Abstract
Background
Hazardous air pollutants are plausible candidate exposures for autism spectrum disorders. They have been explored in recent studies for their role in the development of these disorders.
Methods
We used a prevalent case-control design to screen perinatal exposure to 35 hazardous air pollutants for further investigation in autism etiology. We included 383 children with autism spectrum disorders and, as controls, 2829 children with speech and language impairment. All participants were identified from the records-based surveillance of 8-year-old children conducted by the Autism and Developmental Disabilities Monitoring Network in North Carolina (for children born in 1994 and 1996) and West Virginia (born in 1992 and 1994). Exposures to ambient concentrations of metal, particulate, and volatile organic air pollutants in the census tract of the child’s birth residence were assigned from the 1996 National Air Toxics Assessment annual-average model. We estimated odds ratios (ORs) for autism spectrum disorders and corresponding 95% confidence intervals (CIs), comparing across the 20th and 80th percentiles of log-transformed hazardous air pollutant concentration among the selected controls, using semi-Bayes logistic models and adjusting for sampling variables (surveillance year and state), a priori demographic confounders from the birth certificate and census, and covarying air pollutants.
Results
We estimated many near-null ORs, including those for metals, established human neurodevelopmental toxicants, and several pollutants that were elevated in a similar study in California. Hazardous air pollutants with more precise and elevated OR estimates included methylene chloride, 1.4 (95% CI = 0.7–2.5), quinoline, 1.4 (1.0–2.2), and styrene, 1.8 (1.0–3.1).
Conclusions
Our screening design was limited by exposure misclassification of air pollutants and the use of an alternate developmental disorder as the control group, both of which may have biased results toward the null. Despite these limitations, methylene chloride, quinoline, and styrene emerged (based on this analysis and prior epidemiologic evidence) as candidates that warrant further investigation for a possible role in autism etiology.
Hazardous air pollutants include hundreds of metal, particulate, and volatile organic compounds known to harm human health. Many are plausible candidate exposures in autism etiology because they have neurotoxic or immunotoxic properties,1 and theories of autism etiology include damage to the developing nervous system and immune perturbation.2–4 Airborne chemical exposures may be especially important in contributing to neurodevelopmental disorders because inhaled particles or metals have been found to be delivered directly to the brain through the olfactory bulb.5,6
Although hazardous air pollutants are not routinely monitored in the United States, emissions data have been used to model annual-average census-tract ambient concentrations in the National Air Toxics Assessment (NATA) program. A study in California studied hazardous air pollutants and autism,7 using NATA output to assign exposures. Children with autism spectrum disorders from a state developmental service agency and a large health maintenance organization were compared with children from birth certificate rosters. Hazardous air pollutants were examined individually and combined into composite scores based on chemical structure or mechanism (eg, endocrine disruptors). From a selected list of 25 air pollutants, positive associations were found for the chlorinated solvent group, the metal group, and several individual pollutants.
Despite calls for data to fill gaps in our understanding of associations between environmental agents and autism,8,9 this California study remains one of the few studies contributing actual data. Intense investigation of many environmental agents is time- and cost-prohibitive. The NATA model presents an opportunity to screen a large number of pollutants with biologic plausibility for a role in autism. Results of such screenings can be considered in combination with toxicologic literature to identify the candidates that deserve more indepth research. Semi-Bayes hierarchical methods are useful in multiple-comparisons situations in which investigators must choose which comparisons to investigate further. This is true especially when there is substantial cost associated with future investigations,10,11 as there is with autism and environmental exposures.
We conducted a screening analysis of hazardous air pollutants and autism spectrum disorders using a prevalent case-control design in North Carolina and West Virginia. As in the California study,7 exposure was assigned using output from the NATA model, but we additionally included some design improvements. We included a more complete group of autism spectrum disorders cases by reviewing records from schools in addition to other sources12 and verified residency of our control group at age 8. To account for the fact that hazardous air pollutants are highly correlated and that more than one of them might have effects on autism, we simultaneously adjusted for measured air pollutants and stabilized the estimates using semi-Bayes models.
METHODS
Study Population
Cases with autism spectrum disorders and controls with speech and language impairment were identified from the Autism and Developmental Disabilities Monitoring Network (ADDM) in North Carolina and West Virginia.12 The monitoring network screens developmental records of children in health and educational settings through the 8th year of life, an age at which most affected children have been identified.13 Autism spectrum disorders diagnoses are fairly stable.14 The North Carolina site evaluated children living in the counties of Alamance, Chatham, Davidson, Durham, Forsyth, Guilford, Orange, and Randolph in 2002 and 2004 (born in 1994 and 1996). The West Virginia site evaluated children living in the entire state in 2000 and 2002 (born in 1992 and 1994). We limited participants in this study to children who had resided in the surveillance region at the time of birth, to obtain in-state birth certificate data and to preclude a potential selection bias induced by differential case versus control in-migration from regions with different concentrations of air pollutants.
Cases were all children with developmental records documenting characteristics and behaviors that met a standardized definition for autism spectrum disorders based on the Diagnostic and Statistical Manual of the American Psychiatric Association (DSM-IV-TR),15 regardless of whether a previous diagnosis of autism spectrum disorders had been documented.
Controls were defined as all children in the surveillance system in North Carolina and West Virginia with a school designation of speech and language impairment without documentation of other serious developmental problems (eg, autism spectrum disorders, intellectual disabilities etc). We assumed that speech and language impairments would not be appreciably affected by air pollution. We selected this control group because (1) the children had equivalent access to developmental evaluations, and (2) we could confirm their residency at age 8. Family residential mobility is common, and has been associated with lifestyle factors such as maternal age and income.16 Children who out-migrate may have different air pollution exposures, necessitating the selection of a control group meeting the same residency requirements as cases.
To obtain the residential address in early life and demographic characteristics, we linked each child to corresponding birth certificate data. In West Virginia, we randomly sampled one-third of all children identified with speech and language impairment within each surveillance year to reduce the burden on the vital statistics department performing birth certificate matches.
Hazardous Air Pollutant Exposures
We assigned exposures during the perinatal period because it is a period of susceptibility to exogenous agents in autism etiology.17 Ambient hazardous air pollutant concentrations were not directly measured in North Carolina or West Virginia in the early to mid-1990s, so we assigned exposure using modeled concentrations from the NATA program. NATA was developed as a tool to prioritize geographic areas and individual pollutants requiring study or intervention.18 We selected NATA 1996 estimates over those from 1990 or 1999 because the 1996 model had improved data input compared with earlier models and was closer in time to the birth-years of our cohort.19
NATA-1996 included diesel particulate matter, components of vehicle exhaust of concern to the Office of Transportation and Air Quality (ethylbenzene, hexane, methyl-tert-butyl ether, propionaldehyde, styrene, toluene, and xylenes), and 32 priority pollutants of concern in urban areas (selected based on toxicity-weighted emission levels). Our analytic method required independent exposure groups. Therefore, we selected the combined group of 7 polycyclic aromatic hydrocarbons (PAH) from NATA instead of the encompassing group of 16 compounds termed polycyclic organic matter because previous studies suggesting human neurodevelopmental toxicity have focused on PAH.20
The NATA model assumes Gaussian air dispersion and uses overlapping spatial grids to predict annual-average ambient concentrations of 41 hazardous air pollutants for each census tract.21 The primary source of inputs into the model is the National Emissions Inventory, which includes the location and rate of pollutant release from point sources such as manufacturing, power-generating, and waste incineration facilities, area sources such as dry cleaners and gas stations, and motor vehicles. Inputs also include meteorologic and secondary-pollutant formation data. We used total ambient concentrations obtained by adding modeled concentrations to an invariant clean background level which is known only for a few hazardous air pollutants.
We assigned individual exposures using the modeled concentrations corresponding to the census tract of the residential address from the birth certificate. Census tracts were determined using a variety of methods, including the US Census Internet-based address search feature22 (n = 2321), geocoding in ArcGIS 9.2 (n = 155), Internet delivery databases of the US Postal Service23 (n = 214), identifying rural zip codes that were enclosed by census tracts (n = 156), and assigning to a random location within a zip code when other methods were unsuccessful (n = 366). Some methods provided year 2000 census tracts, which were translated to 1990 boundaries using census tract relationship files.24
Statistical Analysis
We examined distributions of hazardous air pollutant concentrations by state and by level of urbanicity. We examined pairwise Spearman rank correlations for all pollutants.
We estimated prevalence odds ratios for each air pollutant and autism spectrum disorders by calculating odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression. ORs from categorical coding of air pollutants and from quadratic splines of pollutants and autism spectrum disorders suggested monotonic trends. Air pollutant concentrations were log-transformed because resultant ORs were similar in magnitude to ORs with categorical coding of air pollutants but more precise. We calculated ORs corresponding to the odds of autism spectrum disorders for high air pollutant concentrations (80th percentile) over odds estimated for low concentrations (20th percentile). Percentile values were derived from controls from both states combined. All ORs were adjusted for study design factors of surveillance year and state to account for the stratified sampling and the different control sampling fraction in West Virginia.
We used a directed acyclic graph to evaluate covariates for the potential to confound the ORs.25 Adjusted models included suspected autism spectrum disorders risk factors (maternal age26 and smoking in pregnancy27) social class variables potentially related to air pollutants and unknown environmental causes of autism spectrum disorders (marital status, maternal education, race, and census tract median household income) and urbanicity (because air pollution and prevalence of autism28 are higher in urban areas).
Hazardous air pollutants share sources and are therefore correlated with each other. To accurately estimate ORs attributable to a given pollutant, and also to account for multiple comparisons, we simultaneously included all hazardous air pollutants in one semi-Bayes hierarchical model, which improves the plausibility and stability of estimates.29 The semi-Bayes model adjusted the beta coefficient for each hazardous air pollutant toward the mean of its exchangeability group, with the magnitude of shrinkage dependent on the precision of its conventional likelihood estimate and a pre-specified variance of the assumed normal distribution for that parameter (τ2). We selected a τ2 of 0.209 corresponding to expectations that 95% of ORs would fall within a 6-fold range. Semi-Bayes models were fit using SAS IML code developed by Witte et al.30 In this 2-stage hierarchical model, coefficients and covariance matrices from a conventional logistical model were regressed on second-stage data including exchangeability variables and the value for τ2.
We included exchangeability variables expected to predict the magnitude of ORs, considering both determinants of the true OR and determinants of the observed OR, such as exposure misclassification. Hazardous air pollutants were grouped by the following categorical exchangeability variables: an indicator of demonstrated human developmental neurotoxicity,20,31,32 any evidence of placental transfer,33 similarity between NATA model estimates and measured ambient concentrations categorized as low, medium, and high,34 and an indicator that outdoor pollutant concentrations differ from indoor concentrations.35,36 However, including these exchangeability predictors produced results that were similar to those assuming that all estimates arose from one exchangeability group.
We evaluated modification by state because emission inventories are created at the state level and because polluting sources and sample characteristics exhibited variation between North Carolina and West Virginia. We used likelihood ratio tests of cross-products between pollutants and state, and between pollutants and urbanicity, with an alpha level of 0.10 adjusting for a priori demographic confounders in single-pollutant logistic models to evaluate whether estimated prevalence ratios should be combined across these strata. We used likelihood ratio tests and inspection of stratified ORs to examine whether boys or girls were more susceptible to the effects of exposure. We also repeated analyses limiting cases to those with autism spectrum disorders and comorbid intellectual disabilities (n = 161), and autism spectrum disorders without evidence of this comorbidity (n = 234), in case one subgroup was more susceptible to effects of exposure than the other. Finally, we performed a sensitivity analysis of potentially greater misclassification of exposures for children born prior to NATA-1996, by performing analyses separately for North Carolina children born in 1996 and 1994.
RESULTS
In North Carolina, 220 of the 311 children identified with autism spectrum disorders had a matching North Carolina birth certificate. We determined a census tract for all 220, of whom 206 were born in the surveillance counties. In West Virginia, 189 of the 257 children identified with autism spectrum disorders had a West Virginian birth certificate, and we determined a census tract for 177. In North Carolina, 1947 of the 2584 children with speech and language impairment had a matching North Carolina birth certificate. We determined a census tract for 1901, of whom 1733 were born in the surveillance counties. In West Virginia, 4287 children with speech and language impairment were identified. Of these, 1420 were randomly selected for birth certificate matching, 1146 were found, and we determined a census tract for 1096. For North Carolina children with autism spectrum disorders we had evidence that 86% without a matching birth certificate were born out of state, suggesting that most children excluded from further analyses because of a lack of birth certificate match were not part of our intended sample because they were born out of state.
Children with autism spectrum disorders were more likely to be boys, to be firstborn, to have mothers with higher education, and to reside in urban areas, compared with children with speech and language impairment (Table 1). In North Carolina, most children lived in 100% urban or mixed-urbanicity census tracts. In West Virginia, most children lived in 100% rural or mixed census tracts.
TABLE 1.
Percent Distribution of Children With Autism Spectrum Disorders and Speech and Language Impairment Born Inside the Surveillance Regions of the Autism and Developmental Disabilities Monitoring Network by Characteristics of the Child, Family, Census Tract, and Surveillance Year
| North Carolina |
West Virginia |
|||
|---|---|---|---|---|
| Autism Spectrum Disorders (n = 206) % |
Speech and Language Impairment (n = 1733) % |
Autism Spectrum Disorders (n = 189) % |
Speech and Language Impairment (n = 1146) % |
|
| Child characteristics | ||||
| Sex | ||||
| Girl | 13 | 36 | 24 | 35 |
| Boy | 87 | 64 | 76 | 65 |
| Race | ||||
| Non-Hispanic white | 58 | 64 | 95 | 97 |
| Non-Hispanic black | 36 | 28 | 4 | 3 |
| Other | 7 | 7 | 1 | 1 |
| First born | ||||
| No | 47 | 65 | 54 | 64 |
| Yes | 53 | 35 | 46 | 36 |
| Previously diagnosed autism | ||||
| Yes | 77 | 72 | ||
| No | 23 | 28 | ||
| Comorbid intellectual disabilities | ||||
| Yes (IQ ≤70) | 44 | 37 | ||
| No | 50 | 30 | ||
| Missing | 5 | 33 | ||
| Family characteristics | ||||
| Maternal education | ||||
| <High school | 15 | 23 | 17 | 26 |
| High school degree | 51 | 56 | 68 | 65 |
| College degree | 35 | 21 | 15 | 9 |
| Maternal age (years) | ||||
| ≤22 | 18 | 25 | 26 | 35 |
| 23–34 | 68 | 62 | 62 | 56 |
| ≥35 | 14 | 13 | 12 | 8 |
| Married | ||||
| No | 29 | 31 | 27 | 27 |
| Yes | 71 | 69 | 73 | 73 |
| Smoking in pregnancy | ||||
| No | 87 | 81 | 75 | 71 |
| Yes | 13 | 19 | 25 | 29 |
| Census tract characteristics | ||||
| Urban/rural | ||||
| 100% Rural | 10 | 17 | 40 | 46 |
| Mixed | 50 | 47 | 43 | 42 |
| 100% Urban | 39 | 35 | 18 | 12 |
| Median household income ($/year) | ||||
| ≤20,000 | 12 | 14 | 47 | 50 |
| 20,001 ≤ 30,000 | 44 | 43 | 37 | 40 |
| 30,001 ≤ 40,000 | 26 | 33 | 15 | 10 |
| >40,000 | 17 | 11 | 1 | 1 |
| Study design | ||||
| Surveillance year | ||||
| 2000 | 0 | 0 | 43 | 53 |
| 2002 | 42 | 53 | 57 | 47 |
| 2004 | 58 | 47 | 0 | 0 |
We excluded the following air pollutants from further analysis because they had no detectable variation above background levels: carbon tetrachloride, ethylene dibromide, ethylene dichloride, hexachlorobenzene, and polychlorinated biphenyls (Table 2). Coke-oven emissions were present only in West Virginia. Concentrations of all pollutants were higher in urban than nonurban areas. Concentrations were higher in North Carolina than West Virginia for most pollutants even when comparing regions of similar urbanicity. Variability of most pollutants was greater in nonurban areas. Most air pollutants were correlated with all other air pollutants, with Spearman rank correlations of at least 0.5 and often much higher—except for coke oven emissions, which were not correlated with any other pollutant. Air pollutants known to be emitted from mobile sources37 were especially correlated (>0.8).
TABLE 2.
Geometric Means and Geometric Standard Deviations (SDs) of Estimated Ambient Concentrations (ng/m3) of Hazardous Air Pollutants Stratified by State and Urbanicity for Children With Speech and Language Impairment From the Autism and Developmental Disabilities Monitoring Network of North Carolina (2002 and 2004) and West Virginia (2000 and 2002)
| North Carolina |
West Virginia |
||||||
|---|---|---|---|---|---|---|---|
| Clean Air Background |
Urban (n = 613) Mean (SD) |
Not Urban (n = 1120) Mean (SD) |
All NC (n = 1733) Mean (SD) |
Urban (n = 132) Mean (SD) |
Not Urban (n = 964) Mean (SD) |
All WV (n = 1096) Mean (SD) |
|
| Acetaldehyde | 784.1 (1.1) | 559.5 (1.4) | 630.5 (1.4) | 488.7 (1.5) | 208.6 (2.1) | 231.1 (2.1) | |
| Acrolein | 110.1 (1.1) | 85.4 (1.2) | 93.5 (1.2) | 92.8 (1.4) | 52.4 (1.5) | 56.1 (1.6) | |
| Acrylonitrile | 0.9 (2.0) | 0.7 (2.2) | 0.8 (2.1) | 1.9 (4.4) | 0.3 (4.4) | 0.4 (5.0) | |
| Arsenic compounds | 0.22 (1.6) | 0.13 (2.0) | 0.15 (2.0) | 0.11 (2.0) | 0.02 (6.5) | 0.03 (6.3) | |
| Benzene | 480 | 1523 (1.1) | 1066.4 (1.2) | 1209.7 (1.3) | 1388 (1.3) | 790.2 (1.3) | 845.6 (1.4) |
| Beryllium compounds | 0.035 (1.7) | 0.018 (2.1) | 0.023 (2.1) | 0.012 (1.7) | 0.002 (2.6) | 0.002 (3.0) | |
| 1,3-Butadiene | 85.9 (1.3) | 37.8 (1.7) | 50.6 (1.8) | 81.7 (1.6) | 20.9 (2.0) | 24.6 (2.3) | |
| Cadmium compounds | 0.084 (1.7) | 0.047 (1.8) | 0.058 (1.9) | 0.046 (2.1) | 0.010 (3.6) | 0.012 (3.8) | |
| Carbon Tetrachloride | 880 | 880.3 (1.0) | 880.4 (1.0) | 880.3 (1.0) | 880.3 (1.0) | 880.1 (1.0) | 880.1 (1.0) |
| Chloroform | 83 | 85.9 (1.0) | 84.9 (1.0) | 85.2 (1.0) | 87.8 (1.1) | 84.4 (1.0) | 84.8 (1.1) |
| Chromium compounds | 3.4 (3.0) | 1.8 (2.5) | 2.3 (2.8) | 1.0 (5.6) | 0.1 (5.1) | 0.1 (6.0) | |
| Coke oven emissionsa | 0% | 0% | 0% | 38% | 20% | 22% | |
| 1,3-Dichloropropene | 80.2 (1.2) | 33.8 (1.7) | 45.9 (1.8) | 71.8 (1.6) | 13.6 (2.5) | 16.7 (2.8) | |
| Diesel particulate matter | 1788 (1.2) | 1358 (1.2) | 1497 (1.3) | 2793 (1.7) | 1111 (1.7) | 1241 (1.8) | |
| Ethylbenzene | 485.9 (1.2) | 242.6 (1.6) | 310.2 (1.7) | 360.0 (1.5) | 80.2 (2.4) | 96.1 (2.6) | |
| Ethylene dibromide | 7.7 | 7.7 (1.0) | 7.7 (1.0) | 7.7 (1.0) | 7.7 (1.0) | 7.7 (1.0) | 7.7 (1.0) |
| Ethylene dichloride | 61 | 61.2 (1.0) | 61.3 (1.0) | 61.2 (1.0) | 61.1 (1.0) | 61.0 (1.0) | 61.0 (1.0) |
| Ethylene oxide | 8.9 (1.9) | 2.9 (1.9) | 4.3 (2.3) | 6.1 (4.0) | 0.5 (6.7) | 0.6 (7.6) | |
| Formaldehyde | 250 | 1090 (1.1) | 912.7 (1.3) | 972.0 (1.3) | 936.9 (1.3) | 590.4 (1.3) | 624.2 (1.4) |
| Hexachlorobenzene | 0.093 | 0.093 (1.0) | 0.093 (1.0) | 0.093 (1.0) | 0.093 (1.0) | 0.093 (1.0) | 0.093 (1.0) |
| Hexane | 566.3 (1.2) | 264.5 (1.6) | 346.2 (1.7) | 547.6 (1.6) | 119.7 (2.5) | 143.7 (2.8) | |
| Hydrazine | 15 E-5 (3.9) | 4 E-5 (3.6) | 6 E-5 (4.3) | 48 E-5 (9.4) | 0 E-5 (87.8) | 1 E-5 (95.2) | |
| Lead compounds | 3.17 (1.6) | 2.09 (1.9) | 2.42 (1.9) | 1.09 (2.7) | 0.24 (3.5) | 0.29 (3.7) | |
| Manganese compounds | 5.8 (1.7) | 4.9 (2.3) | 5.2 (2.1) | 3.0 (3.8) | 0.4 (4.4) | 0.6 (4.9) | |
| Mercury compounds | 0.309 (1.3) | 0.224 (1.7) | 0.251 (1.6) | 0.187 (1.7) | 0.051 (2.9) | 0.060 (3.0) | |
| Methyl tert-butyl ether (MTBE) | 102.3 (1.3) | 50.2 (1.6) | 64.6 (1.7) | 126.9 (1.7) | 33.9 (2.5) | 39.7 (2.6) | |
| Methylene chloride | 150 | 679.1 (1.9) | 476.0 (1.8) | 539.8 (1.9) | 338.3 (1.3) | 188.6 (1.2) | 202.3 (1.3) |
| Nickel Compounds | 1.9 (1.6) | 0.8 (1.9) | 1.1 (2.0) | 1.5 (7.4) | 0.1 (4.9) | 0.2 (6.3) | |
| Perchloroethylene | 140 | 288.9 (1.1) | 198.9 (1.2) | 227.0 (1.3) | 258.9 (1.3) | 162.9 (1.2) | 172.2 (1.3) |
| Polychlorinated Biphenyls | 0.38 | 0.380 (1.0) | 0.380 (1.0) | 0.380 (1.0) | 0.380 (1.0) | 0.380 (1.0) | 0.380 (1.0) |
| Polycyclic aromatic hydrocarbons group (PAH7) | 6.7 (2.7) | 3.4 (1.8) | 4.3 (2.3) | 3.0 (1.4) | 1.2 (1.6) | 1.4 (1.7) | |
| Propionaldehyde | 222.3 (1.2) | 155.5 (1.4) | 176.5 (1.4) | 105.0 (1.4) | 47.4 (2.0) | 52.1 (2.0) | |
| Propylene dichloride | 0.056 (2.0) | 0.038 (2.2) | 0.044 (2.2) | 0.041 (1.8) | 0.011 (2.9) | 0.013 (3.0) | |
| Quinoline | 0.003 (6.2) | 0.001 (3.5) | 0.001 (4.7) | 0.010 (19.5) | 0.0001 (55.4) | 0.0001 (68.1) | |
| Styrene | 48.4 (1.7) | 15.8 (2.1) | 23.4 (2.3) | 58.4 (2.3) | 7.5 (3.7) | 9.6 (4.2) | |
| 1,1,2,2-Tetrachloroethane | 0.53 (2.3) | 0.37 (2.7) | 0.42 (2.6) | 0.20 (1.8) | 0.08 (2.9) | 0.09 (2.9) | |
| Toluene | 3202 (1.2) | 1672 (1.7) | 2105 (1.7) | 2605 (1.7) | 584.0 (2.3) | 699.2 (2.6) | |
| Trichloroethylene | 81 | 129.1 (1.2) | 113.6 (1.4) | 118.8 (1.3) | 119.0 (1.2) | 87.0 (1.1) | 90.3 (1.2) |
| Vinyl chloride | 1.24 (2.2) | 0.96 (2.3) | 1.05 (2.3) | 1.31 (4.0) | 0.26 (3.6) | 0.32 (4.1) | |
| Xylenes | 2108 (1.2) | 1171 (1.5) | 1441 (1.5) | 1612 (1.5) | 523.9 (1.7) | 599.9 (1.9) | |
Coke oven emissions were mostly 0 and so are shown as percent with any coke oven emissions.
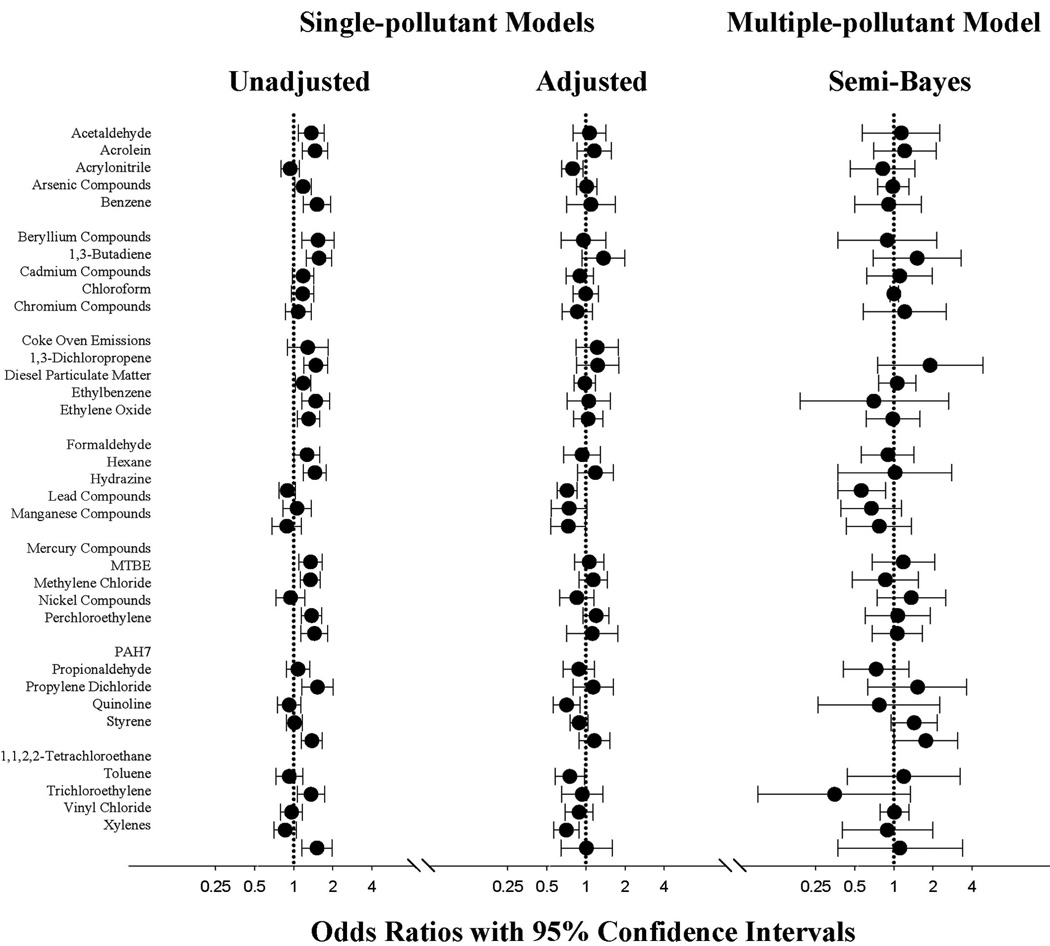
In unadjusted analyses, ORs were elevated above the null for many pollutants (Fig.). After adjustment for a priori demographic confounders, all estimates were attenuated, so that most adjusted estimates were near-null. For example, the OR for beryllium compounds was 1.5 (95% CI = 1.1–2.0) before adjustment and 1.0 (0.6 –1.4) after adjustment. For xylenes, the unadjusted was 1.5 (1.2–2.0) and the adjusted was 1.0 (0.6–1.6). After adjusting for other air pollutants in semi-Bayes models, ORs were less precise and many remained near-null, but some were shifted upwards or downwards. Estimates shifted upward included those for chromium compounds, dichloropropene, methylene chloride, propionaldehyde, quinoline, styrene, and tetrachloroethane. Estimates shifted downward below the null included ethylbenzene and toluene.
FIGURE.
Odds ratios (circles) and 95% confidence intervals (bars) for ambient concentrations of hazardous air pollutants and autism spectrum disorders versus speech and language impairment estimated using different logistic regression models. ORs compare a high exposure (80th percentile) to a low exposure (20th percentile), as shown in Table 3. Single-pollutant estimates include each pollutant in a separate model. Multipollutant models include all listed pollutants except for coke oven emissions. The unadjusted model (n = 3212; 383 cases) controls for surveillance year and state. Adjusted and semi-Bayes models (n = 3177; 374 cases) also include a priori confounders: race (non-Hispanic white, black, other), maternal education (<HS, HS, college), maternal age (quadratic splines with 3 knots), smoking in pregnancy (yes, no), marital status, census tract median household income (quadratic splines with 3 knots), and urbanicity (quadratic splines with 3 knots). Semi-Bayes models specify a prior residual variance (τ2) of 0.209 (6-fold range) and exchangeability predictors of known human developmental neurotoxicant, placental transfer, confidence level in hazardous air pollutant model estimates, and similarity between outdoor and indoor concentrations.
Associations between many pollutants and autism spectrum disorders differed by both state and level of urbanicity when tested in single-pollutant adjusted models. This modification was less pronounced after adjusting for other pollutants in semi-Bayes models (Table 3). Level of urbanicity and state were associated in our sample, with North Carolina having a greater proportion of children living in urban areas than West Virginia. There was a suggestion that urbanicity partly accounted for differences in the magnitude of estimates stratified by state for some pollutants. For example, the ORs for methylene chloride, quinoline, and styrene were more elevated in North Carolina than in West Virginia, and in urban compared with nonurban areas.
TABLE 3.
Associations Between Hazardous Air Pollutants and Autism Spectrum Disorder From Hierarchical Semi-Bayes Modelsa,b by Urban/Rural Classification and State
| Urban/Rural |
State |
||||||
|---|---|---|---|---|---|---|---|
| Unit Changec (ng/m3) |
100% Rural | Mixed | 100% Urban | NC | WV | All | |
| No. Children | 881 | 1446 | 850 | 1931 | 1246 | 3177 | |
| No. Cases | 89 | 177 | 108 | 201 | 173 | 374 | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | ||
| Acetaldehyde | 756 vs. 238 | 1.3 (0.6–2.9) | 1.3 (0.5–3.6) | 0.8 (0.3–2.4) | 1.4 (0.5–4.1) | 1.0 (0.5–2.1) | 1.1 (0.6–2.3) |
| Acrolein | 109 vs. 53 | 1.2 (0.6–2.5) | 1.4 (0.7–2.6) | 0.9 (0.5–1.9) | 1.3 (0.7–2.7) | 1.2 (0.6–2.2) | 1.2 (0.7–2.1) |
| Acrylonitrile | 1.4 vs. 0.2 | 0.9 (0.4–2.0) | 1.0 (0.5–2.4) | 0.8 (0.3–2.5) | 0.6 (0.2–2.4) | 1.0 (0.5–1.8) | 0.8 (0.5–1.5) |
| Arsenic compounds | 0.21 vs. 0.03 | 0.9 (0.5–1.6) | 1.2 (0.8–1.8) | 0.9 (0.2–4.3) | 0.9 (0.3–2.9) | 0.9 (0.7–1.3) | 1.0 (0.8–1.3) |
| Benzene | 1438 vs. 742 | 1.1 (0.6–2.0) | 1.0 (0.5–1.8) | 1.0 (0.5–1.9) | 1.0 (0.5–1.9) | 0.9 (0.5–1.6) | 0.9 (0.5–1.6) |
| Beryllium compounds | 0.031 vs. 0.003 | 0.6 (0.1–2.5) | 1.6 (0.4–5.9) | 2.1 (0.4–11) | 1.1 (0.3–4.9) | 0.9 (0.2–4.6) | 0.9 (0.4–2.1) |
| 1,3-Butadiene | 79 vs. 18 | 1.1 (0.4–3.2) | 1.7 (0.7–4.3) | 1.0 (0.3–3.6) | 1.6 (0.5–4.8) | 1.4 (0.6–3.4) | 1.5 (0.7–3.3) |
| Cadmium compounds | 0.07 vs. 0.01 | 1.2 (0.4–3.5) | 0.8 (0.4–1.9) | 1.2 (0.3–4.7) | 1.2 (0.4–3.2) | 0.6 (0.3–1.6) | 1.1 (0.6–2.0) |
| Chloroform | 90 vs. 83c | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) |
| Chromium compounds | 3.4 vs. 0.2 | 1.1 (0.3–4.2) | 1.4 (0.5–4.4) | 0.7 (0.1–3.6) | 0.7 (0.2–2.9) | 2.0 (0.7–5.4) | 1.2 (0.6–2.5) |
| Coke oven emissionsb | Any vs. none | 1.4 (0.7–2.7) | 0.9 (0.5–1.7) | 1.8 (0.8–4.2) | NE | 1.2 (0.8–1.7) | 1.2 (0.8–1.8) |
| 1,3-Dichloropropene | 71 vs. 14 | 1.7 (0.5–6.4) | 1.8 (0.5–5.9) | 0.9 (0.2–3.5) | 1.2 (0.3–4.2) | 1.7 (0.5–5.2) | 1.9 (0.8–4.8) |
| Diesel particulate matter | 1844 vs. 1025 | 0.9 (0.5–1.6) | 1.0 (0.7–1.6) | 0.9 (0.6–1.5) | 1.1 (0.6–1.9) | 1.0 (0.7–1.5) | 1.1 (0.8–1.5) |
| Ethylbenzene | 441 vs. 82 | 0.6 (0.1–2.3) | 1.2 (0.3–5.0) | 0.9 (0.2–4.2) | 1.5 (0.3–6.6) | 0.5 (0.1–2.0) | 0.7 (0.2–2.6) |
| Ethylene oxide | 8.4 vs. 0.5 | 0.8 (0.3–2.4) | 1.0 (0.5–2.1) | 1.0 (0.3–3.2) | 0.5 (0.1–1.9) | 1.2 (0.6–2.2) | 1.0 (0.6–1.6) |
| Formaldehyde | 1074 vs. 589 | 1.0 (0.6–1.7) | 1.0 (0.6–1.7) | 0.9 (0.5–1.7) | 1.1 (0.6–1.7) | 0.9 (0.5–1.5) | 0.9 (0.6–1.4) |
| Hexane | 523 vs. 120 | 1.4 (0.4–4.9) | 1.1 (0.3–3.3) | 1.0 (0.3–3.6) | 1.1 (0.3–4.2) | 0.9 (0.3–2.5) | 1.0 (0.4–2.8) |
| Hydrazine | 2.4 E-4 vs. 3.4 E-6 | 0.5 (0.2–1.1) | 0.8 (0.4–1.4) | 0.4 (0.1–1.3) | 1.0 (0.3–3.7) | 0.7 (0.4–1.1) | 0.6 (0.4–0.9) |
| Lead compounds | 3.1 vs. 0.3 | 0.9 (0.3–2.7) | 0.7 (0.3–1.5) | 0.3 (0.1–1.3) | 0.6 (0.2–1.5) | 0.9 (0.5–1.9) | 0.7 (0.4–1.1) |
| Manganese compounds | 7.1 vs. 0.4 | 1.2 (0.5–3.3) | 0.7 (0.3–1.6) | 0.6 (0.1–3.4) | 0.7 (0.2–2.6) | 0.8 (0.4–1.8) | 0.8 (0.4–1.4) |
| Mercury compounds | 0.3 vs. 0.1 | 1.2 (0.5–3.1) | 0.9 (0.4–2.2) | 1.0 (0.3–3.2) | 0.9 (0.4–2.2) | 1.1 (0.5–2.4) | 1.2 (0.7–2.1) |
| Methyl tert-butyl ether (MTBE) | 100 vs. 30 | 1.6 (0.7–3.6) | 0.8 (0.4–1.7) | 0.9 (0.4–2.3) | 0.9 (0.4–2.1) | 1.2 (0.6–2.2) | 0.9 (0.5–1.5) |
| Methylene chloride | 640 vs. 183 | 1.2 (0.4–3.3) | 1.2 (0.6–2.5) | 1.9 (0.8–4.7) | 1.5 (0.6–3.3) | 1.0 (0.4–3.0) | 1.4 (0.7–2.5) |
| Nickel compounds | 1.7 vs. 0.1 | 1.2 (0.4–3.4) | 0.9 (0.4–2.0) | 1.8 (0.6–4.9) | 0.8 (0.2–3.3) | 0.9 (0.4–1.8) | 1.1 (0.6–1.9) |
| Perchloroethylene | 271 vs. 155 | 1.1 (0.6–2.0) | 1.2 (0.7–2.0) | 1.0 (0.6–1.7) | 1.1 (0.7–1.9) | 1.1 (0.6–1.8) | 1.1 (0.7–1.7) |
| Polycyclic aromatic hydrocarbons group (PAH7) | 4.9 vs. 1.2 | 0.7 (0.2–3.2) | 0.3 (0.1–1.0) | 1.0 (0.5–1.9) | 0.9 (0.4–1.8) | 0.9 (0.2–3.6) | 0.7 (0.4–1.3) |
| Propionaldehyde | 208 vs. 56 | 1.2 (0.4–3.3) | 1.3 (0.5–3.6) | 1.1 (0.4–3.5) | 1.1 (0.4–3.4) | 1.2 (0.5–3.1) | 1.5 (0.6–3.6) |
| Propylene dichloride | 0.08 vs. 0.01 | 0.7 (0.2–3.2) | 0.9 (0.2–3.2) | 0.8 (0.2–3.9) | 0.8 (0.2–3.7) | 0.6 (0.2–2.4) | 0.8 (0.3–2.3) |
| Quinoline | 0.0051 vs. 0.0001 | 1.5 (0.7–3.3) | 1.1 (0.6–2.1) | 2.1 (0.8–5.6) | 2.1 (0.4–11) | 1.3 (0.8–2.1) | 1.4 (1.0–2.2) |
| Styrene | 41 vs. 5 | 0.8 (0.2–2.7) | 1.1 (0.5–2.8) | 2.0 (0.6–6.1) | 2.0 (0.6–6.9) | 1.6 (0.8–3.2) | 1.8 (1.0–3.1) |
| 1,1,2,2-Tetrachloroethane | 0.8 vs. 0.1 | 1.0 (0.2–5.6) | 1.2 (0.4–3.8) | 0.8 (0.2–3.5) | 0.7 (0.1–3.2) | 1.9 (0.5–7.3) | 1.2 (0.4–3.2) |
| Toluene | 3018 vs. 582 | 0.4 (0.1–2.8) | 0.3 (0.1–1.6) | 0.6 (0.1–3.5) | 0.5 (0.1–3.0) | 0.6 (0.1–3.4) | 0.4 (0.1–1.3) |
| Trichloroethylene | 129 vs. 85 | 1.1 (0.7–1.6) | 1.1 (0.8–1.5) | 1.0 (0.7–1.5) | 1.1 (0.8–1.5) | 1.0 (0.7–1.5) | 1.0 (0.8–1.3) |
| Vinyl chloride | 2.0 vs. 0.3 | 0.9 (0.3–3.3) | 0.8 (0.3–2.5) | 1.3 (0.3–5.0) | 0.8 (0.2–3.3) | 0.9 (0.4–2.4) | 0.9 (0.4–2.0) |
| Xylenes | 1935 vs. 505 | 1.0 (0.3–4.2) | 1.4 (0.4–4.9) | 0.7 (0.2–2.7) | 1.3 (0.3–4.9) | 0.9 (0.3–3.1) | 1.1 (0.4–3.4) |
The semi-Bayes estimates arise from a model including all HAPs except for coke oven emissions and a priori confounders race (non-Hispanic white, black, other), maternal education (<HS, HS, college), maternal age (quadratic splines with 3 knots), smoking in pregnancy (yes, no), marital status, census tract median household income (quadratic splines with 3 knots), and urbanicity (quadratic splines with 3 knots) and also include a second-stage model with a pre-specified residual variance in estimates (τ2) of 0.209 (6-fold range) and exchangeability predictors of known human developmental neurotoxicant, placental transfer, confidence level in HAP model estimates, and similarity between outdoor and indoor concentrations.
Coke oven emissions were not correlated with any other HAP and so were not adjusted for other HAPs or included in semi-Bayes models.
Unit change corresponds to the 80th percentile versus 20th percentile among controls for both states combined, except for chloroform which exhibited low variability and for which we compared the 95th versus the fifth percentile.
NE indicates not estimable.
Most stable ORs from the fully-adjusted semi-Bayes models were near-null overall, in both states, and within levels of urbanicity (Table 3). Pollutants that had more-precise elevated associations overall and within states and levels of urbanicity were methylene chloride, quinoline, and styrene, whereas hydrazine and lead had negative associations.
Sex of the child satisfied our screening criteria for interaction with many pollutants. After adjusting for other pollutants in semi-Bayes models, confidence intervals of ORs stratified by sex were imprecise for girls and exhibited substantial overlap with those for boys, so that differences could be due to chance. Point estimates were not consistently greater for either boys or girls. Some estimates that showed differences were butadiene (boys, 1.5 [0.7–3.4] and girls, 0.9 [0.3–2.9]); mercury compounds (boys, 1.0 [0.5–1.9] and girls, 1.8 [0.7–4.4]); styrene (boys, 2.2 [1.1–4.1] and girls, 0.7 [0.2–2.6]); and tetrachloroethane (boys, 1.6 [0.5–4.6] and girls, 0.7 [0.1–3.9]).
When models were fit separately for autism spectrum disorders with and without comorbid intellectual disabilities, resultant ORs from semi-Bayes models were mostly within the confidence intervals of the estimate from the other case group, with no clear patterns of increased point estimates for one or the other case group. Some estimates with greater differences were coke oven emissions (with intellectual disabilities, 1.9 [1.1–3.2] and without, 1.0 [0.6–1.6]), dichloropropene (with comorbidity, 1.0 [0.3–3.1] and without, 2.2 [0.8–6.2]), mercury compounds (with comorbidity, 1.7 [0.8–3.6] and without, 0.9 [0.5–1.8]), and methylene chloride (with comorbidity, 1.7 [0.8–3.7] and without, 1.0 [0.5–2.1]).
When North Carolina models were fit separately for children born in 1994 and 1996, ORs were very unstable (results not shown).
DISCUSSION
We used semi-Bayes analyses to screen perinatal exposure to 35 hazardous air pollutants for a role in contributing to autism spectrum disorders at age 8. These methods can outperform conventional maximum-likelihood-estimation techniques for prediction,38 and are useful in settings where multiple exposures are evaluated for prioritization in future studies.10 After accounting for family and census-tract social characteristics, urbanicity, and potential confounding by correlated air pollutants, we estimated mostly near-null associations, including pollutants that are established human developmental neurotoxicants: polycyclic aromatic hydrocarbons,20 arsenic, lead, manganese, mercury, and toluene.31 Some pollutants stood out with estimates that were precise but elevated compared with those for all other air pollutants—especially quinoline and styrene. Our results are mostly divergent from those from a previous study of hazardous air pollutants and autism spectrum disorders in California,7 except for methylene chloride.
Methylene chloride is a solvent and propellant widely used in manufacturing, and is emitted from point and area sources. Ambient airborne exposure to methylene chloride was positively associated with autism spectrum disorders prevalence in the previous California study.7 We found an elevated OR for methylene chloride, but with a confidence interval including the null. Chronic exposure to methylene chloride has established central nervous system effects.33 A study examining maternal exposure to solvents including methylene chloride found decrements in child cognitive, language, and behavioral functioning.39 Methylene chloride is a pollutant that should be further studied for a role in autism spectrum disorders etiology, using improved exposure assessment.
Unlike the previous study, we estimated elevated associations for quinoline and styrene. The California study did not examine quinoline because of a lack of evidence of developmental toxicity or endocrine-disrupting characteristics of quinoline. Styrene was examined only as part of a composite score that included 5 aromatic solvents, with a resultant null estimate. It is possible that any effect of styrene in the previous study was diluted by its inclusion in a group with other pollutants, if the other pollutants had null or inverse associations with autism spectrum disorders. Neurodevelopmental effects of quinoline and styrene have not been previously reported. Quinoline is a chemical intermediate used in organic processing such as petroleum refining and coal mining, with emissions primarily from area sources. Traffic is the primary source of styrene emissions.
We estimated near-null associations for several pollutants that had positive associations in previous studies: cadmium, mercury, nickel, trichloroethylene, and vinyl chloride.7,40,41 Estimates for cadmium, mercury, and nickel were elevated prior to adjustment in our study but were attenuated upon adjustment. This suggests that the earlier positive associations for these metals may have been due to confounding by urbanicity, other pollutants, or unknown factors associated with urbanicity or pollutant concentrations. In contrast, trichloroethylene and vinyl chloride estimates were near-null both before and after adjustment for confounding in our study. Low-exposure levels in our study sample could have contributed to null findings, especially for vinyl chloride, for which outdoor concentrations were 10 times higher in the California sample. Overall our study areas were characterized by outdoor pollutant concentrations 2–3 times lower than those in the California study. The range of ambient concentrations of these chemicals that may impair neurodevelopment is largely unknown for these pollutants, limiting our ability to discern the impact of studying potentially low levels. In contrast, null findings could reflect that inhalation exposure to these chemicals from ambient sources is not as important as other routes of exposure for these chemicals. Several of the included pollutants have substantial sources of human exposure from diet, tobacco smoke, and indoor environments.
Differences in results between our study and the California study may also have been influenced by the different control groups. Our control group had similar access to developmental testing and met the same residential requirements as the cases, thereby providing an estimate of exposure for the population source that gave rise to the case group. The age 8 address or access to developmental testing services could not be confirmed for children included from the birth certificate rosters used in the previous study.7 Speech and language impairment was chosen over other disorders included in the ADDM surveillance network because, under this protocol, children designated with speech and language impairment but no other developmental problems never met surveillance criteria for autism spectrum disorders. In addition, speech and language impairments are relatively mild and more common than autism spectrum disorders, and therefore, provide a sufficiently-sized control sample.42
We assumed that isolated speech and language impairments were not caused by exposure to any of the included pollutants. This assumption is consistent with one study examining maternal occupational exposure and speech and language impairment that estimated near-null associations with solvents, pesticides, dusts, and metals.43 However, environmental contributions to speech and language development have been scarcely studied,43 and it remains possible that impairments may be influenced by air pollutants. The below-null estimates for lead and toluene in our study could be due to a positive association between these chemicals and speech and language impairment to a greater extent than for autism spectrum disorders. Furthermore, ORs from our study may underestimate the true association with autism spectrum disorders for any pollutant positively associated with speech and language impairment.
It is possible that only an unknown subgroup of children was susceptible to a given air pollutant. We had little information to define such subgroups, and for the few variables we did have (gender and comorbid intellectual disabilities), we had limited power to detect modification.
Diagnosis of autism spectrum disorders is complex, and detection may vary geographically with access to health or educational services.40 This can complicate studies of autism spectrum disorders when the geographic gradient of environmental exposures may correlate with factors promoting case ascertainment. Our study is less likely to have been affected by an ascertainment bias, in part because our case group was not required to have a prior diagnosis and because case and control selection required similar access to developmental testing (as evidenced by their designation of speech and language impairment). We additionally controlled for urbanicity, which may serve as a proxy of increased access to health services, and is related to autism spectrum disorders prevalence and hazardous air pollutant concentrations.
We lacked information on personal exposure to pollutants during early pregnancy, and so assigned individual exposure to each pollutant using output from a temporally and spatially averaged air pollution model (NATA). NATA addresses hazardous air pollutants of highest health concern and greatest mass of emissions. NATA uses extensive national and local emissions inputs and consequently is more complete than exposure-assignment methods that use only proximity to roads or large polluting sources. Our design took advantage of the ability of NATA to distinguish persons with high exposures from those with low exposures, relying on spatial contrasts more than temporal contrasts.
NATA 1996 output has been found to underpredict monitored ambient concentrations, but to preserve rank order of pollutant level.21,35,44–46 Model accuracy varies by pollutant.46 Discrepancies between model output and measured ambient concentrations are thought to be due primarily to the completeness of emissions inventory inputs. For example, not all businesses report to the Toxic Release Inventory, which is an important source of industrial emissions model inputs. However, inputs for the 1996 model are more complete than those for previous years.19
NATA model performance varies regionally across the United States.21 Both North Carolina and West Virginia were ranked as having the highest level of participation in reporting to the National Toxics Inventory with the lowest proportion (<10%) having uncertain location.46 Still, conclusions about accuracy for our sample are limited by the lack of monitoring of pollutants in North Carolina and West Virginia for the relevant time period, precluding validation studies in these states.
Even where NATA reflects outdoor concentration rankings, there are additional levels of uncertainties in using these outputs to characterize individual exposure. Our design assigned the average concentration for the mother’s residential census tract. This does not account for daily activity patterns that include leaving the tract, geocoding errors, or spatial variability in pollutant concentrations within the tract. All of these can be important determinants of individual exposure.47 Greater errors will arise in assigning exposures for persons residing in high-pollutant areas, especially for pollutants dominated by point sources.46 The ability of model output to serve as a surrogate of personal exposure has been evaluated directly and deemed reasonable for most air pollutants.35 Ability to reflect personal exposure is especially good for mobile source volatile organic compounds (eg, benzene) and less accurate for volatile organic compounds with substantial indoor sources (eg, chloroform).35
Exposure misclassification also arises from temporal averaging. NATA output pertains to an average for 1996, whereas the period of susceptibility may be a small window during gestation. Furthermore, our design assumed that the rank order of air pollutants remained stable from 1992 through 1996, the periods of birth for our subjects. Overall, concentrations of air pollutants during this period were decreasing due to regulations that address point sources and on-road vehicle emissions.48 Concentrations could have changed during these periods in North Carolina or West Virginia, for example, due to the opening or closing of a large polluting source or changes in mountain-top coal removal in West Virginia. We lacked the statistical power to determine if our results were sensitive to potentially greater misclassification for earlier birth years.
We attempted to account for uncertainties in the ability of NATA-model estimates to reflect personal exposure by including 2 exchangeability variables in our semi-Bayes models: similarity between modeled and measured concentrations, and similarity between outdoor and indoor concentrations for a given pollutant. Inclusion of these variables did not influence results. However, these exchangeability variables had limitations. For example, accuracy rankings focused on the ability to predict pollutant concentrations at the mean instead of the ability to differentiate high-exposure from low-exposure areas.35,36
The use of NATA to assign exposure in our study is certainly the largest limitation. However, because autism spectrum disorder cases and speech and language impairment controls met similar residency requirements and were derived from the same data sources, it is unlikely that this misclassification differed by outcome status. Hence, exposure misclassification has likely attenuated the reported estimates toward the null, so that true effects may be larger. In addition, exposure misclassification limited our ability to control for confounding due to correlated pollutants. Simultaneously adjusting for multiple pollutants measured at the ecologic level may have produced unexpected results. For example, outdoor measurements of some criteria pollutants are more correlated with personal exposure to another pollutant than the measured pollutant.49 If this were true in these data, it is possible that we may have inadvertently adjusted a given pollutant for a proxy of itself, thereby biasing estimates toward the null.
In summary, several facets of our study may have produced estimates biased toward the null, including the use of a control group that may have associations with the exposure, exposure misclassification, and our use of semi-Bayes shrinkage estimators. For these reasons our design may have failed to screen in chemicals that could potentially have a role in autism etiology. Despite these limitations, we estimated elevated associations for some hazardous air pollutants. Our results are consistent with previous findings regarding methylene chloride7 and suggest quinoline and styrene as possible targets for follow-up in future investigation of autism etiology.
ACKNOWLEDGMENTS
We thank Barbara Becker-Cottrill, Julie O’Malley, and Tom Leonard for their help with West Virginia surveillance and birth certificate data, Cynthia Cassell and Bob Meyer for assistance with North Carolina birth certificate data, Ted Palma for information regarding the NATA program, and Kristen Rappazzo for geocoding assistance.
Supported in part by grants from the Centers for Disease Control Prevention and the National Institute of Environmental Health Sciences (P30ES10126 and T32 ES007018).
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Environmental Protection Agency. [Accessed July 20, 2009];Health Effects Notebook for Hazardous Air Pollutants. Available at: http://www.epa.gov/ttn/atw/hlthef/hapindex.html.
- 2.Croonenberghs J, Wauters A, Devreese K, et al. Increased serum albumin, gamma globulin, immunoglobulin IgG, and IgG2 and IgG4 in autism. Psychol Med. 2002;32:1457–1463. doi: 10.1017/s0033291702006037. [DOI] [PubMed] [Google Scholar]
- 3.Molloy CA, Morrow AL, Meinzen-Derr J, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172:198–205. doi: 10.1016/j.jneuroim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Zimmerman AW, Jyonouchi H, Comi AM, et al. Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol. 2005;33:195–201. doi: 10.1016/j.pediatrneurol.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, et al. DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol. 2003;31:524–538. doi: 10.1080/01926230390226645. [DOI] [PubMed] [Google Scholar]
- 6.Oberdorster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 7.Windham GC, Zhang L, Gunier R, Croen LA, Grether JK. Autism spectrum disorders in relation to distribution of hazardous air pollutants in the San Francisco bay area. Environ Health Perspect. 2006;114:1438–1444. doi: 10.1289/ehp.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawler CP. The “environment” for autism research: signs of improvement? Environ Health Perspect. 2008;116:A416–A417. doi: 10.1289/ehp.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landrigan PJ. What causes autism? Exploring the environmental contribution. Curr Opin Pediatr. 2010;22:219–225. doi: 10.1097/MOP.0b013e328336eb9a. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S, Robins JM. Empirical-Bayes adjustments for multiple comparisons are sometimes useful. Epidemiology. 1991;2:244–251. doi: 10.1097/00001648-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Steenland K, Bray I, Greenland S, Boffetta P. Empirical Bayes adjustments for multiple results in hypothesis-generating or surveillance studies. Cancer Epidemiol Biomarkers Prev. 2000;9:895–903. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Prevalence of autism spectrum disorders—autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ. 2007;56:12–28. [PubMed]
- 13.Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a US metropolitan area. JAMA. 2003;289:49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- 14.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Arch Gen Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 15.Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association. [Google Scholar]
- 16.Canfield MA, Ramadhani TA, Langlois PH, Waller DK. Residential mobility patterns and exposure misclassification in epidemiologic studies of birth defects. J Expo Sci Environ Epidemiol. 2006;16:538–543. doi: 10.1038/sj.jes.7500501. [DOI] [PubMed] [Google Scholar]
- 17.Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J Comp Neurol. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 18.Environmental Protection Agency. [Accessed July 20, 2009];Technology transfer Network 1996 National-Scale Air Toxics Assessment. Available at: http://www.epa.gov/ttn/atw/nata/index.html.
- 19.Environmental Protection Agency. [Accessed July 20, 2009];Frequently Asked Questions [1996 National-Scale Air Toxics Assessment] Available at: http://www.epa.gov/ttn/atw/nata/natsafaq.html. [Google Scholar]
- 20.Perera FP, Rauh V, Whyatt RM, et al. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first three years of life among inner-city children. Environ Health Perspect. 2006;114:1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenbaum AS, Axelrad DA, Woodruff TJ, Wei YH, Ligocki MP, Cohen JP. National estimates of outdoor air toxics concentrations. J Air Waste Manag Assoc. 1999;49:1138–1152. doi: 10.1080/10473289.1999.10463919. [DOI] [PubMed] [Google Scholar]
- 22.US Census Bureau. [Accessed May 29];Advanced Geography Search. 2007 Available at: http://factfinder.census.gov/servlet/AGSGeoAddressServlet?_lang_en&_program Year_50&_treeId_420.
- 23.Melissa Data. [Accessed December, 2008];US Address Lookup and Verify. Available at: http://www.melissadata.com/lookups/addressverify.asp. [Google Scholar]
- 24.US Census Bureau. [Accessed December, 2008];Census Tract Relationship Files. Available at: http://www.census.gov/geo/www/relate/rel_tract.html. [Google Scholar]
- 25.Greenland S, Brumback B. An overview of relations among causal modelling methods. Int J Epidemiol. 2002;31:1030–1037. doi: 10.1093/ije/31.5.1030. [DOI] [PubMed] [Google Scholar]
- 26.Durkin MS, Maenner MJ, Newschaffer CJ, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol. 2008;168:1268–1276. doi: 10.1093/aje/kwn250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson M, Weiss B, Janson S, Sundell J, Bornehag CG. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology. 2009;30:822–831. doi: 10.1016/j.neuro.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams JG, Higgins JP, Brayne CE. Systematic review of prevalence studies of autism spectrum disorders. Arch Dis Child. 2006;91:8–15. doi: 10.1136/adc.2004.062083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenland S, Poole C. Empirical-Bayes and semi-Bayes approaches to occupational and environmental hazard surveillance. Arch Environ Health. 1994;49:9–16. doi: 10.1080/00039896.1994.9934409. [DOI] [PubMed] [Google Scholar]
- 30.Witte JS, Greenland S, Kim LL. Software for hierarchical modeling of epidemiologic data. Epidemiology. 1998;9:563–566. [PubMed] [Google Scholar]
- 31.Grandjean P, Landrigan PJ. Developmental neurotoxicity of industrial chemicals. Lancet. 2006;368:2167–2178. doi: 10.1016/S0140-6736(06)69665-7. [DOI] [PubMed] [Google Scholar]
- 32.Mendola P, Selevan SG, Gutter S, Rice D. Environmental factors associated with a spectrum of neurodevelopmental deficits. Ment Retard Dev Disabil Res Rev. 2002;8:188–197. doi: 10.1002/mrdd.10033. [DOI] [PubMed] [Google Scholar]
- 33.Toxicological Profile for Methylene Chloride. Atlanta, GA: US Department of Health and Human Services; 2000. Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- 34.Environmental Protection Agency. [Accessed February 9, 2009];Confidence Rankings in 1996 National-Scale Air Toxics Assessment. Available at: http://www.epa.gov/ttn/atw/nata/conf.html.
- 35.Payne-Sturges DC, Burke TA, Breysse P, Diener-West M, Buckley TJ. Personal exposure meets risk assessment: a comparison of measured and modeled exposures and risks in an urban community. Environ Health Perspect. 2004;112:589–598. doi: 10.1289/ehp.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallace LA. Comparison of risks from outdoor and indoor exposure to toxic chemicals. Environ Health Perspect. 1991;95:7–13. doi: 10.1289/ehp.91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cook R, Driver L, Mullen M. [Accessed February 19, 2010];Ann Arbor, MI: US EPA Office of Transportation and Air Quality; Trends in Emissions of Air Toxics from Highway Mobile Sources 1990 to 2002. 2002 Available at: http://www.epa.gov/ttn/chief/conference/ei13/toxics/cook.pdf.
- 38.Efron B, Morris C. Data analysis using Stein’s estimator and its generalizations. J Am Stat Assoc. 1975;70:311–319. [Google Scholar]
- 39.Laslo-Baker D, Barrera M, Knittel-Keren D, et al. Child neurodevelopmental outcome and maternal occupational exposure to solvents. Arch Pediatr Adolesc Med. 2004;158:956–961. doi: 10.1001/archpedi.158.10.956. [DOI] [PubMed] [Google Scholar]
- 40.Palmer RF, Blanchard S, Stein Z, Mandell D, Miller C. Environmental mercury release, special education rates, and autism disorder: an ecological study of Texas. Health Place. 2006;12:203–209. doi: 10.1016/j.healthplace.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 41.Palmer RF, Blanchard S, Wood R. Proximity to point sources of environmental mercury release as a predictor of autism prevalence. Health Place. 2009;15:18–24. doi: 10.1016/j.healthplace.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. J Speech Lang Hear Res. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomblin JB, Smith E, Zhang X. Epidemiology of specific language impairment: prenatal and perinatal risk factors. J Commun Disord. 1997;30:325–343. doi: 10.1016/s0021-9924(97)00015-4. 344. [DOI] [PubMed] [Google Scholar]
- 44.Pratt GC, Palmer K, Wu CY, Oliaei F, Hollerbach C, Fenske MJ. An assessment of air toxics in Minnesota. Environ Health Perspect. 2000;108:815–825. doi: 10.1289/ehp.00108815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.State of New Jersey. [Accessed December 2008];Comparison of 1996 NATA results to measured concentrations in outdoor air in New Jersey. Available at: http://www-.state.nj.us/dep/airmon/airtoxics/natavmon.htm. [Google Scholar]
- 46.Environmental Protection Agency. [Accessed June 19, 2006];Comparison of ASPEN modeling system results to monitored concentrations. Available at: http://www.epa.gov/ttn/atw/nata/mtom_pre.html.
- 47.Ozkaynak H, Palma T, Touma JS, Thurman J. Modeling population exposures to outdoor sources of hazardous air pollutants. J Expo Sci Environ Epidemiol. 2008;18:45–58. doi: 10.1038/sj.jes.7500612. [DOI] [PubMed] [Google Scholar]
- 48.Environmental Protection Agency. [Accessed January 13, 2010];National Air Quality and Emissions Trends Report 1999, Chapter 5—Air Toxics. 1999 Available at: http://www.epa.gov/air/airtrends/aqtrnd99/ [Google Scholar]
- 49.Sarnat JA, Brown KW, Schwartz J, Coull BA, Koutrakis P. Ambient gas concentrations and personal particulate matter exposures: implications for studying the health effects of particles. Epidemiology. 2005;16:385–395. doi: 10.1097/01.ede.0000155505.04775.33. [DOI] [PubMed] [Google Scholar]