Abstract
Paclitaxel has been widely used as an anti-mitotic agent in chemotherapy for a variety of cancers and adds substantial efficacy as the first-line chemotherapeutic regimen for ovarian cancers. However, the frequent occurrence of paclitaxel resistance limits its function in long-term management. Despite abundant clinical and cellular demonstration of paclitaxel resistant tumors, the molecular mechanisms leading to paclitaxel resistance are poorly understood. Using genomic approaches, we have previously identified an association between a BTB/POZ gene, Nac1, and paclitaxel resistance in ovarian cancer. The experiments presented here have applied multiple quantitative proteomic methods to identify protein changes associated with paclitaxel resistance and Nac1 function. The SKOV-3 ovarian serous carcinoma cell line, which has inducible expression of dominant negative Nac1, was used to determine the paclitaxel treatment associated changes in the presence and absence of functional Nac1. Quantitative proteomic analyses were performed using iTRAQ labeling and mass spectrometry. Two label-free quantitative proteomic methods: LC-MS and spectral count were used to increase confidence of proteomic quantification. A total of 1371 proteins were quantified by at least one of the quantitative proteomic methods. Candidate proteins related to paclitaxel and NAC1 function were identified in this study. Go analysis of the protein changes identified upon paclitaxel resistance revealed that cell component enrichment related to mitochondria. Moreover, tubulin and mitochondrial proteins were the major cellular components with changes associated with paclitaxel treatment. This suggests that mitochondria may play a role in paclitaxel resistance.
Keywords: ovarian cancer, paclitaxel, Taxol, mass spectrometry, proteomics
1. Introduction
Paclitaxel (Taxol®) is a potent antimitotic agent which is currently employed for the treatment of many human cancers and as an inflammation deterrent in drug-eluting cardiovascular stents [1]. Paclitaxel is known to induce cytotoxicity by preventing tubulin depolymerization during the metaphase to anaphase transition of mitosis [2, 3] or by triggering apoptosis through regulating the expression of apoptosis-related proteins in both the caspase-dependent and caspase-independent pathways [1]. Unfortunately, while paclitaxel causes initial remission of ovarian cancer, the tumor often acquires resistance and recurs [4]. The molecular mechanisms underlying paclitaxel resistance remain unclear. The experiments presented here attempt to identify the proteins associated with paclitaxel resistance in ovarian cancer cells in order to facilitate the elucidation of molecular mechanisms of paclitaxel induced apoptosis and acquired resistance and discovery of potential drug targets for ovarian cancer with paclitaxel resistance.
Nac1, a member of BTB/POZ gene family, is a transcription repressor that is essential for the growth and survival of tumor cells [5]. We have previously associated Nac1 overexpression with tumor recurrence and paclitaxel resistance in ovarian serous carcinoma [5, 6]. However, the function of Nac1 for paclitaxel resistance is not well understood. To explore this function, we generated the SKOV-3 N130 cell line which is an ovarian serous carcinoma cell line (SKOV-3) with stable transfection of N130/EGFP controlled by tTA (tetracycline-controlled transactivator) [5]. This Tet-OFF inducible system can trigger the expression of N130 by removal of doxycycline which inhibits the function of Nac1.
The relationships between paclitaxel resistance and Nac1 were explored here using iTRAQ (isoabaric tags for relative and absolute quantitation) quantitation method, and also measured by label-free quantitation methods, LC-MS and spectral count. The three methods are among the several high throughput quantitative proteomic methods that have been developed in the past decade. Method development in this field has occurred in two directions: label-dependent and label-free. The label-dependent methods are widely used and include derivitizing methods [7] such as isotope-coded affinity tags (ICAT) [8] and isobaric tags for relative and absolute quantitation (iTRAQ) [9] and non-derivitizing methods such as stable isotope labeling with amino acids in cell culture (SILAC) [10] and 18O labeling [11]. When applied to proteomics, stable isotope labeling allows for the accurate measurement of the relative peptide abundance by direct comparison of light and heavy peptides in the same spectrum.
While label-dependent methods comprise the gold standard for quantitative techniques, only a limited number of samples can be quantified using isotopic derivitization in a single experiment due to the fixed number of channels from the labeling reagents. Thus an alternative direction of method development for quantitative proteomics, that of label-free quantitation methods, has evolved and includes the liquid chromatography-mass spectrometry (LC-MS) method [12, 13] and spectral count [14]. The LC-MS method determines the peptide abundance by comparing the intensity of the same peptide peak in multiple LC-MS runs. Quantitation of protein abundance by spectral count is based on the number of redundant spectra acquired for each protein from different samples in the LC-MS/MS analyses. Label-free quantitative methods are theoretically capable of quantifying an unlimited number of samples in a single study. The limitation of the label-free quantitative method is that the quantitation accuracy relies heavily on the reproducible analyses of different samples in multiple LC-MS and LC-MS/MS analyses [15–18].
Therefore, it is clear that a high-throughput quantitative proteomic method has the potential to identify a large number of protein changes but that these measurements must be validated. The validation of these protein changes with traditional methods such as Western blots or immunohistochemistry is limited due to the availability, expense of the antibodies, and the low throughput of the assays. Therefore, the proteomics study should give the more confident ones for the further immune based validation. The study presented here identified protein changes related to paclitaxel resistance and Nac1 function using most reliable quantitation, iTRAQ labeling. In addition, the two label-free quantitative proteomic methods were used to increase the quantification confidence.
Using the iTRAQ quantitation, most of these changed proteins related to paclitaxel treatment were significantly overrepresented in mitochondria. Our results suggest a new role of mitochondria of ovarian cancer cells in paclitaxel resistance and define potential new targets for treatment of paclitaxel-resistant ovarian cancer. Most protein changes in mitochondria were also identified as up-regulated by the two label-free methods. In addition, we also list the candidate proteins related to NAC1 function.
2 Materials and methods
2.1 Materials
Sequencing grade trypsin was from Promega (Madison, WI); C18 Sep-Pak Vac columns were from Waters (Milford, MA); α-cyano-4-hydroxycinnasmic acid (CHCA) was from Agilent (Palo Alto, CA); iTRAQ reagent and mass calibration standards were from Applied Biosystems (Foster City, CA); BCA assay kit was from Pierce (Rockford, IL); SCX columns and C18 resin were from Sepax (Newark, DE).; Other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
2.2 Treatments of ovarian cancer cells
The N130-inducible SKOV-3 ovarian cancer cell line, stably expressed the inducible construct of N-terminal 130 amino acids (N130) of Nac1 gene and enhanced green fluorescent protein (EGFP), was generated and reported previously [5, 6]. N130-inducible SKOV-3 cells were cultured in G400D2 medium (RPMI medium contained 10% FBS, 1% penicillin/streptomycin, 400 μg/ml geneticin, and 2 μg/ml doxycycline). To induce the expression of N130, the cells were washed with PBS twice and cultured in G400 medium (G400D2 medium without doxycycline). Expression of N130-EGFP was confirmed by fluorescent microscope after 28-hour culture in G400 medium.
The N130-inducible SKOV-3 ovarian adenocarcinoma cells with and without N130 expression by culturing in two different medium as described above, were un-treated or treated with 20nM paclitaxel for 72-hour. The cells that remained alive after paclitaxel treatment were harvested at the end of paclitaxel treatment.
2.3 Peptide extraction
The cell pallets were collected and sonicated. Protein concentration was measured by BCA assay. The same amounts of proteins (1 mg) from each condition were denatured in 8M urea in 0.4M NH4HCO3, 0.1% (w/v) SDS solution (pH8.3), and 10mM TCEP (Tris (2-carboxythyl) phosphine) by incubation at 60°C for 1 hour. Proteins were alkylated with 16mM iodoacetamide by incubation at room temperature in the dark for 30 min. The sample was diluted 4-fold by trypsin digestion buffer (100mM NH4HCO3, pH8.3). Trypsin was added at a 1 to 50 part sample protein excess and allowed to digest at 37°C overnight. SDS-PAGE and silver staining was employed to ensure the completion of tryptic digest. The peptides were purified with C18 Sep-Pak Vac columns and resuspended in water with a final concentration of 10μg/μl.
2.4 iTRAQ labeling
Tryptic peptides (50 μg) from each sample were mixed with 20 μl of dissolution buffer provided with iTRAQ kit. The iTRAQ 4-plex reagents were dissolved in 70μl of methanol respectively and strongly vortexed. Each iTRAQ labeling reagent was then added to the sample and mixed. The mixture was incubated at room temperature for 1 hour followed by cleaning up by SCX column.
2.5 Mass spectrometry analysis
For protein quantification by spectral count, each peptide mixture was analyzed twice by the LTQ ion trap mass spectrometer (Thermo Finnigan, San Jose, CA). For protein identification and quantitative analysis using LC-MS, an ESI-QSTAR mass spectrometer (Applied Biosystems, Foster City, CA) was used. In both systems, 2 μl (2 μg) peptides were injected into a peptide cartridge packed with C18 resin, and then passed through a 10 cm × 75 μm i.d. microcapillary HPLC (μLC) column packed with C18 resin. The effluent from the μLC column entered an electrospray ionization source in which peptides were ionized and passed directly into the mass spectrometers. A linear gradient of acetonitrile from 5%–32% over 100 min at flow rate of ~300 nL/min was applied. During the LC-MS mode, data was acquired in the m/z range of 400 and 2000. The MS/MS was also turned on to collect CID using data dependent mode. Each sample was analyzed three times by QSTAR to increase the accuracy of quantification.
iTRAQ labeled peptide was analyzed by both QSTAR and 2-D LC (Nano, Eksigent, Dublin, CA) MALDI TOF/TOF (ABI 4800, Applied Biosystems, Foster City, CA). The analysis on the QSTAR was performed in the same setting as described above. For the analysis by 2-D Nano LC and MALDI 4800-TOF/TOF, on-line integration of 15-cm-long 300μm strong cation exchange column (SCX) with 15-cm-long 300 μm of C18-reverse phase liquid chromatography (RPLC) was employed. Four SCX fractions of 0, 5, 50 and 500mM KCl and 3–45% linear acetonitrile gradient (containing 0.1% TFA and acetonitrile) of RPLC for each fraction were applied before analysis by MALDI-TOF/TOF. Peptides eluted from columns were directly mixed with CHCA and spotted on a MALDI target plate with 768 spots followed by analysis with MS and MS/MS using the ABI 4800 MALDI-TOF/TOF.
2.6 Peptide identifications
The iTRAQ data analyzed either by QSTAR or MALDI and label-free data from LTQ were searched by ProteinPilot™ software 2.0 [19] against the human International Protein Index database (IPI, version 2.28) using the cut-off probability score of 0.9.
Tandem MS spectra of label-free peptides from the QSTAR were searched with SEQUEST [20] against the same human IPI protein database (version 2.28). The peptide mass tolerance is 2.0Da. Other parameters of database searching are modified as following: cysteine modification (add cysteine 57) and oxidized methionine (add methionine with 16 Da). The output files were evaluated by INTERACT [21] and ProteinProphet [22]. The cutoff of ProteinProphet analysis is the probability score ≥ 0.9 so that low probability protein identifications can be filtered out. For each identified peptide, peptide sequence, protein name, precursor m/z value, peptide mass, charge state, retention time where the MS/MS was acquired, and probability of the peptide identification being correct were recorded and outputted using INTERACT [21].
2.7 Quantitative proteomic analyses
The ratio of the four channels of iTRAQ labeling was determined by the ProteinPilot™ software.
A suite of software tools of SpecArray were used to analyze the LC-MS data as described previously [23]. For each peptide peak, an abundance ratio of matched peptides in different samples was determined for each peptide peak. An in-house Perl script was then used to link the peptide identification from MS/MS spectra to their corresponding MS peaks by matching precursor mass within 1 Da, retention time within 10 min, and charge state of the peptides.
The identified peptides from LTQ with a probability score ≥ 0.9 were used for the spectral count. To determine the number of MS/MS spectra used for identification of each protein in different conditions using our in-house developed software tool. For peptide sequence that could come from multiple proteins, the spectral count is equally distributed to all proteins with the identified peptide. Due to random sampling of mass spectrometer in collecting MS/MS spectra used for spectral count, we only quantified proteins with at least 4 spectral counts in total from the four cell states.
2.8 Evaluation of the cut-off of protein abundance ratio for proteins changes
To correct for any systematic errors of protein ratio introduced by sample handling and to determine the appropriate cut-off for protein changes, the distribution of abundance ratios in different cell states was generated for each quantitative method. Since the majority of proteins were not expressed differently in two cell states, we normalized the ratio based on the distribution of the protein abundance ratios from two cell states. Proteins fell out of the normal distribution from the abundance ratio of two cell states were considered as altered proteins. The threshold to select protein changes was based on the ratio distribution of two cell states. The mean and standard deviation of ratio from two cell states were calculated, and the abundance of proteins with an abundance ratio outside of one standard deviation from the mean were flagged as altered.
2.9 Cellular component classification of changed genes
To classify the changed proteins into cellular component, GO (Gene Ontology) [24] analysis (http://www.godatabase.org/dev) was performed. All the identified and quantified proteins by iTRAQ quantitation were used as background. Protein changes due to paclitaxel quantified by iTRAQ were used as changed proteins. P value was calculated using one-side Fisher exact test. To correct for multiple testing errors, p value was adjusted the minimum P method of Westfall and Young[25].
3 Results and discussion
3.1 Inducible expression of N130 in SKOV-3 cells
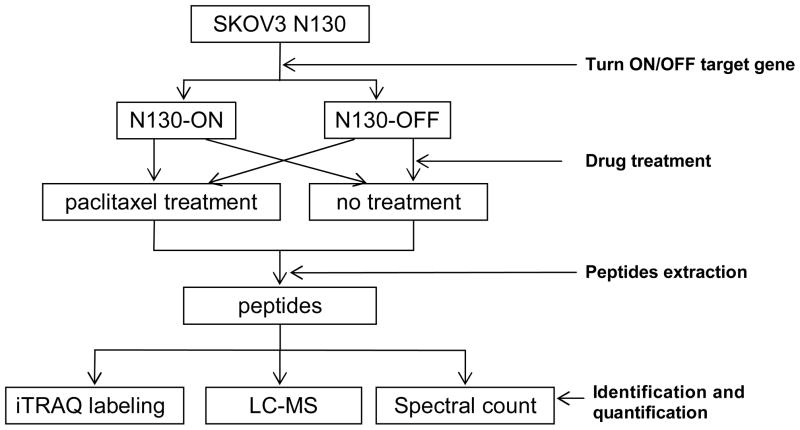
To identify proteins related to paclitaxel treatment and resistance, SKOV-3 cells with inducible expression of N130 protein [6, 26] was used in this study. The quantitative proteomic analyses of paclitaxel treatment for SKOV-3 N130 cells with and without expression of N130 are schematically illustrated in Figure 1 and consist of four steps: 1) two dishes were treated to induce the expression of N130 and two dishes were untreated as controls; 2) One dish with expression of N130 and one dish without expression of N130 were treated with paclitaxel, and the other two dishes were not treated with paclitaxel act as controls; 3) the peptides were extracted from cell lysate of the four cell states by sonication and trypsin digestion; 4) the tryptic peptides were identified and quantified by iTRAQ labeling, LC-MS, and spectral count.
Figure 1.
Flowchart of the quantitative study of Nac1 function and paclitaxel treatment
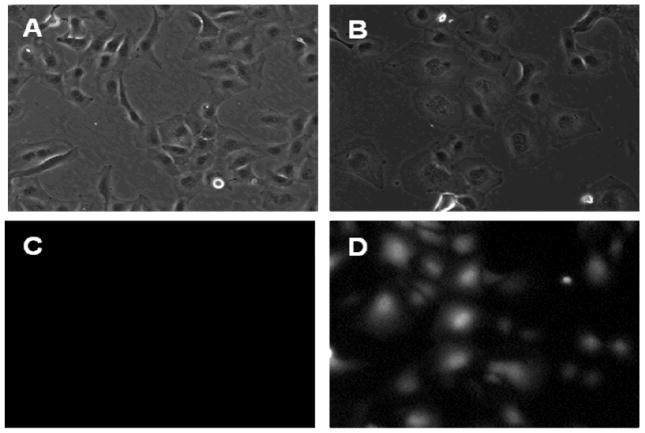
To evaluate the expression of N130, the florescence of EGFP was monitored as an indicator to determine whether N130 was induced after removing doxycycline from culture medium. SKOV-3 N130 cells were observed after 28-hour culture in medium with and without doxycycline according to our previous study [6, 26]. The cells were observed under florescence microscope (Figure 2A and B). The induced expression of N130/EGFP by doxycycline withdrawal was indicated by the green fluorescent (Figure 2D), which was not observed in cells cultured with doxycycline (shown in Figure 2C). Thus the expression of N130 can be robustly induced in SKOV-3 N130 cells.
Figure 2.
Expression of fusion protein N130-EGFP in SKOV-3 ovarian cell line: A) SKOV-3 N130 cultured with doxycycline, the N130-EGFP expression was off; B) SKOV-3 N130 cultured without doxycycline, the N130-EGFP expression was on; C) no fluorescent came from SKOV-3 N130 cultured with doxycycline; D) fluorescent came from SKOV-3 N130 cultured without doxycycline.
3.2 Quantitative proteomic analyses to identify protein changes
To determine the protein changes related to paclitaxel treatment and Nac1 function, the ovarian cancer cells with and without Nac1 function were treated with paclitaxel. After treated with 20nM paclitaxel for 72-hour, around 60 % cells was alive, which were considered as cells resistant to paclitaxel. The cells that remained alive after paclitaxel treatment were harvested at the end of paclitaxel treatment. The cell pallets were sonicated and 1 mg proteins from each cell states were digested by trypsin, followed by quantitative proteomic analysis using iTRAQ. A portion of tryptic peptides (50 μg) from N130-ON without paclitaxel treatment (ON−T), N130-ON with paclitaxel treatment (ON+T), N130-OFF without paclitaxel treatment (OFF−T) and N130-OFF with paclitaxel treatment (OFF+T) cells were labeled with 114, 115, 116 and 117 of iTRAQ reagents and analyzed by LC-MALDI TOF/TOF and LC-QSTAR for quantitative proteomic analysis. We were able to identify and quantify 850 proteins using iTRAQ labeling.
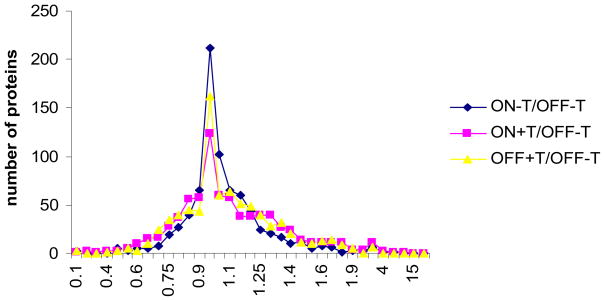
We then determined the protein changes in two cell states quantified by iTRAQ. When cells are induced of N130 expression or treated of paclitaxel, majority of cellular proteins are expected to be not affected and stay in the same level [27]. However, due to errors introduced by analytical procedures, such as sample handling and quantification process, the protein ratio from majority of proteins may be shifted. To determine the proteins with abundance changes in two cell states, histogram was used to generate the number of proteins in different abundance ratio (Figure 3). The threshold was set as < 0.7 and >1.3 for iTRAQ labeling. Majority of proteins (554 proteins, 65%) were distributed within one standard deviation (0.25) from the mean (1.046) and were considered as unchanged. Proteins that fell out of one standard deviation of the normal distribution curve were considered as with changed. A total of 296 proteins were changed due to paclitaxel treatment (160 proteins) or Nac1 inactivation (93 proteins) or both of paclitaxel treatment and Nac1 inactivation (181 proteins).
Figure 3.
The histogram analysis of peptide ratios of different cell states quantified by iTRAQ
Nac1 was determined as changed upon the induced expression of N130. A total of seven peptides were identified and quantified from Nac1, and all the identified peptides were located in the N-terminus 1-130 amino acids (with 71% sequence coverage of N 130), indicating that the identified peptides were likely from overexpressed N130 instead of endogenous Nac1 protein. The amount of N130 in N130 ON cells was measured as about 10-fold higher than it in N130 OFF cells (Table 1). The quantitative results of N130 expression confirmed that: 1) the inducible Tet-OFF system was efficient in inducing N130 expression; 2) the iTRAQ quantitative methods was able to determine the relative abundance of proteins and could be used for identification of other protein changes.
Table 1.
Overexpression of N130 determined by three quantitative proteomic methods. For iTRAQ quantitation, all ratios are normalized to the reporter ion at m/z 116 (OFF-T). For LC-MS quantitation, the number showed the peak intensities.
| Methods | OFF−T | OFF+T | ON−T | ON+T |
|---|---|---|---|---|
| iTRAQ | 1.00 | 1.08±0.41 | 10.73±2.87 | 12.13±5.21 |
| LC-MS | 0.00±0.00 | 0.00±0.00 | 2628.84±167.78 | 3244.69±335.38 |
| Spectral count | 0.00 | 5.00 | 80.13 | 83.00 |
3.3 Altered proteins related to Nac1 function and paclitaxel treatment
The 296 unique protein alterations determined by iTRAQ labeling were listed in Table 2 and grouped into 3 classes: 1) Protein changes upon paclitaxel treatment (OFF−T vs. OFF+T). These include proteins elevated upon paclitaxel treatment such as tubulin beta-5 chain, tubulin alpha-4 chain, mitochondrial proteins such as cytochrome c and ATP synthase, mitochondrial inner membrane protein, acute-phase proteins such as hemoglobin, cell surface antigens such as CD44 and 4F2 cell surface antigen, etc., and proteins with decreased abundance upon paclitaxel treatment such as seven subunits of ribosomal proteins, proteins regulating cell meiosis, mitosis and postmitotic functions such as mitogen-activated protein kinase3, etc. 2) Changed protein expression upon inactivation of Nac1 (OFF−T vs. ON−T). Induced expression of N130 (ON−T) inhibits the function of Nac1. Since Nac1 is a potential transcriptional repressor [26], the proteins with altered expression after Nac1 inhibition could be controlled by Nac1. These proteins include Ras-related protein Rab-8, transcription repressor, eukaryotic translation initiation factor 3, etc. 3) Changes of protein abundance upon paclitaxel treatment and induced N130 expression (OFF−T vs. ON+T), and proteins in this class might associate with the function of Nac1 gene in the response to paclitaxel treatment. Proteins in this class include Ras GTPase-activating-like protein IQGAP1, polyadenylate-binding protein 1, etc.
Table 2.
Proteins associated with paclitaxel treatment with and without N130 overexpression determined by iTRAQ
| IPI | ProteinName | Swiss-Prot | % Cov | OFF+T/OFF−T | ON−T/OFF−T | ON+T/OFF−T |
|---|---|---|---|---|---|---|
| Proteins regulated upon paclitaxel treatment | ||||||
| IPI00298971 | Vitronectin (S-protein) (V75) | P04004 | 3.1 | 2.54 | 1.44 | 2.50 |
| IPI00328696 | Hemoglobin alpha chain | P01922 | 21.2 | 2.45 | 1.20 | 2.78 |
| IPI00305185 | Stromal cell protein | Q9BRV3 | 10.9 | 2.37 | 1.67 | 3.15 |
| IPI00028481 | Ras-related protein Rab-8 | P24407 | 12.1 | 2.32 | 3.32 | 2.16 |
| IPI00160897 | Hypothetical protein | Q969E5 | 37.6 | 2.05 | 1.08 | 2.27 |
| IPI00396589 | Interleukin enhancer binding factor 2, 45kD | Q9BWD4 | 11.5 | 2.01 | 0.88 | 1.63 |
| IPI00026087 | Barrier-to-autointegration factor | O75531 | 15.7 | 1.84 | 2.47 | |
| IPI00299149 | Ubiquitin-like protein SMT3B | P55855 | 32.6 | 1.83 | 1.48 | 1.08 |
| IPI00218816 | beta globin | P02023 | 19.7 | 1.81 | 2.10 | 2.49 |
| IPI00219219 | beta-galactosidase binding lectin | P09382 | 65.2 | 1.81 | 1.14 | 1.39 |
| IPI00030131 | Splice isoform Beta of P42167 Thymopoietin, isoforms beta/gamma | P42167 | 14.1 | 1.81 | 1.23 | 2.11 |
| IPI00009346 | Protein C6orf53 | Q9P0S9 | 13.4 | 1.80 | ||
| IPI00002520 | Serine hydroymethyltransferase, mitochondrial | P34897 | 12.5 | 1.79 | 1.13 | 1.34 |
| IPI00013452 | Bifunctional aminoacyl-tRNA synthetase | P07814 | 4.2 | 1.78 | 1.30 | 1.28 |
| IPI00027192 | Procollagen-lysine,2-ooglutarate 5-dioygenase 1 | Q02809 | 3.9 | 1.77 | 2.10 | 0.84 |
| IPI00329705 | KIAA1363 protein | Q86WZ1 | 9.8 | 1.76 | 1.18 | 2.77 |
| IPI00374657 | vesicle-associated membrane protein-associated protein A isoform 1 | 4.9 | 1.74 | 1.49 | 0.97 | |
| IPI00236879 | Atain-2 related domain protein | Q8WWM7 | 7.1 | 1.74 | 1.51 | 1.84 |
| IPI00219291 | ATP synthase f chain, mitochondrial | P56134 | 37.9 | 1.73 | 1.15 | 1.73 |
| IPI00015786 | Spectrin alpha chain, brain | Q13813 | 11.2 | 1.71 | 1.41 | 1.89 |
| IPI00382733 | Transcription repressor | O75799 | 6.6 | 1.68 | 1.84 | 1.19 |
| IPI00025273 | Trifunctional purine biosynthetic protein adenosine-3 | P22102 | 10.1 | 1.68 | 1.43 | 1.57 |
| IPI00006558 | CGI-61 protein | Q9NR47 | 10.1 | 1.67 | 1.32 | 0.75 |
| IPI00011229 | Cathepsin D | P07339 | 14.1 | 1.66 | 1.02 | 1.61 |
| IPI00305064 | CD44 | P16070 | 6.3 | 1.65 | 1.26 | 1.65 |
| IPI00025874 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 67 kDa subunit | P04843 | 15.7 | 1.65 | 1.20 | 1.48 |
| IPI00029557 | GrpE protein homolog 1, mitochondrial | Q9HAV7 | 18.4 | 1.65 | 1.30 | 1.46 |
| IPI00290889 | DNA topoisomerase I | P11387 | 2.4 | 1.64 | 1.01 | 1.27 |
| IPI00009960 | Mitochondrial inner membrane protein | Q16891 | 7.4 | 1.64 | 1.20 | 1.05 |
| IPI00218682 | P13674 Prolyl 4-hydroylase alpha-1 subunit | P13674 | 5.4 | 1.62 | 0.82 | 1.21 |
| IPI00015148 | Ras-related protein Rap-1b | P09526 | 14.7 | 1.61 | 1.11 | 1.44 |
| IPI00215916 | cytochrome c | P00001 | 29.5 | 1.61 | 1.21 | 1.73 |
| IPI00016572 | Small nuclear ribonucleoprotein G | Q15357 | 17.1 | 1.60 | 1.52 | 1.77 |
| IPI00218019 | Basigin long isoform | Q8IZL7 | 9.6 | 1.59 | 0.84 | 1.57 |
| IPI00005202 | Membrane associated progesterone receptor component 2 | O15173 | 10.3 | 1.59 | 1.14 | 2.17 |
| IPI00009236 | Caveolin-1 | Q03135 | 24.2 | 1.56 | 1.22 | 1.66 |
| IPI00019472 | Neutral amino acid transporter B(0) | Q15758 | 7.4 | 1.55 | 0.68 | 1.19 |
| IPI00031522 | Trifunctional enzyme alpha subunit, mitochondrial | P40939 | 4.7 | 1.55 | 0.80 | 1.19 |
| IPI00216492 | P31942 Heterogeneous nuclear ribonucleoprotein H3 | P31942 | 10.9 | 1.55 | 1.14 | 1.35 |
| IPI00219835 | P04895 Guanine nucleotide-binding protein G(S), alpha subunit | P04895 | 23.9 | 1.55 | 1.06 | 1.64 |
| IPI00016447 | Hypothetical protein FLJ20502 | Q9N08 | 13.1 | 1.53 | 1.08 | 2.08 |
| IPI00220739 | progesterone receptor membrane component 1 | O00264 | 27.7 | 1.52 | 1.08 | 1.42 |
| IPI00025019 | Proteasome subunit beta type 1 | P20618 | 21.2 | 1.52 | 1.00 | 0.64 |
| IPI00217952 | Glucosamine--fructose-6-phosphate aminotransferase [isomerizing] 1 | Q06210 | 8.6 | 1.51 | 0.96 | 1.52 |
| IPI00014238 | Lysyl-tRNA synthetase | Q15046 | 6.7 | 1.50 | 1.49 | |
| IPI00385566 | Hypothetical protein FLJ30014 | Q969M3 | 3.5 | 1.50 | 1.62 | 1.56 |
| IPI00300074 | Phenylalanyl-tRNA synthetase beta chain | Q9NSD9 | 10.7 | 1.48 | 1.17 | 0.97 |
| IPI00141318 | P63 protein | Q07065 | 17.6 | 1.47 | 1.07 | 1.25 |
| IPI00142634 | Tubulin beta-5 chain | P05218 | 43 | 1.47 | 1.00 | 1.47 |
| IPI00017726 | 3-hydroyacyl-CoA dehydrogenase type II | Q99714 | 15.3 | 1.47 | 1.04 | 1.74 |
| IPI00014230 | Complement component 1, Q subcomponent binding protein, mitochondrial | Q07021 | 19.9 | 1.47 | 1.17 | 1.25 |
| IPI00005159 | Actin-like protein 2 | O15142 | 19.3 | 1.47 | 1.22 | 1.00 |
| IPI00107117 | Peptidylprolyl isomerase B | P23284 | 25.9 | 1.47 | 1.32 | 1.72 |
| IPI00024911 | Endoplasmic reticulum protein ERp29 | P30040 | 5 | 1.46 | 1.10 | 1.27 |
| IPI00220985 | Keratin, type I cytoskeletal 18 | P05783 | 39.6 | 1.46 | 0.94 | 1.49 |
| IPI00180128 | Similar to KIAA0005 gene product | Q9BUY0 | 10.4 | 1.45 | 1.11 | 1.29 |
| IPI00383671 | serologically defined breast cancer antigen 84 isoform a | Q9Y282 | 7.2 | 1.45 | 1.21 | 1.10 |
| IPI00395917 | ferritin heavy chain | P02794 | 14.8 | 1.44 | 0.87 | 1.87 |
| IPI00374410 | cytochrome b5 reductase soluble isoform | P003781-2 | 26.6 | 1.43 | 0.90 | 1.26 |
| IPI00031479 | Protein disulfide isomerase A5 | Q14554 | 2.3 | 1.43 | 0.88 | 1.54 |
| IPI00028055 | Transmembrane protein Tmp21 | P49755 | 22.4 | 1.41 | 1.11 | 1.32 |
| IPI00303882 | Cargo selection protein TIP47 | O60664 | 27.2 | 1.41 | 1.26 | 1.60 |
| IPI00384489 | Similar to adaptor-related protein comple 1, beta 1 subunit | Q10567 | 8.7 | 1.41 | 1.14 | 1.24 |
| IPI00021766 | Splice isoform 1 of Q9NQC3 Reticulon 4 | Q9NQC3 | 7.1 | 1.41 | 1.09 | 1.32 |
| IPI00011937 | Peroiredoin 4 | Q13162 | 17.7 | 1.41 | 1.73 | |
| IPI00003965 | Ubiquitin carboyl-terminal hydrolase 7 | Q93009 | 1.9 | 1.40 | 1.08 | 1.65 |
| IPI00024919 | Thioredoin-dependent peroide reductase, mitochondrial | P30048 | 25.4 | 1.40 | 1.05 | 1.39 |
| IPI00386755 | ERO1 (S. cerevisiae)-like | Q96HE7 | 3 | 1.39 | 1.06 | 1.21 |
| IPI00219604 | mitogen-activated protein kinase kinase 1 | Q02750 | 7.6 | 1.39 | 0.99 | 1.36 |
| IPI00009407 | Defender against cell death 1 | P46966 | 14.2 | 1.39 | 1.13 | 1.94 |
| IPI00004902 | Electron transfer flavoprotein beta-subunit | P38117 | 14.1 | 1.38 | 1.03 | 1.06 |
| IPI00024993 | Enoyl-CoA hydratase, mitochondrial | P30084 | 14.1 | 1.38 | 0.81 | 1.12 |
| IPI00046828 | similar to CG15881-PB | Q4VC31 | 14.5 | 1.37 | 1.33 | |
| IPI00021954 | Golgi-specific brefeldin A-resistance guanine nucleotide echange factor 1 | Q92538 | 7 | 1.37 | 0.87 | 1.43 |
| IPI00165092 | Hypothetical protein FLJ13995 | Q9H817 | 6.3 | 1.37 | 0.87 | 1.61 |
| IPI00027493 | 4F2 cell-surface antigen heavy chain | P08195 | 22.9 | 1.37 | 0.97 | 1.36 |
| IPI00007750 | Tubulin alpha-4 chain | P05215 | 40 | 1.37 | 1.04 | 1.29 |
| IPI00009329 | Utrophin | P46939 | 1.3 | 1.37 | 1.44 | 0.92 |
| IPI00216393 | Splice isoform Non-brain of P09496 Clathrin light chain A | P09496 | 9.6 | 1.37 | 1.37 | 1.09 |
| IPI00337814 | Hypothetical protein | Q9BWL4 | 11.7 | 1.37 | 1.16 | 1.65 |
| IPI00026167 | NHP2-like protein 1 | P55769 | 24.2 | 1.37 | 0.86 | 1.46 |
| IPI00396321 | Hypothetical protein | Q9P189 | 25.7 | 1.36 | 1.00 | 1.17 |
| IPI00386621 | Similar to calmodulin 2 | Q9BRL5 | 16.3 | 1.36 | 1.44 | 1.10 |
| IPI00029574 | Putative S100 calcium-binding protein A11 pseudogene | O60417 | 10.8 | 1.36 | 0.85 | 1.26 |
| IPI00027423 | Serine/threonine protein phosphatase PP1-alpha 1 catalytic subunit | P08129 | 10.6 | 1.36 | 1.07 | 1.05 |
| IPI00007682 | Vacuolar ATP synthase catalytic subunit A, ubiquitous isoform | P38606 | 6.3 | 1.35 | 0.90 | 1.07 |
| IPI00160021 | HIRA-interacting protein 5 | Q9UMS0 | 10.2 | 1.35 | 1.17 | 1.10 |
| IPI00291005 | cytosolic malate dehydrogenase | P40925 | 9.9 | 1.34 | 0.46 | 0.95 |
| IPI00007928 | PRP8 protein | O14547 | 6.2 | 1.34 | 1.02 | 0.96 |
| IPI00220834 | ATP-dependant DNA helicase II | P13010 | 14.1 | 1.34 | 1.38 | 0.91 |
| IPI00016513 | Ras-related protein Rab-10 | O88386 | 8 | 1.34 | 1.07 | 1.18 |
| IPI00009950 | Vesicular integral-membrane protein VIP36 | Q12907 | 6.7 | 1.34 | 0.92 | 1.17 |
| IPI00029079 | GMP synthase [glutamine-hydrolyzing] | P49915 | 9.6 | 1.33 | 2.70 | 2.50 |
| IPI00297084 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit | P39656 | 12.7 | 1.33 | 1.32 | 1.41 |
| IPI00290142 | CTP synthase | P17812 | 12.5 | 1.33 | 1.10 | 1.42 |
| IPI00009922 | DC50 | Q9GZT3 | 12.8 | 1.33 | 1.01 | 1.42 |
| IPI00006865 | Vesicle trafficking protein SEC22B | O75396 | 11.2 | 1.33 | 1.61 | |
| IPI00293102 | Splice isoform 2 of Q15257 Protein phosphatase 2A, regulatory subunit B′ | Q15257 | 12.8 | 1.33 | 1.05 | 1.45 |
| IPI00008240 | Methionyl-tRNA synthetase | P56192 | 7.8 | 1.33 | 1.09 | 1.20 |
| IPI00220855 | Similar to H2A histone family, member O | Q9BTM1 | 58.9 | 1.33 | 0.89 | 1.42 |
| IPI00186338 | unnamed protein | 41.9 | 1.33 | 1.09 | 1.30 | |
| IPI00329351 | 60 kDa heat shock protein, mitochondrial | P10809 | 31 | 1.33 | 1.05 | 1.31 |
| IPI00007765 | Stress-70 protein, mitochondrial | P38646 | 28.4 | 1.33 | 1.05 | 1.12 |
| IPI00026328 | Thioredoin-like protein p19 | O95881 | 5.2 | 1.33 | 1.30 | 1.32 |
| IPI00027434 | Transforming protein RhoC | P08134 | 19.7 | 1.32 | 1.22 | 1.09 |
| IPI00005270 | Hypothetical protein | Q9BVD2 | 1.8 | 1.32 | 2.46 | 1.41 |
| IPI00003815 | Rho GDP-dissociation inhibitor 1 | P52565 | 16.7 | 1.32 | 0.97 | 1.22 |
| IPI00024364 | Importin beta-2 subunit | Q92973 | 7.3 | 1.31 | 0.61 | 1.21 |
| IPI00006482 | Sodium/potassium-transporting ATPase alpha-1 chain | P05023 | 10.8 | 1.31 | 1.31 | 1.33 |
| IPI00021785 | Cytochrome c oidase polypeptide Vb, mitochondrial | P10606 | 25.6 | 1.31 | 1.38 | 2.03 |
| IPI00304802 | Dihydrolipoamide succinyltransferase component of 2-ooglutarate dehydrogenase comple, mitochondrial | P36957 | 11.9 | 1.31 | 0.92 | 1.23 |
| IPI00027252 | repressor of estrogen receptor activity | Q9BV3 | 15.1 | 1.31 | 0.96 | 1.54 |
| IPI00328188 | fatty acid synthase | Q96IT0 | 9.8 | 1.31 | 0.96 | 1.11 |
| IPI00027717 | Component of gems 4 | P57678 | 4.6 | 1.30 | 1.17 | 1.76 |
| IPI00022793 | Trifunctional enzyme beta subunit, mitochondrial | P55084 | 13.5 | 1.30 | 1.29 | 1.35 |
| IPI00021978 | Peroisome assembly factor | O96011 | 6.2 | 1.30 | 0.77 | 0.78 |
| IPI00008167 | Sodium/potassium-transporting ATPase beta-3 chain | P54709 | 10.4 | 1.30 | 0.96 | 1.26 |
| IPI00334713 | Heterogeneous nuclear ribonucleoprotein A/B | Q99729 | 21.4 | 0.69 | 0.00 | 1.21 |
| IPI00219158 | ribosomal protein L29 | P47914 | 30.8 | 0.69 | 1.17 | 0.53 |
| IPI00033904 | similar to ribosomal protein S3a | P61247 | 26.5 | 0.69 | 1.03 | 0.63 |
| IPI00004860 | Arginyl-tRNA synthetase | P54136 | 6.8 | 0.69 | 1.02 | 0.88 |
| IPI00219446 | prostatic binding protein | P30086 | 20.3 | 0.68 | 0.43 | 0.54 |
| IPI00374260 | ribosomal protein L10 | P27635 | 30.4 | 0.68 | 0.98 | 0.69 |
| IPI00052229 | Hypothetical protein | Q9UG74 | 6.7 | 0.68 | 0.98 | 2.48 |
| IPI00005680 | Hypothetical protein KIAA0095 | Q14705 | 6.2 | 0.68 | 1.48 | 0.73 |
| IPI00385244 | phosphoglycerate mutase 1 (brain) | P18669 | 23.2 | 0.68 | 1.15 | 1.20 |
| IPI00002149 | GTP-binding protein SAR1b | Q9Y6B6 | 7.1 | 0.67 | 1.21 | 0.81 |
| IPI00165164 | Similar to ubiquitin-conjugating enzyme E2I | Q9BQ25 | 16.8 | 0.67 | 0.90 | 1.32 |
| IPI00215719 | 60S ribosomal protein L18 | Q07020 | 31 | 0.67 | 1.10 | 0.55 |
| IPI00027463 | Calcyclin | P06703 | 24.4 | 0.66 | 1.11 | 0.58 |
| IPI00021828 | Cystatin B | P04080 | 44.9 | 0.66 | 0.84 | 0.72 |
| IPI00182728 | SKD1 protein | O75351 | 8.2 | 0.66 | 0.93 | 1.24 |
| IPI00008524 | Polyadenylate-binding protein 1 | P11940 | 23.3 | 0.66 | 0.97 | 0.56 |
| IPI00216237 | ribosomal protein L36 | Q9Y3U8 | 14.3 | 0.66 | 1.04 | 0.80 |
| IPI00219520 | UNR protein | O75534 | 5.6 | 0.66 | 1.03 | 0.63 |
| IPI00395748 | Cytosolic acyl coenzyme A thioester hydrolase | O00154 | 14.8 | 0.65 | 1.04 | 0.84 |
| IPI00186712 | 40S ribosomal protein S26 | P02383 | 20.6 | 0.65 | 1.20 | 0.65 |
| IPI00170935 | Hypothetical protein KIAA1185 | Q8N1G4 | 28.3 | 0.65 | 1.14 | 0.98 |
| IPI00032826 | Hsc70-interacting protein | P50502 | 13 | 0.65 | 1.02 | 0.60 |
| IPI00220067 | leucine aminopeptidase | P28838 | 8.1 | 0.65 | 0.80 | 0.87 |
| IPI00215790 | 60S ribosomal protein L38 | P23411 | 19.3 | 0.65 | 0.93 | 0.62 |
| IPI00395865 | Histone acetyltransferase type B subunit 2 | Q16576 | 5 | 0.64 | 0.69 | 0.84 |
| IPI00000861 | LIM and SH3 domain protein 1 | Q14847 | 9.2 | 0.64 | 0.71 | 0.48 |
| IPI00165486 | similar to ribosomal protein S2 | 9.6 | 0.63 | 0.93 | 0.28 | |
| IPI00184821 | Bifunctional coenzyme A synthase (CoA synthase) | Q13057 | 11.7 | 0.63 | 0.45 | 1.26 |
| IPI00217709 | DNA topoisomerase II, beta isozyme | Q02880 | 6.2 | 0.63 | 1.08 | 1.00 |
| IPI00018768 | Translin | Q15631 | 5.3 | 0.63 | 0.99 | 0.71 |
| IPI00011603 | 26S proteasome non-ATPase regulatory subunit 3 | O43242 | 8.6 | 0.60 | 1.01 | 0.37 |
| IPI00396417 | MHC class I antigen | Q861B7 | 6.3 | 0.57 | 1.20 | 1.31 |
| IPI00334922 | Hypothetical protein FLJ10519 | Q9NVT5 | 8.8 | 0.57 | 0.69 | 0.90 |
| IPI00382700 | Filamin B | O75369 | 7.1 | 0.55 | 0.90 | 0.66 |
| IPI00296635 | 1,4-alpha-glucan branching enzyme | Q04446 | 2.5 | 0.52 | 0.97 | 0.69 |
| IPI00219486 | 40S ribosomal protein S24 | P16632 | 9.2 | 0.52 | 1.05 | 0.56 |
| IPI00163230 | COP9 signalosome subunit 6 | O15387 | 12.8 | 0.52 | 0.59 | 0.60 |
| IPI00303063 | KIAA0648 protein | Q96DB6 | 8.9 | 0.51 | 0.92 | 0.78 |
| IPI00382617 | P37 AUF1 | Q12771 | 11.5 | 0.50 | 0.77 | 0.71 |
| IPI00384261 | Muscleblind-like protein EP40s | Q86UV9 | 7.3 | 0.43 | 1.24 | 0.89 |
| IPI00295386 | carbonyl reductase 1 | P16152 | 10.5 | 0.33 | 0.77 | 1.17 |
| IPI00385399 | mitogen-activated protein kinase 3 | P27361 | 18.7 | 0.05 | 0.06 | 0.11 |
| IPI00218547 | Delta 1-pyrroline-5-carboylate synthetase | P54886 | 13 | 0.00 | 0.82 | 0.00 |
| Proteins regulated upon inactivation of Nac1 | ||||||
| IPI00028481 | Ras-related protein Rab-8 | P24407 | 12.1 | 2.32 | 3.32 | 2.16 |
| IPI00029079 | GMP synthase [glutamine-hydrolyzing] | P49915 | 4.5 | 1.33 | 2.70 | 2.50 |
| IPI00026087 | Barrier-to-autointegration factor | O75531 | 15.7 | 1.84 | 2.47 | |
| IPI00005270 | Hypothetical protein | Q9BVD2 | 1.8 | 1.32 | 2.46 | 1.41 |
| IPI00027192 | Procollagen-lysine,2-oxoglutarate 5-dioxygenase 1 | Q02809 | 3.9 | 1.77 | 2.10 | 0.84 |
| IPI00218816 | beta globin | P02023 | 19.7 | 1.81 | 2.10 | 2.49 |
| IPI00259901 | similar to peptidylprolyl isomerase A (cyclophilin A) | Q68J44 | 8.5 | 0.88 | 2.07 | 0.59 |
| IPI00290416 | Splice isoform 1 of Q9NTK5 Putative GTP-binding protein PTD004 | Q9NTK5 | 9.1 | 1.17 | 1.92 | 1.02 |
| IPI00382733 | Transcription repressor | O75799 | 6.6 | 1.68 | 1.84 | 1.19 |
| IPI00015953 | Nucleolar RNA helicase II | Q9NR30 | 18.7 | 0.95 | 1.82 | 1.43 |
| IPI00011937 | Peroxiredoxin 4 | Q13162 | 17.7 | 1.41 | 1.73 | |
| IPI00215736 | Alpha enolase | P06733 | 45 | 1.00 | 1.67 | 0.83 |
| IPI00305185 | Stromal cell protein | Q9BRV3 | 10.9 | 2.37 | 1.67 | 3.15 |
| IPI00005648 | Scaffold attachment factor B2 | Q14151 | 5 | 1.13 | 1.67 | 1.25 |
| IPI00003565 | 26S proteasome non-ATPase regulatory subunit 10 | O75832 | 4 | 0.90 | 1.63 | 1.05 |
| IPI00385566 | Hypothetical protein FLJ30014 | Q969M3 | 3.5 | 1.50 | 1.62 | 1.56 |
| IPI00006865 | Vesicle trafficking protein SEC22B | O75396 | 11.2 | 1.33 | 1.61 | |
| IPI00016339 | Ras-related protein Rab-5C | P51148 | 27.3 | 0.96 | 1.60 | 0.86 |
| IPI00021383 | Heterogeneous nuclear ribonucleoprotein A3 | P51991 | 19 | 1.03 | 1.58 | 0.67 |
| IPI00029485 | Splice isoform p150 of Q14203 Dynactin 1 | Q14203 | 5.8 | 1.07 | 1.56 | |
| IPI00302925 | T-complex protein 1, theta subunit | P50990 | 24.1 | 1.02 | 1.54 | 0.86 |
| IPI00008918 | Splice isoform Beta of Q9UHB6 Epithelial protein lost in neoplasm | Q9UHB6 | 7.2 | 0.98 | 1.52 | 0.64 |
| IPI00016572 | Small nuclear ribonucleoprotein G | Q15357 | 17.1 | 1.60 | 1.52 | 1.77 |
| IPI00236879 | Ataxin-2 related domain protein | Q8WWM7 | 7.1 | 1.74 | 1.51 | 1.84 |
| IPI00301434 | similar to My016 protein | Q9H3K6 | 13.8 | 1.03 | 1.51 | |
| IPI00014238 | Lysyl-tRNA synthetase | Q15046 | 6.7 | 1.50 | 1.49 | |
| IPI00374657 | vesicle-associated membrane protein-associated protein A isoform 1 | 4.9 | 1.74 | 1.49 | 0.97 | |
| IPI00176799 | similar to hypothetical protein | Q8N3B3 | 12.2 | 1.04 | 1.48 | 0.88 |
| IPI00299149 | Ubiquitin-like protein SMT3B | P55855 | 32.6 | 1.83 | 1.48 | 1.08 |
| IPI00005680 | Hypothetical protein KIAA0095 | Q14705 | 6.2 | 0.68 | 1.48 | 0.73 |
| IPI00215802 | Splice isoform Short of P23152 Splicing factor, arginine/serine- rich 3 | P23152 | 29 | 0.79 | 1.44 | 0.81 |
| IPI00009329 | Utrophin | P46939 | 1.3 | 1.37 | 1.44 | 0.92 |
| IPI00298971 | Vitronectin (Serum spreading factor) (S-protein) (V75) | P04004 | 8.2 | 2.54 | 1.44 | 2.50 |
| IPI00295589 | Eukaryotic translation initiation factor 4GI | Q96I65 | 9.2 | 1.12 | 1.44 | 1.63 |
| IPI00386621 | Similar to calmodulin 2 | Q9BRL5 | 16.3 | 1.36 | 1.44 | 1.10 |
| IPI00025273 | Splice isoform Long of P22102 Trifunctional purine biosynthetic pro | P22102 | 10.1 | 1.68 | 1.43 | 1.57 |
| IPI00015947 | DnaJ homolog subfamily B member 1 | P25685 | 11.2 | 0.94 | 1.42 | 0.80 |
| IPI00148062 | Nuclear-associated protein SPAN-Xb | Q9NS25 | 43.7 | 1.25 | 1.42 | 1.36 |
| IPI00382644 | Putative eukaryotic translation initiation factor 1A | O75642 | 13.3 | 0.99 | 1.41 | 0.92 |
| IPI00015786 | Spectrin alpha chain, brain | Q13813 | 11.2 | 1.71 | 1.41 | 1.89 |
| IPI00107357 | Cleft lip and palate associated transmembrane protein 1 | Q9BSS5 | 8.2 | 0.99 | 1.40 | 1.08 |
| IPI00307162 | VCL isoform meta-VCL | P18206 | 12.1 | 0.92 | 1.40 | 0.79 |
| IPI00219156 | ribosomal protein L30 | P04645 | 10.4 | 0.83 | 1.39 | 1.13 |
| IPI00168388 | Splice isoform 1 of Q9UHB9 Signal recognition particle 68 kDa protein | Q9UHB9 | 9.3 | 1.15 | 1.39 | 1.23 |
| IPI00021785 | Cytochrome c oxidase polypeptide Vb, mitochondrial | P10606 | 25.6 | 1.31 | 1.38 | 2.03 |
| IPI00220834 | ATP-dependant DNA helicase II | P13010 | 13.3 | 1.34 | 1.38 | 0.91 |
| IPI00017596 | Microtubule-associated protein RP/EB family member 1 | Q15691 | 10.4 | 0.97 | 1.37 | 0.96 |
| IPI00006328 | ATPase inhibitor, mitochondrial | Q9UII2 | 17.9 | 1.13 | 1.37 | 0.94 |
| IPI00186711 | Similar to plectin 1, intermediate filament binding protein, 500kD | Q96IE3 | 22.2 | 0.98 | 1.37 | 1.03 |
| IPI00216393 | Splice isoform Non-brain of P09496 Clathrin light chain A | P09496 | 9.6 | 1.37 | 1.37 | 1.09 |
| IPI00293350 | Translin-associated protein X | Q99598 | 5.5 | 1.05 | 1.36 | 1.17 |
| IPI00008552 | Thioredoxin-like protein 2 | O76003 | 12.8 | 1.02 | 1.35 | 0.71 |
| IPI00332570 | Polyadenylate-binding protein 2 | Q15097 | 9.2 | 1.01 | 1.34 | 1.27 |
| IPI00218606 | 40S ribosomal protein S23 | P39028 | 23.2 | 0.90 | 1.34 | 0.77 |
| IPI00377199 | Histone H2B.d | Q99877 | 48.7 | 1.01 | 1.34 | 0.99 |
| IPI00304925 | Heat shock 70 kDa protein 1 | P08107 | 36.3 | 0.97 | 1.34 | 0.81 |
| IPI00182373 | Splice isoform IIa of O15460 Prolyl 4-hydroxylase alpha-2 subunit | O15460 | 9.8 | 0.97 | 1.34 | 0.70 |
| IPI00084495 | similar to ribosomal protein S15 | 18.3 | 1.09 | 1.33 | 1.19 | |
| IPI00217468 | H1 histone family, member 5 | P16401 | 19.5 | 0.98 | 1.33 | 0.68 |
| IPI00383500 | Splice isoform 2 of Q96AC1 Pleckstrin homology domain contain | Q96AC1 | 1.7 | 1.08 | 1.33 | 1.29 |
| IPI00107117 | Peptidylprolyl isomerase B | P23284 | 25.9 | 1.47 | 1.32 | 1.72 |
| IPI00006558 | CGI-61 protein | Q9NR47 | 10.1 | 1.67 | 1.32 | 0.75 |
| IPI00297084 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 k | P39656 | 12.7 | 1.33 | 1.32 | 1.41 |
| IPI00240812 | Hypothetical protein KIAA0979 | Q9Y2I5 | 7.4 | 0.85 | 1.32 | 0.71 |
| IPI00376295 | mitogen-activated protein kinase 1 | P28482 | 13.9 | 0.95 | 1.32 | 1.22 |
| IPI00396171 | microtubule-associated protein 4 isoform 2 | P27816-1 | 11.6 | 0.81 | 1.31 | 1.14 |
| IPI00006482 | Splice isoform Long of P05023 Sodium | P05023 | 10.8 | 1.31 | 1.31 | 1.33 |
| IPI00026328 | Thioredoxin-like protein p19 | O95881 | 5.2 | 1.33 | 1.30 | 1.32 |
| IPI00029557 | GrpE protein homolog 1, mitochondrial | Q9HAV7 | 18.4 | 1.65 | 1.30 | 1.46 |
| IPI00032313 | Placental calcium-binding protein | P26447 | 29.7 | 1.08 | 0.69 | 0.87 |
| IPI00395865 | Histone acetyltransferase type B subunit 2 | Q16576 | 5 | 0.64 | 0.69 | 0.84 |
| IPI00005728 | RER1 protein | O15258 | 7.7 | 1.09 | 0.69 | 0.94 |
| IPI00334922 | Hypothetical protein FLJ10519 | Q9NVT5 | 8.8 | 0.57 | 0.69 | 0.90 |
| IPI00019472 | Neutral amino acid transporter B(0) | Q15758 | 6.7 | 1.55 | 0.68 | 1.19 |
| IPI00017292 | Splice isoform 1 of P35222 Beta-catenin | P35222 | 9.1 | 1.09 | 0.68 | 0.68 |
| IPI00014197 | Hypothetical protein | Q9UKY7 | 11.2 | 0.93 | 0.66 | 0.73 |
| IPI00010810 | Electron transfer flavoprotein alpha-subunit, mitochondrial | P13804 | 24.6 | 1.21 | 0.63 | 1.62 |
| IPI00013068 | Eukaryotic translation initiation factor 3 subunit 6 | Q64252 | 4.5 | 0.85 | 0.63 | 1.01 |
| IPI00396373 | BLOCK 23 | Q8NHW5 | 4.1 | 1.20 | 0.62 | 0.92 |
| IPI00024364 | Importin beta-2 subunit | Q92973 | 7.3 | 1.31 | 0.61 | 1.21 |
| IPI00216298 | thioredoxin | P10599 | 41 | 0.71 | 0.61 | 1.09 |
| IPI00032406 | DnaJ homolog subfamily A | O60884 | 5.3 | 0.76 | 0.60 | 0.78 |
| IPI00163230 | member 2 COP9 signalosome subunit 6 | O15387 | 12.8 | 0.52 | 0.59 | 0.60 |
| IPI00030940 | Protein KIAA0052 | P42285 | 2.6 | 0.74 | 0.59 | 1.18 |
| IPI00154645 | Similar to hypothetical protein FLJ12085 | Q9HA83 | 2.3 | 0.94 | 0.57 | 0.92 |
| IPI00395750 | Splice isoform Long of O75083 WD-repeat protein 1 | O75083 | 9.2 | 0.86 | 0.52 | 0.93 |
| IPI00328193 | Hypothetical protein | Q8WVM8 | 5.9 | 1.00 | 0.50 | 1.00 |
| IPI00291005 | cytosolic malate dehydrogenase | P40925 | 3.9 | 1.34 | 0.46 | 0.95 |
| IPI00184821 | Bifunctional coenzyme A synthase (CoA synthase) (NBP) (POV-2) | Q13057 | 11.7 | 0.63 | 0.45 | 1.26 |
| IPI00219624 | proteasome alpha 3 subunit isoform 1 | P25788 | 4.7 | 1.29 | 0.45 | 1.22 |
| IPI00219446 | prostatic binding protein | P30086 | 20.3 | 0.68 | 0.43 | 0.54 |
| IPI00385399 | mitogen-activated protein kinase 3 | 18.7 | 0.05 | 0.06 | 0.11 | |
| IPI00334713 | Splice isoform 3 of Q99729 Heterogeneous nuclear ribonucleoprotein A/B | Q99729 | 24.9 | 0.69 | 0.00 | 1.21 |
| Proteins regulated upon paclitaxel treatment and induced N130 expression | ||||||
| IPI00168812 | Transmembrane receptor PTK7-4 | Q8NFA6 | 7 | 1.07 | 0.72 | 6.54 |
| IPI00305185 | Stromal cell protein | Q9BRV3 | 10.9 | 2.37 | 1.67 | 3.15 |
| IPI00328696 | Hemoglobin alpha chain | P01922 | 17 | 2.45 | 1.20 | 2.78 |
| IPI00329705 | KIAA1363 protein | Q86WZ1 | 9.8 | 1.76 | 1.18 | 2.77 |
| IPI00029079 | GMP synthase [glutamine-hydrolyzing] | P49915 | 4.5 | 1.33 | 2.70 | 2.50 |
| IPI00298971 | Vitronectin (Serum spreading factor) (S-protein) (V75) | P04004 | 8.2 | 2.54 | 1.44 | 2.50 |
| IPI00218816 | beta globin | P02023 | 19.7 | 1.81 | 2.10 | 2.49 |
| IPI00052229 | Hypothetical protein | Q9UG74 | 6.7 | 0.68 | 0.98 | 2.48 |
| IPI00160897 | Hypothetical protein | Q969E5 | 37.6 | 2.05 | 1.08 | 2.27 |
| IPI00005202 | Membrane associated progesterone receptor component 2 | O15173 | 10.3 | 1.59 | 1.14 | 2.17 |
| IPI00028481 | Ras-related protein Rab-8 | P24407 | 12.1 | 2.32 | 3.32 | 2.16 |
| IPI00030131 | Splice isoform Beta of P42167 Thymopoietin, isoforms beta/gamma | P42167 | 14.1 | 1.81 | 1.23 | 2.11 |
| IPI00016447 | Hypothetical protein FLJ20502 | Q9NX08 | 13.1 | 1.53 | 1.08 | 2.08 |
| IPI00021785 | Cytochrome c oxidase polypeptide Vb, mitochondrial | P10606 | 25.6 | 1.31 | 1.38 | 2.03 |
| IPI00010157 | S-adenosylmethionine synthetase gamma form | P31153 | 8.4 | 0.75 | 1.28 | 1.97 |
| IPI00009407 | Defender against cell death 1 | P46966 | 14.2 | 1.39 | 1.13 | 1.94 |
| IPI00026154 | Protein kinase C substrate, 80 kDa protein, heavy chain | P14314 | 10.2 | 1.21 | 0.78 | 1.90 |
| IPI00021187 | RuvB-like 1 | Q9Y265 | 10.1 | 1.04 | 1.29 | 1.89 |
| IPI00015786 | Spectrin alpha chain, brain | Q13813 | 11.2 | 1.71 | 1.41 | 1.89 |
| IPI00395917 | ferritin heavy chain | P02794 | 14.8 | 1.44 | 0.87 | 1.87 |
| IPI00236879 | Ataxin-2 related domain protein | Q8WWM7 | 7.1 | 1.74 | 1.51 | 1.84 |
| IPI00377175 | similar to Esterase D | Q9BVJ2 | 6.9 | 0.89 | 1.08 | 1.78 |
| IPI00032825 | Hypothetical protein CGI-109 | Q9Y3B3 | 6.5 | 1.14 | 1.04 | 1.77 |
| IPI00016572 | Small nuclear ribonucleoprotein G | Q15357 | 17.1 | 1.60 | 1.52 | 1.77 |
| IPI00027717 | Component of gems 4 | P57678 | 4.6 | 1.30 | 1.17 | 1.76 |
| IPI00008453 | Coronin 1C | Q9ULV4 | 10.1 | 1.07 | 1.28 | 1.75 |
| IPI00017726 | Splice isoform 1 of Q99714 3- hydroxyacyl-CoA dehydrogenase type II | Q99714 | 15.3 | 1.47 | 1.04 | 1.74 |
| IPI00215916 | cytochrome c | P00001 | 29.5 | 1.61 | 1.21 | 1.73 |
| IPI00219291 | Splice isoform 2 of P56134 ATP synthase f chain, mitochondrial | P56134 | 37.9 | 1.73 | 1.15 | 1.73 |
| IPI00003927 | 40 kDa peptidyl-prolyl cis-trans isomerase | Q08752 | 3.5 | 1.02 | 1.09 | 1.73 |
| IPI00216172 | Splice isoform LAMP-2B of P13473 Lysosome-associated | P13473 | 4.9 | 1.29 | 1.18 | 1.73 |
| IPI00107117 | Peptidylprolyl isomerase B | P23284 | 25.9 | 1.47 | 1.32 | 1.72 |
| IPI00011274 | JKTBP2 | O14979 | 18.8 | 1.05 | 1.20 | 1.69 |
| IPI00021439 | Actin, cytoplasmic 1 | P02570 | 59.5 | 1.62 | 1.50 | 1.68 |
| IPI00009236 | Caveolin-1 | Q03135 | 24.2 | 1.56 | 1.22 | 1.66 |
| IPI00305064 | Splice isoform CD44 of P16070 CD44 antigen | P16070 | 6.3 | 1.65 | 1.26 | 1.65 |
| IPI00337814 | Hypothetical protein | Q9BWL4 | 11.7 | 1.37 | 1.16 | 1.65 |
| IPI00003965 | Ubiquitin carboxyl-terminal hydrolase 7 | Q93009 | 1.9 | 1.40 | 1.08 | 1.65 |
| IPI00219835 | Splice isoform Alpha-S1 of P04895 Guanine nucleotide-binding protein G(S), | P04895 | 23.9 | 1.55 | 1.06 | 1.64 |
| IPI00295589 | Eukaryotic translation initiation factor 4GI | Q96I65 | 9.2 | 1.12 | 1.44 | 1.63 |
| IPI00396589 | Interleukin enhancer binding factor 2, 45kD | Q9BWD4 | 19.2 | 2.01 | 0.88 | 1.63 |
| IPI00010810 | Electron transfer flavoprotein alpha-subunit, mitochondrial | P13804 | 24.6 | 1.21 | 0.63 | 1.62 |
| IPI00165092 | Hypothetical protein FLJ13995 | Q9H817 | 6.3 | 1.37 | 0.87 | 1.61 |
| IPI00011229 | Cathepsin D | P07339 | 14.1 | 1.66 | 1.02 | 1.61 |
| IPI00303882 | Splice isoform B of O60664 Cargo selection protein TIP47 | O60664 | 27.2 | 1.41 | 1.26 | 1.60 |
| IPI00249267 | similar to H2A histone family, member Z | 39.1 | 1.05 | 0.91 | 1.57 | |
| IPI00025273 | Splice isoform Long of P22102 Trifunctional purine biosynthetic protein adenosine-3 | P22102 | 10.1 | 1.68 | 1.43 | 1.57 |
| IPI00218019 | Basigin long isoform | Q8IZL7 | 9.6 | 1.59 | 0.84 | 1.57 |
| IPI00385566 | Hypothetical protein FLJ30014 | Q969M3 | 3.5 | 1.50 | 1.62 | 1.56 |
| IPI00385098 | MSTP086 | Q7Z4F2 | 7.1 | 0.83 | 0.99 | 1.54 |
| IPI00027252 | repressor of estrogen receptor activity | Q9BXV3 | 15.1 | 1.31 | 0.96 | 1.54 |
| IPI00291006 | Malate dehydrogenase, mitochondrial | P40926 | 26.9 | 1.28 | 1.00 | 1.54 |
| IPI00031479 | Protein disulfide isomerase A5 | Q14554 | 2.3 | 1.43 | 0.88 | 1.54 |
| IPI00395769 | ATP synthase gamma chain, mitochondrial | P36542 | 14 | 1.15 | 1.15 | 1.54 |
| IPI00217952 | Splice isoform 1 of Q06210 | Q06210 | 8.6 | 1.51 | 0.96 | 1.52 |
| IPI00329629 | Glucosamine--fructose-6-phosphate DnaJ homolog subfamily C member 7 | Q99615 | 5.3 | 0.86 | 0.72 | 1.50 |
| IPI00003833 | HSPC032 | Q9Y6C9 | 16.8 | 0.83 | 1.26 | 1.49 |
| IPI00297982 | eukaryotic translation initiation factor 2, subunit 3 gamma, 52kDa | P41091 | 21.8 | 1.20 | 1.20 | 1.49 |
| IPI00220985 | Keratin, type I cytoskeletal 18 | P05783 | 39.6 | 1.46 | 0.94 | 1.49 |
| IPI00025095 | Cellular nucleic acid binding protein | P20694 | 8.5 | 1.14 | 1.15 | 1.49 |
| IPI00025874 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 67 kDa subunit | P04843 | 15.7 | 1.65 | 1.20 | 1.48 |
| IPI00142634 | Tubulin beta-5 chain | P05218 | 43 | 1.47 | 1.00 | 1.47 |
| IPI00007188 | ADP, ATP carrier protein, fibroblast isoform | P05141 | 46.6 | 1.24 | 0.94 | 1.46 |
| IPI00026167 | NHP2-like protein 1 | P55769 | 24.2 | 1.37 | 0.86 | 1.46 |
| IPI00029557 | GrpE protein homolog 1, mitochondrial | Q9HAV7 | 18.4 | 1.65 | 1.30 | 1.46 |
| IPI00164305 | Membrane associated protein SLP-2 | Q9UJZ1 | 11.8 | 1.24 | 1.10 | 1.45 |
| IPI00293102 | Splice isoform 2 of Q15257 Protein phosphatase 2A, regulatory subunit B′ | Q15257 | 12.8 | 1.33 | 1.05 | 1.45 |
| IPI00015148 | Ras-related protein Rap-1b | P09526 | 14.7 | 1.61 | 1.11 | 1.44 |
| IPI00003519 | 116 kDa U5 small nuclear ribonucleoprotein component | Q15029 | 7.2 | 1.17 | 0.83 | 1.44 |
| IPI00176903 | Leucine-zipper protein FKSG13 | O00535 | 15.9 | 1.14 | 1.08 | 1.44 |
| IPI00021954 | Golgi-specific brefeldin A-resistance guanine nucleotide exchange factor 1 | Q92538 | 7 | 1.37 | 0.87 | 1.43 |
| IPI00015953 | Nucleolar RNA helicase II | Q9NR30 | 18.7 | 0.95 | 1.82 | 1.43 |
| IPI00171626 | hypothetical protein FLJ12443 | Q7Z4G6 | 9.9 | 1.28 | 0.99 | 1.43 |
| IPI00009922 | DC50 | Q9GZT3 | 12.8 | 1.33 | 1.01 | 1.42 |
| IPI00220855 | Similar to H2A histone family, member O | Q9BTM1 | 58.9 | 1.33 | 0.89 | 1.42 |
| IPI00220739 | progesterone receptor membrane component 1 | O00264 | 16.9 | 1.52 | 1.08 | 1.42 |
| IPI00290142 | CTP synthase | P17812 | 12.5 | 1.33 | 1.10 | 1.42 |
| IPI00297084 | Dolichyl-diphosphooligosaccharide--protein glycosyltransferase 48 kDa subunit | P39656 | 12.7 | 1.33 | 1.32 | 1.41 |
| IPI00005270 | Hypothetical protein | Q9BVD2 | 1.8 | 1.32 | 2.46 | 1.41 |
| IPI00019927 | 26S proteasome non-ATPase regulatory subunit 7 | P51665 | 11.4 | 1.25 | 1.17 | 1.41 |
| IPI00333010 | SR-related CTD associated factor 6 | Q8WU30 | 8 | 1.18 | 1.03 | 1.40 |
| IPI00028091 | Actin-like protein 3 | P32391 | 6.7 | 0.80 | 1.16 | 1.40 |
| IPI00386685 | citrate synthase isoform a | Q96FZ8 | 17 | 1.24 | 1.06 | 1.40 |
| IPI00007824 | ABP125 | Q9UM06 | 4.2 | 1.08 | 0.78 | 1.40 |
| IPI00219219 | beta-galactosidase binding lectin | P09382 | 65.2 | 1.81 | 1.14 | 1.39 |
| IPI00021440 | Actin, cytoplasmic 2 | P02571 | 59.5 | 1.27 | 0.48 | 1.39 |
| IPI00024919 | Thioredoxin-dependent peroxide reductase, mitochondrial | P30048 | 25.4 | 1.40 | 1.05 | 1.39 |
| IPI00020984 | Calnexin | P27824 | 17.1 | 1.29 | 1.09 | 1.38 |
| IPI00386803 | LIM and SH3 protein 1 | Q96IG0 | 10.2 | 1.16 | 1.02 | 1.38 |
| IPI00012578 | Importin alpha-4 subunit | O00629 | 13.4 | 1.24 | 0.96 | 1.37 |
| IPI00215918 | ADP-ribosylation factor 4 | P18085 | 22.2 | 1.17 | 0.81 | 1.37 |
| IPI00019345 | Ras-related protein Rap-1A | P10113 | 14.7 | 1.21 | 0.75 | 1.37 |
| IPI00023542 | gp25L2 protein | Q9BVK6 | 20.9 | 1.23 | 1.12 | 1.37 |
| IPI00009328 | Probable ATP-dependent helicase DDX48 | P38919 | 15.8 | 1.10 | 1.01 | 1.37 |
| IPI00291467 | ADP, ATP carrier protein, liver isoform T2 | P12236 | 44.3 | 1.29 | 1.00 | 1.36 |
| IPI00219604 | mitogen-activated protein kinase kinase 1 | Q02750 | 7.6 | 1.39 | 0.99 | 1.36 |
| IPI00009342 | Ras GTPase-activating-like protein IQGAP1 | P46940 | 11.9 | 1.08 | 0.95 | 1.36 |
| IPI00027493 | 4F2 cell-surface antigen heavy chain | P08195 | 22.9 | 1.37 | 0.97 | 1.36 |
| IPI00333383 | Adapter-related protein complex 2 beta 1 subunit | P21851 | 2.8 | 1.13 | 1.09 | 1.36 |
| IPI00148062 | Nuclear-associated protein SPAN-Xb | Q9NS25 | 43.7 | 1.25 | 1.42 | 1.36 |
| IPI00396304 | tubulin, alpha, ubiquitous | P68363 | 39.7 | 1.27 | 0.88 | 1.36 |
| IPI00034283 | Similar to tubulin, beta, 4 | Q9BUF5 | 34.8 | 1.07 | 1.12 | 1.35 |
| IPI00018206 | Aspartate aminotransferase, mitochondrial | P00505 | 8.6 | 1.00 | 0.79 | 1.35 |
| IPI00002134 | 26S proteasome non-ATPase regulatory subunit 5 | Q16401 | 19.4 | 1.23 | 1.25 | 1.35 |
| IPI00022793 | Trifunctional enzyme beta subunit, mitochondrial (TP- beta) | P55084 | 13.5 | 1.30 | 1.29 | 1.35 |
| IPI00216492 | Splice isoform 2 of P31942 Heterogeneous nuclear ribonucleoprotein H3 | P31942 | 8.2 | 1.55 | 1.14 | 1.35 |
| IPI00002520 | Serine hydroxymethyltransferase, mitochondrial | P34897 | 12.5 | 1.79 | 1.13 | 1.34 |
| IPI00221012 | Splice isoform Long of Q93008 Probable ubiquitin carboxyl-terminal hydrolase FAF-X | Q93008 | 6.1 | 1.11 | 1.02 | 1.34 |
| IPI00217466 | H1 histone family, member 3 | P16402 | 29 | 1.18 | 0.98 | 1.34 |
| IPI00216312 | vimentin | P08670 | 53.4 | 1.25 | 1.15 | 1.34 |
| IPI00215914 | ADP-ribosylation factor 1 | P32889 | 47.5 | 1.00 | 0.89 | 1.33 |
| IPI00046828 | similar to CG15881-PB | Q4VC31 | 14.5 | 1.37 | 1.33 | |
| IPI00026111 | Hypothetical protein | Q9BZS3 | 14.3 | 1.15 | 1.03 | 1.33 |
| IPI00016638 | ATP synthase alpha chain, mitochondrial | P25705 | 18.1 | 1.21 | 0.95 | 1.33 |
| IPI00215920 | ADP-ribosylation factor 6 | P26438 | 10.9 | 1.00 | 1.13 | 1.33 |
| IPI00218889 | Splice isoform 2 of P50570 Dynamin 2 | P50570 | 8.7 | 1.23 | 0.95 | 1.33 |
| IPI00218343 | Tubulin alpha-6 chain | Q9BQE3 | 59.8 | 1.25 | 0.95 | 1.33 |
| IPI00006482 | Splice isoform Long of P05023 | P05023 | 10.8 | 1.31 | 1.31 | 1.33 |
| IPI00165164 | Sodium/Similar to ubiquitin-conjugating enzyme E2I | Q9BQ25 | 16.8 | 0.67 | 0.90 | 1.32 |
| IPI00185600 | Annexin A11 | P50995 | 8.3 | 0.85 | 0.74 | 1.32 |
| IPI00021766 | Splice isoform 1 of Q9NQC3 Reticulon 4 | Q9NQC3 | 7.1 | 1.41 | 1.09 | 1.32 |
| IPI00028055 | Transmembrane protein Tmp21 | P49755 | 22.4 | 1.41 | 1.11 | 1.32 |
| IPI00026328 | Thioredoxin-like protein p19 | O95881 | 5.2 | 1.33 | 1.30 | 1.32 |
| IPI00220362 | 10 kDa heat shock protein, mitochondrial | Q04984 | 14.2 | 1.14 | 1.07 | 1.32 |
| IPI00008708 | PBK1 protein | Q8WUZ1 | 8.3 | 1.00 | 1.07 | 1.32 |
| IPI00306667 | Splice isoform CNPII of P09543 2′,3′-cyclic nucleotide 3′-phosphodiesterase | P09543 | 4.1 | 0.99 | 1.13 | 1.31 |
| IPI00329351 | 60 kDa heat shock protein, mitochondrial | P10809 | 31 | 1.33 | 1.05 | 1.31 |
| IPI00027230 | Endoplasmin | P14625 | 22.4 | 1.21 | 1.04 | 1.31 |
| IPI00004968 | Nuclear matrix protein NMP200 | Q9UMS4 | 6.9 | 1.20 | 0.98 | 1.31 |
| IPI00396417 | MHC class I antigen | Q861B7 | 6.3 | 0.57 | 1.20 | 1.31 |
| IPI00215884 | splicing factor, arginine/serine-rich 1 (splicing factor 2, alternate splicing factor) | Q07955 | 27 | 1.18 | 1.11 | 1.30 |
| IPI00186338 | unnamed protein | 41.9 | 1.33 | 1.09 | 1.30 | |
| IPI00374260 | ribosomal protein L10 | P27635 | 30.4 | 0.68 | 0.98 | 0.69 |
| IPI00296635 | 1,4-alpha-glucan branching enzyme | Q04446 | 2.5 | 0.52 | 0.97 | 0.69 |
| IPI00027681 | Nicotinamide N-methyltransferase | P40261 | 13.3 | 0.82 | 0.89 | 0.69 |
| IPI00217468 | H1 histone family, member 5 | P16401 | 21.2 | 0.98 | 1.33 | 0.68 |
| IPI00021840 | 40S ribosomal protein S6 | P10660 | 23.3 | 0.75 | 1.04 | 0.68 |
| IPI00017292 | Splice isoform 1 of P35222 Beta-catenin | P35222 | 9.1 | 1.09 | 0.68 | 0.68 |
| IPI00021383 | Heterogeneous nuclear ribonucleoprotein A3 | P51991 | 19 | 1.03 | 1.58 | 0.67 |
| IPI00386590 | DJ423B22.4 (Ribosomal protein S27 | Q9BQZ7 | 13.1 | 0.90 | 1.19 | 0.66 |
| IPI00382700 | Splice isoform 6 of O75369 Filamin B | O75369 | 7.1 | 0.55 | 0.90 | 0.66 |
| IPI00396660 | Elongation factor 1-beta | P24534 | 17.4 | 0.93 | 1.17 | 0.66 |
| IPI00014808 | Platelet-activating factor acetylhydrolase IB gamma subunit | Q15102 | 27.3 | 0.92 | 0.93 | 0.66 |
| IPI00219757 | Glutathione S-transferase P | P09211 | 31.5 | 0.73 | 0.92 | 0.65 |
| IPI00186712 | 40S ribosomal protein S26 | P02383 | 20.6 | 0.65 | 1.20 | 0.65 |
| IPI00015952 | Eukaryotic translation initiation factor 4 gamma 2 | P78344 | 5.6 | 0.71 | 1.13 | 0.65 |
| IPI00216320 | Splice isoform 2 of O00764 Pyridoxal kinase | O00764 | 14.8 | 0.88 | 0.93 | 0.64 |
| IPI00217223 | Multifunctional protein ADE2 [Includes: | P22234 | 9.8 | 0.74 | 0.75 | 0.64 |
| IPI00025019 | Proteasome subunit beta type 1 | P20618 | 15.8 | 1.52 | 1.00 | 0.64 |
| IPI00386491 | Splice isoform Short of Q00839 Heterogenous nuclear ribonucleoprotein U | Q00839 | 21 | 0.72 | 0.94 | 0.64 |
| IPI00008918 | Splice isoform Beta of Q9UHB6 Epithelial protein lost in neoplasm | Q9UHB6 | 7.2 | 0.98 | 1.52 | 0.64 |
| IPI00332371 | 6-phosphofructokinase, liver type | P17858 | 16.5 | 0.70 | 0.81 | 0.63 |
| IPI00219520 | Splice isoform Short of O75534 UNR protein | O75534 | 5.6 | 0.66 | 1.03 | 0.63 |
| IPI00033904 | similar to ribosomal protein S3a | P61247 | 26.5 | 0.69 | 1.03 | 0.63 |
| IPI00013184 | N-terminal acetyltransferase complex ARD1 subunit homolog | P41227 | 4.7 | 1.13 | 1.15 | 0.63 |
| IPI00336008 | aldehyde dehydrogenase 5A1 isoform 1 | Q8N3W7 | 8.2 | 1.22 | 1.12 | 0.62 |
| IPI00215790 | 60S ribosomal protein L38 | P23411 | 19.3 | 0.65 | 0.93 | 0.62 |
| IPI00000874 | Peroxiredoxin 1 | Q06830 | 41.2 | 0.81 | 1.00 | 0.62 |
| IPI00018219 | Transforming growth factor-beta induced protein IG-H3 | Q15582 | 7.2 | 0.96 | 0.81 | 0.60 |
| IPI00032826 | Hsc70-interacting protein | P50502 | 13 | 0.65 | 1.02 | 0.60 |
| IPI00163230 | COP9 signalosome subunit 6 | O15387 | 12.8 | 0.52 | 0.59 | 0.60 |
| IPI00000051 | Prefoldin subunit 1 | O60925 | 23.8 | 0.81 | 1.17 | 0.59 |
| IPI00259901 | similar to peptidylprolyl isomerase A (cyclophilin A) | Q68J44 | 8.5 | 0.88 | 2.07 | 0.59 |
| IPI00027463 | Calcyclin | P06703 | 24.4 | 0.66 | 1.11 | 0.58 |
| IPI00374119 | smooth muscle and non-muscle myosin alkali light chain isoform 3 | 33.8 | 0.72 | 1.09 | 0.58 | |
| IPI00025512 | Heat shock 27 kDa protein | P04792 | 38.5 | 0.73 | 0.87 | 0.57 |
| IPI00302850 | Small nuclear ribonucleoprotein Sm D1 | P13641 | 31.5 | 0.84 | 0.85 | 0.57 |
| IPI00008524 | Polyadenylate-binding protein 1 | P11940 | 23.3 | 0.66 | 0.97 | 0.56 |
| IPI00219486 | Splice isoform 2 of P16632 40S ribosomal protein S24 | P16632 | 9.2 | 0.52 | 1.05 | 0.56 |
| IPI00004656 | Beta-2-microglobulin | P01884 | 21.8 | 0.84 | 0.73 | 0.55 |
| IPI00215719 | 60S ribosomal protein L18 | Q07020 | 31 | 0.67 | 1.10 | 0.55 |
| IPI00219446 | prostatic binding protein | P30086 | 20.3 | 0.68 | 0.43 | 0.54 |
| IPI00219158 | ribosomal protein L29 | P47914 | 30.8 | 0.69 | 1.17 | 0.53 |
| IPI00375511 | Similar to RIKEN cDNA 2510008H07 gene | Q8N6E1 | 21.5 | 0.77 | 1.15 | 0.50 |
| IPI00000861 | LIM and SH3 domain protein 1 | Q14847 | 9.2 | 0.64 | 0.71 | 0.48 |
| IPI00026271 | 40S ribosomal protein S14 | P06366 | 24.5 | 0.73 | 1.13 | 0.47 |
| IPI00011603 | 26S proteasome non-ATPase regulatory subunit 3 | O43242 | 8.6 | 0.60 | 1.01 | 0.37 |
| IPI00165486 | similar to ribosomal protein S2 | 9.6 | 0.63 | 0.93 | 0.28 | |
| IPI00021700 | Proliferating cell nuclear antigen | P12004 | 8.8 | 0.72 | 1.26 | 0.19 |
| IPI00385399 | mitogen-activated protein kinase 3 | P27361 | 18.7 | 0.05 | 0.06 | 0.11 |
| IPI00218547 | Delta 1-pyrroline-5-carboxylate synthetase (P5CS) | P54886 | 4.7 | 0.00 | 0.82 | 0.00 |
Although the proteins identified in this proteomic study need further investigation to facilitate the understanding of the biological mechanism of Nac1 function or paclitaxel treatment, the results provide a list of proteins and cellular machinery, including ribosomal complexes, cell surface antigens, and stress response proteins, such as heat shock proteins and acute-phase proteins. These protein changes associated with Nac1 or paclitaxel resistance can be exploited as targets for treatment of paclitaxel resistance.
To further analyze the relationship of the protein changes upon paclitaxel treatment, the GO categories of protein changes were classified. Cellular components analysis revealed that the protein changes are significantly overrepresented in mitochondrion in the set of all proteins identified by iTRAQ (p value of Fisher’s product: 8.8 e-5, p value corrected by multiple testing: 0.024).
Furthermore, we found some interesting co-regulation of tubulin and mitochondrial proteins after paclitaxel treatment. Tubulin is a well-known target for paclitaxel function and responsible for paclitaxel induced cell death [33]. One of the mechanisms of paclitaxel function is believed to induce cell death by altering microtubule assembly through the binding to the microtubule polymer so as to stabilize microtubules [34], as a result, it disrupts the normal re-assembling of microtubule network which is required by mitosis and cell proliferation [3]. Another protein, cytochrome c, was reported previously of release from mitochondrion thus inducing cell apoptosis upon paclitaxel treatment [35]. However, how cytochrome c was released upon paclitaxel treatment is not clear. Interestingly, in this study, both α-4 and β-5 subunit of tubulin were observed of up-regulated after paclitaxel treatment (Table 1), so were many mitochondrial proteins including mitochondrial trifunctional enzyme, mitochondrial ATP synthase, cytochrome c, Serine hydroymethyltransferase, GrpE protein homolog 1, Mitochondrial inner membrane protein, Complement component 1 Q subcomponent binding protein, Thioredoin-dependent peroide reductase, and mitochondrial malate dehydrogenase, etc.
This observation suggests a regulation of mitochondrial function associated with paclitaxel treatment and tubulins. The regulation of mitochondrial function by tubulins was also reported by several studies recently. The regulation might be the result of direct interaction of the voltage-dependent anion channel (VDAC) on mitochondrial outer membrane with tubulin [36–38]. Taken together, a hypothesis is that mitochondria may be involved in the response to paclitaxel treatment. Mitochondrial function is the key player for cell apoptosis, and the mechanism of paclitaxel treatment might be to induce apoptosis through tubulin polymerization and regulation of mitochondrial function.
3.4 protein changes determined by label-free quantitation
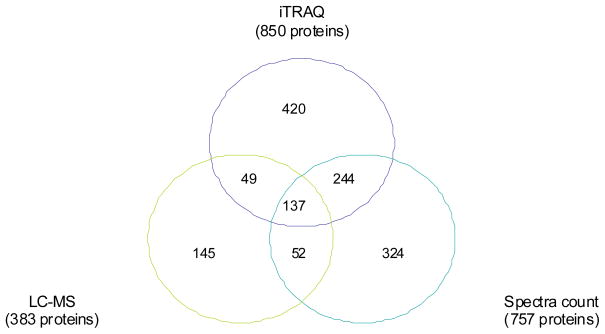
Quantitative analysis using different quantitative proteomic methods may increase the confidence of the protein changes if the proteins could be identified and quantified by multiple methods consistently. To this end, label free quantitation methods were also employed in this study. The tryptic peptides from the four cell states without iTRAQ labeling were analyzed three times with the QSTAR for the LC-MS quantitative analysis and two times with the LTQ for spectral count (Figure 1). A total of 383 proteins were quantified by the LC-MS method, and 757 were quantified by spectral count (Figure 4).
Figure 4.
Venn diagram depicts the number of proteins quantified by each quantitation method
We then determined the proteins that were changed in two cell states quantified by LC-MS and spectral count. Similar as iTRAQ quantitation, proteins that fell out of one standard deviation of the normal distribution curve were considered as with changed expression. The thresholds were determined as <0.75 and >1.15 for both spectral count and LC-MS.
Since N130 was induced expressed in cells, N130 was the perfect internal control for quantitation. N130 should be over-expressed in N130 ON cells compared to the N130 OFF cells. All the three quantitation showed the higher abundance of N130 in N130 ON cells (Table 1). However, the detection limitation varied among the three methods. The N130 overexpression were undetectable in N130 OFF cells for LC-MS and spectral count (Table 1), which may come from different instrumentations with various dynamic range and background. Further work and experiments will help define the most accurate quantifications along with better standards for calibrating the ratio of protein abundance from different methods. This is needed in proteomics to improve quantitative accuracy [28]. Nevertheless, the quantitative results of N130 expression confirmed that the three quantitative proteomic methods could be used to increase the confidence of quantitation.
For the mitochondria protein changes upon piclitaxel treatment, 7 out of 14 proteins determined by iTRAQ were also measured by the label free methods in the same track, e.g. ATP synthase, cytochrome c, Trifunctional enzyme, and Enoyl-CoA hydratase, etc (Table 3). Those proteins consistently determined by the two label-free methods confirmed the real changes of mitochondrial proteins upon piclitaxel treatment. This study represents the first proteomic study to discover the association of paclitaxel treatment and mitochondria protein changes in ovarian cancer cells, which may offer a new direction for studying the mechanism of drug resistance of cancer cells.
Table 3.
Mitochondrial protein changes related to paclitaxel quantified by iTRAQ were also measured by label-free quantitation methods
| IPI | ProteinName | Swiss-Prot | methods | OFF+T/OFF−T | ON−T/OFF−T | ON+T/OFF−T |
|---|---|---|---|---|---|---|
| IPI00002520 | Serine hydroxymethyltransferase, mitochondrial | P34897 | iTRAQ | 1.79 | 1.13 | 1.34 |
| IPI00219291 | ATP synthase f chain, mitochondrial | P56134 | iTRAQ | 1.73 | 1.15 | 1.73 |
| LC-MS | 1.70 | 1.16 | 1.99 | |||
| IPI00029557 | GrpE protein homolog 1, mitochondrial | Q9HAV7 | iTRAQ | 1.65 | 1.30 | 1.46 |
| IPI00009960 | Mitochondrial inner membrane protein | Q16891 | iTRAQ | 1.64 | 1.20 | 1.05 |
| IPI00215916 | cytochrome c | P00001 | iTRAQ | 1.61 | 1.21 | 1.73 |
| LC-MS | 1.19 | 1.00 | 1.27 | |||
| IPI00031522 | Trifunctional enzyme alpha subunit, mitochondrial | P40939 | iTRAQ | 1.55 | 0.80 | 1.19 |
| SC | 3.01 | 3.00 | 6.06 | |||
| IPI00014230 | Complement component 1, mitochondrial | Q07021 | iTRAQ | 1.47 | 1.17 | 1.25 |
| SC | 1.59 | 0.60 | 0.40 | |||
| IPI00024919 | Thioredoxin-dependent peroxide reductase, mitochondrial | P30048 | iTRAQ | 1.40 | 1.05 | 1.39 |
| SC | 1.25 | 0.50 | 1.00 | |||
| IPI00024993 | Enoyl-CoA hydratase, mitochondrial | P30084 | iTRAQ | 1.38 | 0.81 | 1.12 |
| SC | 1.67 | 1.33 | 1.33 | |||
| IPI00329351 | 60 kDa heat shock protein, mitochondrial | P10809 | iTRAQ | 1.33 | 1.05 | 1.31 |
| IPI00007765 | Stress-70 protein, mitochondrial | P38646 | iTRAQ | 1.33 | 1.05 | 1.12 |
| LC-MS | 1.71 | 1.21 | 1.47 | |||
| SC | 1.75 | 1.50 | 1.65 | |||
| IPI00021785 | Cytochrome c oxidase polypeptide Vb, mitochondrial | P10606 | iTRAQ | 1.31 | 1.38 | 2.03 |
| IPI00304802 | Dihydrolipoamide succinyltransferase component of 2-oxoglutarate dehydrogenase complex, mitochondrial | P36957 | iTRAQ | 1.31 | 0.92 | 1.23 |
| IPI00022793 | Trifunctional enzyme beta subunit, mitochondrial | P55084 | iTRAQ | 1.30 | 1.29 | 1.35 |
4 Concluding remarks
In this study, 1371 proteins were identified and quantified from Nac1 dominant negative model, SKOV-3 N130 cell line, associated with paclitaxel resistance and Nac1 function using iTRAQ quantitation, LC-MS method and spectral count. Candidate proteins related to paclitaxel resistance and NAC1 function were determined. Go analysis of the protein changes upon paclitaxel resistance revealed that protein changes significantly overrepresented in mitochondria. The co-regulation of tubulins and mitochondrial proteins was found, which suggests the roles of mitochondria in response to paclitaxel treatment. The identified proteins will be useful for further study of biological functions of Nac1 and elucidation of the molecular mechanism of paclitaxel treatment and resistance.
Supplementary Material
Acknowledgments
This work was supported by federal funds from the National Cancer Institute, National Institutes of Health, by grant R21-CA-114852 and RO1-CA-103937 (IMS) and Early Detection and Research Network (EDRN). We gratefully acknowledge the support from the Mass Spectrometry Facility at the Johns Hopkins University and the support from Applied Biosystems.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Khayat D, Antoine EC, Coeffic D. Taxol in the management of cancers of the breast and the ovary. Cancer Invest. 2000;18:242–260. doi: 10.3109/07357900009031828. [DOI] [PubMed] [Google Scholar]
- 2.Jordan MA, Wendell K, Gardiner S, Derry WB, et al. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825. [PubMed] [Google Scholar]
- 3.Amos LA, Lowe J. How Taxol stabilises microtubule structure. Chem Biol. 1999;6:R65–69. doi: 10.1016/s1074-5521(99)89002-4. [DOI] [PubMed] [Google Scholar]
- 4.Sangrajrang S, Fellous A. Taxol resistance. Chemotherapy. 2000;46:327–334. doi: 10.1159/000007306. [DOI] [PubMed] [Google Scholar]
- 5.Ishibashi M, Nakayama K, Yeasmin S, Katagiri A, et al. A BTB/POZ gene, NAC-1, a tumor recurrence-associated gene, as a potential target for Taxol resistance in ovarian cancer. Clin Cancer Res. 2008;14:3149–3155. doi: 10.1158/1078-0432.CCR-07-4358. [DOI] [PubMed] [Google Scholar]
- 6.Nakayama K, Nakayama N, Davidson B, Sheu JJ, et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci U S A. 2006;103:18739–18744. doi: 10.1073/pnas.0604083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Li XJ, Martin DB, Aebersold R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat Biotechnol. 2003;21:660–666. doi: 10.1038/nbt827. [DOI] [PubMed] [Google Scholar]
- 8.Gygi SP, Rist B, Gerber SA, Turecek F, et al. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. Nat Biotechnol. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 9.Ross PL, Huang YN, Marchese JN, Williamson B, et al. Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics. 2004;3:1154–1169. doi: 10.1074/mcp.M400129-MCP200. [DOI] [PubMed] [Google Scholar]
- 10.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1:376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 11.Yao X, Freas A, Ramirez J, Demirev PA, Fenselau C. Proteolytic 18O labeling for comparative proteomics: model studies with two serotypes of adenovirus. Anal Chem. 2001;73:2836–2842. doi: 10.1021/ac001404c. [DOI] [PubMed] [Google Scholar]
- 12.Bondarenko PV, Chelius D, Shaler TA. Identification and relative quantitation of protein mixtures by enzymatic digestion followed by capillary reversed-phase liquid chromatography-tandem mass spectrometry. Anal Chem. 2002;74:4741–4749. doi: 10.1021/ac0256991. [DOI] [PubMed] [Google Scholar]
- 13.Chelius D, Bondarenko PV. Quantitative profiling of proteins in complex mixtures using liquid chromatography and mass spectrometry. J Proteome Res. 2002;1:317–323. doi: 10.1021/pr025517j. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Sadygov RG, Yates JR., 3rd A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76:4193–4201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Yi EC, Li XJ, Mallick P, et al. High throughput quantitative analysis of serum proteins using glycopeptide capture and liquid chromatography mass spectrometry. Mol Cell Proteomics. 2005;4:144–155. doi: 10.1074/mcp.M400090-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Olson MT, Blank PS, Sackett DL, Yergey AL. Evaluating reproducibility and similarity of mass and intensity data in complex spectra--applications to tubulin. J Am Soc Mass Spectrom. 2008;19:367–374. doi: 10.1016/j.jasms.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Frewen BE, Merrihew GE, Wu CC, Noble WS, MacCoss MJ. Analysis of peptide MS/MS spectra from large-scale proteomics experiments using spectrum libraries. Anal Chem. 2006;78:5678–5684. doi: 10.1021/ac060279n. [DOI] [PubMed] [Google Scholar]
- 18.Lam H, Deutsch EW, Eddes JS, Eng JK, et al. Development and validation of a spectral library searching method for peptide identification from MS/MS. Proteomics. 2007;7:655–667. doi: 10.1002/pmic.200600625. [DOI] [PubMed] [Google Scholar]
- 19.Shilov IV, Seymour SL, Patel AA, Loboda A, et al. The Paragon Algorithm, a next generation search engine that uses sequence temperature values and feature probabilities to identify peptides from tandem mass spectra. Mol Cell Proteomics. 2007;6:1638–1655. doi: 10.1074/mcp.T600050-MCP200. [DOI] [PubMed] [Google Scholar]
- 20.Eng JM, AL, Yates JR., 3rd An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:13. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- 21.Han DK, Eng J, Zhou H, Aebersold R. Quantitative profiling of differentiation-induced microsomal proteins using isotope-coded affinity tags and mass spectrometry. Nat Biotechnol. 2001;19:946–951. doi: 10.1038/nbt1001-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 23.Li XJ, Yi EC, Kemp CJ, Zhang H, Aebersold R. A software suite for the generation and comparison of peptide arrays from sets of data collected by liquid chromatography-mass spectrometry. Mol Cell Proteomics. 2005;4:1328–1340. doi: 10.1074/mcp.M500141-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner M, Ball CA, Blake JA, Botstein D, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Westfall PaYS. Resampling-Based Multiple Testing: Examples and Methods for p-Value Adjustment. 1993. [Google Scholar]
- 26.Nakayama K, Nakayama N, Wang TL, Shih Ie M. NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007;67:8058–8064. doi: 10.1158/0008-5472.CAN-07-1357. [DOI] [PubMed] [Google Scholar]
- 27.Li XJ, Zhang H, Ranish JA, Aebersold R. Automated statistical analysis of protein abundance ratios from data generated by stable-isotope dilution and tandem mass spectrometry. Anal Chem. 2003;75:6648–6657. doi: 10.1021/ac034633i. [DOI] [PubMed] [Google Scholar]
- 28.Lau KW, Jones AR, Swainston N, Siepen JA, Hubbard SJ. Capture and analysis of quantitative proteomic data. Proteomics. 2007;7:2787–2799. doi: 10.1002/pmic.200700127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Sokoll LJ, Rush J, Zou N, Chan DW. Targeted detection of prostate cancer proteins in serum using heavy peptide standards and MALDI-TOF/TOF. Proteomics-Clinical Applications. 2009 In press. [Google Scholar]
- 30.Stahl-Zeng J, Lange V, Ossola R, Eckhardt K, et al. High sensitivity detection of plasma proteins by multiple reaction monitoring of N-glycosites. Mol Cell Proteomics. 2007;6:1809–1817. doi: 10.1074/mcp.M700132-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2007;6:2212–2229. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson L, Hunter CL. Quantitative mass spectrometric multiple reaction monitoring assays for major plasma proteins. Mol Cell Proteomics. 2006;5:573–588. doi: 10.1074/mcp.M500331-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Umezu T, Shibata K, Kajiyama H, Terauchi M, et al. Taxol resistance among the different histological subtypes of ovarian cancer may be associated with the expression of class III beta-tubulin. Int J Gynecol Pathol. 2008;27:207–212. doi: 10.1097/PGP.0b013e318156c838. [DOI] [PubMed] [Google Scholar]
- 34.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuo HC, Lee HJ, Hu CC, Shun HI, Tseng TH. Enhancement of esculetin on Taxol-induced apoptosis in human hepatoma HepG2 cells. Toxicol Appl Pharmacol. 2006;210:55–62. doi: 10.1016/j.taap.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 36.Rostovtseva TK, Sheldon KL, Hassanzadeh E, Monge C, et al. Tubulin binding blocks mitochondrial voltage-dependent anion channel and regulates respiration. Proc Natl Acad Sci U S A. 2008;105:18746–18751. doi: 10.1073/pnas.0806303105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carre M, Andre N, Carles G, Borghi H, et al. Tubulin is an inherent component of mitochondrial membranes that interacts with the voltage-dependent anion channel. J Biol Chem. 2002;277:33664–33669. doi: 10.1074/jbc.M203834200. [DOI] [PubMed] [Google Scholar]
- 38.Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.