Abstract
BACKGROUND AND PURPOSE
Metabolic and cardiovascular abnormalities accompanying metabolic syndrome, such as obesity, insulin resistance and hypertension, are all associated with endothelial dysfunction and are independent risk factors for erectile dysfunction. The purpose of the present study was to investigate the vascular effects of insulin in penile arteries and whether these effects are impaired in a rat model of insulin resistance and metabolic syndrome.
EXPERIMENTAL APPROACH
Penile arteries from obese Zucker rats (OZR) and their counterpart, lean Zucker rats (LZR), were mounted on microvascular myographs and the effects of insulin were assessed in the absence and presence of endothelium and of specific inhibitors of nitric oxide (NO) synthesis, phosphatidylinositol 3-kinase (PI3K) and mitogen-activated protein kinase (MAPK). Insulin-induced changes in intracellular Ca2+ concentration [Ca2+]i were also examined.
KEY RESULTS
OZR exhibited mild hyperglycaemia, hypercholesterolemia, hypertryglyceridemia and hyperinsulinemia. Insulin induced endothelium- and NO-dependent relaxations in LZR that were impaired in OZR. Inhibition of PI3K reduced relaxation induced by insulin and by the β-adrenoceptor agonist isoprenaline, mainly in arteries from LZR. Antagonism of endothelin 1 (ET-1) receptors did not alter insulin-induced relaxation in either LZR or OZR, but MAPK blockade increased the responses in OZR. Insulin decreased [Ca2+]i, a response impaired in OZR.
CONCLUSIONS AND IMPLICATIONS
Insulin-induced relaxation was impaired in penile arteries of OZR due to altered NO release through the PI3K pathway and unmasking of a MAPK-mediated vasoconstriction. This vascular insulin resistance is likely to contribute to the endothelial dysfunction and erectile dysfunction associated with insulin resistant states.
Keywords: penile arteries, insulin resistance, endothelial dysfunction, β-adrenoceptors, obese Zucker rat
Introduction
Erectile dysfunction (ED) is currently considered as an early sign of subclinical vascular disease and is a highly prevalent condition in patients with cardiovascular risk factors such as hypertension, dyslipidemia, central obesity and insulin resistance (Montorsi et al., 2003). The cluster of these metabolic and cardiovascular abnormalities, jointly referred to as metabolic syndrome, further increases the risk for the development of ED (Fonseca and Jawa, 2005; Jackson, 2006).
Insulin resistance and hyperinsulinemia play a major role in the pathogenesis of type 2 diabetes and have been associated with the development of cardiovascular disease and hypertension (De Fronzo and Ferrannini, 1991). Insulin resistance is typically defined as a decreased sensitivity to the metabolic actions of insulin such as insulin-mediated glucose disposal. However, in addition to its classical physiological actions, insulin has vascular actions that couple the metabolic and haemodynamic homeostasis under healthy conditions (Kim et al., 2006). The association between insulin resistance and cardiovascular disease was initially ascribed to the actions of insulin on vascular smooth muscle growth and extracellular matrix production (Tamaroglio and Lo, 1994). However, experimental evidence shows that endothelial function is impaired in insulin resistance and diabetic patients and diminished sensitivity or resistance to the actions of insulin in the vascular endothelium also contribute to the phenotype of the insulin-resistant states (Baron et al., 1991; Steinberg et al., 1996; Kim et al., 2006).
Insulin binding to its receptor at the endothelial cells activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway that in turns phosphorylates endothelial nitric oxide synthase (eNOS) at Ser 1177 and induces nitric oxide (NO) release and vasodilatation (Zeng et al., 2000). Stimulation of endothelial insulin receptors can also lead to the activation of the Ras-Raf-mitogen-activated protein kinase (MAPK) pathway that is associated with gene expression, cell growth and the production of vasoconstrictor endothelin 1 (ET-1) (Cardillo et al., 1999). In obese insulin-resistant men and patients with type 2 diabetes, the insulin-mediated activation of the PI3K/Akt pathway underlying both the metabolic and the vascular actions of the hormone is impaired, and endothelial dysfunction and blunted vasodilatation have been reported (Steinberg et al., 1996; Cusi et al., 2000).
The metabolic and cardiovascular abnormalities accompanying metabolic syndrome, that is, glucose intolerance, dyslipidemia, insulin resistance and hypertension, act as independent risk factors for ED and are all associated to some extent with endothelial dysfunction (Fonseca and Jawa, 2005), although the mechanisms for this dysfunction in the penile vasculature are not completely understood. Novel studies in an animal model of type 1 diabetes-associated ED have demonstrated an impairment of the PI3K/Akt/eNOS pathway in the erectile tissue associated with a decreased erectile capability (Musicki et al., 2005). However, the effects of insulin on the penile vasculature of both healthy individuals and of type 2 diabetes and insulin resistance-associated ED models are unknown. In the present study, we investigated the mechanisms underlying the vasoactive actions of insulin in penile arteries from the obese Zucker rat (OZR), a well-established genetic model of insulin resistance and metabolic syndrome caused by a dysfunctional gene of the leptin receptor. These animals progressively develop obesity, type 2 diabetes and hypertension (Guerre-Millo 1997). We have recently demonstrated profound alterations in vascular structure and endothelial dysfunction in dorsal penile arteries from the OZR (Villalba et al., 2009), data consistent with the ED reported in these animals (Wingard et al., 2007). Here, we aimed to further investigate whether the vascular effects of insulin are impaired in penile arteries under conditions of insulin resistance in the OZR.
Methods
Animal model
All animal care and experimental protocols conform to European Union guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the Complutense University of Madrid. Male OZR (n = 47) and lean Zucker rats (LZR, n = 46) were obtained from Charles River Laboratories (Barcelona, Spain) and used for study at 17–18 weeks of age.
Experimental procedures for the functional experiments
On the day of the experiment, blood samples were obtained from the tail vein before death and plasma was frozen for determination of non-fasting glucose, cholesterol, triglycerides and insulin plasma levels. Glucose, total triglyceride and total cholesterol levels were determined by using commercially available kits. Plasma insulin levels were measured by specific enzyme-linked immunosorbent assay.
Immediately after killing of the animal (cervical dislocation and exsanguination), the penis was removed and placed in cold physiological saline solution (PSS) of the following composition (mM): NaCl 119, NaHCO3 25, KCl 4.7, K2H2PO4 1.17, MgSO4 1.18, CaCl2 1.5, EDTA 0.027 and glucose 11. The penile arteries, first- or second-order branches of the dorsal penile artery from LZR and OZR were carefully dissected by removing the connective and fat tissue, as described previously (Villalba et al., 2009; Sánchez et al., 2010). These arteries were used in order to assess erectile tissue function as they can supply blood to the cavernous tissue and are of a size in the rat that allows handling without damaging the vascular endothelium (Villalba et al., 2009). One arterial segment from a LZR and another from an OZR were mounted in parallel on double microvascular myographs (DMT, Denmark) and stretched to their optimal internal lumen diameter l1 corresponding to 90% of the diameter the arteries would have in situ under an internal transmural pressure of 100 mmHg (Villalba et al., 2009). Aortic rings from LZR and OZR were also used in order to compare the vascular effects of insulin with those on penile arteries. Thoracic aortas were isolated and cleaned from the fatty and connective tissue, and arterial rings were mounted in vascular myographs and a passive initial tension of 10 mN applied.
At the beginning of each experiment, vasoconstrictor responses to 120 mM K+ (high K+-physiological saline solution, KPSS) confirmed vessel viability. The vasoactive effects of insulin were evaluated by adding cumulative concentrations of the hormone to arteries precontracted with phenylephrine (1 µM), in the absence or presence of specific inhibitors of NOS (Nω-nitro-L-arginine, L-NNA, 100 µM), PI3K (LY-294002, 3 µM), cyclooxygenase (indomethacin, 1 µM), MAPK (PD-98059, 5 µM) and the non-selective antagonist of ET-1 receptors (bosentan, 10 µM). Because insulin effects were not reproducible in a second stimulation, two consecutive arterial segments of the same animal were used, one as a control and in the second the treatment was applied. The role of the vascular endothelium was assessed in arteries where the endothelium was mechanically removed by passing a human hair through the vessel lumen. The absence of functional endothelium was confirmed by the lack of relaxation to acetylcholine (ACh, 10 µM). The effects of the β-adrenoceptor agonist isoprenaline were also evaluated in the absence and presence of LY-294002. In order to assess the cAMP-dependent relaxations in penile arteries, concentration–response curves for the adenylate cyclase activator forskolin were also performed.
Measurements of intracellular Ca2+ ([Ca2+]i)
Simultaneous measurements of [Ca2+]i and force were performed in intact penile arteries as previously described (Villalba et al., 2007). The myograph was mounted on an inverted microscope (Axiovert S100 TV) equipped for dual excitation wavelength microfluorimetry. Penile arteries were incubated in the dark in PSS with 8 µM Fura 2-acetoxymethyl ester for 3 h at 37°C, changing the solution after 1.5 h. Then arteries were washed for 45 min to remove remaining Fura 2-acetoxymethyl ester. After loading, the arteries were illuminated with alternating 340 and 380 nm light using a monochromator-based system (Deltascan, PTI). The fluorescence emission was detected at a wavelength of 510 nm. At the end of each experiment, Ca2+-insensitive signals were determined after quenching with 25 mM Mn2+, and the values obtained were substracted from those obtained during the experiment. The ratio (R) F340/F380 was taken as a measure of [Ca2+]i.
Arteries were initially stimulated with KPSS in order to test vessel viability. Then, the effect of a single dose of insulin (0. 1 µM) was evaluated in arteries pre-contracted with phenylephrine (1 µM). Finally, the effect of ACh (10 µM) was also tested.
Data analysis
Values are expressed as means ± SEM of n (number of arteries, 1–2 per animal). Relaxants responses to insulin are given as a percentage of precontraction induced by phenylephrine. The sensitivity of the arteries to the vasoactive agonists is expressed in terms of pEC50 values, where pEC50 is −logEC50. EC50 is the concentration of the agonist required to produce 50% of the response and was calculated by using standard computer software (Prism 5.0, Graphpad, San Diego, CA, USA). The statistical differences between means were analysed by either unpaired Student's t-test or by one-way anova. Probability levels lower than 5% were considered significant.
Materials
The sources of the compounds used in this MS were as follows: L-NNA, indomethacin, insulin, PD98059, isoprenaline, acetylcholine, forskolin and phenylephrine were from Sigma Chemicals (Madrid, Spain). LY 294002 and endothelin1 (ET-1) were from Tocris, Cookson, (Bristol, UK); Fura 2-acetoxymethyl ester (Fura-2-AM) was from Molecular Probes (OR, USA). Bosentan was a generous gift of F. Hoffman La Roche Laboratories (Basel, Switzerland). All drug and molecular target nomenclature follows Alexander et al., (2009).
Results
General parameters
At the time of the experiment, the OZR were significantly heavier than the LZR and exhibited mild hyperglycaemia, hyperinsulinemia and dyslipidemia with elevated total cholesterol and triglyceride levels (Table 1). In penile arteries from the OZR group, contractions to KPSS were reduced (2.11 ± 0.14 Nm−1 in LZR, n = 46; and 1.62 ± 0.10 Nm−1 in OZR, n = 47, P < 0.01), and the ACh-induced relaxations were impaired (58 ± 4% in LZR, n = 27; and 43 ± 5% in OZR, n = 38, P < 0.05), indicating reduced contractility and endothelial dysfunction respectively.
Table 1.
Metabolic parameters of LZR and OZR
| LZR | N | OZR | n | |
|---|---|---|---|---|
| Body weight (g) | 384 ± 7 | 46 | 474 ± 7*** | 47 |
| Weeks | 17–18 | 17–18 | ||
| [Glucose]blood (mg/ml) | 1.16 ± 0.13 | 12 | 1.75 ± 0.20* | 12 |
| [Insulin]plasma (ng/ml) | 1.10 ± 0.20 | 6 | 4.30 ± 0.50*** | 6 |
| [Cholesterol]plasma (mg/ml) | 0.92 ± 0.04 | 12 | 1.83 ± 0.13*** | 12 |
| [Triglycerides]plasma (mg/ml) | 0.99 ± 0.13 | 12 | 3.34 ± 0.33*** | 12 |
Data are means ± SEM of the number n of animals. Significant differences were analyzed by an unpaired Student t-test. *P < 0.05; **p < 0.001; ***P < 0.0001 vs LZR. LZR, lean Zucker rat; OZR, obese Zucker rat.
Structure of penile arteries
Normalized internal lumen diameters (l1) of penile arteries were significantly smaller in OZR (129 ± 4 µm, n = 36) than in LZR (155 ± 6 µm, n = 38, P < 0.001), indicating inward vascular remodelling in insulin-resistant animals. Penile weights were significantly lower in OZR than LZR (0.5 ± 0.1 g and 0.7 ± 0.1 g, n = 7, P < 0.01, respectively), also suggesting structural remodelling of penile erectile tissue. Masson's trichrome staining confirmed wall thickening and smaller internal diameters in arteries from OZR (not shown).
Effect of insulin on penile arteries from LZR and OZR
Exogenously applied insulin (0.1–100 nM) induced concentration-dependent relaxations in penile arteries of LZR pre-contracted with phenylephrine (Figure 1). The pEC50 and response to the highest concentration of insulin used (100 nM) was 8.02 ± 0.12 and 24.3 ± 3.6% (n = 27) of the phenylephrine-induced contraction respectively (Figure 1A,C). The relaxant responses to insulin were impaired in penile arteries from OZR (insulin-induced (100 nM) relaxation of 7.5 ± 2.5%, n = 31, P < 0.01 vs. LZR) (Figure 1B,C). In aortas from OZR, responses to insulin were also blunted and, in some samples, turned into contractions, the response to 100 nM insulin being −4.1 ± 10.9% (n = 10) of the phenylephrine-induced contraction. In LZR aortas, the corresponding response to insulin was greater (24.0 ± 7.2%, n = 11) of the phenylephrine-induced contraction (P < 0.01).
Figure 1.
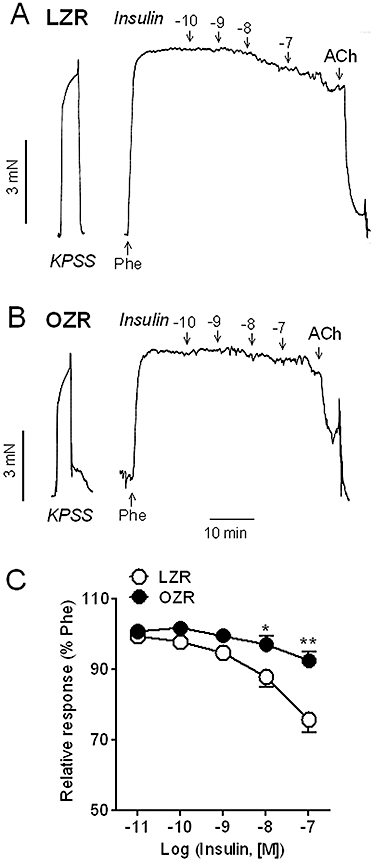
Relaxant responses to insulin were significantly impaired in penile arteries from OZR compared with LZR. (A, B) Representative traces showing the insulin-induced relaxations in penile arteries from (A) LZR and (B) OZR. (C) Average concentration–response curves for the relaxant effect of insulin in arteries from LZR and OZR. Data are shown as the mean ± SEM of 26 arteries (24 animals, LZR) and 28 arteries (25 animals, OZR). *P < 0.05, **P < 0.01 versus LZR. Vertical bars show force in mN and horizontal bars show time in min. ACh, acetylcholine; KPSS, high K+-physiological saline solution; LZR, lean Zucker rat; OZR, obese Zucker rat; Phe, phenylephrine.
Effects of endothelial cell removal on insulin relaxant responses
Mechanical removal of the endothelium impaired insulin-induced relaxations in penile arteries from LZR but did not significantly alter responses in OZR (Figure 2A,B, Table 2). There was a significant linear correlation between the 100 nM insulin-induced relaxation and that elicited by the endothelial cell agonist ACh (10 µM) in both LZR and OZR (Figure 2C,D). The absence of functional endothelium was verified by the blunted vasodilator effect of ACh after endothelial cell removal (Figure 2E,F, Table 2).
Figure 2.
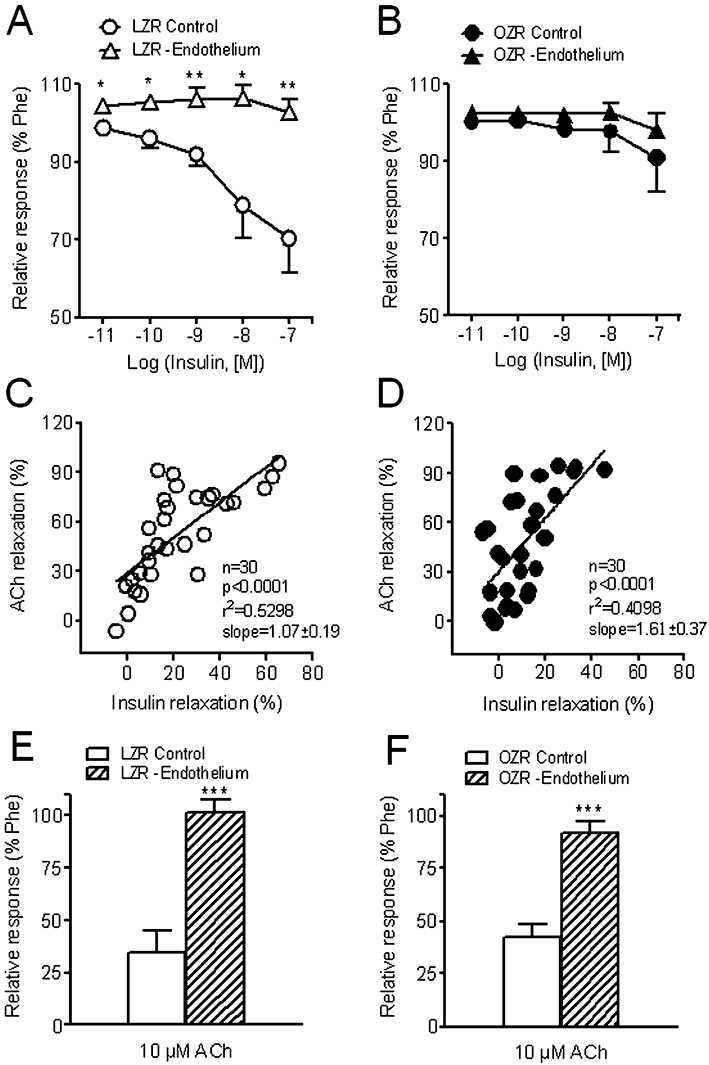
Effect of endothelial cell removal on the relaxant effects of insulin in penile arteries from LZR and OZR. (A, B) Average relaxant responses to insulin in endothelium-intact and endothelium-denuded penile arteries from (A) LZR and (B) OZR. (C, D) There was a significant linear correlation between insulin-dependent and ACh-dependent relaxations in both (C) LZR and (D) OZR. (E, F) Average ACh (10 µM) relaxant responses in endothelium-intact and endothelium-denuded penile arteries from (E) LZR and (F) OZR. Data are expressed as the mean ± SEM of 6–7 arteries (A, B, E, F) and 30 arteries (C, D), 1–2 per animal. *P < 0.05, **P < 0.01, ***P < 0.0001 versus endothelium-intact arteries. Unpaired Student's t-test. ACh, acetylcholine; LZR, lean Zucker rat; OZR, obese Zucker rat; Phe, phenylephrine.
Table 2.
Effects of endothelium removal (-Endo) and of the inhibitors of NOS, L-NNA (100 µM), and PI3K, LY-294002 (1 µM) on the relaxant responses to insulin and to acetylcholine in penile arteries from LZR and OZR
| Insulin (100 nM) | Acetylcholine (10 µM) | |||||||
|---|---|---|---|---|---|---|---|---|
| LZR | N | OZR | n | LZR | n | OZR | n | |
| Control | 29.7 ± 8.9 | 7 | 9.1 ± 9.0 | 7 | 65.1 ± 9.9 | 7 | 57.4 ± 6.1 | 7 |
| -Endo | −2.7 ± 3.3b | 7 | 2.0 ± 4.2 | 6 | 4.5 ± 4.1c | 7 | 7.8 ± 5.2c | 6 |
| Control | 34.9 ± 6.8 | 8 | 16.0 ± 8.8 | 6 | 53.8 ± 9.9 | 8 | 43.1 ± 15.3 | 6 |
| L-NNA | 7.4 ± 8.4a | 7 | 8.0 ± 5.3 | 7 | 29.3 ±8.5a | 7 | 16.8 ± 6.1a | 7 |
| Control | 20.2 ± 4.8 | 6 | 12.6 ± 3.4 | 7 | 58.9 ± 6.9 | 10 | 57.3 ± 10.9 | 10 |
| LY-294002 | 2.0 ± 1.5c | 7 | 3.7 ± 0.9b | 8 | 44.7 ± 12.1 | 10 | 45.6 ± 12.1 | 11 |
Values represent mean ± SEM of the number n of individual arteries, 1–2 per animal. The relaxant responses to insulin and acetylcholine are expressed as percentage of the contraction induced by phenylephrine (1 µM), prior to the addition of the agonist in each artery. Significant differences from controls were assessed by an unpaired Student's t-test.
P < 0.05
P < 0.01
P < 0.001.
L-NNA, Nω-nitro-L-arginine; LZR, lean Zucker rat; NOS, nitric oxide synthase; OZR, obese Zucker rat; PI3K, phosphatidylinositol 3-kinase.
Effects of eNOS and PI3K inhibitors on insulin relaxant responses
Treatment with the NOS blocker L-NNA (100 µM) produced a significant inhibition of the insulin-induced relaxation in penile arteries of LZR but not of OZR (Figure 3A,B, Table 2). The maximal ACh relaxant responses were significantly diminished in both LZR and OZR after NOS blockade (Figure 3C,D, Table 2).
Figure 3.
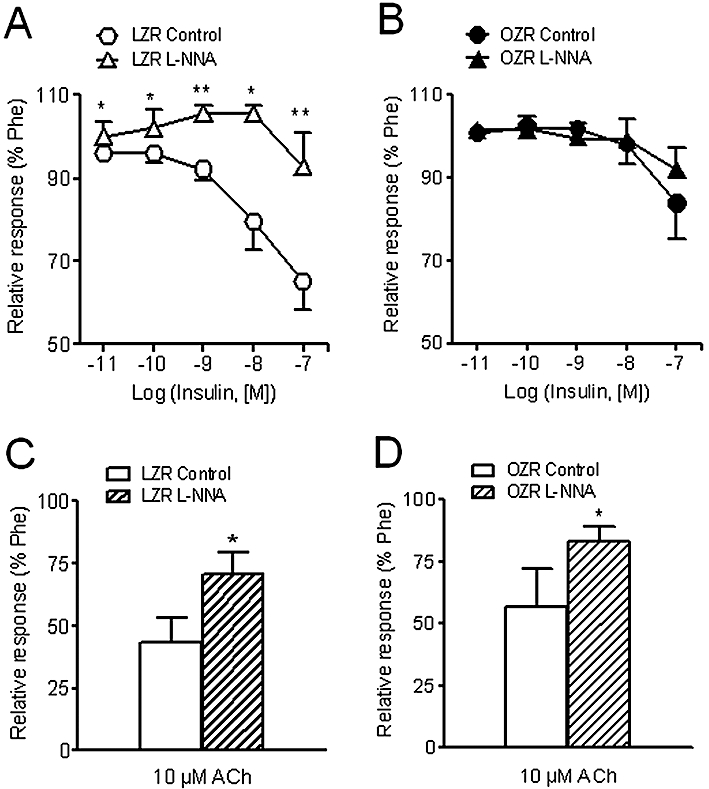
Effects of the NOS inhibitor, L-NNA (100 µM) on the relaxant responses to insulin (A, B) and (C, D) ACh (10 µM) in penile arteries from LZR and OZR. Data are shown as the means ± SEM of 6–8 arteries, 1–2 per animal. *P < 0.05, **P < 0.01, versus control arteries. Unpaired Student's t-test. ACh, acetylcholine; L-NNA, Nω-nitro-L-arginine; LZR, lean Zucker rat; NOS, nitric oxide synthase; OZR, obese Zucker rat; Phe, phenylephrine.
Inhibition of PI3K with LY-294002 (1 µM) markedly reduced the relaxant effect of insulin in arteries of LZR, and to a lesser extent in OZR (Figure 4A,B, Table 2), without significantly altering the relaxant effects of ACh in either LZR or OZR (Figure 4C,D, Table 2). Interestingly, LY-294002 also inhibited the relaxant responses induced by the β-adrenoceptor agonist isoprenaline in LZR but not in OZR (Figure 5A,B). The maximal relaxant effect of isoprenaline was significantly impaired in OZR compared with controls (Figure 5C). However, the relaxant effect of the adenylate cyclase activator forskolin was not different in penile arteries from OZR compared with LZR (Figure 5D), pEC50 being 6.18 ± 0.21 and 6.30 ± 0.21 (n = 15, not significantly different) respectively.
Figure 4.
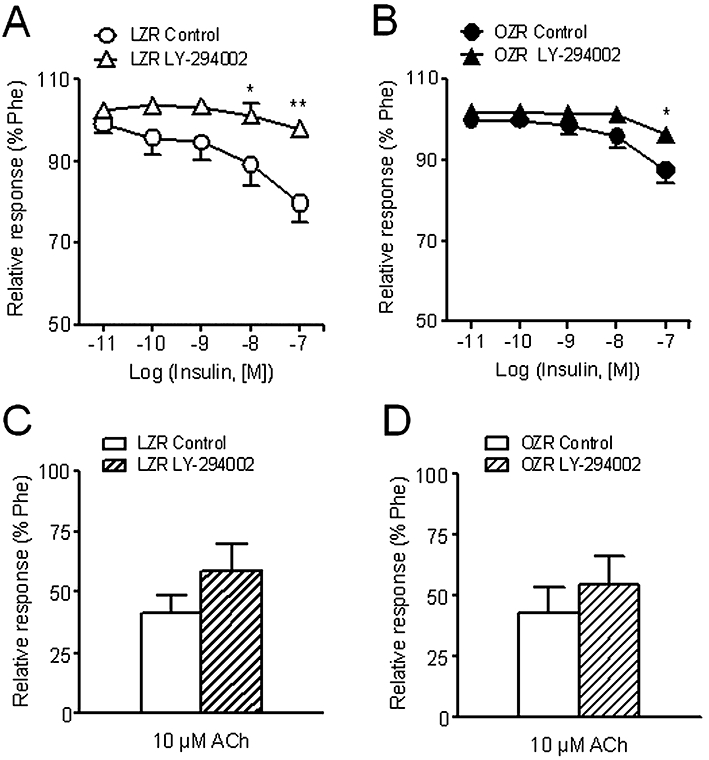
Effect of the PI3K inhibitor LY-294002 (3 µM) on the vasodilator responses induced by insulin (A, B) and ACh (C, D) in penile arteries from LZR and OZR. Data are shown as the means ± SEM of 7–8 arteries, 1–2 per animal. *P < 0.05, **P < 0.01, versus control arteries. Unpaired Student's t-test. ACh, acetylcholine; LZR, lean Zucker rat; OZR, obese Zucker rat; Phe, phenylephrine.
Figure 5.
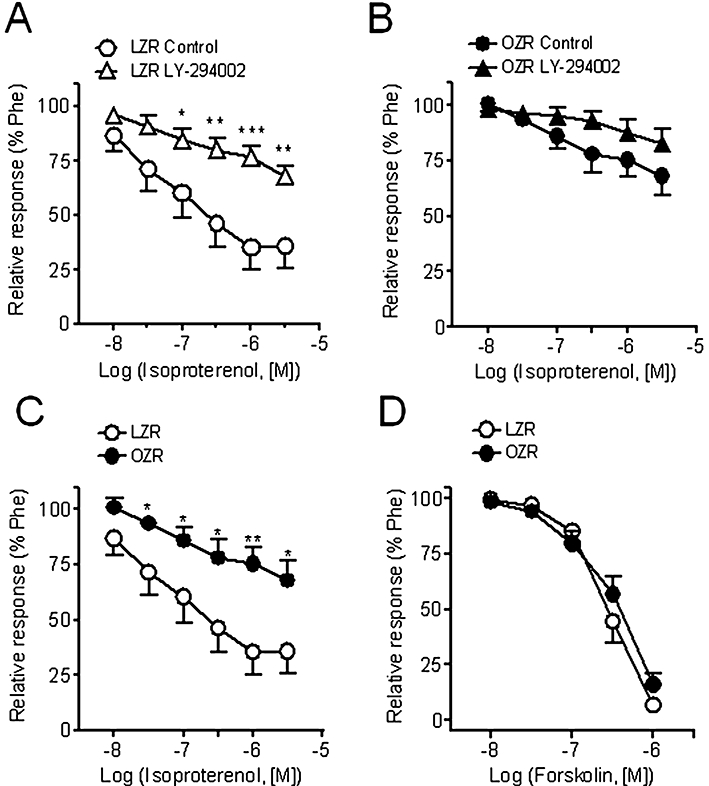
The relaxant responses to the β-adrenoceptor agonist isoprenaline were partly dependent on the PI3K/Akt pathway in penile arteries from LZR but not from OZR. (A, B) Effect of the PI3K inhibitor LY-294002 (3 µM) on the vasodilator responses induced by isoprenaline in penile arteries from LZR (A) and OZR (B). (C, D) Relaxations induced by isoprenaline (C) and by the adenylate cyclase activator forskolin (D) in penile arteries from LZR and OZR. Data are shown as the means ± SEM of 10–15 arteries, 1–2 per animal. *P < 0.05, **P < 0.01, ***P < 0.0001. Unpaired Student's t-test. LZR, lean Zucker rat; OZR, obese Zucker rat; Phe, phenylephrine.
Effect of ET-1 receptor antagonist and MAPK inhibitors on arterial responses to insulin
The antagonist of ET-1 receptors, bosentan, did not modify the relaxant responses to insulin (Figure 6A,B) in penile arteries from either LZR or OZR. However, bosentan inhibited the contractions induced by ET-1 in both LZR (Figure 6C,D) (pEC50 values: 8.94 ± 0.13, n = 8, vs. 7.23 ± 0.12, n = 10, P < 0.0001, in control and treated LZR arteries, respectively) and OZR (pEC50 9.20 ± 0.34, n = 7, vs. 7.23 ± 0.11, n = 12, P < 0.01, in control and treated arteries, respectively).
Figure 6.
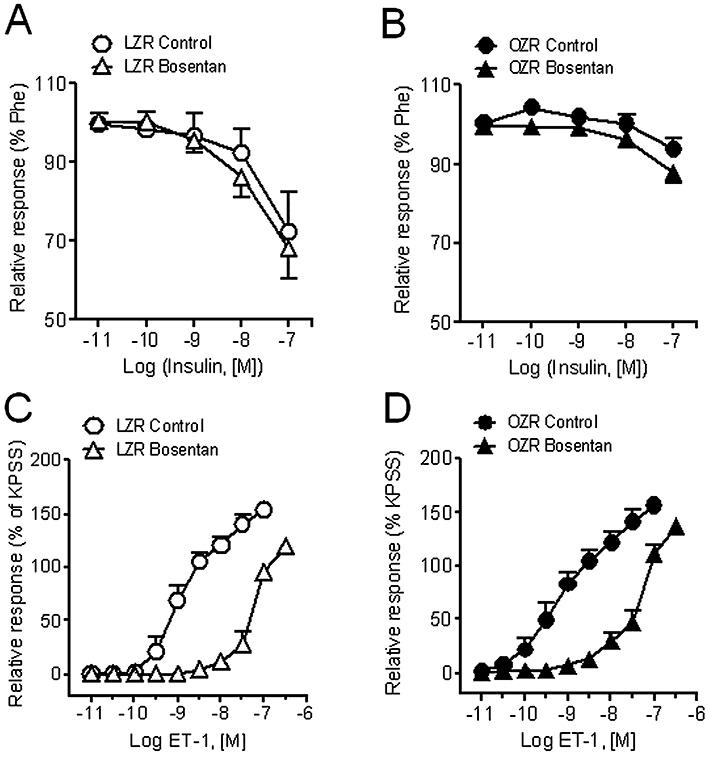
Effect of the non-selective ET-1 receptor antagonist, bosentan (10 µM) on the relaxant responses to insulin (A, B) and on the contractions elicited by ET-1 (C, D) of penile arteries from LZR and OZR. Data are shown as the means ± SEM of 6–12 arteries, 1–2 per animal. ET-1, endothelin 1; KPSS, high K+-physiological saline solution; LZR, lean Zucker rat; OZR, obese Zucker rat; Phe, phenylephrine.
The MAPK inhibitor, PD-98059, did not alter the relaxant responses to insulin in LZR (Figure 7A), but significantly enhanced those in OZR (Figure 7B). The relaxant responses to insulin in the presence of PD-98059 were significantly inhibited by NOS blockade in LZR (Figure 7C), but blunted only after combined blockade of NOS and cyclooxygenase in OZR (Figure 7D).
Figure 7.
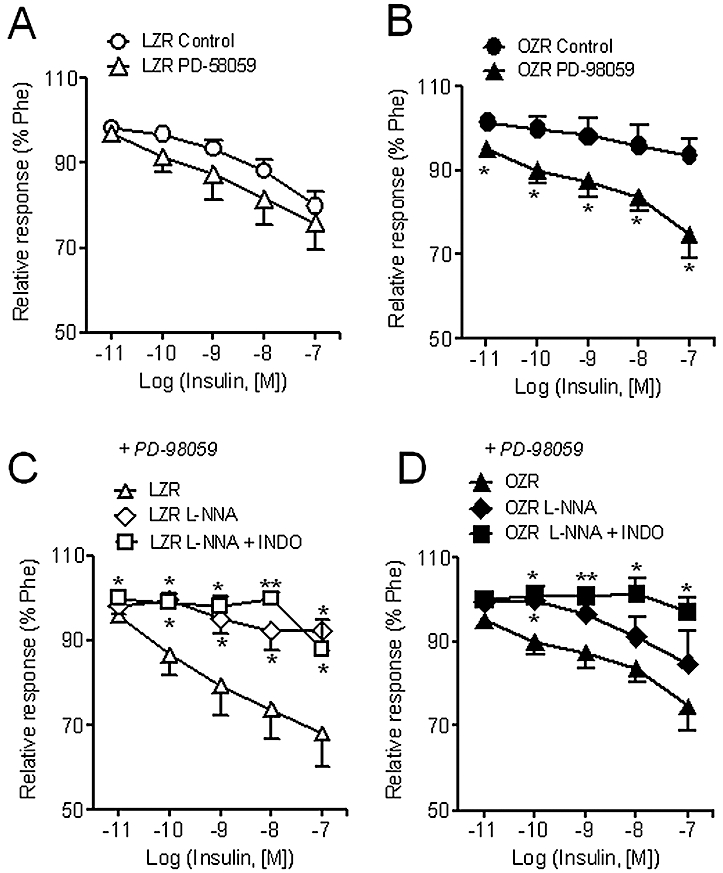
Effect of the MAPK inhibitor, PD-98059 (5 µM) on the relaxant responses to insulin in penile arteries from LZR and OZR (A, B). Effect of the NOS inhibitor, L-NNA (100 µM) and the cyclooxygenase inhibitor indomethacin (1 µM) on the relaxant responses to insulin in penile arteries from LZR (C) and OZR (D) treated with PD-98059. Data are shown as the means ± SEM of 6–12 arteries, 1–2 per animal. *P < 0.05, **P < 0.01 versus control arteries. (A, B) Unpaired Student's t-test. (C, D) One-way anova. L-NNA, Nω-nitro-L-arginine; LZR, lean Zucker rat; MAPK, mitogen-activated protein kinase; OZR, obese Zucker rat; Phe, phenylephrine.
Effect of insulin on [Ca2+]i
Stimulation of penile arteries from LZR and OZR with a high K+ solution (KPSS) induced simultaneous increases in both [Ca2+]i[Δ(F340/F380) = 0.51 ± 0.05, n = 7, in LZR, and 0.35 ± 0.04, n = 7, in OZR, P < 0.05] and tension (1.17 ± 0.16 Nm−1, n = 7 in LZR and 0.95 ± 0.18 Nm−1, n = 7, in OZR, P < 0.01) (Figure 8A,C). The α1-adrenoceptor agonist phenylephrine (1 µM) also evoked sustained increases in [Ca2+]i[Δ(F340/F380) = 0.38 ± 0.06, n = 7, in LZR and 0.26 ± 0.04, n = 7, in OZR, P < 0.05] and tension (2.49 ± 0.26 Nm−1, n = 7 in LZR and 1.31 ± 0.24 Nm−1, n = 7, in OZR, P < 0.01) (Figure 8A,C).
Figure 8.
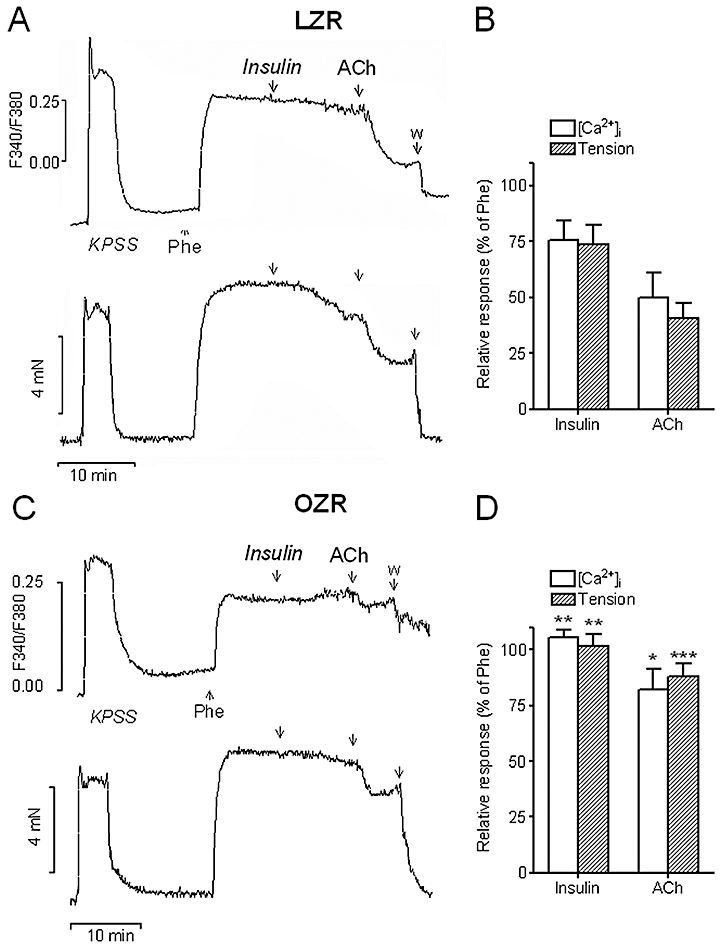
Simultaneous recordings of [Ca2+]i (A, C top) and tension (A, C bottom) showing the effects of a single dose of insulin (10−7 M) and ACh (10−5 M) on penile arteries from LZR (A) and OZR (C) pre-contracted with Phe (1 µM). Relaxations were accompanied by a substantial decrease of [Ca2+]i. in LZR (A) and both relaxation and decrease in [Ca2+]i were blunted in OZR (C,D). (B, D) Average effects of insulin and ACh on the increases in [Ca2+]i and tension induced by Phe in penile arteries from LZR (B) and OZR (D). Data are shown as the means ± SEM of 5–7 arteries, 1 per animal. *P < 0.05, **P < 0.01 versus LZR. Unpaired Student's t-test. ACh, acetylcholine; KPSS, high K+-physiological saline solution; LZR, lean Zucker rat; OZR, obese Zucker rat; Phe, phenylephrine.
The relaxations induced by a single dose of both insulin (100 nM) and ACh (10 µM) were accompanied by simultaneous decreases in [Ca2+]i in penile arteries from LZR pre-contracted with phenylephrine (Figure 8A,B). These decreases in [Ca2+]i were impaired, along with relaxation, in arteries from OZR (Figure 8C,D). In some arteries from OZR, stimulation with insulin was eventually accompanied by increases in [Ca2+]i (Figure 8C).
Discussion
The present study demonstrates that insulin induced endothelium-dependent relaxations in penile arteries through the release of NO via the PI3K/eNOS pathway. This pathway was impaired in arteries from insulin-resistant OZR thus unmasking MAPK-dependent vasoconstrictor effects of insulin. Penile endothelial dysfunction in insulin resistant animals, measured here as a decreased insulin-stimulated, NO-mediated, relaxation of the penile arterial rings, correlates with abnormal structure and vascular remodelling (Villalba et al., 2009), and probably contributes to the decreased erectile capability reported for this animal model (Wingard et al., 2007) and to the ED associated to insulin-resistant states, such as diabetes, obesity and hypertension (Fonseca and Jawa, 2005).
The impaired relaxant effect of insulin in penile arteries of OZR was also found in the aorta of the same animals and is in agreement with the reported impairment of the insulin-mediated vasodilatation in obese subjects (Baron et al., 1991; Steinberg et al., 1996) and in skeletal muscle arterioles (Eringa et al., 2007) and coronary arteries (Katakam et al., 2005) from OZR. The present experiments showed that insulin-induced relaxation in penile arteries was endothelium-dependent and NO-mediated in healthy animals, as shown by the inhibitory effect of endothelium removal and NOS blockers, and also by the significant correlation between the relaxant effect of insulin and that of the endothelial agonist ACh. Furthermore, our data provide pharmacological evidence for the involvement of the PI3K/Akt pathway in the vasoactive effects of insulin in penile arteries. This pathway plays a key role in the physiology of erection, as it is responsible for the sustained production of NO and full erection upon stimulation of eNOS by shear stress, after the increased blood flow to the corpus cavernosum caused by nerve-derived NO released by sexual stimuli (Hurt et al., 2002; Prieto, 2008).
The functional significance of the NO-mediated vascular effects of insulin via the PI3K/Akt/eNOS pathway has earlier been reported in studies showing vascular protection by the hormone, in the coronary circulation. Thus, in vivo administration of insulin improved endothelium-dependent vasodilatation and reduced post-ischaemic myocardial apoptotic death (Gao et al., 2002; Ma et al., 2006). On the other hand, the endothelial dysfunction reported for insulin-resistant aging animals was associated with impaired arterial insulin-induced vasorelaxation and eNOS phosphorylation (Li et al., 2009). The activity of the PI3K/Akt pathway and phosphorylation of eNOS have been reported to be also impaired in models of type 1 diabetes-associated ED due to the O-linked glycosylation and inactivation of eNOS by hyperglycaemia (Federici et al., 2002; Musicki et al., 2005). In the present study, we further demonstrated that the PI3K/Akt pathway is impaired in penile tissue from an animal model of insulin resistance/metabolic syndrome, as shown by the blunted vasodilator effects elicited not only by insulin but also by the β-adrenoceptor agonist isoprenaline, and by the reduced effect of the selective PI3K inhibitor on these relaxations in penile arteries from OZR. In contrast to that reported for type 1 diabetes models, impairment of the PI3K/Akt pathway is unlikely to be due to hyperglycaemia, because the insulin-resistant OZR animals used in our study were hyperinsulinemic but they only exhibited mild hyperglycaemia. On the other hand, decreased insulin-stimulated production of NO and impaired vasodilatation have been ascribed to decreased eNOS expression in skeletal muscle arterioles from OZR (Eringa et al., 2007), but in penile arteries we have recently shown that eNOS protein content is unaltered (Villalba et al., 2009). The functional data of the present study confirm earlier biochemical studies demonstrating that insulin-stimulated PI3K activity and serine phosphorylation of eNOS were markedly reduced in microvessels from OZR (Jiang et al., 1999). Moreover, reduced insulin-stimulated vasodilatation (Kobayashi et al., 2004) coupled to reduced phosphorylation and expression of Akt also occurs in arteries from type 2 diabetic patients (Okon et al., 2005) and rodent models (Kobayashi et al., 2004). The present data therefore suggest that penile endothelial dysfunction and impaired NO-mediated vasodilatation was in part due to the impaired PI3K/eNOS pathway in penile arteries from insulin-resistant OZR. In a recent study in which the contribution from vascular insulin signalling to eNOS in regulating endothelial function and blood pressure was assessed, high levels of free fatty acids rather that defective upstream signalling via Akt were demonstrated to be responsible for the impaired insulin-stimulated eNOS phosphorylation, abnormal endothelial function and hypertension in mice with diet-induced obesity (Symons et al., 2009). The 17–18-week-old hyperinsulinemic OZR used in the present study exhibited penile endothelial dysfunction and vascular insulin resistance, along with ED (Wingard et al., 2007) and abnormal structure but before the onset of hypertension (Villalba et al., 2009). These findings reinforce the view of ED as an early sign of subclinical vascular disease (Montorsi et al., 2003).
Interestingly, the endothelial dysfunction involving the PI3K/eNOS pathway observed in the present study affected the vasodilator effect of β-adrenoceptor agonists, as shown by the blunted relaxations elicited by isoprenaline in penile arteries from OZR. This impairment could initially be ascribed to a failure of the cAMP/protein kinase A pathway. However, relaxations to the adenylate cyclase activator forskolin were unaffected, whereas inhibition of PI3K did not further reduce relaxations to isoprenaline in arteries from insulin-resistant animals suggesting an impairment of the PI3K/eNOS pathway. Our findings are consistent with those of a recent study showing reduced cardiac output and depressor responses to isoprenaline in OZR, due to an impairment of the β-adrenoceptor signalling pathway in both cardiac and vascular myocytes from OZR (D'Angelo et al., 2006). Thus, impairment of the β-adrenoceptor-mediated vasodilatation results in an impaired ability to regulate blood pressure after stress, in pre-diabetic OZR. The β2-adrenoceptor is the predominant receptor subtype in most vascular smooth muscles and mediates relaxations that are completely or partly dependent on the release of endothelial NO through activation of the PI3K/Akt pathway (Ferro et al., 2004). Concerning erectile tissue, β2-adrenoceptors are involved in the relaxation of penile arteries (Simonsen et al., 1997) and β-adrenoceptor-mediated vasodilatation has been proposed to play a role in the erectile response to the adrenaline release associated with sexual activity (Simonsen et al., 1997; Prieto, 2008). Therefore, the blunted responses to β-adrenoceptor agonists found for the first time in penile arteries from OZR would further contribute to the endothelial and ED associated with insulin-resistant states.
The MAPK-dependent branch of the insulin signalling pathway promotes pro-hypertensive and pro-atherogenic actions in part mediated through the production of ET-1, but usually opposed by the PI3K/Akt pathway and NO in healthy individuals (Kim et al., 2006). In insulin-resistant states, there is a selective impairment of the PI3K/Akt branch (Jiang et al., 1999) and the compensatory hyperinsulinemia activates the MAPK pathway and enhances pro-hypertensive and pro-atherogenic actions of insulin (Kim et al., 2006). Accordingly, in the present study, inhibition of MAPK significantly increased the relaxant effect of insulin in penile arteries from hyperinsulinemic OZR but not LZR, thus suggesting that insulin could exert constrictor effects through the MAPK branch, probably unmasked by the impairment of the PI3K/eNOS pathway. However, antagonism of ET-1 receptors did not change insulin relaxant responses in penile arteries of either LZR or OZR. This is in contrast to the findings in skeletal muscle arterioles, where insulin lacked a net vasoactive effect in arteries from LZR, but ET-1 antagonism uncovered insulin-induced vasodilatation and inhibits insulin-induced vasoconstriction in arteries from OZR (Eringa et al., 2007). The discrepancies between our results on the insulin signalling pathways in penile arteries and those in skeletal muscle arteries are likely to reflect tissue-specific differences in the regulation of blood flow by the hormone. On the other hand, the relaxant responses uncovered by MAPK blockade in penile arteries from OZR were blunted by combined blockade of NOS and cyclooxygenase, suggesting the release of a relaxant prostanoid probably to compensate the impaired PI3K/NO pathway. Insulin-induced endothelium-dependent relaxation in healthy rabbit corpus cavernosum involves the release of prostacyclin but not NO (Myung et al., 2006), whereas in penile arteries from healthy LZR, this relaxation is mainly due to NO release, which suggests differences in the signalling pathways of insulin between penile erectile tissues. Furthermore, in small mesenteric arteries from Wistar rats, insulin-induced endothelium-dependent relaxations were unaffected by NOS inhibitors but were blunted by cyclooxygenase blockade and were due to prostacyclin release (Miller et al., 2002). In insulin resistant fructose-fed rats, insulin-induced vasodilatation was impaired and unmasked by ETA receptor blockade, but the hormone-evoked release of prostacyclin persisted (Miller et al., 2002). These data again indicate differences in the signalling pathways of insulin among the different vascular beds, in both healthy and insulin resistant animals.
Earlier studies have demonstrated that insulin attenuates vascular smooth muscle cells (VSMC) contractility by reducing vasoconstrictor agonists-induced increases in [Ca2+]i through voltage-dependent Ca2+ channels (Standley et al., 1991; 1993; Kahn et al., 1994) and by altering the activity of mysosin light chain phosphatases (Begum et al., 2002). Insulin relaxant responses in the corpus cavernosum have been reported to be due to an inhibition of L-type calcium channels (Myung et al., 2006). The present data further demonstrate that the insulin vasodilator effect is paralleled by a decrease in [Ca2+]i in arterial smooth muscle of healthy penile arteries precontracted with phenylephrine. Because α1-adrenoceptor-mediated vasoconstriction is mostly dependent on extracellular Ca2+ influx in penile arteries (Villalba et al., 2007), the effect of insulin on [Ca2+]i is probably due to an attenuation of Ca2+ influx. The decrease in [Ca2+]i induced by insulin was found to be blunted and even turned into increase in [Ca2+]i along with impaired relaxation in penile arteries from OZR. In isolated VSMC from OZR aorta, however, Ca2+ entry induced by vasopressin was similarly attenuated by insulin in LZR and OZR, which suggests that the ability of insulin to reduce Ca2+ influx in VSMC is preserved in arteries from insulin resistant animals (Standley et al., 1993). Because the experiments of the present study were performed in arteries with intact endothelium, our results suggest that the blunted effects of insulin on [Ca2+]i of penile arteries from OZR are likely to reflect the impaired release/effects of endothelial mediators of insulin relaxation, namely NO. Accordingly, the relaxations induced by ACh, which are mediated by NO and prostanoids (Villalba et al., 2009; Sánchez et al., 2010), were found to be impaired along with the ACh-induced decreases in [Ca2+]i in penile arteries from OZR.
In summary, the present results demonstrate that the relaxant responses to insulin were blunted due to altered NO release through the PI3K/eNOS pathway, in penile arteries from the OZR, a model of insulin resistance/metabolic syndrome-associated ED. This endothelial dysfunction further affected the β-adrenoceptor-mediated vasodilatation and correlated with structural abnormalities in penile arteries. Thus, insulin resistance in the penile vasculature is likely to contribute to the endothelial dysfunction observed in insulin-resistant states such as diabetes, obesity and hypertension, all of which act as independent risk factors and jointly increase the risk for ED.
Acknowledgments
This work was supported by grant n° SAF2006-09191 and SAF2009-10448 of the Spanish Ministry of Science and Education (MEC). Cristina Contreras is a research fellow of MEC (FPI grant BES-2007-15205).
Glossary
Abbreviations
- ED
erectile dysfunction
- ET-1
endothelin 1
- KPSS
high K+-physiological saline solution
- L-NNA
Nω-nitro-L-arginine
- LZR
lean Zucker rat
- MAPK
mitogen-activated protein kinase
- NOS
nitric oxide synthase
- OZR
obese Zucker rat
- PI3K
phosphatidylinositol 3-kinase
- PSS
physiological saline solution
- VSMC
vascular smooth muscle cells
Conflict of interest
None to disclose.
Supplemental material
Supporting Information: Teaching Materials; Figs 1–8 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br. J. Pharmacol. (4th edn) 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron AD, Laakso M, Brechtel G, Edelman SV. Mechanism of insulin resistance in insulin-dependent diabetes mellitus: a major role for reduced skeletal muscle blood flow. Clin Endocrinol Metab. 1991;73:637–643. doi: 10.1210/jcem-73-3-637. [DOI] [PubMed] [Google Scholar]
- Begum N, Sandu OA, Duddy N. Negative regulation of rho signaling by insulin and its impact on actin cytoskeleton organization in vascular smooth muscle cells: role of nitric oxide and cyclic guanosine monophosphate signaling pathways. Diabetes. 2002;51:2256–2263. doi: 10.2337/diabetes.51.7.2256. [DOI] [PubMed] [Google Scholar]
- Cardillo C, Nambi SS, Kilcoyne CM, Choucair WK, Katz A, Quon MJ, et al. Insulin stimulates both endothelin and nitric oxide activity in the human forearm. Circulation. 1999;100:820–825. doi: 10.1161/01.cir.100.8.820. [DOI] [PubMed] [Google Scholar]
- Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, et al. Insulin resistance differentially affects the PI3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 2000;105:311–320. doi: 10.1172/JCI7535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Angelo G, Mintz JD, Tidwell JE, Schreihofer AM, Pollock DM, Stepp DW. Exaggerated cardiovascular stress responses and impaired beta-adrenergic-mediated pressor recovery in obese Zucker rats. Hypertension. 2006;48:1109–1115. doi: 10.1161/01.HYP.0000247306.53547.d4. [DOI] [PubMed] [Google Scholar]
- De Fronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab. 2007;293:E1134–E1139. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- Federici M, Menghini R, Mauriello A, Hribal ML, Ferrelli F, Lauro D, et al. Insulin-dependent activation of endothelial nitric oxide synthase is impaired by O-linked glycosylation modification of signaling proteins in human coronary endothelial cells. Circulation. 2002;106:466–472. doi: 10.1161/01.cir.0000023043.02648.51. [DOI] [PubMed] [Google Scholar]
- Ferro A, Coash M, Yamamoto T, Rob J, Ji Y, Queen L. Nitric oxide-dependent beta2-adrenergic dilatation of rat aorta is mediated through activation of both protein kinase A and Akt. Br J Pharmacol. 2004;143:397–403. doi: 10.1038/sj.bjp.0705933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca VA, Jawa A. Endothelial and erectile dysfunction, diabetes mellitus, and the metabolic syndrome: common pathways and treatments? Am J Cardiol. 2005;96:13M–18M. doi: 10.1016/j.amjcard.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gao F, Gao E, Yue TL, Ohlstein EH, Lopez BL, Christopher TA, et al. Nitric oxide mediates the antiapoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:1497–1502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M. Regulation of ob gene overexpression in obesity. Biomed Pharmacother. 1997;51:318–323. doi: 10.1016/S0753-3322(97)88048-1. [DOI] [PubMed] [Google Scholar]
- Hurt KJ, Musicki B, Palese MA, Crone JK, Becker RE, Moriarity JL, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G. The metabolic syndrome and erectile dysfunction: multiple vascular risk factors and hypogonadism. Eur Urol. 2006;50:426–427. doi: 10.1016/j.eururo.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, et al. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. J Clin Invest. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn AM, Allen JC, Seidel CL, Song T. Insulin inhibits serotonin-induced Ca2+ influx in vascular smooth muscle. Circulation. 1994;90:384–390. doi: 10.1161/01.cir.90.1.384. [DOI] [PubMed] [Google Scholar]
- Katakam PV, Tulbert CD, Snipes JA, Erdös B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol. 2005;288:H854–H860. doi: 10.1152/ajpheart.00715.2004. [DOI] [PubMed] [Google Scholar]
- Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation. 2006;113:1888–1904. doi: 10.1161/CIRCULATIONAHA.105.563213. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004;44:956–962. doi: 10.1161/01.HYP.0000147559.10261.a7. [DOI] [PubMed] [Google Scholar]
- Li QX, Xiong ZY, Hu BP, Tian ZJ, Zhang HF, Gou WY, et al. Aging-associated insulin resistance predisposes to hypertension and its reversal by exercise: the role of vascular vasorelaxation to insulin. Basic Res Cardiol. 2009;104:269–284. doi: 10.1007/s00395-008-0754-8. [DOI] [PubMed] [Google Scholar]
- Ma H, Zhang HF, Yu L, Zhang QJ, Li J, Huo JH, et al. Vasculoprotective effect of insulin in the ischemic/reperfused canine heart: role of Akt-stimulated NO production. Cardiovasc Res. 2006;69:4–6. doi: 10.1016/j.cardiores.2005.08.019. [DOI] [PubMed] [Google Scholar]
- Miller AW, Tulbert C, Puskar M, Busija DW. Enhanced endothelin activity prevents vasodilation to insulin in insulin resistance. Hypertension. 2002;40:78–82. doi: 10.1161/01.hyp.0000022806.87281.62. [DOI] [PubMed] [Google Scholar]
- Montorsi P, Montorsi F, Schulman CC. Is erectile dysfunction the ‘tip of the iceberg’ of a systemic vascular disorder? Eur Urol. 2003;44:352–354. doi: 10.1016/s0302-2838(03)00307-5. [DOI] [PubMed] [Google Scholar]
- Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2005;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung SC, Keum EM, Park SY, Lee MY, Kim SC. Vasomotor action of insulin in rabbit normal corpum cavernosum. Eur J Pharmacol. 2006;536:142–147. doi: 10.1016/j.ejphar.2006.01.046. [DOI] [PubMed] [Google Scholar]
- Okon EB, Chung AW, Rauniyar P, Padilla E, Tejerina T, McManus BM, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes. 2005;54:2415–2423. doi: 10.2337/diabetes.54.8.2415. [DOI] [PubMed] [Google Scholar]
- Prieto D. Physiological regulation of penile arteries and veins. Int J Impot Res. 2008;20:17–29. doi: 10.1038/sj.ijir.3901581. [DOI] [PubMed] [Google Scholar]
- Sánchez A, Contreras C, Villalba N, Martínez P, Martínez AC, Briones A, et al. Altered arachidonic acid metabolism via COX-1 and COX-2 contributes to the endothelial dysfunction of penile arteries from obese Zucker rats. Br J Pharmacol. 2010;159:604–616. doi: 10.1111/j.1476-5381.2009.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen U, Prieto D, Hernández M, Sáenz de Tejada I, García-Sacristán A. Adrenoceptor-mediated regulation of the contractility in horse penile resistance arteries. J Vasc Res. 1997;34:90–102. doi: 10.1159/000159206. [DOI] [PubMed] [Google Scholar]
- Standley PR, Zhang F, Ram JL, Zemel MB, Sowers JR. Insulin attenuates vasopressin-induced calcium transients and a voltage-dependent calcium response in rat vascular smooth muscle cells. J Clin Invest. 1991;88:1230–1236. doi: 10.1172/JCI115426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley PR, Ram JL, Sowers JR. Insulin attenuation of vasopressin-induced calcium responses in arterial smooth muscle from Zucker rats. Endocrinology. 1993;133:1693–1699. doi: 10.1210/endo.133.4.8404611. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JD, McMillin SL, Riehle C, Tanner J, Palionyte M, Hillas E, et al. Contribution of insulin and Akt1 signaling to endothelial nitric oxide synthase in the regulation of endothelial function and blood pressure. Circ Res. 2009;104:1085–1094. doi: 10.1161/CIRCRESAHA.108.189316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaroglio TA, Lo CS. Regulation of fibronectin by insulin-like growth factor-I in cultured rat thoracic aortic smooth muscle cells and glomerular mesangial cells. Exp Cell Res. 1994;215:338–346. doi: 10.1006/excr.1994.1350. [DOI] [PubMed] [Google Scholar]
- Villalba N, Stankevicius E, García-Sacristán A, Simonsen U, Prieto D, et al. Contribution of both Ca2+ entry and Ca2+ sensitization to the alpha1-adrenergic vasoconstriction of rat penile small arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1157–H1169. doi: 10.1152/ajpheart.01034.2006. [DOI] [PubMed] [Google Scholar]
- Villalba N, Martinez MP, Briones AM, Sánchez A, Salaíces M, García-Sacristán A, et al. Differential structural and functional changes of penile and coronary arteries from obese Zucker rats. Am J Physiol Heart Circ Physiol. 2009;297:H696–H707. doi: 10.1152/ajpheart.01308.2008. [DOI] [PubMed] [Google Scholar]
- Wingard C, Fulton D, Husain S. Altered penile vascular reactivity and erection in the Zucker obese-diabetic rat. J Sex Med. 2007;4:348–462. doi: 10.1111/j.1743-6109.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski H, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–1545. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


