Abstract
BACKGROUND AND PURPOSE
Advanced glycation endproducts (AGEs) represent one of the many types of chemical modifications that occur with age in long-lived proteins. AGEs also accumulate in pathologies such as diabetes, cardiovascular diseases, neurodegeneration and cancer. Mast cells are major effectors of acute inflammatory responses that also contribute to the progression of chronic diseases. Here we investigated interactions between AGEs and mast cells.
EXPERIMENTAL APPROACHES
Histamine secretion from AGEs-stimulated mast cells was measured. Involvement of a receptor for AGEs, RAGE, was assessed by PCR, immunostaining and use of inhibitors of RAGE. Production of reactive oxygen species (ROS) and cytokines was measured.
KEY RESULTS
Advanced glycation endproducts dose-dependently induced mast cell exocytosis with maximal effects being obtained within 20 s. RAGE mRNA was detected and intact cells were immunostained by a specific anti-RAGE monoclonal antibody. AGEs-induced exocytosis was inhibited by an anti-RAGE antibody and by low molecular weight heparin, a known RAGE antagonist. RAGE expression levels were unaltered after 3 h treatment with AGEs. AGE-RAGE signalling in mast cells involves Pertussis toxin-sensitive Gi-proteins and intracellular Ca2+ increases as pretreatment with Pertussis toxin, caffeine, 2-APB and BAPTA-AM inhibited AGE-induced exocytosis. AGEs also rapidly stimulated ROS production. After 6 h treatment with AGEs, the pattern of cytokine secretion was unaltered compared with controls.
CONCLUSIONS AND IMPLICATIONS
Advanced glycation endproducts activated mast cells and may contribute to a vicious cycle involving generation of ROS, increased formation of AGEs, activation of RAGE and to the increased low-grade inflammation typical of chronic diseases.
Keywords: mast cells, AGEs, RAGE, exocytosis, inflammation
Introduction
Advanced glycation endproducts (AGEs) form a heterogeneous group of irreversible adducts resulting from non-enzymatic glycation and oxidation of proteins (Baynes, 2001). Reducing sugars such as glucose, react non-enzymatically with amino groups of proteins, lipids and nucleic acids to produce AGEs via the Maillard reaction that involves a series of reactions forming Schiff bases and Amadori products (Singh et al., 2001). AGEs accumulate in diverse pathological conditions including hyperglycaemia and diabetes (Brownlee, 1992), amyloidosis (Yan et al., 1996), tumours (Taguchi et al., 2000), diabetic vascular dysfunction (Park et al., 1998) and cardiac diastolic dysfunction (Li et al., 2005), oxidant stress and inflammation (Ramasamy et al., 2005). It has also been suggested that AGEs form during natural aging, in particular as a consequence of exposure of long-lived proteins to homeostatic levels of glucose. The development of increased insulin resistance in the aging population is also believed to favour generation of AGEs (Ramasamy et al., 2005).
Many receptors for AGEs have been identified, including scavenger receptors SR-A type I and type II, SR-BI, CD36 (Ohgami et al., 2002), galectin-3 and RAGE (Neeper et al., 1992; Schmidt et al., 1992; El Khoury et al., 1994; Vlassara et al., 1995; Li et al., 1996). Such receptors have been linked to a number of different functions including detoxification/removal of AGEs and modulation of gene expression by activation of receptor-triggered signal transduction pathways (Hudson et al., 2003). Among these AGE-binding proteins, RAGE has been clearly identified as a signal transduction receptor. RAGE belongs to the immunoglobulin superfamily of receptors and is a multiligand receptor. In addition to AGEs, RAGE is also a receptor for amphoterin (Hori et al., 1995), S100/calgranulins (Hofmann et al., 1999), amyloid-β peptide and β-sheet fibrils (Yan et al., 1996). The AGE-RAGE interaction was shown to activate the NF-κB pathway (Schmidt et al., 2001). NF-κB is a critical transcription factor transducing a variety of inflammatory and pro- or anti-apoptotic signals in the cell, depending on the time course, site and chronicity of stimulus. Importantly, one consequence of RAGE-dependent activation of NF-κB is the up-regulation of RAGE itself (Li and Schmidt, 1997). Thus, the AGE-RAGE system is likely to sustain the chronic inflammatory state that characterizes many AGE-related pathologies (Schmidt et al., 2001).
Chronic inflammation is a major causative factor in a wide range of human pathologies such as cardiovascular diseases, neurodegeneration and cancer. An increased number of mast cells at sites of chronic inflammation was first described over a hundred years ago (Ehrlich, 1879). Mast cells are tissue dwelling cells that play a pivotal role in allergic reactions and take part in other pathophysiological conditions such as innate and acquired immunity, autoimmune diseases, wound healing, fibrosis and tumours (Gurish and Austen, 2001; Bischoff, 2007). Activated mast cells release stored and de novo synthesized mediators including histamine, cytokines, leukotrienes, prostaglandins and proteases (Marshall, 2004). Mast cell degranulation can thus initiate an acute inflammatory response that might contribute to the progression of chronic diseases. Mast cells could therefore represent a major actor in the low-grade chronic inflammatory state observed in pathologies characterized by a strong accumulation of AGEs. However, to date, the involvement of mast cells in diabetes, cardiovascular diseases, neurodegeneration or cancers is poorly studied.
We thus investigated the possible stimulatory effects of AGEs on mast cells. Here, we show for the first time that AGEs rapidly induce secretion of histamine from rat peritoneal mast cells. Pretreatment with an anti-RAGE monoclonal antibody (mAb) and with low molecular weight heparin, an antagonist of RAGE, inhibits AGE-induced degranulation. Pretreatment with Pertussis toxin also inhibited AGE-stimulated secretion, consistent with RAGE signalling involving Gi-proteins. RAGE-mediated exocytosis required the mobilization of intracellular calcium pools. We also found that AGEs stimulated the production of reactive oxygen species (ROS) in mast cells. Taken together, our results indicate that mast cells may play a key role in AGE-mediated inflammatory processes.
Methods
Isolation and purification of mast cells
All animal care and experimental procedures were in accordance with Institutional policies (N° D-67-218-26, Direction Départementale des Services Vétérinaires du Bas-Rhin). Mature mast cells were isolated as previously described (Ferry et al., 2001). Briefly, male Wistar rats (250–350 g, 12–14 weeks old), raised in the animal house facilities in the Faculty of Pharmacy, were anesthetized with sodium pentobarbital (50 mg·kg−1 ip), before exsanguination. Buffer (10 mL of 137 mM NaCl, 2.7 mM KCl, 0.3 mM CaCl2, 1.0 mM MgCl2, 0.4 mM NaH2PO4, 5.6 mM glucose, 10 mM HEPES and NaOH to pH 7.4) supplemented with 0.1% BSA was injected i.p. Peritoneal fluid was collected after gentle abdominal massage and centrifuged for 3 min at 180×g. The pellet of mixed peritoneal cells was resuspended in the same buffer and mast cells were purified by centrifugation for 10 min at 220×g on a discontinuous BSA gradient (30% and 40%, w/v). The approximate yield of mast cells was 1–1.5 × 106 cells per animal. The pellet was then resuspended and mast cells were examined under a light microscope for viability (>95%) and purity (>97%) using Trypan blue and toluidine blue respectively.
RNA extraction and RT-PCR
Total RNA was extracted from mast cells with PureZOL™ reagent (Bio-Rad, Hercules, CA, USA) according to the manufacturer's recommendations. Reverse transcription (RT) was performed using 500 ng total RNA with the SuperScript™III First-strand synthesis system (Invitrogen, Paisley, UK) according to the manufacturer's protocol. Amplification was assessed using 1 µL RT products in a mixture containing 200 µM of each dNTP, 0.5 µM oligonucleotide primer, 1 × Phusion HF buffer and 0.02 U·µL−1 Phusion DNA polymerase (Finnzymes, Espoo, Finland). PCR primers 5′-GGAATTGTCGATGAGGGGAC-3′ (forward) and 5′-CAACAGCTGAATGCCCTCTG-3′ (reverse) were used to detect rat RAGE mRNA [25], and 5′-ATGACCACAGTCCATGCCAT-3′ (forward) and 5′-TTCAGCTCTGGGATGACCTT-3′ (reverse) for rat GAPDH mRNA. Cycling parameters were: 98°C for 30 s, 60°C for 30 s and 72°C for 30 s for 30 cycles, followed by a final elongation at 72°C for 5 min. PCR products were run on 2% agarose gels stained with 1 µg·mL−1 ethidium bromide.
Immunofluorescence microscopy
Purified mast cells were allowed to adhere to glass coverslips for 1 h at 37°C. Cells were fixed for 10 min at −20°C with 100% methanol. Non-specific binding sites were blocked with 2% BSA/PBS for 1 h at room temperature under gentle agitation. Mast cells were incubated with a primary monoclonal antibody (mAb) directed against RAGE (10 µg·mL−1) for 1 h at room temperature under gentle agitation. This mAb targets an extracellular epitope of RAGE, as it blocks AGE binding to RAGE. Mast cells were then incubated for 1 h at room temperature with the secondary Ab (FITC-coupled rabbit anti-goat IgG, 1 µg·mL−1) under gentle agitation. Coverslips were mounted and observed using an epifluorescence microscope (Nikon Diaphot). As control, no fluorescence was observed from cells treated only with the secondary FITC-coupled Ab.
Quantification of mast cell exocytosis
Purified mast cells (2.5 × 104 cells/assay) were pre-incubated for 5 min at 37°C before challenge with different agents for 10 min at 37°C. Reactions were stopped by adding ice-cold buffer. Exocytosis of mast cells was quantified by determining the amount of secreted histamine by spectrofluorimetry, as previously described (Ferry et al., 2001; Sick et al., 2009). Values for stimulated histamine release in the supernatant were expressed as a percentage of the total cellular histamine content obtained after cell lysis (supernatant/(supernatant + pellet) × 100) and were corrected for the basal release of histamine (similarly calculated) that occurred in the absence of any stimulus. Basal histamine release from control non-stimulated cells was less than 5% of total content.
Measurement of intracellular calcium
Intracellular Ca2+ was measured from Fura-2-loaded mast cell suspensions using a spectrofluorimeter (Hitachi F-2000) essentially as previously described (Kassel et al., 1995). Briefly, mast cells (1 × 106 cells·mL−1) were incubated with 1 µM Fura-2/AM for 15 min at room temperature in HEPES buffer. Cells were then washed twice in HEPES buffer and re-suspended in the same buffer at 1 × 106 cells·mL−1 in a 1 cm quartz cuvette. Cells were continuously stirred and sequentially excited at 340 and 380 nm for 1 s periods at room temperature; emitted fluorescence was measured at 510 nm (Lynch et al., 1994).
Assay of cytokine secretion
Mast cells (5 × 105 cells·mL−1) were challenged with the AGE mimetic, glucosamide-bovine serum albumin (Glu-BSA) (10 µM), peptide 4N1 (100 µM) or compound 48/80 (1 µg·mL−1) and incubated for 6 h at 37°C. After centrifugation, the supernatant was incubated overnight at 4°C on a membrane array assay coated with immobilized, capture anti-cytokine mAbs (RayBio® Rat Cytokine Antibody Array, RayBiotech, Norcross, GA, USA). Determination and quantification of cytokines were done according to the manufacturer's instructions.
Determination of reactive oxygen species (ROS)
Mast cells (106·mL−1) were preincubated for 5 min at 37°C. In total, 100 µL aliquots were stimulated with 10 µM Glu-BSA for 5 min. ROS were immediately determined by a chemiluminescent assay as previously described (Andréet al., 2005).
Data analysis
Results are presented as mean ± SEM. from at least three independent experiments. Data were analysed with one-way anova (followed by Bonferroni's post hoc test when appropriate). Significant differences between means were assumed when P < 0.05
Materials
2-aminoethoxydiphenyl borate (2-APB) and Fura-2/AM were from Calbiochem (San Diego, CA, USA). Galactosamide-BSA (Gal-BSA), glucosamide-BSA (Glu-BSA), p-aminophenylmannopyranoside-BSA (Man-BSA), maltosyl-BSA (Malt-BSA), lactosyl-BSA (Lact-BSA), EDTA, BAPTA-AM, caffeine, pertussis toxin (PTX), luminol, low molecular weight heparin (LMWH), compound 48/80 and FITC-coupled rabbit anti-goat IgG were from Sigma (St. Louis, MO, USA). Peptide 4N1 was from Bachem (Bubendorf, Switzerland). Anti-RAGE mAb was from R&D Systems (Minneapolis, MN, USA) and anti-CD48 mAb (clone OX45) was from Serotec (Oxford, UK).
Results
AGEs induce rapid mast cell exocytosis
A number of prototype AGEs were tested for their capacity to activate mast cell exocytosis. Glu-BSA is a compound widely used to mimic AGEs (Li et al., 1996; Kuniyasu et al., 2003; Mercer et al., 2004; Valencia et al., 2004; Ishiguro et al., 2005) and Gal-BSA has also been used in some studies (Wang et al., 2002). We found that stimulation of rat isolated peritoneal mast cells by Glu-BSA, Man-BSA and Gal-BSA induced exocytosis in a dose-dependent manner, as quantified by secreted histamine. A maximal effect of 65–75% release of total histamine content was reached at 10 µM with an EC50 close to 1 µM (Figure 1A). In contrast, Lact-BSA and Malt-BSA were without effect. The kinetics of exocytosis induced by Glu-BSA were rapid, with maximal effects being obtained within 20 s (Figure 1B).
Figure 1.
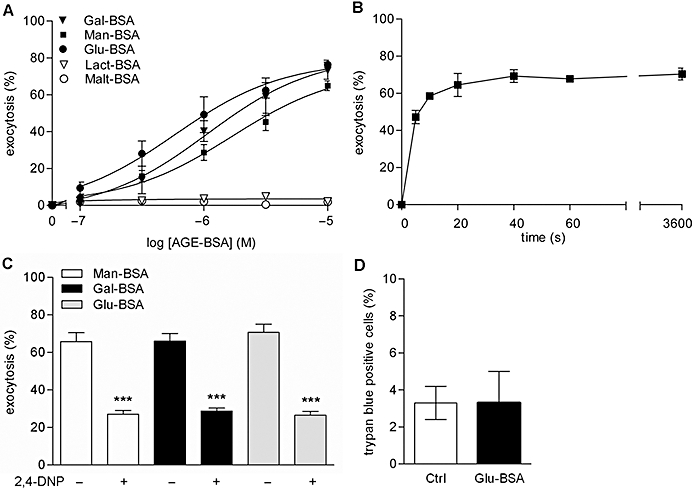
Dose-response curves for AGEs-induced exocytosis from rat peritoneal mast cells (A). Cells were stimulated for 10 min at 37°C with the different compounds and exocytosis was assessed by measuring released histamine. Data are mean ± SEM from five independent experiments. Time course of exocytosis induced by Glu-BSA (B). Cells were pre-incubated for 5 min at 37°C and then stimulated for the indicated times with 10 µM Glu-BSA at 37°C. Data are mean ± SEM from three independent experiments. Effect of pretreatment with 2,4-dinitrophenol and 2-deoxyglucose on exocytosis induced by AGEs (C). Cells were pre-incubated with 0.1 mM 2,4-dinitrophenol and 5 mM 2-deoxyglucose (shown as 2,4-DNP) for 1 h at 37°C before stimulation with three different AGEs, all at 10 µM. Values are mean ± SEM for three independent experiments. ***P < 0.001, significantly different compared with the absence of DNP). Effect of Glu-BSA on cell viability (D). Cells were stimulated for 10 min at 37°C with 10 µM Glu-BSA. Cell viability was assessed by Trypan blue staining, immediately after stimulation. Cells were visualized on a microscope and counted manually, without knowledge of the treatments. Values are mean ± SEM from three independent experiments. AGEs, advanced glycation endproducts.
In order to verify that the stimulatory effects of AGEs were not of cytotoxic nature (e.g. non specific cell lysis), mast cells were incubated in the presence of two metabolic inhibitors (2,4-dinitrophenol, deoxyglucose). This treatment has been long ago shown to inhibit increases in Ca2+ uptake and exocytotic responses from mast cells stimulated by IgE or compound 48/80 (Cochrane and Distel, 1982; Mohr and Fewtrell, 1990) but not by the Ca2+ ionophore A23187 (Cochrane and Distel, 1982). As shown in Figure 1C, pre-incubation of mast cells with metabolic inhibitors resulted in a significant decrease in exocytosis induced by Glu-BSA, Man-BSA and Gal-BSA. This indicates that AGE-induced exocytosis from mast cells does not result from a non-specific increase in Ca2+ subsequent for example to lysis of cell membranes. Cell viability was also assessed by Trypan blue staining, with the percentage of living mast cells after Glu-BSA treatment being similar to that observed in resting mast cells, both of them being greater then 96% (Figure 1D). This confirmed that AGE-induced exocytosis did not arise from cytotoxicity.
Role of RAGE in AGE-induced mast cell activation
To our knowledge, the presence of RAGE in rat peritoneal mast cells has never been previously described. Therefore, we first checked the expression of RAGE in mast cells by using specific PCR primers for the detection of RAGE mRNA (Figure 2A). A PCR product of 453 bp in size was found, as expected for rat RAGE (Sorci et al., 2003). RAGE expression on fixed, intact rat peritoneal mast cells was also assessed by immunostaining using a specific anti-RAGE mAb that targets an extracellular eptitope on RAGE and standard epifluorescence microscopy. The observed pattern of staining is consistent with RAGE being expressed at the cell membrane (Figure 2B). According to the manufacturer, 5–25 µg·mL−1 of this function-blocking mAb blocks 50% of the binding of AGE-BSA to RAGE, with ≥95% inhibition of binding being achieved at a concentration of 166 µg·mL−1. Thus, the effects of this anti-RAGE mAb were tested at 10 and 100 µg·mL−1. Glu-BSA-induced exocytosis was dose-dependently inhibited by anti-RAGE mAb (40% inhibition at 10 µg·mL−1 and 60% inhibition at 100 µg·mL−1; Figure 2C). Pretreatment with an isotype control IgG (10 and 100 µg·mL−1 anti-CD48 Ab OX45) was without effect on Glu-BSA-induced exocytosis (Figure 2C), consistent with a lack of non-specific action of the anti-RAGE mAb. Basal exocytosis from cells in the absence of Glu-BSA was unaffected by pretreatment with 100 µg·mL−1 anti-RAGE mAb (data not shown). Low molecular weight heparin (LMWH), an antagonist of RAGE (Myint et al., 2006), was also tested. Mast cell exocytosis was dose-dependently inhibited by LMWH (Figure 2D). The maximal inhibitory effect against Glu-BSA-induced exocytosis obtained after pre-incubation with LMWH (5 IU·mL−1) was greater than 75%.
Figure 2.

Receptor for advanced glycation endproduct (RAGE) expression in rat peritoneal mast cells. mRNA for RAGE was detected by first strand cDNA PCR (A). Immunocytochemical determination of RAGE on intact, fixed mast cells (B). Effect of pretreatment with anti-RAGE mAb on exocytosis induced by Glu-BSA (C). Cells were pre-incubated with anti-RAGE mAb (1 h, 37°C) and then stimulated with 10 µM Glu-BSA for 10 min at 37°C. Pretreatment with an isotype control IgG (10 and 100 µg·mL−1 anti-CD48 Ab OX45) was without effect on Glu-BSA secretory responses. Data are mean ± SEM for three independent experiments. ***P < 0.001, significantly different compared with untreated cells. Effect of pretreatment with low molecular weight heparin (LMWH) on exocytosis induced by Glu-BSA (D). Cells were pre-incubated with LMWH (5 min, 37°C) and then stimulated for 10 min with 1 µM Glu-BSA at 37°C. Data are mean ± SEM from three independent experiments. Effect of pretreatment with Pertussis toxin (PTX) on exocytosis induced by AGEs (E). Cells were pre-incubated with PTX (20 ng·mL−1, 2 h, 37°C) and then stimulated for 10 min at 37°C with three different AGEs, all at 10 µM. Data are mean ± SEM for three independent experiments. ***P < 0.001, significantly different compared with the absence of PTX. AGEs, advanced glycation endproducts.
Signalling pathway for AGE-induced exocytosis
A few previous studies have addressed the downstream signalling pathways consequent to RAGE activation. For example, engagement of RAGE has been shown to trigger p21ras, p44/p42 MAP kinases, p38 MAP kinases, rho GTPases, PI3K and the JAK/STAT pathway, leading to downstream consequences such as the activation of NF-κB and CREB (Yan et al., 2003). Other reports have suggested a possible coupling of RAGE with a pertussis toxin-sensitive G-protein (Degryse et al., 2001; Yang et al., 2007). In addition to the classical activation pathway involving high affinity IgE receptor FcεRI binding, mast cells are also activated by a Gi-protein dependent pathway (Aridor et al., 1993; Ferry et al., 2002). We therefore assessed the possible involvement of G-proteins following stimulation of RAGE by AGEs. As illustrated in Figure 2E, pre-incubation of mast cells with Pertussis toxin resulted in a significant decrease in exocytosis induced by Glu-BSA, Man-BSA and Gal-BSA. These results support the implication of G-proteins in downstream RAGE signalling, in agreement with previously published reports (Degryse et al., 2001; Yang et al., 2007).
Mast cell exocytosis requires an increase in intracellular Ca2+ (Nakamura and Ui, 1985; Mousli et al., 1989; Bueb et al., 1992; Ferry et al., 2001). In order to determine whether AGE-induced histamine secretion was Ca2+-dependent, mast cells were loaded with Fura-2 and stimulated with 10 µM Glu-BSA. As illustrated in Figure 3A, application of Glu-BSA induced a rapid increase in intracellular Ca2+. In contrast, Lact-BSA was without effect on intracellular Ca2+, consistent with the absence of histamine secretion after pretreatment with this AGE prototype (Figure 3B). To confirm that a rise in intracellular Ca2+ was necessary for AGE-induced secretion, mast cells were pretreated with BAPTA-AM (Gianonne et al., 2004). The resulting dose-dependent chelation of intracellular Ca2+ was mirrored by a similar inhibition of Glu-BSA stimulated secretion of histamine (Figure 3C). As increase in intracellular Ca2+ can either result from an external influx and/or release from internal Ca2+ stores, histamine secretion induced by Glu-BSA was measured in Ca2+-free buffer (Figure 3D). Histamine secretion induced by Glu-BSA in Ca2+-free buffer was similar to that obtained in Ca2+-containing HEPES buffer. These data indicate that Glu-BSA-induced exocytosis is exclusively dependent from internal calcium stores. In order to confirm this observation, mast cells were pretreated with caffeine and 2-APB, compounds that interfere with release of Ca2+ from internal stores by acting as antagonists of the inositol trisphosphate receptor (IP3R; Maruyama et al., 1997; Teraoka et al., 1997). Both caffeine and 2-APB produced inhibition of Glu-BSA induced secretion in a dose-dependent manner (Figures 3E, F). Although 2-APB has more recently been described to inhibit Ca2+ influx through transient receptor potential (TRP) channels (Togashi et al., 2008), the observed Glu-BSA induced secretion in the absence of extracellular Ca2+ is consistent with the inhibitory effect of 2-APB arising from its action on Ca2+ release from internal stores.
Figure 3.

Changes in emitted fluorescence (at 510 nm) from Fura-2-loaded mast cells excited alternately at 340 and 380 nm upon stimulation (arrow) with 10 µM Glu-BSA (A) and 10 µM Lact-BSA (B). Traces are representative of three independent experiments. Effect of pretreatment with BAPTA-AM (C) on exocytosis induced by Glu-BSA. Cells were pre-incubated with BAPTA-AM at the indicated concentrations (15 min, 37°C) and then stimulated for 10 min with 1 µM Glu-BSA. Data are mean ± SEM from three independent experiments. Effect of Glu-BSA on mast cell exocytosis in Ca2+-free HEPES buffer (D). Cells were stimulated for 10 min at 37°C with 10 µM Glu-BSA. Data are mean ± SEM from four independent experiments. Effects of caffeine (E) and 2-APB (F) on exocytosis induced by Glu-BSA. Cells were pre-incubated with the different agents at the indicated concentrations (15 min, 37°C) and then stimulated for 10 min with 10 µM (E) or 1 µM Glu-BSA (F) at 37°C. Data are mean ± SEM from three independent experiments.
AGEs induce ROS production in mast cells
Because reactive oxygen species (ROS) strongly sustain both inflammatory reactions and generation of AGEs (Ramasamy et al., 2005), we determined whether Glu-BSA was capable of increasing ROS production in mast cells. As shown in Figure 4A, we found using a chemiluminescent assay that Glu-BSA dose-dependently increased ROS production in mast cells, with a fourfold increase being obtained at 10 µM Glu-BSA. The kinetics of ROS production induced by Glu-BSA were rapid, with maximal effects being obtained within 30 s (Figure 4B). Pretreatment with a function blocking anti-RAGE mAb significantly inhibited ROS production stimulated by Glu-BSA, with measured levels of ROS being not different from those produced by control, untreated, cells (Figure 4C). These data are consistent with RAGE being the receptor responsible for AGE-stimulated ROS production in mast cells.
Figure 4.

Production of reactive oxygen species (ROS) induced by Glu-BSA (A). Cells were pre-incubated (5 min, 37°C) and then stimulated for 1 min with the indicated concentrations of Glu-BSA at 37°C. ROS were assessed using a chemiluminescent assay (RLU, relative light units). Data are mean ± SEM for four independent experiments. ***P < 0.001,significantly different compared with non-stimulated cells. Time course of ROS production induced by Glu-BSA (B). Cells were pre-incubated (5 min, 37°C) and then stimulated for the indicated times with 10 µM Glu-BSA at 37°C. Data are mean ± SEM for four independent experiments. ***P < 0.001, significantly different compared with time 0. Effect of pretreatment with anti-RAGE mAb on ROS production induced by Glu-BSA (C). Cells were pre-incubated with anti-RAGE mAb (100 µg·mL−1, 1 h, 37°C) and then stimulated with 10 µM Glu-BSA for 1 min at 37°C. Data are mean ± SEM for four independent experiments. ***P < 0.001, significantly different compared with non-stimulated cells; ###significantly different compared with the absence of blocking Ab.
Cytokine secretion and RAGE expression are unchanged in AGE-stimulated mast cells
Several studies have suggested that ligand-RAGE interactions in endothelial cells, smooth muscle cells, monocytes/macrophages and neurons result in activation of the NF-κB pathway, thereby inducing the subsequent secretion of TNF-α, IL-6 or IL-1β (Ramasamy et al., 2005). We therefore assessed whether cytokine secretion from mast cells was increased following stimulation by Glu-BSA. Using an array assay for 19 different cytokines, no significant differences were found between the level of cytokines secreted basally from non-stimulated mast cells and the level of cytokines secreted after 6 h pretreatment with 10 µM Glu-BSA (Figure 5A). We also tested for cytokine secretion induced by compound 48/80 (1 µg·mL−1; Figure 5B) and peptide 4N1 (100 µM; Figure 5C), two other non IgE-related compounds known to cause histamine release (Mousli et al., 1989; Miller et al., 1995; Sick et al., 2009). No significant differences were found between the level of cytokines secreted basally from non stimulated mast cells and the level of cytokines secreted after pretreatment with these compounds.
Figure 5.

Effect of pretreatment with Glu-BSA (A), compound 48/80 (B) and peptide 4N1 (C) on cytokine secretion from mast cells. Cells were incubated with 10 µM Glu-BSA (A), 1 µg·mL−1 48/80 (B) or 100 µM 4N1 (C) for 6 h at 37°C or left untreated (control). Cytokines were assessed using an array assay with the cytokine-specific mAbs shown in the table (pos: positive control; neg: negative control). Blots were quantified following manufacturer's instructions and are presented after normalization to values for positive controls for untreated and treated cells. The data are representative of two independent experiments.
As the NF-κB pathway was also identified as mediating RAGE overexpression after AGE-RAGE ligation, mast cells were incubated with 10 µM Glu-BSA for 3 h at 37°C and transcript levels for RAGE were assessed by PCR. No differences were found between the basal mRNA levels of RAGE in control cells compared with those in Glu-BSA treated cells (data not shown).
Discussion
To our knowledge, our data demonstrate for the first time that AGEs are capable of activating secretion of histamine from mast cells. Mast cells can be divided in two distinct classes: mucosal mast cells and serosal (or connective tissue) mast cells. Rat peritoneal mast cells belong to the serosal mast cell class. Serosal mast cells are mainly distributed in the sub-mucosa of the gastrointestinal tract, in the skin, in the brain and also in close proximity to blood vessels (Marshall, 2004). In particular, AGEs are known to accumulate in the vessel wall, especially in diabetes. Moreover, AGEs have been reported to accumulate in the brain during normal aging and also in several neurodegenerative disorders, including Alzheimer's disease and amyotrophic lateral sclerosis (Yan et al., 2003; Ramasamy et al., 2005). Thus, serosal mast cells were tested for their reactivity to AGEs because of their critical location surrounding vessel walls and in the brain, where they are more likely to encounter AGEs compared with mucosal mast cells. Furthermore, serosal mast cells are long-lived cells, unlike mucosal mast cells, and may therefore play a critical role in age-related inflammation.
We found that AGE-BSA activated mast cells with an apparent selectivity depending on the sugar attached to the BSA (Figure 1). Indeed, Glu-BSA, Gal-BSA and Man-BSA (all monosaccharide-containing compounds) induced mast cell exocytosis whereas Lact-BSA and Malt-BSA (which are disaccharide-containing compounds) were unable to activate mast cells. Physiological accumulation of such disaccharide-containing compounds may not occur because of enzymatic degradation occurring during assimilation. In any case, the concentrations of AGEs-BSA used here are in the same range as recently reported (Lappas et al., 2007; Nah et al., 2007; Rüster et al., 2008). The time course of exocytosis induced by 10 µM Glu-BSA was rapid, with a plateau response being reached within 10 to 20 s. These rapid kinetics indicate that the AGE-mediated pathway is different from the IgE/FcεRI pathway for which stimulated exocytosis reaches a maximum only after a few minutes (Blank and Rivera, 2004). Note that the time course of histamine release induced by Glu-BSA is similar to that obtained after treatment of serosal mast cells with many other physiological secretagogues including substance P, bradykinin, neuropeptide Y or natural polyamines such as agmatine, spermine, spermidine (Mousli et al., 1989; Bueb et al., 1992; Ferry et al., 2002). We confirmed that histamine secretion induced by AGEs resulted from exocytosis and not lytic processes.
In addition to histamine, which alters vascular permeability, mast cells release other co-localized granule-associated mediators including heparin, chondroitin sulphate and proteases such as tryptase and chymase. These additionally released mediators take part in enhancing the effects of chemokines or cytokines and therefore contribute in the resultant remodelling of surrounding tissues and the recruitment of various effector cells (Marshall, 2004). Indeed, histamine and proteases promote for example the migration, maturation, differentiation and function of immune cells such as T cells, B cells, monocyes/macrophages, dendritic cells and granulocytes (Galli et al., 2008). Therefore, mast cells are of particular interest because they are very likely to contribute in a crucial manner in controlling the balance between pro- and anti-inflammatory signals that are known to be disturbed in cardiovascular diseases, neurodegeneration or cancers.
In the current study, we demonstrate for the first time that RAGE, a receptor for AGEs, is expressed in rat peritoneal mast cells (Figure 2). A recent report showed that RAGE was also expressed in other mucosal types of mast cells, namely RBL-2H3 cells and BMMC (bone marrow-derived mast cells) and it was proposed that RAGE might mediate, in part, galectin-3-induced apoptosis in these cells (Suzuki et al., 2008). Our data indicated that RAGE mediated AGE-stimulated exocytosis of histamine from peritoneal mast cells because pretreatment with anti-RAGE mAb and low molecular weight heparin, a known antagonist having high affinity for RAGE (Hanford et al., 2004: Myint et al. 2006; Liu et al. 2009), inhibited Glu-BSA-induced histamine secretion. It should be noted that given the current lack of other RAGE antagonists, use of function-blocking Abs is widely used to verify the involvement of RAGE in AGE-induced effects (for example: Yan et al. 1996; Origlia et al. 2008; Touréet al. 2008; Tanikawa et al. 2009). We nevertheless attempted experiments using siRNA-mediated knock-down of RAGE to confirm that RAGE is responsible for AGE-stimulated exocytosis. However, we found that freshly isolated, mature, rat peritoneal mast cells could not be maintained alone in culture for more than 2 days, unfortunately precluding siRNA-based experiments in these cells.
We found that downstream signalling by RAGE in mast cells involves Gi-proteins, as demonstrated by sensitivity to Pertussis toxin. Our results are in agreement with the few reports suggesting a possible coupling of RAGE with Gi-proteins: in human dendritic cells and in rat smooth muscle cells, activation of RAGE by amphoterin was inhibited by Pertussis toxin (Degryse et al. 2001; Yang et al. 2007). As mentioned in those studies, we cannot rule out that RAGE might contribute in targeting AGEs to a heptahelical G-protein-coupled receptor. Nevertheless, accumulating evidence indicates that heterotrimeric G-proteins can also be activated by some non-heptahelical receptors. For example, this property has been attributed to some transmembrane proteins having or not cytosolic kinase activity, and also for some receptors belonging to the class of glycosylphosphatidylinositol (GPI)-anchored proteins (Landry et al. 2006).
Stimulation of histamine secretion by AGEs also requires an increase in intracellular Ca2+ released from internal stores and not influx, from extracellular sources, as Glu-BSA induced histamine occurred in the absence of extracellular Ca2+ (Figure 3). This is in agreement with the well-described signalling pathway leading to mast cell exocytosis that involves Gi-proteins and intracellular Ca2+ increases (Nakamura and Ui, 1985; Mousli et al. 1989; 1992; Bueb et al. 1992; Ferry et al. 2001). However, our data represent the first indication that RAGE may also act via this signalling pathway.
We showed that AGEs induced a dose-dependent increase in ROS production in mast cells with a maximal effect being obtained within 30 s (Figure 4). Our data indicate that RAGE mediates AGE-stimulated ROS production from peritoneal mast cells because pretreatment with anti-RAGE mAb inhibited Glu-BSA-induced ROS production. A large body of evidence suggests that ROS generation, in part via the activation of NADPH oxidase, is a consequence of AGE-RAGE interactions (Yan et al. 1994; Lander et al. 1997; Wautier et al. 2001). Recent studies have underscored the notion that Ca2+ and ROS signalling systems are intimately interrelated. Indeed, a number of ROS-generating and anti-oxidant systems, such as the cell-surface NADPH oxidase, have been shown to be Ca2+-dependent. Cell surface NADPH-oxidases, with rapid kinetics of activation and inactivation, are the most important multienzyme complexes in receptor-mediated signalling cascades that result in the generation of ROS (Brookes et al. 2004). We propose that intracellular Ca2+ increases in mast cells also leads to ROS generation. In support, it has been shown that rat peritoneal mast cells produce ROS in response to the Ca2+ ionophore A23187 (Niu et al. 1996) and that upon degranulation induced via the IgE/antigen pathway, both human and rodent mast cells generate ROS. Both IgE-mediated release of 5-HT and generation of ROS were inhibited by diphenyleneiodonium, a flavoenzyme inhibitor (Swindle et al. 2004). Furthermore, compounds known to induce exocytosis in mast cells such as nerve growth factor (NGF), compound 48/80 and substance P have also been found to cause ROS generation in rat peritoneal mast cells (Brooks et al. 1999). These data are consistent with ROS generation being tightly related to intracellular Ca2+ increase and exocytosis in mast cells. It can also be suggested that ROS production is tightly related to increased formation of AGEs. For example, in endothelial cells, glucose-induced increases in mitochondrial superoxide have been described to be a key factor in generation of AGEs (Nishikawa et al. 2000). The formation of AGEs stimulated by oxidative stress and ROS generation, inflammatory stimuli, physical injury and hyperglycaemia is believed to be a key first step in broad array of injury settings (Ramasamy et al. 2005). Therefore, AGEs may participate in a vicious cycle whereby ROS generation leads to amplified generation of AGEs and activation of RAGE, particularly in inflammation and aging. In agreement, during aging, naturally occurring AGEs accumulate, oxidative damage increases and antioxidant levels decrease (see Dröge and Schipper, 2007). These age-related dysfunctions, together with the low-grade chronic inflammation observed in aging people, are consistent with inflammatory cells, in particular mast cells, being key actors in controlling ROS production, enhanced generation of AGEs and RAGE activation.
Our data show that RAGE-mediated activation of mast cells results in specific short time-course responses like histamine release and ROS production. Indeed, as reviewed recently, differential release of mast cell mediators can occur depending on the stimulus (Galli et al. 2005; Theoharides et al. 2007). Thus, histamine release is not necessarily accompanied by de novo synthesis of cytokines and their subsequent secretion. In our case, stimulation of mast cells with AGE-BSA rapidly leads to histamine release and production of ROS. Responses occurring on a longer time scale (like synthesis and secretion of cytokines or changes in RAGE levels) were absent (Figure 5).
We also show that compound 48/80, a classical mast cell activator, and peptide 4N1, a CD47 receptor agonist, do not trigger cytokine release from rat peritoneal mast cells (Figure 5). However, both 48/80 and CD47-mediated activation of mast cells result in histamine release and ROS production (Brooks et al. 1999; Sick et al. 2009). Note that 48/80 and CD47-mediated signalling pathways share several common features with AGE-mediated mast cell activation: degranulation being essentially complete within 20 s, exclusive release of preformed mediators and lack of dependence on extracellular Ca2+. These are hallmarks of the so-called IgE-independent mast cell activation pathway (see Gies et al. 1993; Metcalfe et al. 1997; Ferry et al. 2002). In contrast, mast cells stimulated by lipopysaccharide (LPS) or IgE/FcεRI interaction release both preformed and newly formed mediators (Leal-Berumen et al. 1994). Indeed, both LPS and IgE trigger histamine release and secretion of interleukin(IL)-6. Interestingly, IL-6 secretion induced by LPS or IgE is totally inhibited in the absence of extracellular Ca2+. On the other hand, in response to the calcium ionophore A23187, mast cells release histamine but not IL-6. Furthermore, FcεRI aggregation requires extracellular Ca2+ as well as the activation of several signalling kinases, including phosphatidyl inositol 3-kinase (PI3K), extracellular signal regulated kinase (ERK), protein kinase C (PKC), c-Jun N terminal kinase (JNK), and of NF-κB. By contrast, IL-6 secretion induced by IL-1 does not require extracellular Ca2+ but involves p38 MAPK, NF-κB and PKCθ activation (Theoharides et al. 2007). Overall, as suggested previously (Galli et al. 2005; Theoharides et al. 2007), mast cells are tunable effectors with individual activation pathways and associated downstream signalling differentially conditioning secretory responses.
In conclusion, we show that AGEs rapidly induced exocytosis of histamine and production of ROS in mast cells. These actions of AGEs in mast cells were mediated by RAGE and involved Gi-proteins (Figure 6). We propose that mast cells may contribute to the regulation of the amounts of AGEs via feedback mechanisms involving ROS generation. Therefore, mast cells may well play a key role in establishing and maintaining the chronic, low-grade inflammatory state that characterizes aging and pathologies like cancer, neurodegeneration and cardiovascular diseases.
Figure 6.

Representation of a postulated positive-feedback loop whereby ROS generation induced by AGE-RAGE interaction leads to increased formation of AGEs. Together with AGE-stimulated histamine release, increased ROS production would contribute to the establishment and maintenance of the chronic inflammatory state. AGEs, advanced glycation endproducts; RAGE, receptor for AGEs; ROS, reactive oxygen species.
Acknowledgements This work was in part supported by the CNRS and the Ligue Contre le Cancer (grant to JPG, from the Comités du Haut-Rhin et du Bas-Rhin). ES was recipient of a Fellowship from the Ministère de la Recherche.
Glossary
Abbreviations
- 2-APB
2-aminoethoxydiphenyl borate
- AGEs
advanced glycation endproducts
- Gal-BSA
galactosamide-bovine serum albumin adduct
- Glu-BSA
glucosamide-BSA
- Lact-BSA
lactosyl-BSA
- LMWH
low molecular weight heparin
- mAb
monoclonal antibody
- Man-BSA
p-aminophenylmannopyranoside-BSA
- Malt-BSA
maltosyl-BSA
- PTX
Pertussis toxin
- RAGE
receptor for AGEs
- ROS
reactive oxygen species
Conflict of interest
The authors state no conflict of interest.
Supplemental material
Supporting Information: Teaching Materials; Figs 1–6 as PowerPoint slide.
References
- André P, Metzger C, Petey S, Muller D, Vidon DJ. Chemiluminescence of enterococci isolates from freshwater. FEMS Microbiol Lett. 2005;245:123–129. doi: 10.1016/j.femsle.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Aridor M, Rajmilevich G, Beaven MA, Sagi-Eisenberg R. Activation of exocytosis by the heterotrimeric G protein Gi3. Science. 1993;262:1569–1572. doi: 10.1126/science.7504324. [DOI] [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: causation or correlation. Exp Gerontol. 2001;36:1527–1532. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robothan JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Brooks AC, Whelan CJ, Purcell WM. Reactive oxygen species generation and histamine release by activated mast cells: modulation by nitric oxide synthase inhibition. Br J Pharmacol. 1999;128:585–590. doi: 10.1038/sj.bjp.0702838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M. Glycation products and the pathogenesis of diabetes. Diabetes Care. 1992;15:1835–1843. doi: 10.2337/diacare.15.12.1835. [DOI] [PubMed] [Google Scholar]
- Bueb JL, Da Silva A, Mousli M, Landry Y. Natural polyamines stimulate G-proteins. Biochem J. 1992;282:545–550. doi: 10.1042/bj2820545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane DE, Distel DL. Association of 45calcium with rat mast cells stimulated by 48/80: effects of inactivation, calcium ad metabolic inhibition. J Physiol (Lond) 1982;330:413–427. doi: 10.1113/jphysiol.1982.sp014348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, et al. The High Mobility Group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dröge W, Schipper M. Oxidative stress and aberrant signalling in aging and cognitive decline. Aging Cell. 2007;6:361–370. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich P. Beiträge zur Kenntniss der granulirten Bindegewebszellen und der eosinophilen Leukocythen. Arch Anat Physiol. 1879;3:166–169. [Google Scholar]
- El Khoury J, Thomas CA, Loike JD, Hickman SE, Cao L, Silverstein SC. Macrophages adhere to glucose-modified basement membrane collagen IV via their scavenger receptors. J Biol Chem. 1994;269:10197–10200. [PubMed] [Google Scholar]
- Ferry X, Eichwald V, Daeffler L, Landry Y. Activation of βγ subunits of Gi2 and Gi3 proteins by basic secretagogues induce exocytosis through phospholipase Cβ and arachidonate release through phospholipase Cβ in mast cells. J Immunol. 2001;167:4805–4813. doi: 10.4049/jimmunol.167.9.4805. [DOI] [PubMed] [Google Scholar]
- Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cells by peptides and basic secretagogues. Peptides. 2002;23:1507–1515. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CMM, Tsai M. Mast cells as ‘tunable’ effector and immunoregulatory cells: recent advances. Annu Rev Immunol. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Grimbalderson M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianonne G, Ronde P, Gaire M, Beaudouin J, Haiech J, Ellenberg J, et al. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem. 2004;279:28715–28723. doi: 10.1074/jbc.M404054200. [DOI] [PubMed] [Google Scholar]
- Gies JP, Landry Y, Mousli M. Receptor-independent activation of mast cells by bradykinin and related peptides. Trends Neurosci. 1993;16:498–999. doi: 10.1016/0166-2236(93)90191-n. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Austen KF. The diverse roles of mast cells. J Exp Med. 2001;194:1–5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford LE, Enghild JJ, Valnickova Z, Petersen SV, Schaefer LM, Schaefer TM, et al. Purification and characterization of mouse soluble receptor for advanced glycation end products (sRAGE) J Biol Chem. 2004;279:50019–50024. doi: 10.1074/jbc.M409782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Hori O, Brett J, Slattery T, Cao R, Zhang J, Chen JX, et al. The receptor for advanced glycation end products (RAGE) is a cellular binding site for amphoterin. J Biol Chem. 1995;270:25752–25761. doi: 10.1074/jbc.270.43.25752. [DOI] [PubMed] [Google Scholar]
- Hudson BI, Bucciarelli LG, Wendt T, Sakaguchi T, Lalla E, Qu W, et al. Blockade of receptor for advanced glycation endproducts: a new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys. 2003;419:80–88. doi: 10.1016/j.abb.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92–100. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- Kassel O, Amrani Y, Landry Y, Bronner C. Mast cell activation involves membrane potential- and thapsigargin- sensitive intracellular calcium pools. Fund Clin Pharmacol. 1995;9:531–539. doi: 10.1111/j.1472-8206.1995.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Kuniyasu H, Chihara Y, Kondo H. Differential effects between amphoterin and advanced glycation end products on colon cancer cells. Int J Cancer. 2003;104:722–727. doi: 10.1002/ijc.11016. [DOI] [PubMed] [Google Scholar]
- Lander HL, Tauras JM, Ogiste JS, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation endproducts triggers a MAP kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- Landry Y, Niederhoffer N, Sick E, Gies JP. Heptahelical and other G-protein-coupled receptors (GPCRs) signalling. Curr Med Chem. 2006;13:51–63. [PubMed] [Google Scholar]
- Lappas M, Permezel M, Rice GE. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-kappaB and extracellular signal-regulated kinase 1/2. J Endocrinol. 2007;193:269–277. doi: 10.1677/JOE-06-0081. [DOI] [PubMed] [Google Scholar]
- Leal-Berumen I, Conlon P, Marshall JS. IL-6 production by rat peritoneal mast cells is not necessarily preceded by histamine release and can be induced by bacterial lipopolysaccharide. J Immunol. 1994;152:5468–5476. [PubMed] [Google Scholar]
- Li SY, Du M, Dolence EK, Fang CX, Mayer GE, Ceylan-Isik AF, et al. Aging induces cardiac diastolic dysfunction, oxidative stress, accumulation of advanced glycation endproducts and protein modification. Aging Cell. 2005;4:57–64. doi: 10.1111/j.1474-9728.2005.00146.x. [DOI] [PubMed] [Google Scholar]
- Li YM, Mitsuhashi T, Wojciechowicz D, Shimizu N, Li J, Stitt A, et al. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci USA. 1996;93:11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schmidt AM. Characterization and functional analysis of the promoter of RAGE, the receptor for advanced glycation endproducts. J Biol Chem. 1997;272:16498–16506. doi: 10.1074/jbc.272.26.16498. [DOI] [PubMed] [Google Scholar]
- Liu R, Mori S, Wake H, Zhang J, Liu K, Izushi Y, et al. Establishment of in vitro binding assay of high mobility group box-1 and S100A12 to receptor for advanced glycation endproducts: heparin's effect on binding. Acta Med Okayama. 2009;63:203–211. doi: 10.18926/AMO/31812. [DOI] [PubMed] [Google Scholar]
- Lynch JW, Lemos VS, Bucher B, Stoclet JC, Takeda K. A Pertussis toxin-sensitive calcium influx mediated by neuropeptide Y2 receptors in a human neuroblastoma cell line. J Biol Chem. 1994;269:8226–8233. [PubMed] [Google Scholar]
- Marshall JS. Mast-cell responses to pathogens. Nat Rev Immunol. 2004;4:787–799. doi: 10.1038/nri1460. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2. Release J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Mercer N, Ahmed H, McCarthy AD, Etcheverry SB, Vasta GR, Cortizo AM. AGE-R3/galectin-3 expression in osteoblast-like cells: regulation by AGEs. Mol Cell Biochem. 2004;266:17–24. doi: 10.1023/b:mcbi.0000049128.71095.ac. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Miller LA, Cochrane DE, Carraway RE, Feldberg RS. Blockade of mast cell histamine secretion in response to neurotensin by SR 48692, a nonpeptide antagonist of the neurotensin brain receptor. Br J Pharmacol. 1995;114:1466–1470. doi: 10.1111/j.1476-5381.1995.tb13371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr FC, Fewtrell C. The effect of mitochondrial inhibitors on calcium homeostasis in tumor mast cells. Am J Physiol. 1990;258:C217–C226. doi: 10.1152/ajpcell.1990.258.2.C217. [DOI] [PubMed] [Google Scholar]
- Mousli M, Bronner C, Bueb JL, Tschirhart E, Gies JP, Landry Y. Activation of rat peritoneal mast cells by substance P and mastoparan. J Pharmacol Exp Ther. 1989;250:329–335. [PubMed] [Google Scholar]
- Mousli M, Hugli TE, Landry Y, Bronner C. A mechanism of action for anaphylatoxin C3a stimulation of mast cells. J Immunol. 1992;148:2456–2461. [PubMed] [Google Scholar]
- Myint KM, Yamamoto Y, Doi T, Kato I, Harashima A, Yonekura H, et al. RAGE control of diabetic nephropathy in a mouse model. Diabetes. 2006;55:2510–2522. doi: 10.2337/db06-0221. [DOI] [PubMed] [Google Scholar]
- Nah SS, Choi IY, Yoo B, Kim YG, Moon HB, Lee CK. Advanced glycation end products increases matrix metalloproteinase-1, -3, and -13, and TNF-α in human osteoarthritic chondrocytes. FEBS Lett. 2007;581:1928–1932. doi: 10.1016/j.febslet.2007.03.090. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985;260:3584–3593. [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Nishikawa T, Du Edelstein DXL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Niu XF, Ibbotson G, Kubes P. A balance between nitric oxide and oxidants regulates mast cell-dependent neutrophil-endothelial cell interactions. Circ Res. 1996;79:992–999. doi: 10.1161/01.res.79.5.992. [DOI] [PubMed] [Google Scholar]
- Ohgami N, Nagai R, Ikemoto M, Arai H, Miyazaki A, Hakamata H, et al. CD36 serves as a receptor for advanced glycation endproducts (AGE) J Diabetes Complic. 2002;16:56–59. doi: 10.1016/s1056-8727(01)00208-2. [DOI] [PubMed] [Google Scholar]
- Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci. 2008;28:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ, Chow WS, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Vannucci SJ, Yan SSD, Herold K, Yan SF, Schmidt AM. Arguing for the motion: yes, RAGE is a receptor for advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration and inflammation. Glycobiology. 2005;15:1111–1115. doi: 10.1093/glycob/cwi053. [DOI] [PubMed] [Google Scholar]
- Rüster C, Bondeva T, Franke S, Förster M, Wolf G. Advanced glycation end-products induce cell cycle arrest and hypertrophy in podocytes. Nephrol Dial Transplant. 2008;23:2179–2191. doi: 10.1093/ndt/gfn085. [DOI] [PubMed] [Google Scholar]
- Schmidt AM, Vianna M, Gerlach M, Brett J, Ryan J, Kao J, et al. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992;267:14987–14997. [PubMed] [Google Scholar]
- Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:16R–28R. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sick E, Niederhoffer N, Takeda K, Landry Y, Gies JP. Activation of CD47 receptors causes histamine secretion from mast cells. Cell Mol Life Sci. 2009;66:1271–1282. doi: 10.1007/s00018-009-8778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- Sorci G, Riuzzi F, Agneletti AL, Marchetti C, Donato R. S100B inhibits myogenic differentiation and myotube formation in a RAGE-independent manner. Mol Cell Biol. 2003;23:4870–4881. doi: 10.1128/MCB.23.14.4870-4881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Inoue T, Yoshimaru T, Ra C. Galectin-3 but not galectin-1 induces mast cell death by oxidative stress and mitochondrial permeability transition. Biochim Biophys Acta. 2008;1783:924–934. doi: 10.1016/j.bbamcr.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J Biol Chem. 2004;279:48751–48759. doi: 10.1074/jbc.M409738200. [DOI] [PubMed] [Google Scholar]
- Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signaling suppresses tumour growth and metastases. Nature. 2000;405:354–360. doi: 10.1038/35012626. [DOI] [PubMed] [Google Scholar]
- Tanikawa T, Okada Y, Tanikawa R, Tanaka Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46:572–580. doi: 10.1159/000226225. [DOI] [PubMed] [Google Scholar]
- Teraoka H, Akiba H, Takai R, Taneike T, Hiraga T, Ohga A. Inhibitory effects of caffeine on Ca2+ influx and histamine secretion independent of cAMP in rat peritoneal mast cells. Gen Pharmacol. 1997;28:237–243. doi: 10.1016/s0306-3623(96)00186-3. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Togashi K, Inada H, Tominaga M. Inhibition of the transient potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touré F, Zahm JM, Garnotel R, Lambert E, Bonnet N, Schmidt AM, et al. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem J. 2008;416:255–261. doi: 10.1042/BJ20080054. [DOI] [PubMed] [Google Scholar]
- Valencia JV, Weldon SC, Quinn D, Kiers GH, DeGroot J, TeKoppele JM, et al. Advanced glycation end product ligands for the receptor for advanced glycation end products: biochemical characterization and formation kinetics. Anal Biochem. 2004;324:68–78. doi: 10.1016/j.ab.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Vlassara H, Li YM, Imani F, Wojciechowicz D, Yang Z, Liu FT, et al. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995;1:634–646. [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Li D, Liang Y, Wang D, Cai N. Activation of astrocytes by advanced glycation end products: cytokines induction and nitric oxide release. Acta Pharmacol Sin. 2002;23:974–980. [PubMed] [Google Scholar]
- Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by advanced glycation endproducts (AGEs) links oxidant stress to altered gene expression via RAGE. Am J Physiol. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:674–675. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Yan SD, Schmidt AM, Anderson G, Zhang J, Brett J, Zou YS, et al. Enhanced cellular oxidant stress by the interaction of advanced glycation endproducts with their receptors/binding protein. J Biol Chem. 1994;269:9889–9897. [PubMed] [Google Scholar]
- Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Re. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- Yang D, Chen Q, Yang H, Tracey KJ, Bustin M, Oppenheim JJ. High mobility group box-1 protein induces the migration and activation of human dendritic cells and acts as an alarmin. J Leukoc Biol. 2007;81:59–66. doi: 10.1189/jlb.0306180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


