Abstract
BACKGROUND AND PURPOSE
The histaminergic neurotransmitter system is currently under investigation as a target for drug treatment of cognitive deficits in clinical disorders. The therapeutic potential of new drugs may initially be screened using a model of histaminergic dysfunction, for example, as associated with the use of centrally active antihistamines. Of the selective second generation antihistamines, cetirizine has been found to have central nervous system effects. The aim of the present study was to determine whether cetirizine can be used as a tool to model cognitive deficits associated with histaminergic hypofunction.
EXPERIMENTAL APPROACH
The study was conducted according to a three-way, double-blind, cross-over design. Treatments were single oral doses of cetirizine 10 and 20 mg and placebo. Effects on cognition were assessed using tests of word learning, memory scanning, vigilance, divided attention, tracking and visual information processing speed.
KEY RESULTS
Cetirizine 10 mg impaired tracking performance and both doses impaired memory scanning speed. None of the other measures indicated impaired performance.
CONCLUSION AND IMPLICATIONS
Cetirizine affects information processing speed, but these effects were not sufficient to serve as a model for cognitive deficits in clinical disorders.
Keywords: cetirizine, working memory, psychomotor performance, episodic memory, antihistamine
Introduction
The histaminergic neurotransmitter system has recently been discovered as a possible target for the pharmacological treatment of cognitive deficits in clinical disorders. Over the past years evidence has accumulated that histamine may play an important role in such disorders. However, the findings are still controversial. For example, decreased H1-receptor binding and histamine content have been found in brains of patients with Alzheimer's disease as compared with age- matched controls (Panula et al., 1998; Higuchi et al., 2000). Conversely, an increased histamine content has also been found in the brains (Cacabelos et al., 1989) and blood serum (Cacabelos et al., 1992) of Alzheimer's patients. Evidence for histamine as a cognition promoting substance has mainly come from animal studies. These studies have shown that H3-antagonists improve performance in models of many cognitive deficits and clinical disorders, like Alzheimer's disease, attention deficit hyperactivity disorder and schizophrenia (for review see: Esbenshade et al., 2006).
If histamine hypofunction is involved in cognitive deficits seen in clinical disorders, an artificial histaminergic hypofunction may reveal what cognitive functions are vulnerable in these disorders. Most studies performed in animals show that a decrease in histamine neurotransmission results in impaired performance. However, some studies have shown stimulating effects of decreased histamine neurotransmission, induced by the administration of H1-antagonists (Theunissen et al., 2006c). Yet, such effects have never been confirmed and underlying mechanisms have been investigated, but not found (Theunissen et al., 2006a). Alternatively, decreased histamine neurotransmission may be achieved using H3-agonists or H2-antagonists. However, these substances are not easily accessible for use in healthy humans. Histamine H1-receptor antagonists are easily accessible and decrease histaminergic activity and may therefore be used as a tool to model histamine hypofunction and the resulting cognitive impairments.
Recent studies have attempted to investigate the specific effects of H1-receptor blockade in humans (Turner et al., 2006; van Ruitenbeek et al., 2008). Although, some of the first generation antihistamines used in these studies, like dexchlorpheniramine, are relatively selective (van Ruitenbeek et al., 2008; Wiech and Martin, 1982), second generation antihistamines are even more selective for H1-receptors. The concentration of cetirizine producing 50% inhibition of radioligand binding (IC50) at H1-receptors is 0.65 µmol·L−1, whereas the IC50 at calcium channels, α1, D2, 5-HT2 and M1 receptors is more than 10 µmol·L−1 (Campoli-Richards et al., 1990). Although common held opinion is that second generation antihistamines cross the blood-brain barrier to a much lesser extent, which would make them less suitable as a tool drug, one possible exception is cetirizine. Tashiro et al. (2002; 2004) found that after a double therapeutic dose of 20 mg, cetirizine occupied 20% to 50% of the H1-receptors in the brain. In comparison, 2 mg dexchlorpheniramine occupies 77% of the H1-receptors and has been shown to induce clear behavioural effects and sedation (van Ruitenbeek et al., 2008). The 50% receptor occupancy in the central nervous system by cetirizine may be sufficient to induce behavioural effects. Taken together, after dexchlorpheniramine, cetirizine is the second in line as a candidate tool drug to induce histamine hypofunction.
Studies investigating the behavioural effects of cetirizine show conflicting results. On the one hand, there are several studies showing effects of cetirizine on performance and measures of alertness (Gengo and Gabos, 1987; Ramaekers et al., 1992; Patat et al., 1995; Sannita et al., 1996; Nicholson and Turner, 1998; Vermeeren et al., 2002; Gupta et al., 2004; Vacchiano et al., 2008). On the other hand, there are also studies that did not find behavioural effects of cetirizine (Seidel et al., 1987; Gengo et al., 1990; Volkerts et al., 1992; Shamsi et al., 2001; Theunissen et al., 2004). Many of the studies that failed to find effects of cetirizine used the recommended therapeutic dose of 10 mg, which should be insufficient to induce reliable behavioural effects. Twice this dose was found to increase subjective drowsiness (Gengo and Gabos, 1987) and objective drowsiness, as measured by theta and lower alpha frequency band power in the electroencephalography (Sannita et al., 1996). Only a few authors found no effects of cetirizine 20 mg on behavioural performance (Gengo and Gabos, 1987; Gengo et al. 1987; 1990), which is double the therapeutic dose. As the receptor occupancy may only reach 50%, the effects may be mild enough to identify the most sensitive cognitive functions. In order to identify these functions, both cetirizine 10 mg and 20 mg are included in the present study and it is expected that cetirizine's effect on cognitive performance will increase with increasing dose.
Another reason why the effects of cetirizine have been inconsistent relates to the time of peak impairment. Peak blood-plasma concentrations (Tmax) of cetirizine have been shown to be at approximately 1 h after oral dosing (Campoli-Richards et al., 1990) and the elimination half-life (t1/2β) is approximately 7 to 11 h (Simons and Simons, 1991). There is some evidence that the behavioural effects occur later than Tmax. Volkerts et al. (1992) and Theunissen et al. (2004) failed to find effects of cetirizine 10 mg on driving performance at 1 h after dosing, i.e. at Tmax, whereas, Ramaekers et al. (1992) and Vermeeren et al. (2002) found impaired driving performance of the same dose between 3 and 4 h after its administration. In line with this, a study in guinea-pigs found that there is a delay in the Tmax of levocetirizine in the brain as compared with blood plasma (Gupta et al., 2007). Therefore, in the present study behavioural effects were assessed at both 1 h (i.e. around Tmax) and 3 h after treatment.
To detect cognitive deficits, tasks were used that have been shown to be sensitive to central H1 blockade and that cover a range of important cognitive functions. The critical flicker/fusion frequency is a sensitive measure used to detect sedation caused by centrally active antihistamines (Hou et al., 2007). The visual vigilance test (Nuechterlein et al., 1983; O'Hanlon and Vermeeren, 1988) was added to the battery of tasks as a measure of vigilance, which is known to be affected by sedating agents (Kay, 2000). The critical tracking task and divided attention task are sensitive measures used to detect slowing in sensorimotor performance and impaired attention (van Ruitenbeek et al., 2008). Explicit short-term and long-term memory was assessed using a word learning task (Rey, 1964). Finally, a memory scanning task was used, in which speed of memory search can be separated from perceptual and motor processes (Sternberg, 1969).
The present study showed that the effects of cetirizine are present but small and therefore indicate that memory scanning speed, divided attention and psychomotor performance are sensitive to histaminergic dysfunction.
Methods
Subjects
The number of subjects for the present within subjects designed experiment was based on the effects of a low dose of the antihistamine dexchlorpheniramine (2 mg) on the critical tracking task, which was shown to be sensitive to sedative effects in a previous study (van Ruitenbeek et al., 2008). Power calculation using the G*Power (V3.1.2) computer program (Faul et al., 2007) showed 12 subjects are sufficient to observe a significant difference with a power of 0.80. However, in the present study less sensitive tasks were included. Therefore, eighteen (9 female) healthy volunteers were recruited for this study and were paid to participate. One female subject withdrew from the study for reasons unrelated to treatments. The mean± SD age of the 17 remaining subjects was 23 ± 2.6 years. Subject's health was screened using a medical history questionnaire and a physical examination. Exclusion criteria were hypertension, body mass index outside the limits of 18 and 28 kg·m−2, history of alcohol and drug abuse, history of psychiatric disorders, presence of cardiovascular, respiratory, renal, hepatic, metabolic or endocrine disorders, history of glaucoma, overt allergy, history of allergic reactions to antihistamine drug or any sensory or motor impairment. Subjects were not allowed to smoke, use caffeinated beverages or alcohol on treatment days or take any medication during or between treatments, except oral contraceptives, aspirin and acetaminophen.
All subjects received written information and were given the opportunity to ask questions. They signed a written informed consent prior to enrolment. The study was approved by the Ethics Committee of Maastricht University and University Hospital Maastricht and was carried out in accordance with the World Medical Association Declaration of Helsinki and its amendments (World Medical Association, 1964).
Study design and treatments
The study was conducted according to a 3 × 2, double blind, cross over design. The two factors were Treatment (3 levels) and Time of testing (2 levels). Treatments were single oral doses of cetirizine 10 mg, cetirizine 20 mg and placebo. Subjects were tested twice on each testday, at 1 and 3 h after drug administration. All test days were separated by a washout period of 1 week. The order of treatments was counterbalanced using six independent 3 × 3 Latin squares.
Procedure
Subjects were trained on two separate occasions to perform the tasks until their performance reached plateau levels. On treatment days subjects were instructed to arrive at the test facility well rested. Drug administration occurred at 9:00 h, at least 3 h after the subjects had consumed a meal. Test sessions started 1 h (T1) and 3 h (T3) after drug administration. The duration of the session was 1 h and consisted of a 15-word learning task, a critical flicker/fusion frequency test, a visual vigilance task, a critical tracking task, a divided attention task and a memory scanning task presented in the order mentioned. Critical flicker/fusion frequency was measured both at the beginning and end of each test session. Ten minutes before the first test session subjects consumed a light meal (see Figure 1).
Figure 1.
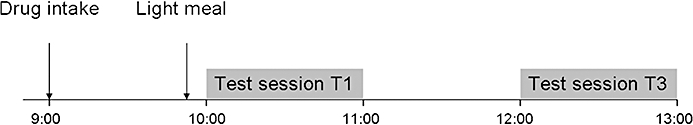
The procedure during a test day with time displayed on the horizontal axis. Oral doses of cetirizine 10 mg, 20 mg or placebo was administered at 9:00 am, which was followed by testbatteries at 10:00 (T1) and 12:00 (T3). Test sessions consisted of 15-word learning task, a critical flicker/fusion frequency test, a visual vigilance task, a critical tracking task, a divided attention task and a memory scanning task and a critical flicker/fusion frequency test again.
Behavioural assessments
The 15-word learning task
The 15-word learning task (Rey, 1964; Riedel et al., 1995) assesses short- and long-term verbal memory. Fifteen Dutch monosyllabic meaningful nouns and adjectives are presented for 1000 ms at a rate of 1 per 2 s and subjects are required to read them aloud. When the presentation ends, subjects are required to verbally recall as many words as possible (immediate recall). This procedure is repeated five times, with the same words presented in the same sequence. After a 20 min delay subjects are requested again to recall as many words as possible (delayed recall). Dependent variables were the sum of the number of words correctly recalled on the five immediate recall trials and the number of correctly recalled words after the 20 min delay.
Memory scanning task
Sternberg's memory scanning task (Sternberg, 1969), adjusted by Riedel et al. (1995) measures the time it takes to scan items held in memory as part of working memory integrity, separating it from other processes required to respond. When subjects judge whether a test symbol is contained in a short memorized sequence of symbols, their mean reaction time increases linearly with the length of the sequence. The linearity and slope of the function imply the existence of an internal serial comparison process whose average rate is between 20 and 30 items per second. In this test the subjects are presented with a set of one, two or four consonants, which they are asked to memorize. Hereafter a series of 90 consonants is presented on a computer screen of which 45 are targets and 45 are non-targets. The subject's task is to indicate as fast as possible whether or not the letter presented was one from the memory set by pressing one of two buttons. The task consists of three blocks of 90 stimuli with memory sets of one, two and four digits. The average reaction time for correct responses (detections and rejections) was recorded and used to calculate individual linear regression lines of reaction time on memory set size. The slope of this line is a measure of speed of scanning short-term memory, whereas the intercept is a measure of psychomotor speed. Both slope (ms per letter) and intercept (ms) are outcome measures.
Critical flicker/fusion frequency
The critical flicker/fusion frequency test measures the frequency threshold, which separates the perception of light flickering from fusion and light constancy. The threshold is fundamentally determined by the speed of information processing of the visual system, which can be influenced by sedative drugs. Subjects discriminate the flicker from fusion of a flickering of four light emitting diodes, held at 0.75 m from the subject's eye, using the Leeds Psychomotor Tester. The threshold (Hz) was determined by averaging three ascending and three descending frequency trials (Hindmarch, 1980). A lower threshold indicates slower visual information processing speed.
Visual vigilance task
The visual vigilance task (Nuechterlein et al., 1983), adjusted by O'Hanlon and Vermeeren (1988), consists of rapidly presenting visual stimuli for 8 min and was used to assess sustained visual discrimination. The stimuli were presented for 34 ms at a rate of 1 per second and consisted of digits (0, 2, 3, 5, 6, 8 and 9) of which the ‘0’ was considered the target and was presented at a 25% target rate. To visually degrade the stimuli a glass diffusion screen was positioned between the subject and the display. Upon appearance of the target subjects were instructed to press a response button as fast as possible. The reaction times of the false and correct detections were measured. From these measures the perceptual sensitivity index d′ and the response criterion β were calculated to determine the effects on stimulus and response-related processes respectively.
Critical tracking task
The critical tracking task measures the ability to control an unstable triangle, which is displayed on a horizontal axis on a computer screen, using a joystick (Jex et al., 1966). An error signal causes the triangle to become increasingly unstable and therefore tends to diverge from the centre of the axis. The subject has to make compensatory movements to null the error in order to keep the triangle in the middle of the screen. As the correction frequency of the cursor deviations increases as a stochastic function of time, the subject is required to make compensatory movements with an increasingly higher frequency to the limit of his or her ability, whereupon control is lost. This frequency decreases under the influence of sedating drugs. The dependent measure is the average frequency at which control is lost of five trials, after removing the lowest and highest score. This is called the ‘critical frequency’ or ‘lambdac’ (rad·s−1).
Divided attention task
The divided attention task (Moskowitz, 1973) assesses the ability to perform two tasks simultaneously and evaluates cognitive processing resources. The primary task is similar to the critical tracking task described above, with the exception that the level of difficulty is held constant at 50% of that, which is just controllable, by the subject. Tracking error is measured by the absolute distance (in mm) between the cursor's position and the centre. The secondary task involves the monitoring of 24 digits (0–9) that are arranged around the display's periphery. The digits change asynchronously every 5 s. Subjects were required to respond as rapidly as possible by lifting the foot from a pedal anytime the digit ‘2’ appears. The average reaction time (in ms) to targets is recorded as the response measure in this task. Average reaction times and tracking error of each measure were transformed to z-scores using data from all subjects, test days and test sessions. Second, the standardized scores of the subtasks were summed to yield an overall performance score for each subject, test day and test session. Overall scores were used for further analysis.
Statistical analysis
All variables were screened for normality of the distribution. There were no signs of non-normal distributions. All dependent variables were analysed according to a 3 × 2 factorial model with Treatment (cetirizine 10 mg, cetirizine 20 mg, placebo) and Time of testing (T1, T3) as factors using analysis of variance for repeated measures. The critical flicker/fusion frequency data were analysed according to a 3 × 2 × 2 factorial model with Treatment (3 levels), Time of testing (2 levels) and Time in session (2 levels: begin, end) as factors. Regardless of the outcome of the overall F-tests, three planned univariate comparisons were carried out between the treatments and placebo for T1 and T3 separately, which is a legitimate procedure as the comparisons are suggested by the theoretical basis of the experiment (Winer, 1971). When there was a significant interaction between Treatment and Time of testing, the data were analysed further per level of Time of testing. All data were analysed using SPSS 15.0 for the Windows operating system.
Results
A summary of mean (±SEM) performance scores in tasks assessing verbal memory, critical flicker-fusion frequency, vigilance, critical tracking and divided attention is presented in Table 1.
Table 1.
Mean (±SEM) performance scores after treatment with cetirizine 10 mg (CET10), 20 mg (CET20) and placebo (PLA)
| Overall analysis Effect of treatment | Effect of time of testing | Interaction between treatment × time of testing | Test session | Mean (±SEM) scores Treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F (2, 15) = | P = | F (1, 16) = | P = | F (2, 15)= | P = | PLA | CET10 | CET20 | ||
| Word learning task | ||||||||||
| Immediate recall (no. correct) | <1 | 0.815 | 3.0 | 0.100 | <1 | 0.535 | T1 | 58.1 ± 1.7 | 55.9 ± 2.0 | 56.2 ± 2.0 |
| T3 | 54.1 ± 2.1 | 53.9 ± 2.0 | 54.9 ± 2.0 | |||||||
| Delayed recall (no. correct) | 1.3 | 0.304 | 3.2 | 0.091 | 1.2 | 0.327 | T1 | 12.0 ± 0.6 | 11.7 ± 0.7 | 12.2 ± 0.8 |
| T3 | 11.7 ± 0.6 | 10.1 ± 0.8 | 10.9 ± 0.7 | |||||||
| Critical flicker/fusion task | ||||||||||
| Critical frequency (Hz) | 1.4 | 0.280 | <1 | 0.366 | <1 | 0.865 | T1begin | 29.2 ± 0.8 | 28.7 ± 0.7 | 29.1 ± 0.8 |
| T1end | 28.4 ± 0.8 | 28.3 ± 0.8 | 28.4 ± 0.8 | |||||||
| T3begin | 28.8 ± 0.9 | 28.9 ± 0.8 | 29.0 ± 0.8 | |||||||
| T3end | 28.4 ± 0.9 | 27.9 ± 0.7 | 28.5 ± 0.8 | |||||||
| Visual vigilance task | ||||||||||
| Hits (%) | 1.5 | 0.262 | 3.1 | 0.096 | <1 | 0.800 | T1 | 79.1 ± 3.5 | 75.1 ± 5.1 | 67.4 ± 5.4 |
| T3 | 75.5 ± 4.4 | 73.2 ± 5.4 | 65.9 ± 5.5 | |||||||
| Reaction time (ms) | 1.9 | 0.187 | 2.3 | 0.147 | <1 | 0.647 | T1 | 503 ± 16 | 517 ± 9 | 522 ± 18 |
| T3 | 492 ± 11 | 502 ± 8 | 520 ± 24 | |||||||
| Sensitivity d′ | 2.2 | 0.150 | <1 | 0.393 | <1 | 0.590 | T1 | 2.6 ± 0.3 | 2.8 ± 0.2 | 2.3 ± 0.3 |
| T3 | 2.8 ± 0.2 | 2.7 ± 0.2 | 2.1 ± 0.3 | |||||||
| Criterion β | <1 | 0.589 | <1 | 0.672 | 1.2 | 0.316 | T1 | 5.0 ± 1.0 | 7.1 ± 2.0 | 6.8 ± 1.7 |
| T3 | 6.5 ± 1.3 | 5.9 ± 1.6 | 7.2 ± 2.2 | |||||||
| Critical tracking task | ||||||||||
| Lambda (rad·s−1) | 4.2 | 0.035 | <1 | 0.752 | 1.1 | 0.363 | T1 | 5.4 ± 0.2 | 5.1 ± 0.21 | 5.4 ± 0.2 |
| T3 | 5.4 ± 0.3 | 5.2 ± 0.2 | 5.2 ± 0.3 | |||||||
| Divided attention task | ||||||||||
| Overall performance (z-score) | <1 | 0.664 | 2.6 | 0.129 | <1 | 0.416 | T1 | −0.12 ± 0.3 | 0.02 ± 0.4 | −0.26 ± 0.4 |
| T3 | −0.05 ± 0.4 | 0.32 ± 0.4 | 0.09 ± 0.4 | |||||||
| Tracking error (mm) | 1.8 | 0.199 | 2.4 | 0.144 | 2.4 | 0.124 | T1 | 16.9 ± 1.3 | 19.3 ± 1.4 | 17.9 ± 1.6 |
| T3 | 17.9 ± 1.6 | 18.7 ± 1.5 | 19.2 ± 1.7 | |||||||
| Reaction time (ms) | <1 | 0.629 | 1.5 | 0.237 | 3.0 | 0.083 | T1 | 1811 ± 81 | 1723 ± 102 | 1701 ± 76 |
| T3 | 1771 ± 102 | 1862 ± 86 | 1750 ± 78 | |||||||
Bold P-values indicate significant main effects or interaction.
Significant treatment–placebo contrasts.
The 15-word learning task
Analysis of immediate and delayed recall scores showed no significant differences between the treatments or times of testing. Delayed recall scores were lower at T3 as compared with T1, but the difference was not significant (F(1,16) = 3.2, P = n.s.).
Memory scanning task
The slope of the regression line of reaction time on memory load differed between treatments, but did not reach significance (F(2,15) = 3.1, P = n.s.). Nevertheless, drug-placebo comparison indicated that both 10 and 20 mg cetirizine increased the slope significantly, indicating a slowing of memory scanning speed (F(1,16) = 5.2, P = 0.037 and F(1,16) = 4.8, P = 0.044 respectively; Figure 2). There were no overall differences in slopes between T1 and T3. There were no significant effects on intercepts of the regression lines.
Figure 2.
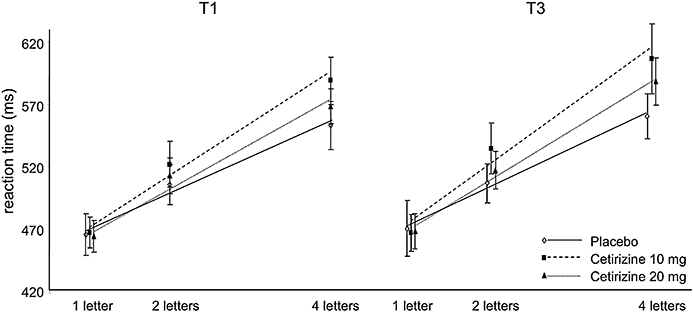
The slope of the regression line of reaction time on memory load in Sternberg's memory scanning task increased significantly after administration of cetirizine 10 mg and after cetirizine 20 mg.
Critical flicker/fusion frequency
The critical flicker/fusion frequency was significantly lower at the end of every test session as compared with the beginning, indicated by the significant effects of Time in session (F(1,16) = 14.1, P = 0.002). Visual information processing speed was slower at the end of every test session. The differences between the treatments and times of testing were not significant.
Visual vigilance task
The percentage of hits, reaction time, perceptual sensitivity and response criterion in the vigilance test did not differ between the treatments and times of testing.
Critical tracking task
Tracking performance was significantly different between treatments (F(2,15) = 4.2, P = 0.03). Drug-placebo comparisons showed that 10 mg cetirizine impaired tracking performance (F(1,16) = 7.1, P = 0.017). There were no differences between T1 and T3.
Divided attention task
The overall performance scores, tracking errors and reaction times in the divided attention task did not differ between treatments or times of testing.
Discussion and conclusions
The aim of the present study was to investigate if cetirizine at doses of 10 or 20 mg would be a suitable tool to induce histamine hypofunction and the associated cognitive impairments. Histamine is known to be involved in attention, psychomotor functioning and possibly memory. It was therefore hypothesized that cetirizine would affect performance measures of these functions. In the present study cetirizine only tended to affect psychomotor performance, as measured by the critical tracking task, and it affected memory scanning speed. In contrast, cetirizine did not affect word learning, vigilance and divided attention.
Memory function as measured with the memory scanning task was impaired in this study, but not as measured with the word learning task. Its impairing effects on speed of memory scanning are in accordance with results from a study by Ramaekers et al. (1992), who found an increased variation in memory scanning speed after administration of cetirizine 10 mg. In a previous study, van Ruitenbeek et al. (2008) also found a mean increase in memory scanning speed after the administration of dexchlorpheniramine 4 mg, but the difference failed to reach significance. The lack of effects of cetirizine on word learning and recall is also in line with results from previous studies that failing to find significant effects of first generation antihistamines on performance in similar word learning tests (Turner et al., 2006; van Ruitenbeek et al., 2008; 2010). The differential effects of antihistamines on performance in memory scanning and word learning tasks may be due to differences in the degree to which they involve speeded information processing. In the memory scanning task performance is solely dependent on speed of information processing at the millisecond level. In contrast, performance in the word learning task depends primarily on the subject's ability to organize and store information in working memory under time pressure on a larger scale (i.e. 15 words presented at a rate of one word per 2 s). Second, the memory loads in the memory scanning task did not exceed memory span capacity of seven independent items, whereas 15 words were presented in the word learning task. Any impairment on working memory storage capacity would be detected by the word learning task. Taken together, it is likely that antihistamines primarily affect the speed of information processing, but not the integrity of information processing in working memory.
The role of histamine in memory functioning remains unclear. On the one hand, recent studies have shown that H1-recepter knockout animals and H2-receptor knockout animals performed worse on maze tasks and object recognition as compared with wild-type mice (Dai et al., 2007; Zlomuzica et al., 2009), which suggests a memory promoting role for histamine. On the other hand, Knoche et al. (2003) have shown decreased hippocampal functioning after histamine administration to freely moving rats and an increased functioning after the administration of H1-antagonists. Furthermore, Liu et al. (2007) have shown that learning and memory improved in histidine decarboxylase knockout mice, which decreases histamine synthesis. In humans stimulating effects of some H1-antagonists have been suggested, but a mechanism has never been found (Theunissen et al., 2006a).
In the present study, it was assumed that histamine neurotransmission in the central nervous system is mediated by the histamine H1, H2 and H3 receptors and that blockade of H1 and/or H2 receptors leads to decreased histamine transmission. It may be that the H1-receptor is not involved in memory formation, but that the H2-receptor plays an exclusive role in memory processes. Histamine H2-receptors are present in the hippocampus and have been shown to regulate the excitability of hippocampal cells (for review see Brown et al., 2001). In addition, Flood et al. (1998) have shown that infusion of a H2-agonist and H2-antagonist into the septum of mice led to improved and impaired memory performance respectively. However, centrally active H2 drugs are not available to be used in humans. Their availability would make studying the role of the H2-receptor in human memory possible.
In the present study slower information processing speed was observed, which may also explain the impaired tracking performance. The critical tracking task is a complex, sensitive and frequently used measure of drug-induced psychomotor impairment (van Ruitenbeek et al., 2008). Performance on the critical tracking task involves continuous interaction between perceptual and motor processes, but also central executive processes like anticipation and inhibition of responses, in case the anticipated response is not required to be executed. A delay in any process, including such central processes, can result in impaired tracking performance. Indeed, this study showed that tracking performance was impaired 1 h after cetirizine 10 mg administration. In accordance with this result, Nicholson and Turner (1998) and Patat et al. (1995) found impaired tracking performance after the administration of cetirizine. In contrast, Ramaekers et al. (1992), Theunissen et al. (2004, 2006b) and Shamsi et al. (2001) found no impairments. This may indicate that the effects of cetirizine are small and are therefore not always detected. Ramaekers et al. (1992) suggested that some subjects are very sensitive to effects of cetirizine, while others are not. Inspection of the individual data in our study did not support this hypothesis. Almost all subjects performed worse after cetirizine administration as compared with placebo and standard errors of measurement were of comparable size, which does not support large differences in individual sensitivity to the effects of cetirizine.
Perceptual processing and motor-related information processing did not seem to be affected by cetirizine. In contrast to the effects on the slope of the regression line, the intercept in the Sternberg's memory scanning task remained unaffected. The intercept is a measure of all perceptual and motor-related processes involved in this task. A lack of effect of cetirizine on perceptual processing is supported by the non-significant effects on the perceptual sensitivity index (d prime) in the vigilance test and on the critical flicker/fusion frequency, which is primarily a measure of perceptual processing speed. In contrast, results from a previous study suggested that sensory processes were affected by an oral dose of dexchlorpheniramine 4 mg (van Ruitenbeek et al., 2009). As the H1-receptor occupancy by cetirizine is not as high (Tashiro et al., 2009) as that by dexchlorpheniramine (Yanai et al., 1995), it is possible that sensory processes are not the most sensitive measure of antihistamine effects. From the present results it was concluded that cetirizine affected information processing speed, rather than perceptual or motor processes.
From the literature (Ramaekers et al., 1992; Vermeeren et al., 2002), it was suggested that the effects of cetirizine may not coincide with the Tmax of approximately 1 h after drug administration and that the behavioural effects are delayed. This study therefore assessed the effects both at 1 and 3 h after oral administration of cetirizine. Results did not show differential effects of cetirizine at T1 and T3. Only reaction time in the divided attention test tended to be slower at T3 as compared with T1 after cetirizine, whereas subjects administered the placebo tended to respond faster at T3 as compared with T1. However, the interaction was only marginally significant. No other measure showed such an interaction. Therefore, our data do not suggest that behavioural effects of cetirizine lag behind Tmax.
Overall, this study showed only marginal effects of cetirizine, which suggested that H1-receptor occupancy in the brain was not sufficient to produce clear, measurable effects on performance that may serve as a model for cognitive dysfunction associated with histaminergic hypofunction. Yanai et al. (1999) reported that H1-receptor occupancy in the brain is significantly correlated with reported measures of sleepiness. Non-sedating antihistamines were associated with receptor occupancies of approximately 30%, while sedating antihistamines were associated with occupancy of 70% or higher. Tashiro et al. (2002; 2004) studied receptor occupancy and sedation associated with use of cetirizine. In an initial study they showed that cetirizine 20 mg occupied approximately 20 to 50% of the central H1-receptors and slowed reaction times (Tashiro et al., 2002). In a later study, however, they found that cetirizine 20 mg only tended to induce sedation, which was in line with the relatively low H1-receptor occupancy of approximately 26% found in that study (Tashiro et al., 2004). In addition to the marginal sedative effects, it may be concluded that cetirizine 20 mg in our study occupied only a moderate percentage of the central H1-receptors and therefore, induced only marginal effects on cognitive measures.
In summary, cetirizine after single oral doses of 10 mg and 20 mg was found to impair speed of memory scanning and critical tracking, which may be due to common effects on speed of central processes. The effects did not differ between the doses of 10 mg and 20 mg. Furthermore, there was no evidence that cetirizine's effects were delayed as compared with Tmax. From our findings, memory scanning appears to be the most sensitive to the effects of cetirizine. However, on a larger scale the effects of cetirizine 10 and 20 mg are not sufficient for it to be used as a model for histamine hypofunction.
Acknowledgments
None.
Conflict of interest
The study was entirely conducted at, paid by and reported within the Maastricht University.
Supplemental material
Supporting Information: Teaching Materials; Figs 1–2 as PowerPoint slide.
References
- Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- Cacabelos R, Yamatodani A, Niigawa H, Hariguchi S, Tada K, Nishimura T, et al. Brain histamine in Alzheimer's disease. Methods Find Exp Clin Pharmacol. 1989;11:353–360. [PubMed] [Google Scholar]
- Cacabelos R, Fernandez-Novoa L, Perez-Trullen JM, Franco-Maside A, Alvarez XA. Serum histamine in Alzheimer's disease and multi-infarct dementia. Methods Find Exp Clin Pharmacol. 1992;14:711–715. [PubMed] [Google Scholar]
- Campoli-Richards DM, Buckley MM, Fitton A. Cetirizine. A review of its pharmacological properties and clinical potential in allergic rhinitis, pollen-induced asthma, and chronic urticaria. Drugs. 1990;40:762–781. doi: 10.2165/00003495-199040050-00009. [DOI] [PubMed] [Google Scholar]
- Dai H, Kaneko K, Kato H, Fujii S, Jing Y, Xu A, et al. Selective cognitive dysfunction in mice lacking histamine H1 and H2 receptors. Neurosci Res. 2007;57:306–313. doi: 10.1016/j.neures.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Esbenshade TA, Fox GB, Cowart MD. Histamine H3 receptor antagonists: preclinical promise for treating obesity and cognitive disorders. Mol Interv. 2006;6:77–88. doi: 10.1124/mi.6.2.5. 59. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Flood JF, Uezu K, Morley JE. Effect of histamine H2 and H3 receptor modulation in the septum on post-training memory processing. Psychopharmacology (Berl) 1998;140:279–284. doi: 10.1007/s002130050768. [DOI] [PubMed] [Google Scholar]
- Gengo FM, Gabos C. Antihistamines, drowsiness, and psychomotor impairment: central nervous system effect of cetirizine. Ann Allergy. 1987;59(Pt 2):53–57. [PubMed] [Google Scholar]
- Gengo FM, Dabronzo J, Yurchak A, Love S, Miller JK. The relative antihistaminic and psychomotor effects of hydroxyzine and cetirizine. Clin Pharmacol Ther. 1987;42:265–272. doi: 10.1038/clpt.1987.145. [DOI] [PubMed] [Google Scholar]
- Gengo FM, Gabos C, Mechtler L. Quantitative effects of cetirizine and diphenhydramine on mental performance measured using an automobile driving simulator. Ann Allergy. 1990;64:520–526. [PubMed] [Google Scholar]
- Gupta S, Kapoor B, Gillani Z, Kapoor V, Gupta BM. Effects of fexofenadine, cetirizine and diphenhydramine on psychomotor performance in adult healthy volunteer. JK Sci. 2004;6:201–205. [Google Scholar]
- Gupta A, Gillard M, Christophe B, Chatelain P, Massingham R, Hammarlund-Udenaes M. Peripheral and central H1 histamine receptor occupancy by levocetirizine, a non-sedating antihistamine; a time course study in the guinea pig. Br J Pharmacol. 2007;151:1129–1136. doi: 10.1038/sj.bjp.0707318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Yanai K, Okamura N, Meguro K, Arai H, Itoh M, et al. Histamine H1 receptors in patients with Alzheimer's disease assessed by positron emission tomography. Neuroscience. 2000;99:721–729. doi: 10.1016/s0306-4522(00)00230-x. [DOI] [PubMed] [Google Scholar]
- Hindmarch I. Psychomotor function and psychoactive drugs. Br J Clin Pharmacol. 1980;10:189–209. doi: 10.1111/j.1365-2125.1980.tb01745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou RH, Langley RW, Szabadi E, Bradshaw CM. Comparison of diphenhydramine and modafinil on arousal and autonomic functions in healthy volunteers. J Psychopharmacol. 2007;21:567–578. doi: 10.1177/0269881106071022. [DOI] [PubMed] [Google Scholar]
- Jex HR, McDonnell JD, Phatak AV. A ‘critical’ tracking task for man-machine research related to the operator's effective delay time. I. Theory and experiments with a first-order divergent controlled element. NASA CR-616. 1966. NASA Contract Rep NASA CR 1-105. [PubMed]
- Kay GG. The effects of antihistamines on cognition and performance. J Allergy Clin Immunol. 2000;105(Pt 2):S622–S627. doi: 10.1067/mai.2000.106153. [DOI] [PubMed] [Google Scholar]
- Knoche A, Yokoyama H, Ponomarenko A, Frisch C, Huston J, Haas HL. High-frequency oscillation in the hippocampus of the behaving rat and its modulation by the histaminergic system. Hippocampus. 2003;13:273–280. doi: 10.1002/hipo.10057. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang S, Zhu Y, Fu Q, Zhu Y, Gong Y, et al. Improved learning and memory of contextual fear conditioning and hippocampal CA1 long-term potentiation in histidine decarboxylase knock-out mice. Hippocampus. 2007;17:634–641. doi: 10.1002/hipo.20305. [DOI] [PubMed] [Google Scholar]
- Moskowitz H. Laboratory studies of the effects of alcohol on some variables related to driving. J Safety Res. 1973;5:185–199. [Google Scholar]
- Nicholson AN, Turner C. Central effects of the H1-antihistamine, cetirizine. Aviat Space Environ Med. 1998;69:166–171. [PubMed] [Google Scholar]
- Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science. 1983;220:327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- O'Hanlon JF, Vermeeren A. Effects of Ro 15-1788 on the vigilance performance of sleep deprived men. Human Psychopharmacol. 1988;3:267–274. [Google Scholar]
- Panula P, Rinne J, Kuokkanen K, Eriksson KS, Sallmen T, Kalimo H, et al. Neuronal histamine deficit in Alzheimer's disease. Neuroscience. 1998;82:993–997. doi: 10.1016/s0306-4522(97)00353-9. [DOI] [PubMed] [Google Scholar]
- Patat A, Stubbs D, Dunmore C, Ulliac N, Sexton B, Zieleniuk I, et al. Lack of interaction between two antihistamines, mizolastine and cetirizine, and ethanol in psychomotor and driving performance in healthy subjects. Eur J Clin Pharmacol. 1995;48:143–150. doi: 10.1007/BF00192740. [DOI] [PubMed] [Google Scholar]
- Ramaekers JG, Uiterwijk MM, O'Hanlon JF. Effects of loratadine and cetirizine on actual driving and psychometric test performance, and EEG during driving. Eur J Clin Pharmacol. 1992;42:363–369. doi: 10.1007/BF00280119. [DOI] [PubMed] [Google Scholar]
- Rey A. L'exam Psychologique Dans Les Cas D'encéphalopathie Traumatique. 1964. Presses Universitaires de France.
- Riedel WJ, Hogervorst E, Leboux R, Verhey F, van Praag H, Jolles J. Caffeine attenuates scopolamine-induced memory impairment in humans. Psychopharmacology (Berl) 1995;122:158–168. doi: 10.1007/BF02246090. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Riedel WJ. Histamine H1-receptor blockade in humans affects psychomotor performance but not memory. J Psychopharmacol. 2008;22:663–672. doi: 10.1177/0269881107081526. [DOI] [PubMed] [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Smulders FT, Sambeth A, Riedel WJ. Histamine H1 receptor blockade predominantly impairs sensory processes in human sensorimotor performance. Br J Pharmacol. 2009;157:76–85. doi: 10.1111/j.1476-5381.2008.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ruitenbeek P, Vermeeren A, Riedel WJ. Memory in humans is unaffected by central H1-antagonism, while objectively and subjectively measured sedation is increased. Eur Neuropsychopharmacol. 2010;20:226–235. doi: 10.1016/j.euroneuro.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Sannita WG, Crimi E, Riela S, Rosadini G, Brusasco V. Cutaneous antihistaminic action of cetirizine and dose-related EEG concomitants of sedation in man. Eur J Pharmacol. 1996;300:33–41. doi: 10.1016/0014-2999(95)00756-3. [DOI] [PubMed] [Google Scholar]
- Seidel WF, Cohen S, Bliwise NG, Dement WC. Cetirizine effects on objective measures of daytime sleepiness and performance. Ann Allergy. 1987;59(Pt 2):58–62. [PubMed] [Google Scholar]
- Shamsi Z, Kimber S, Hindmarch I. An investigation into the effects of cetirizine on cognitive function and psychomotor performance in healthy volunteers. Eur J Clin Pharmacol. 2001;56:865–871. doi: 10.1007/s002280000257. [DOI] [PubMed] [Google Scholar]
- Simons FE, Simons KJ. Pharmacokinetic optimisation of histamine H1-receptor antagonist therapy. Clin Pharmacokinet. 1991;21:372–393. doi: 10.2165/00003088-199121050-00005. [DOI] [PubMed] [Google Scholar]
- Sternberg S. Memory-scanning: mental processes revealed by reaction-time experiments. Am Sci. 1969;57:421–457. [PubMed] [Google Scholar]
- Tashiro M, Mochizuki H, Iwabuchi K, Sakurada Y, Itoh M, Watanabe T, et al. Roles of histamine in regulation of arousal and cognition: functional neuroimaging of histamine H1 receptors in human brain. Life Sci. 2002;72:409–414. doi: 10.1016/s0024-3205(02)02276-2. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Sakurada Y, Iwabuchi K, Mochizuki H, Kato M, Aoki M, et al. Central effects of fexofenadine and cetirizine: measurement of psychomotor performance, subjective sleepiness, and brain histamine H1-receptor occupancy using 11C-doxepin positron emission tomography. J Clin Pharmacol. 2004;44:890–900. doi: 10.1177/0091270004267590. [DOI] [PubMed] [Google Scholar]
- Tashiro M, Kato M, Miyake M, Watanuki S, Funaki Y, Ishikawa Y, et al. Dose dependency of brain histamine H(1) receptor occupancy following oral administration of cetirizine hydrochloride measured using PET with [11C]doxepin. Hum Psychopharmacol. 2009;24:540–548. doi: 10.1002/hup.1051. [DOI] [PubMed] [Google Scholar]
- Theunissen EL, Vermeeren A, van Oers AC, van Maris I, Ramaekers JG. A dose-ranging study of the effects of mequitazine on actual driving, memory and psychomotor performance as compared to dexchlorpheniramine, cetirizine and placebo. Clin Exp Allergy. 2004;34:250–258. doi: 10.1111/j.1365-2222.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- Theunissen EL, van Kroonenburgh MJ, van Deursen JA, Blom-Coenjaerts C, Ramaekers JG. Stimulating effects of the antihistamine fexofenadine: testing the dopamine transporter hypothesis. Psychopharmacology (Berl) 2006a;187:95–102. doi: 10.1007/s00213-006-0406-3. [DOI] [PubMed] [Google Scholar]
- Theunissen EL, Vermeeren A, Ramaekers JG. Repeated-dose effects of mequitazine, cetirizine and dexchlorpheniramine on driving and psychomotor performance. Br J Clin Pharmacol. 2006b;61:79–86. doi: 10.1111/j.1365-2125.2005.02524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theunissen EL, Vermeeren A, Vuurman EF, Ramaekers JG. Stimulating effects of H1-antagonists. Curr Pharm Des. 2006c;12:2501–2509. doi: 10.2174/138161206777698800. [DOI] [PubMed] [Google Scholar]
- Turner C, Handford AD, Nicholson AN. Sedation and memory: studies with a histamine H-1 receptor antagonist. J Psychopharmacol. 2006;20:506–517. doi: 10.1177/0269881106059804. [DOI] [PubMed] [Google Scholar]
- Vacchiano C, Moore J, Rice GM, Crawley G. Fexofenadine effects on cognitive performance in aviators at ground level and simulated altitude. Aviat Space Environ Med. 2008;79:754–760. doi: 10.3357/asem.2212.2008. [DOI] [PubMed] [Google Scholar]
- Vermeeren A, Ramaekers JG, O'Hanlon JF. Effects of emedastine and cetirizine, alone and with alcohol, on actual driving of males and females. J Psychopharmacol. 2002;16:57–64. doi: 10.1177/026988110201600104. [DOI] [PubMed] [Google Scholar]
- Volkerts ER, Van Willigenburg AP, Van Laar MW, Maes RA. Does cetirizine belong to the new generation of antihistamines? An investigation into its acute and subchronic effects on highway driving, psychometric test performance and daytime sleepiness. Human Psychopharmacol Clin Exp. 1992;7:227–238. [Google Scholar]
- Wiech NL, Martin JS. Absence of an effect of terfenadine on guinea pig brain histamine H1-receptors in vivo determined by receptor binding techniques. Arzneimittelforschung. 1982;32:1167–1170. [PubMed] [Google Scholar]
- Winer J. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- World Medical Association (Producer) 1964. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Available at http://www.wma.net/en/30publications/10policies/b3/index.html (accessed 12 May 2010)
- Yanai K, Ryu JH, Watanabe T, Iwata R, Ido T, Sawai Y, et al. Histamine H1 receptor occupancy in human brains after single oral doses of histamine H1 antagonists measured by positron emission tomography. Br J Pharmacol. 1995;116:1649–1655. doi: 10.1111/j.1476-5381.1995.tb16386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai K, Okamura N, Tagawa M, Itoh M, Watanabe T. New findings in pharmacological effects induced by antihistamines: from PET studies to knock-out mice. Clin Exp Allergy. 1999;29(Suppl 3):29–36. discussion 37–28. [PubMed] [Google Scholar]
- Zlomuzica A, Ruocco LA, Sadile AG, Huston JP, Dere E. Histamine H1 receptor knockout mice exhibit impaired spatial memory in the eight-arm radial maze. Br J Pharmacol. 2009;157:86–91. doi: 10.1111/j.1476-5381.2009.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


