Abstract
Objectives
To determine the combined effect of age and comorbidity on receipt of chemotherapy and its impact on survival in elderly patients with stage III colorectal cancer (CRC).
Materials and methods
All patients over age 65 with Stage III CRC diagnosed 1996–2006 were identified from the Barnes-Jewish Hospital Oncology Data Services registry. An age/comorbidity staging system was created using the ACE-27 comorbidity index and data from both Stage II and III CRC. The staging system was then applied to patients with Stage III CRC. Odds of receiving chemotherapy were calculated, and survival analyses determined the impact of chemotherapy on overall survival in each age/comorbidity stage.
Results
435 patients with Stage III CRC were evaluated [median age 75 years (range 65–99)]. Advancing age/comorbidity stage (Alpha, Beta, Gamma) was associated with decreasing odds of receiving chemotherapy for Stage III CRC [Odds Ratio 0.83 (95% CI, 0.51–1.35) for Beta and 0.14 (95% CI, 0.08–0.24) for Gamma, compared to Alpha]. Chemotherapy was associated with lower risk of death in each of the age/comorbidity stages, compared to those who underwent surgery only. The hazard ratio for death in patients who did not receive chemotherapy, relative to those who did, within each age/comorbidity stage was 1.8 [95%CI 1.06–3.06] for Alpha, 2.24 [95%CI 1.38–3.63] for Beta and 2.10 [95% CI 1.23–3.57] for Gamma.
Conclusion
While stage III CRC patients with increasing age and comorbidity are less likely to receive chemotherapy, receipt of chemotherapy is associated with a lower risk of death.
Keywords: Elderly, Geriatric Oncology, Colorectal cancer, Comorbidity Chemotherapy, Adjuvant chemotherapy, Survival
1. Introduction
The elderly population is disproportionately affected by cancer, with cancer incidence rates in persons aged 65–74 years being two to three times higher than in those aged 50–64. The rapidly growing elderly population in the United States, coupled with the increased incidence of cancer among the elderly, is expected to cause cancer incidence to double its 2000 value by 2050.1 Despite the increasing burden of cancer in the elderly, optimal management of an older individual remains undefined for many types of cancer. Older adults with cancer are consistently under-enrolled in clinical trials, 2–4 resulting in a lack of definitive data on elderly-specific cancer treatment. Additionally, there is a clear age bias in the treatment of elderly cancer patients, with older patients being less likely to receive standard therapy.5–8 Comorbid medical conditions increase in prevalence and severity with advancing age,9 further complicating the treatment of elderly cancer patients. The presence of comorbidities influences treatment decisions10 and is associated with poor tolerance of chemotherapy.11
Colorectal cancer (CRC) is the second leading cause of cancer death among all ages, with nearly 150,000 incident cases and 50,000 deaths in the United States in 2009.12 Over two-thirds of CRC cases occur in individuals aged ≥ 65 years,13 with a median age of incidence of 72.1 Overall, 36% of colorectal cancer patients will die within 5 years of diagnosis.12
In the past decade, major advances in the treatment of patients with CRC have improved outcomes, even in patients with advanced stages of disease. The 5 year overall survival (OS) for patients with regional colorectal cancer is 68%.12 Randomized controlled trials demonstrate the benefit of adjuvant chemotherapy in both stage III colon14–18 and rectal19–22 cancer. However, older patients were underrepresented in these trials, wherein the median age of participants was typically 60 years, and adjuvant chemotherapy is often not administered in the elderly population, ostensibly due to concerns about toxicity, comorbidities, and quality of life. Both chronological age and comorbidity independently influence the rate of adjuvant chemotherapy use in patients with stage III CRC 6, 7, 23–25. In one recent population-based study, only 50% of patients over the age of 75 with stage III CRC received adjuvant chemotherapy 25. While encouraging evidence is emerging on the effectiveness of adjuvant chemotherapy in elderly patients with stage III CRC,24, 26–30 no study to date has examined the combined effects of age and comorbidity on the decision to administer adjuvant chemotherapy and the impact of this decision on survival.
Thus, we examined the combined effect of age and comorbidity on treatment in elderly patients with stage III CRC and determined the impact of chemotherapy on survival. The knowledge gained regarding the impact of treatment decisions on outcomes specific to the elderly population provides the basis for improved decision-making in senior adults with CRC.
2. Methods
2.1. Data Sources
From the Barnes-Jewish Hospital Oncology Data Service cancer registry, we identified all adults aged 65 years and older with locally advanced cancer of the colon or rectum diagnosed and initially treated between 1996 and 2006 at the Washington University School of Medicine Siteman Cancer Center, a tertiary care institution affiliated with Barnes-Jewish Hospital. The institution’s cancer registrars retrospectively collected clinical, demographic, and survival data in accordance with the American College of Surgeons Commission on Cancer (CoC) guidelines.
In addition to the data collected under CoC guidelines, registrars also comprehensively recorded information on comorbid medical diagnoses present at the time of diagnosis of cancer using the Adult Comorbidity Evaluation-27 (ACE-27). The ACE-27, developed and validated by Piccirillo et al31 based on previous research by Kaplan and Feinstein 32, is a comorbidity collection and scoring system which assigns a comorbidity severity score of none, mild, moderate or severe based on the number and severity of comorbid ailments, as detailed in the patient’s medical record (See Table 1 for example). The ACE-27 measures 27 different comorbid ailments and is useful in predicting prognosis and survival in cancer patients33, 34. The ACE-27 can be viewed at http://oto2.wustl.edu/clinepi/comorbid.html.
Table 1.
Baseline characteristics of 936 patients with stage II and III colorectal cancer
| Characteristic | Colon Cancer Patients (N=647) | Rectal Cancer Patients (N=289) | All patients (N=936) | Unadjusted HR for death | 95% CI |
|---|---|---|---|---|---|
| Age Group, years | |||||
| 65–74 | 276 (43%) | 153 (53%) | 429 (46%) | 1.00 | Reference |
| 75–84 | 287 (44%) | 112 (39%) | 399 (43%) | 1.56 | 1.27–1.91 |
| 85+ | 84 (13%) | 24 (8%) | 108 (11%) | 3.02 | 2.30–3.97 |
| Gender | |||||
| Female | 361 (56%) | 129 (45%) | 490 (52%) | 1.00 | Reference |
| Male | 286 (44%) | 160 (55%) | 446 (48%) | 1.19 | 0.99–1.43 |
| Race | |||||
| White | 488 (75%) | 257 (89%) | 745 (80%) | 1.00 | -- |
| Black | 159 (25%) | 32 (11%) | 191 (20%) | 1.23 | 0.99–1.54 |
| Comorbidity (ACE-27) | |||||
| None | 131 (20%) | 70 (24%) | 201 (21%) | 1.00 | Reference |
| Mild | 227 (35%) | 125 (43%) | 352 (38%) | 1.43 | 1.08–1.89 |
| Moderate | 193 (30%) | 68 (24%) | 261 (28%) | 2.13 | 1.60–2.82 |
| Severe | 96 (15%) | 26 (9%) | 122 (13%) | 2.48 | 1.78–3.44 |
| Treatment | |||||
| No chemotherapy | 426 (66%) | 153 (53%) | 579 (62%) | 1.00 | Reference |
| Chemotherapy | 221 (34%) | 136 (47%) | 357 (38%) | 0.89 | 0.73–1.07 |
| TNM Stage | |||||
| II | 373 (58%) | 128 (44%) | 501 (53%) | 1.00 | Reference |
| III | 274 (42%) | 161 (56%) | 435 (47%) | 1.66 | 1.38–2.00 |
Note: All the characteristics were significantly related to cancer site
HR: hazard ratio; CI: confidence intervals; ACE-27: Adult Comorbidity Evaluation-27
The primary endpoint in this study was overall survival (OS). Duration of survival was calculated from the date of diagnosis and censored at time of last follow-up. Patient mortality data was obtained from follow-up information gathered as standard practice by the cancer registrars. Demographic information (age, gender and race) and the ACE-27 comorbidity severity score were identified as probable predictors of prognosis, patient mortality and treatment decisions based on prior studies7, 27, 35, 36. Age at diagnosis was divided a priori into three categories: 65–74, 75–84 and 85 and older. Patient race was classified as white, black or other; analyses were confined to comparisons of those of white or black race.
Staging was either clinical or pathologic, depending on available data and whether the patient received neoadjuvant therapy. If the patient received radiation or chemotherapy prior to surgery and were downstaged by pathology, the clinical stage was used. Cancer site was categorized as colon or rectal. Patients who did not undergo surgery were excluded. Treatment was classified as no chemotherapy if they underwent surgery only or surgery plus radiation. Treatment was classified as chemotherapy if they received either neoadjuvant or adjuvant chemotherapy in addition to surgery. Data on the chemotherapeutic agents and doses administered were not available.
2.2 Data Analysis
2.2.1. Cox Proportional Hazards Regression
The impact of covariates on overall survival was evaluated using Cox proportional hazards modeling. This regression modeled the dependent variable of time until death from the independent covariates: age group, race, gender, comorbidity severity score, treatment and stage. Unadjusted hazards ratios (HR) for death and corresponding 95% confidence intervals (CI) and p values were obtained for each covariate according to reference groups for each variable. The log minus log plot of survival confirmed that the proportional hazard assumption was met for all the variables included in the model.
2.2.2. Conjunctive Consolidation
Conjunctive consolidation was used to create a staging system that combined age group and comorbidity severity score. To study the combined effect of age and comorbidity on treatment decisions and outcomes, the two variables must be incorporated in some fashion. Simple linear combination results in patients being stratified according to values of each covariate. This, however, results in many strata and therefore a reduction in sample size for each group, minimizing the power of further statistical analysis. Alternately, in conjunctive consolidation, by examining the conjoined effect of these two variables on survival through cross table analysis, a new staging system can be derived.37
To create a new age/comorbidity staging system, cox proportional hazard modeling was used to calculate the adjusted hazards ratios for each of the 12 combinations of age group and comorbidity severity scores, controlling for race, gender, stage and cancer site. Data from both stage II and stage III patients (N=936) were initially used to increase sample size and thus the statistical validity within the stratifications. Cells with similar HR were grouped, maintaining an equitable distribution of patients throughout all age/comorbidity stages. This grouping generated the stages Alpha, Beta and Gamma for the newly created age/comorbidity staging system.
After creation of the new age/comorbidity staging system, analyses focused on stage III patients only (N=435). The behavior of this new model was compared to that of the model containing the age and comorbidity groups as separate covariates, uncombined. In order to compare the goodness of fit of the two models accurately, Bayesian Information Criteria (BIC) were used. BIC penalizes models for increasing free parameters, thus standardizing the likelihood ratio chi-square38, 39. When used to examine the effectiveness of two models, linear regression is performed and BIC are assigned to each model. The model with the lowest BIC values for the likelihood ratio chi-square is the more accurate fit of the data.40 The behavior of this model was further tested by comparing the significance of the various covariates when entered either independently or along with the new age/comorbidity stages into a Cox proportional hazards regression model.
The adjusted HR for death and corresponding 95% confidence intervals were calculated using the new age/comorbidity stage, race and gender as covariates, and stratified by cancer site. The combined effect of age and comorbidity on treatment received (no chemotherapy vs. chemotherapy) and OS was examined. The odds of receiving chemotherapy were calculated for each age/comorbidity group, controlling for gender, race and cancer site. The adjusted HR for death for each treatment was calculated within each age/comorbidity stage, controlling for gender, race and cancer site. Adjusted Kaplan-Meier survival curves were generated from the Cox proportional hazards regression model for the two different treatment courses within each of the age/comorbidity stages. Corresponding log-rank chi-square values were calculated.
2.2.3. Propensity Scoring
Propensity score analysis41 was also used to test the relationship between treatment choice and outcomes. Using a logistic regression model controlling for race, gender and age/comorbidity stage (all factors known to influence the rate of receiving adjuvant chemotherapy) all stage III patients were assigned a propensity score from 0 to 1, representing the probability of receiving chemotherapy. All patients were then stratified into quintiles based on their propensity score. These quintiles were used to examine treatment decisions and outcomes in a similar fashion to the age/comorbidity stages. The frequency of each treatment as well as overall survival (controlling for age/comorbidity stage, race and gender) were calculated for each treatment choice within each of the quintiles.
All tabulation, sorting, coding, and analyses were performed using the SAS® system release 9.1 (SAS Institute Inc., Cary, NC). For all tests, the criterion for statistical significance was set at p<0.05 The Washington University School of Medicine Human Studies Committee reviewed this study and deemed it exempt.
3. Results
3.1. Patients
The demographic and clinical characteristics of the 936 stage II and III CRC patients aged 65 and older are presented in Table 2. The patients were similarly distributed across the 65–74 and 75–84 age groups, with 11% comprising the 85+ age group. Comorbidity severity scores were approximately evenly distributed between none, mild and moderate with 13% classified as severe.
Table 2.
Age/comorbidity staging system: Conjunctive consolidation of age group and comorbidity severity with strata sample size and Hazard Ratioa (N=936)
| Age Group | Comorbidity Category | |||
|---|---|---|---|---|
| None | Mild | Moderate | Severe | |
| 65–74 | N=104 HR 1.0 |
N=163 1.28 |
N=119 2.29 |
N=43 2.45 |
| 75–84 | N=81 1.64 |
N=145 2.15 |
N=111 2.94 |
N=62 3.86 |
| 85+ | N=16 3.52 |
N=44 5.04 |
N=31 6.02 |
N=17 5.37 |
| α = 348 | β = 307 | γ = 281 | ||
Adjusted Hazards Ratios for death (controlling for race, gender, stage and cancer site)
The unadjusted impact of age, gender, race, comorbidity, treatment and stage on risk of death for the stage II and III patients is also shown in Table 2. Increasing age, severity of comorbidity and stage III were associated with significantly increased risk of death.
3.2. Conjunctive Consolidation: Creation of Age/Comorbidity Staging System
The results of the conjunctive consolidation process are illustrated in Table 3. All 12 of the possible combinations of the three age groups and the four comorbidity severity scores are shown with corresponding adjusted HR for death and sample sizes. The final grouping of the Alpha, Beta and Gamma stages of the new age/comorbidity staging system is shown. The Alpha stage corresponds to the younger patients with lower severity of comorbidities. The Beta group includes the younger elderly with moderate to severe comorbidities and the mid-elderly with mild comorbidities. The Gamma group corresponds with the combination of the most senior patients and the mid-elderly with moderate or severe comorbidities. The number of patients was equitably distributed among the Alpha, Beta and Gamma groups, accounting for 37.2%, 32.8% and 30.0% percent of the study patients, respectively.
Table 3.
Impact of age/comorbidity staging system on survival in stage III patients (N=435)
| Characteristic | N (%) | Adjusted HR for deatha | 95% CI |
|---|---|---|---|
| All Stage III Patients | |||
| Gender | |||
| Female | 218 (50) | 1.00 | Reference |
| Male | 217 (50) | 1.27 | 0.99–1.64 |
| Race | |||
| White | 359 (83) | 1.00 | Reference |
| Black | 76 (17) | 1.32 | 0.96–1.82 |
| Age/Comorbidity stage | |||
| Alpha | 162 (37) | 1.00 | Reference |
| Beta | 150 (35) | 1.71 | 1.24–2.36 |
| Gamma | 123 (28) | 2.67 | 1.93–3.68 |
| Colon cancer | |||
| Gender | |||
| Female | 156 (57) | 1.00 | Reference |
| Male | 118 (43) | 1.35 | 1.0–1.84 |
| Race | |||
| White | 217 (79) | 1.00 | Reference |
| Black | 57 (21) | 1.31 | 0.91–1.88 |
| Age/Comorbidity stage | |||
| Alpha | 91 (33) | 1.00 | Reference |
| Beta | 91 (33) | 1.76 | 1.17–2.64 |
| Gamma | 92 (34) | 2.76 | 1.86–4.10 |
| Rectal cancer | |||
| Gender | |||
| Female | 62 (39) | 1.00 | Reference |
| Male | 99 (61) | 1.34 | 0.82–2.18 |
| Race | |||
| White | 142 (88) | 1.00 | Reference |
| Black | 19 (12) | 1.29 | 0.65–2.54 |
| Age/Comorbidity stage | |||
| Alpha | 71 (44) | 1.00 | Reference |
| Beta | 59 (37) | 1.57 | 0.92–2.67 |
| Gamma | 31 (19) | 2.06 | 1.12–3.80 |
Adjusted for other predictors in the model.
HR: hazard ratio; CI: confidence interval
3.3. Behavior of Age/Comorbidity Staging System in Stage III CRC Patients
The age/comorbidity staging system was then applied to patients with Stage III CRC only [Table 4]. Overall, the HR for death increased with increasing age/comorbidity stage (1.71 in stage Beta, 2.67 in stage Gamma, relative to stage Alpha). Further, the age/comorbidity staging system remained predictive of death when stratified by cancer site (colon or rectal cancer), with the exception of stage Beta with rectal cancer. Among patients with stage III colon cancer, the adjusted HR for death compared to stage Alpha was 1.76 in stage Beta and 2.76 in stage Gamma. In patients with stage III rectal cancer, the adjusted HR for death was 1.57 in stage Beta and 2.06 in stage Gamma, compared to stage Alpha.
Table 4.
Impact of treatment on risk of death and 3 year overall survival in stage III colorectal cancer, stratified by age/comorbidity stage (N=435)
| Characteristic | N (%) | 3-year OS | Adjusted HR for deatha | 95% CI |
|---|---|---|---|---|
| All stage III patients (N=435) | ||||
| Chemotherapy | 255 (59) | 70.4% | 1.00 | Reference |
| No Chemotherapy | 180 (41) | 41.7% | 2.28 | 1.76–2.96 |
| Alpha (N=162) | ||||
| Chemotherapy | 116 (72) | 71.4% | 1.00 | Reference |
| No Chemotherapy | 46 (28) | 58.5% | 1.80 | 1.06–3.06 |
| Beta (N=150) | ||||
| Chemotherapy | 103 (69) | 72.0% | 1.00 | Reference |
| No Chemotherapy | 47 (31) | 38.6% | 2.24 | 1.38–3.63 |
| Gamma (N=123) | ||||
| Chemotherapy | 36 (29) | 61.5% | 1.00 | Reference |
| No Chemotherapy | 87 (71) | 34.9% | 2.10 | 1.23–3.57 |
Controlling for gender, race, cancer site
HR: hazard ratio; CI: confidence interval; OS: overall survival
The superiority (goodness of fit) of the new age/comorbidity staging system over the model stratified by age and comorbidity was confirmed by BIC.
3.4. Odds of Receiving Chemotherapy
Advancing age/comorbidity stage was associated with decreasing odds of receiving chemotherapy in patients with Stage III CRC. In Stage Alpha, 71.6% of patients received chemotherapy, while in Stage Beta 68.7% received chemotherapy and only 29.3% of patients in stage Gamma received chemotherapy. Adjusted for gender, race and cancer site, the odds ratio of receiving chemotherapy was 0.83 [95% Confidence Intervals (CI) 0.51–1.35] in stage Beta and 0.14 [95% CI 0.08–0.24] in stage Gamma, compared to stage Alpha.
3.5. Survival by Treatment and Age/Comorbidity Stage
At the time of last follow-up, 195 (45%) of the patients were alive. Overall, the median survival time was 33.9 months (range 4 days–143 months). Of the patients that were alive at the time of last follow-up, the median survival time was 48.6 (range 5–143) months.
Receipt of chemotherapy was associated with lower risk of death in each of the age/comorbidity stages, compared to those who did not receive chemotherapy. The adjusted HR for death for patients who underwent surgery only compared to those who received chemotherapy was 1.8 in stage Alpha, 2.24 in stage Beta and 2.1 in stage Gamma [Table 5]. The 3 year survival rate was higher in those who received chemotherapy, overall and in each of the age/comorbidity stages.
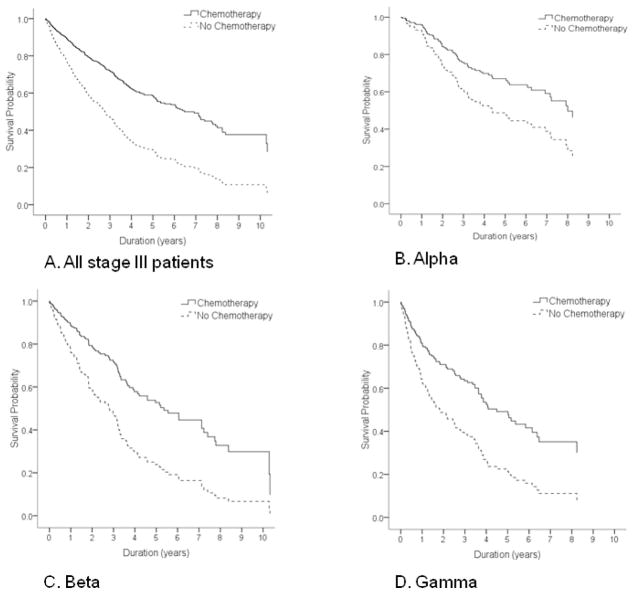
The improvement in survival is illustrated in Figure 1. In the overall cohort and in each stage of the age/comorbidity stages, patients receiving chemotherapy had significantly greater overall survival compared to those not receiving chemotherapy. Although older patients with more severe comorbidity were less likely than patients in stage Alpha to receive adjuvant therapy, those who did receive chemotherapy had improved survival relative to those who did not.
FIGURE 1. Impact of treatment on survivala in stage III colorectal cancer.
(A) All stage III patients
(B) Stage Alpha
(C) Stage Beta
(D) Stage Gamma
aAll curves adjusted for gender, race and cancer site.
Propensity score analysis confirmed these results. Within each quintile, chemotherapy was associated with increased survival. There was no significant difference in survival for those who received chemotherapy across the quintiles (data not shown).
4. Discussion
While patients with increasing age and comorbidity are less likely to receive chemotherapy, we demonstrate that a dramatic survival benefit associated with chemotherapy persists across age and comorbidity categories in patients with stage III CRC. This raises the question whether some older patients with more severe comorbidities may fail to receive a potential survival benefit with chemotherapy.
Our findings are consistent with previous reports that increasing age and comorbidity reduce the rate of administration of chemotherapy in elderly patients with stage III colon 25, 35, 42, 43 and rectal cancer7. Additionally, our results support the findings of other studies which demonstrate the OS benefit of adjuvant chemotherapy in elderly patients with stage III CRC,24, 26–29 even among patients with significant comorbidity42.
We have extended previous analyses to examine the combined effect of age and comorbidity. By using conjunctive consolidation, we combined age and comorbidity into a single predictive variable and created a model that better fits the data and, clinically, more precisely delineates prognosis in the heterogeneous population of older adults. We then demonstrated that the age/comorbidity staging system predicts likelihood of receiving chemotherapy in patients with Stage III CRC, and that the survival benefit of chemotherapy relative to surgery alone is maintained independent of the age/comorbidity status and propensity to receive chemotherapy. Importantly, our sample size allowed us to evaluate the survival benefit of chemotherapy in the most elderly group (age >85), a subset of patients in whom data are sparse.
The observed survival benefit of chemotherapy among the very old and those with severe comorbidities suggest that some elderly patients are not receiving optimal care. There are a number of barriers to the administration of chemotherapy in older patients. Advancing age, male gender, black race, higher comorbidity scores44 and Medicaid insurance23 are associated with lower chance of consulting with a medical oncologist. Age and comorbidity significantly impact whether a medical oncologist recommends adjuvant chemotherapy.45, 46 Life expectancy is an important consideration in estimating the potential benefit of adjuvant therapy. Even if offered, a patient may decline chemotherapy due to concerns about a negative impact on quality of life. However, patients with stage III CRC who receive adjuvant therapy report health-related quality of life similar to those who do not receive adjuvant therapy.47 In a recent population-based cohort study, patients over 65 years were more likely to discontinue chemotherapy early, though the rate of clinical adverse events was similar across the age spectrum. 25 This underscores the importance of quality of life in older patients undergoing chemotherapy, and the need to better understand the factors that influence an older individual’s decision to proceed with chemotherapy or to discontinue it prematurely.
The principal limitations of this single-institution study are those inherent with observational research. Without randomization, the bias due to unmeasured covariates on treatment outcome cannot be removed. We do not have data on the functional status of the cohort. As functional status and comorbidities are independent of each other,48, 49 there may be differences in functional status between the groups to which the difference in survival may be attributed. Further, data on the precise chemotherapeutic regimens administered are not available from the institution’s cancer registry. Presumably, all patients received standard-of-care 5-fluorouracil based regimens. More recently, regimens adding oxaliplatin to fluorouracil (FOLFOX) have supplanted earlier regimens, with superior disease-free17 and overall survival50. FOLFOX was found to be safe and effective in a pooled analysis of 4 clinical trials of older adults with colon cancer.29 However, a recent study of over 12,500 patients who received newer adjuvant regimens (including regimens containing oxaliplatin, irinotecan or oral 5FU) for stage II or III colon cancer found that patients over the age of 70 years did not receive the same survival benefit from the newer agents than did those under the age of 70.51
In conclusion, increasing age and comorbidity are associated with a lower likelihood of receiving chemotherapy and increased risk of death in patients with stage III CRC. Despite this disparity in treatment, older patients with more severe comorbidity who do receive chemotherapy have a lower risk of death relative to similar patients who do not receive chemotherapy. These findings should encourage clinicians to reconsider biases that impact their decision to offer adjuvant chemotherapy. Our new age/comorbidity staging system will aid clinicians and patients in assessing the combined effect of age and comorbidity. Future study will determine whether this survival benefit persists with more modern chemotherapeutic regimens, and should include additional parameters relevant to a geriatric oncology population, particularly functional status.
Acknowledgments
Research support: This publication was made possible by Grant Number K30 RR022251 and UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
Biography
Tanya Wildes, MD, is a geriatric oncologist and Instructor of Medicine at Washington University School of Medicine. She completed fellowships in hematology, oncology and geriatrics at Washington University. During her fellowships, she was a scholar in the Clinical Research Training Center Postdoctoral Program. Through this program, she completed the research detailed in this manuscript. She is interested in the impact patient-specific factors, such as comorbidities, functional status and other geriatric syndromes, on tolerance of therapy, prognosis and survival in senior adults with cancer.
Footnotes
Contributions:
Dr. Wildes contributed to the conception and design of the manuscript, data collection, analysis and interpretation, and manuscript writing. Dr. Kallogjeri contributed to data collection, analysis and interpretation of data and manuscript writing. Mr. Powers contributed to the conception and design of the manuscript, analysis and interpretation of data, and manuscript writing. Ms. Vlahiotis contributed to the conception and design, analysis and interpretation of data. Dr. Mutch contributed to study conception and design and manuscript writing. Dr. Spitznagel contributed to the conception and design, analysis and interpretation of data and manuscript writing. Dr. Tan contributed to the conception and design of the manuscript, analysis and interpretation of data, and manuscript writing. Dr. Piccirillo contributed to the conception and design, data collection of, analysis and interpretation of data and manuscript writing. All authors provided final approval of the manuscript.
Disclosures: The authors have no conflict of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Edwards BK, Howe HL, Ries LAG, Thun MJ, Rosenberg HM, Yancik R, et al. Annual Report to the Nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94(10):2766–92. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 2.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Soares HP, Balducci L, Djulbegovic B. Treatment tolerance and efficacy in geriatric oncology: a systematic review of phase III randomized trials conducted by five National Cancer Institute-sponsored cooperative groups. J Clin Oncol. 2007;25(10):1272–6. doi: 10.1200/JCO.2006.09.2759. [DOI] [PubMed] [Google Scholar]
- 4.Lewis JH, Kilgore ML, Goldman DP, Trimble EL, Kaplan R, Montello MJ, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21(7):1383–9. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Bouchardy C, Rapiti E, Fioretta G, Laissue P, Neyroud-Caspar I, Schafer P, et al. Undertreatment strongly decreases prognosis of breast cancer in elderly women. J Clin Oncol. 2003;21(19):3580–7. doi: 10.1200/JCO.2003.02.046. [DOI] [PubMed] [Google Scholar]
- 6.Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg. 2000;135(2):182–5. doi: 10.1001/archsurg.135.2.182. [DOI] [PubMed] [Google Scholar]
- 7.Neugut AI, Fleischauer AT, Sundararajan V, Mitra N, Heitjan DF, Jacobson JS, et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: a population-based study. J Clin Oncol. 2002;20(11):2643–50. doi: 10.1200/JCO.2002.08.062. [DOI] [PubMed] [Google Scholar]
- 8.Sundararajan V, Hershman D, Grann VR, Jacobson JS, Neugut AI. Variations in the use of chemotherapy for elderly patients with advanced ovarian cancer: a population-based study. J Clin Oncol. 2002;20(1):173–8. doi: 10.1200/JCO.2002.20.1.173. [DOI] [PubMed] [Google Scholar]
- 9.Coebergh JW, Janssen-Heijnen ML, Post PN, Razenberg PP. Serious co-morbidity among unselected cancer patients newly diagnosed in the southeastern part of the Netherlands in 1993-1996. J Clin Epidemiol. 1999;52(12):1131–6. doi: 10.1016/s0895-4356(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 10.Yancik R, Wesley MN, Ries LA, Havlik RJ, Edwards BK, Yates JW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. JAMA. 2001;285(7):885–92. doi: 10.1001/jama.285.7.885. [DOI] [PubMed] [Google Scholar]
- 11.Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18(13):2529–36. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 12.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 13.Rim SH, Seeff L, Ahmed F, King JB, Coughlin SS. Colorectal cancer incidence in the United States, 1999–2004. Cancer. 2009;115(9):1967–76. doi: 10.1002/cncr.24216. [DOI] [PubMed] [Google Scholar]
- 14.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11(10):1879–87. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 15.Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122(5):321–6. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell MJ, Mailliard JA, Kahn MJ, Macdonald JS, Haller DG, Mayer RJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol. 1997;15(1):246–50. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 17.Andre T, Boni C, Mounedji-Boudiaf L, Navarro M, Tabernero J, Hickish T, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350(23):2343–51. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 18.Kuebler JP, Wieand HS, O’Connell MJ, Smith RE, Colangelo LH, Yothers G, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol. 2007;25(16):2198–204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 19.Akasu T, Moriya Y, Ohashi Y, Yoshida S, Shirao K, Kodaira S. Adjuvant chemotherapy with uracil-tegafur for pathological stage III rectal cancer after mesorectal excision with selective lateral pelvic lymphadenectomy: a multicenter randomized controlled trial. Jpn J Clin Oncol. 2006;36(4):237–44. doi: 10.1093/jjco/hyl014. [DOI] [PubMed] [Google Scholar]
- 20.Douglass HO, Jr, Moertel CG, Mayer RJ, Thomas PR, Lindblad AS, Mittleman A, et al. Survival after postoperative combination treatment of rectal cancer. N Engl J Med. 1986;315(20):1294–5. doi: 10.1056/NEJM198611133152014. [DOI] [PubMed] [Google Scholar]
- 21.Fisher B, Wolmark N, Rockette H, Redmond C, Deutsch M, Wickerham DL, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst. 1988;80(1):21–9. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 22.Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med. 1991;324(11):709–15. doi: 10.1056/NEJM199103143241101. [DOI] [PubMed] [Google Scholar]
- 23.Bradley CJ, Given CW, Dahman B, Fitzgerald TL. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med. 2008;168(5):521–9. doi: 10.1001/archinternmed.2007.82. [DOI] [PubMed] [Google Scholar]
- 24.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 25.Kahn KL, Adams JL, Weeks JC, Chrischilles EA, Schrag D, Ayanian JZ, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. Jama. 2010;303(11):1037–45. doi: 10.1001/jama.2010.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwashyna TJ, Lamont EB. Effectiveness of adjuvant fluorouracil in clinical practice: a population-based cohort study of elderly patients with stage III colon cancer. J Clin Oncol. 2002;20(19):3992–8. doi: 10.1200/JCO.2002.03.083. [DOI] [PubMed] [Google Scholar]
- 27.Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136(5):349–57. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 28.Jessup JM, Stewart A, Greene FL, Minsky BD. Adjuvant Chemotherapy for Stage III Colon Cancer: Implications of Race/Ethnicity, Age, and Differentiation. JAMA. 2005;294(21):2703–11. doi: 10.1001/jama.294.21.2703. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg RM, Tabah-Fisch I, Bleiberg H, de Gramont A, Tournigand C, Andre T, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24(25):4085–91. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 30.Zuckerman IH, Davidoff AJ, Onukwugha E, Pandya N, Gardner JF, Seal B, et al. Effect of age on survival benefit of adjuvant chemotherapy in elderly stage III colon cancer patients: a population-based analysis. J Clin Oncol (Meeting Abstracts) 2008;26(15S):4014. (Abstract) [Google Scholar]
- 31.Piccirillo J, Creech C, Zequeira R, Anderson S, Johnston A. Inclusion of comorbidity into oncology data registries. J Registry Manage. 1999;26(2):66–70. [Google Scholar]
- 32.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluatin the outcome of diabetes mellitus. J Chronic Dis. 1974;27(7–8):387–404. doi: 10.1016/0021-9681(74)90017-4. [DOI] [PubMed] [Google Scholar]
- 33.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441–7. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 34.Read WL, Tierney RM, Page NC, Costas I, Govindan R, Spitznagel EL, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22(15):3099–103. doi: 10.1200/JCO.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 35.Lemmens VE, Janssen-Heijnen ML, Verheij CD, Houterman S, Repelaer van Driel OJ, Coebergh JW. Co-morbidity leads to altered treatment and worse survival of elderly patients with colorectal cancer. Br J Surg. 2005;92(5):615–23. doi: 10.1002/bjs.4913. [DOI] [PubMed] [Google Scholar]
- 36.Lemmens VE, van Halteren AH, Janssen-Heijnen ML, Vreugdenhil G, Repelaer van Driel OJ, Coebergh JW. Adjuvant treatment for elderly patients with stage III colon cancer in the southern Netherlands is affected by socioeconomic status, gender, and comorbidity. Ann Oncol. 2005;16(5):767–72. doi: 10.1093/annonc/mdi159. [DOI] [PubMed] [Google Scholar]
- 37.Feinstein AR. Principles of Medical Statistics. Boca Raton, FL: Chapman & Hall/CRC Press; 2002. [Google Scholar]
- 38.Kass RE, Raftery AE. Bayes Factors. Journal of the American Statistical Association. 1995;90(430):773–95. [Google Scholar]
- 39.Volinksy C, Raftery AE. Bayesian information criterion for censored survival models. Biometrics. 2000;56(1):256–62. doi: 10.1111/j.0006-341x.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6:461–64. [Google Scholar]
- 41.Rosenbaum P, Rubin D. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika. 1983;70(1):41–55. [Google Scholar]
- 42.Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109(12):2410–9. doi: 10.1002/cncr.22726. [DOI] [PubMed] [Google Scholar]
- 43.Ananda S, Field KM, Kosmider S, Compston D, Desai J, Lim LC, et al. Patient age and comorbidity are major determinants of adjuvant chemotherapy use for stage III colon cancer in routine clinical practice. J Clin Oncol. 2008;26(27):4516–7. doi: 10.1200/JCO.2008.18.7443. [DOI] [PubMed] [Google Scholar]
- 44.Luo R, Giordano SH, Freeman JL, Zhang D, Goodwin JS. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006;11(9):1025–33. doi: 10.1634/theoncologist.11-9-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keating NL, Landrum MB, Klabunde CN, Fletcher RH, Rogers SO, Doucette WR, et al. Adjuvant chemotherapy for stage III colon cancer: do physicians agree about the importance of patient age and comorbidity? J Clin Oncol. 2008;26(15):2532–7. doi: 10.1200/JCO.2007.15.9434. [DOI] [PubMed] [Google Scholar]
- 46.Krzyzanowska MK, Regan MM, Powell M, Earle CC, Weeks JC. Impact of patient age and comorbidity on surgeon versus oncologist preferences for adjuvant chemotherapy for stage III colon cancer. J Am Coll Surg. 2009;208(2):202–9. doi: 10.1016/j.jamcollsurg.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 47.Bouvier A-M, Jooste V, Bonnetain F, Cottet V, Bizollon M-H, Bernard M-P, et al. Adjuvant treatments do not alter the quality of life in elderly patients with colorectal cancer. Cancer. 2008;113(4):879–86. doi: 10.1002/cncr.23629. [DOI] [PubMed] [Google Scholar]
- 48.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–7. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 49.Repetto L, Fratino L, Audisio RA, Venturino A, Gianni W, Vercelli M, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002;20(2):494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 50.de Gramont A, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, et al. Oxaliplatin/5FU/LV in adjuvant colon cancer: Updated efficacy results of the MOSAIC trial, including survival, with a median follow-up of six years. J Clin Oncol. 2007;25(18S):4007. (Abstract) [Google Scholar]
- 51.McCleary NA, Meyerhardt JA, Green E, Yothers G, de Gramont A, Van Cutsem E, et al. Impact of older age on the efficacy of newer adjuvant therapies in >12,500 patients (pts) with stage II/III colon cancer: Findings from the ACCENT Database. J Clin Oncol. 2009;27(15S):4010. doi: 10.1200/JCO.2013.49.6638. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]