623200. [DOI] [PubMed] [Google Scholar]
- Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T, et al. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. Biochem J. 2002b;365(Pt 3):617–628. doi: 10.1042/BJ20020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumura A, Carbone LD, Yoshioka Y, Morishige J, Kikuchi M, Postlethwaite A, et al. Elevated serum levels of arachidonoyl-lysophosphatidic acid and sphingosine 1-phosphate in systemic sclerosis. Int J Med Sci. 2009;6:168–176. doi: 10.7150/ijms.6.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar A, George SP, Mathew S, Khurana S. Differential effects of lysophosphatidic acid and phosphatidylinositol 4,5-bisphosphate on actin dynamics by direct association with the actin-binding protein villin. J Biol Chem. 2009;284:35278–35282. doi: 10.1074/jbc.C109.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MH, Hall A, Stacey DW. Inhibition by phospholipids of the interaction between R-ras, rho, and their GTPase-activating proteins. Mol Cell Biol. 1989;9:5260–5264. doi: 10.1128/mcb.9.11.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda S, Okudaira S, Moriya-Ito K, Shimamoto C, Tanaka M, Aoki J, et al. Cyclic phosphatidic acid is produced by autotaxin in blood. J Biol Chem. 2006;281:26081–26088. doi: 10.1074/jbc.M602925200. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Tsukahara R, Yasuda S, Makarova N, Valentine WJ, Allison P, et al. Different residues mediate recognition of 1-O-oleyllysophosphatidic acid and rosiglitazone in the ligand binding domain of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:3398–3407. doi: 10.1074/jbc.M510843200. [DOI] [PubMed] [Google Scholar]
- Tsukahara T, Tsukahara R, Fujiwara Y, Yue J, Cheng Y, Guo H, et al. Phospholipase D2-dependent Inhibition of the Nuclear Hormone Receptor PPARγ by Cyclic Phosphatidic Acid. Mol Cell. 2010;38 doi: 10.1016/j.molcel.2010.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome M, Nishio M, Ito M, Tanahashi S, Kawano M, Komada H, et al. Effects of hemagglutinin-neuraminidase protein mutations on cell-cell fusion mediated by human parainfluenza type 2 virus. J Virol. 2008;82:8283–8295. doi: 10.1128/JVI.00460-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama A, Mukai M, Fujiwara Y, Kobayashi S, Kawai N, Murofushi H, et al. Inhibition of transcellular tumor cell migration and metastasis by novel carba-derivatives of cyclic phosphatidic acid. Biochim Biophys Acta. 2007;1771:103–112. doi: 10.1016/j.bbalip.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H. Molecular mechanisms of neuropathic pain-phenotypic switch and initiation mechanisms. Pharmacol Ther. 2006;109:57–77. doi: 10.1016/j.pharmthera.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine WJ, Fells JI, Perygin DH, Mujahid S, Yokoyama K, Fujiwara Y, et al. Subtype-specific residues involved in ligand activation of the endothelial differentiation gene family lysophosphatidic acid receptors. J Biol Chem. 2008a;283:12175–12187. doi: 10.1074/jbc.M708847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine WJ, Fujiwara Y, Tsukahara R, Tigyi G. Lysophospholipid signaling: beyond the EDGs. Biochim Biophys Acta. 2008b;1780:597–605. doi: 10.1016/j.bbagen.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Zuniga LM, Cabello-Verrugio C, Canon P, Fadic R, Brandan E. Skeletal muscle cells express the profibrotic cytokine connective tissue growth factor (CTGF/CCN2), which induces their dedifferentiation. J Cell Physiol. 2008;215:410–421. doi: 10.1002/jcp.21324. [DOI] [PubMed] [Google Scholar]
- Vidot S, Witham J, Agarwal R, Greenhough S, Bamrah HS, Tigyi GJ, et al. Autotaxin delays apoptosis induced by carboplatin in ovarian cancer cells. Cell Signal. 2010;22:926–935. doi: 10.1016/j.cellsig.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Vincent MS, Leslie DS, Gumperz JE, Xiong X, Grant EP, Brenner MB. CD1-dependent dendritic cell instruction. Nat Immunol. 2002;3:1163–1168. doi: 10.1038/ni851. [DOI] [PubMed] [Google Scholar]
- Vincent MS, Gumperz JE, Brenner MB. Understanding the function of CD1-restricted T cells. Nat Immunol. 2003;4:517–523. doi: 10.1038/ni0603-517. [DOI] [PubMed] [Google Scholar]
- Wallace VC, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Loo R, Chen Z, Dennis EA. Regiospecificity and catalytic triad of lysophospholipase I. J Biol Chem. 1997;272:12723–12729. doi: 10.1074/jbc.272.35.22030. [DOI] [PubMed] [Google Scholar]
- Wang DA, Du H, Jaggar JH, Brindley DN, Tigyi GJ, Watsky MA. Injury-elicited differential transcriptional regulation of phospholipid growth factor receptors in the cornea. Am J Physiol Cell Physiol. 2002;283:C1646–C1654. doi: 10.1152/ajpcell.00323.2002. [DOI] [PubMed] [Google Scholar]
- Wasserman MJ, Corson TW, Sibony D, Cooke RG, Parikh SV, Pennefather PS, et al. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology. 2004;29:759–769. doi: 10.1038/sj.npp.1300400. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007a;41:616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Tomiya T, et al. Plasma lysophosphatidic acid level and serum autotaxin activity are increased in liver injury in rats in relation to its severity. Life Sci. 2007b;81:1009–1015. doi: 10.1016/j.lfs.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Weiner JA, Hecht JH, Chun J. Lysophosphatidic acid receptor gene vzg-1/lpA1/edg-2 is expressed by mature oligodendrocytes during myelination in the postnatal murine brain. J Comp Neurol. 1998;398:587–598. [PubMed] [Google Scholar]
- Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: Rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta. 2009;1791:501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter JA, Revankar C, Hanson BJ. Utilization of the Tango beta-arrestin recruitment technology for cell-based EDG receptor assay development and interrogation. J Biomol Screen. 2009;14:1134–1141. doi: 10.1177/1087057109343809. [DOI] [PubMed] [Google Scholar]
- Wiedmaier N, Muller S, Koberle M, Manncke B, Krejci J, Autenrieth IB, et al. Bacteria induce CTGF and CYR61 expression in epithelial cells in a lysophosphatidic acid receptor-dependent manner. Int J Med Microbiol. 2008;298:231–243. doi: 10.1016/j.ijmm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Williams JR, Khandoga AL, Goyal P, Fells JI, Perygin DH, Siess W, et al. Unique ligand selectivity of the GPR92/LPA5 lysophosphatidate receptor indicates role in human platelet activation. J Biol Chem. 2009;284:17304–17319. doi: 10.1074/jbc.M109.003194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Gibbs TC, Mukhin YV, Meier KE. Role for 18:1 lysophosphatidic acid as an autocrine mediator in prostate cancer cells. J Biol Chem. 2002;277:32516–32526. doi: 10.1074/jbc.M203864200. [DOI] [PubMed] [Google Scholar]
- Xie W, Matsumoto M, Chun J, Ueda H. Involvement of LPA1 receptor signaling in the reorganization of spinal input through Abeta-fibers in mice with partial sciatic nerve injury. Mol Pain. 2008;4:46. doi: 10.1186/1744-8069-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang X-J SA, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995;1:1223–1232. [PubMed] [Google Scholar]
- Xu MY, Porte J, Knox AJ, Weinreb PH, Maher TM, Violette SM, et al. Lysophosphatidic acid induces alphavbeta6 integrin-mediated TGF-beta activation via the LPA2 receptor and the small G protein G alpha(q) Am J Pathol. 2009;174:1264–1279. doi: 10.2353/ajpath.2009.080160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubu MA, Leffler CW. Regulation of ET-1 biosynthesis in cerebral microvascular endothelial cells by vasoactive agents and PKC. Am J Physiol. 1999;276(2 Pt 1):C300–C305. doi: 10.1152/ajpcell.1999.276.2.C300. [DOI] [PubMed] [Google Scholar]
- Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain-containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Ishii S, Hamano F, Noguchi K, Shimizu T. LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J Biol Chem. 2007;282:5814–5824. doi: 10.1074/jbc.M610767200. [DOI] [PubMed] [Google Scholar]
- Yanagida K, Masago K, Nakanishi H, Kihara Y, Hamano F, Tajima Y, et al. Identification and characterization of a novel lysophosphatidic acid receptor, p2y5/LPA6. J Biol Chem. 2009;284:17731–17741. doi: 10.1074/jbc.M808506200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Andrews DA, Low PS. Lysophosphatidic acid opens a Ca(++) channel in human erythrocytes. Blood. 2000;95:2420–2425. [PubMed] [Google Scholar]
- Ye X. Lysophospholipid signaling in the function and pathology of the reproductive system. Hum Reprod Update. 2008;14:519–536. doi: 10.1093/humupd/dmn023. [DOI] [PubMed] [Google Scholar]
- Ye X, Hama K, Contos JJ, Anliker B, Inoue A, Skinner MK, et al. LPA3-mediated lysophosphatidic acid signalling in embryo implantation and spacing. Nature. 2005;435:104–108. doi: 10.1038/nature03505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Skinner MK, Kennedy G, Chun J. Age-dependent loss of sperm production in mice via impaired lysophosphatidic acid signaling. Biol Reprod. 2008;79:328–336. doi: 10.1095/biolreprod.108.068783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Watsky MA. Chloride channel activity in human lung fibroblasts and myofibroblasts. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1110–L1116. doi: 10.1152/ajplung.00344.2004. [DOI] [PubMed] [Google Scholar]
- Yin Z, Tong Y, Zhu H, Watsky MA. ClC-3 is required for LPA-activated Cl- current activity and fibroblast-to-myofibroblast differentiation. Am J Physiol Cell Physiol. 2008;294:C535–C542. doi: 10.1152/ajpcell.00291.2007. [DOI] [PubMed] [Google Scholar]
- Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, et al. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem. 2009;284:12328–12338. doi: 10.1074/jbc.M806516200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Nishida W, Hayashi K, Ohkawa Y, Ogawa A, Aoki J, et al. Vascular remodeling induced by naturally occurring unsaturated lysophosphatidic acid in vivo. Circulation. 2003;108:1746–1752. doi: 10.1161/01.CIR.0000089374.35455.F3. [DOI] [PubMed] [Google Scholar]
- Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008;100:1630–1642. doi: 10.1093/jnci/djn378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue J, Yokoyama K, Balazs L, Baker DL, Smalley D, Pilquil C, et al. Mice with transgenic overexpression of lipid phosphate phosphatase-1 display multiple organotypic deficits without alteration in circulating lysophosphatidate level. Cell Signal. 2004;16:385–399. doi: 10.1016/j.cellsig.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Yuelling LM, Fuss B. Autotaxin (ATX): a multi-functional and multi-modular protein possessing enzymatic lysoPLD activity and matricellular properties. Biochim Biophys Acta. 2008;1781:525–530. doi: 10.1016/j.bbalip.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowski BK, Liu W, Ramirez K, Akagi Y, Mills GB, Ellis LM. Markedly elevated levels of vascular endothelial growth factor in malignant ascites. Ann Surg Oncol. 1999;6:373–378. doi: 10.1007/s10434-999-0373-0. [DOI] [PubMed] [Google Scholar]
- Zhang C, Baker DL, Yasuda S, Makarova N, Balazs L, Johnson LR, et al. Lysophosphatidic acid induces neointima formation through PPARgamma activation. J Exp Med. 2004;199:763–774. doi: 10.1084/jem.20031619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bialkowska A, Rusovici R, Chanchevalap S, Shim H, Katz JP, et al. Lysophosphatidic acid facilitates proliferation of colon cancer cells via induction of Kruppel-like factor 5. J Biol Chem. 2007a;282:15541–15549. doi: 10.1074/jbc.M700702200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang D, Sun H, Hall RA, Yun CC. MAGI-3 regulates LPA-induced activation of Erk and RhoA. Cell Signal. 2007b;19:261–268. doi: 10.1016/j.cellsig.2006.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Xu X, Gajewiak J, Tsukahara R, Fujiwara Y, Liu J, et al. Dual activity lysophosphatidic acid receptor pan-antagonist/autotaxin inhibitor reduces breast cancer cell migration in vitro and causes tumor regression in vivo. Cancer Res. 2009a;69:5441–5449. doi: 10.1158/0008-5472.CAN-09-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qian Y, Lu W, Chen X. The G protein-coupled receptor 87 is necessary for p53-dependent cell survival in response to genotoxic stress. Cancer Res. 2009b;69:6049–6056. doi: 10.1158/0008-5472.CAN-09-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Usatyuk PV, Cummings R, Saatian B, He D, Watkins T, et al. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid-induced calcium release, NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem J. 2005;385(Pt 2):493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Voice JK, Kong Y, Goetzl EJ. Altered expression and functional profile of lysophosphatidic acid receptors in mitogen-activated human blood T lymphocytes. FASEB J. 2000;14:2387–2389. doi: 10.1096/fj.00-0492fje. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Kong Y, Goetzl EJ. Lysophosphatidic acid receptor-selective effects on Jurkat T cell migration through a Matrigel model basement membrane. J Immunol. 2001;166:2317–2322. doi: 10.4049/jimmunol.166.4.2317. [DOI] [PubMed] [Google Scholar]
- Zhou D, Luini W, Bernasconi S, Diomede L, Salmona M, Mantovani A, et al. Phosphatidic acid and lysophosphatidic acid induce haptotacic migration of human monocytes. J Biol Chem. 1995;270:25549–25556. doi: 10.1074/jbc.270.43.25549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


