Abstract
Background:
Early definitive diagnosis and effective treatment are mandatory in rheumatoid arthritis (RA) as it can halt the disease progression and subsequent joints destruction.
Objective:
To investigate the diagnostic and prognostic value of anti-mutated citrullinated vimentin (anti-MCV) and its correlation with disease activity, peripheral and axial skeleton affection in RA patients.
Patients and methods:
A total of 123 patients with different rheumatic diseases were enrolled in a prospective-two year study at Ain Shams University hospital: 64 patients with RA and 59 patients with other rheumatic diseases as controls. RA patients were fulfilling the traditional and the new ACR/EULAR diagnostic criteria for RA. They have been followed up for two years. At baseline, all RA patients were subjected to: Clinical assessment of disease activity by taking full histories, general and local examination, measurement of 28 joint count of tender and swollen joints with calculation of disease activity score (DAS-28) for each patient. Complete blood count, erythrocytes sedimentation rate, C-reactive protein and rheumatoid factor titers were performed. Anti-MCV IgG immunoglobulins’ assay was performed at the study endpoint by ELISA. RA patients were then classified into; anti-MCV positive and anti-MCV negative groups for statistical comparison. Plain X-ray was performed on the peripheral joints and scored by the Simple Erosion Narrowing score (SEN-score). Magnetic Resonance Imaging (MRI) scans were carried out to 22 RA patients on cervical and lumbosacral regions.
Results:
Anti-MCV antibodies were found to be of high sensitivity (79.6%) and specificity (96.6%) in diagnosing RA. The area under the curve was 0.893 at 95% confidence interval (CI), confers an odds ratio of 23.5. Anti-MCV positive RA patients had significantly higher DAS-28 and SEN-scores than anti-MCV negative patients; who were found to have more benign disease with lower incidence of erosions (P < 0.05). MRI scans revealed that; 17/22 (77%) had cervical joints involvement while, 8 (36%) had lumbo-sacral joint lesions (P < 0.05), both were correlated significantly with aggressive peripheral joint disease.
Conclusion:
Anti-MCV antibodies are promising diagnostic and prognostic marker in RA, with high sensitivity and specificity. They may identify a subset of RA patients with aggressive early erosive disease. The axial skeleton—especially the cervical spine—could be affected in RA and this was correlated with aggressive peripheral joints’ disease. MRI scanning is a sensitive method for detecting axial skeleton involvement in RA, in attempt for better disease control and outcomes.
Keywords: rheumatoid arthritis, RA, anti-mutated citrullinated vimentin, anti-MCV
Introduction
Rheumatoid arthritis (RA) is a heterogenous disease of multifactorial origin that has variable outcomes. Early definitive diagnosis is essential in RA patients, as they have a true chance for achieving a “cure” of the disease if they are treated early and aggressively in the “window of opportunity” period. However, this needs sensitive clinical and laboratory diagnostic tools.1
Recently, the diagnosis of RA has been substantially improved by the introduction of standardized immunoassays for the detection of auto-antibodies against different citrullinated antigens.2 Despite the excellent performance of these immunoassays, anti-cyclic citrullinated peptide antibodies (anti-CCP1, 2 and 3) provide a sensitivity less than that of rheumatoid factor (RF) ∼55%–60% and many cases with clinically definitive RA lack these antibodies. Additionally, the correlation of anti-CCP with RA disease activity yielded conflicting results.3
Although the peptide/antigen used in the first generation anti-CCP1 test has been clearly described. The antigen used for the second and third generation anti-CCP tests (anti-CCP2 and anti-CCP3) have been described by the manufacturer as being confidential. The confidential nature of the antigen used in these tests has its limitations. We do not know whether these anti-CCP tests measure true auto-antibody reactivity or not. This provides a cautionary note for clinicians interpreting the anti-CCP test results, since the exact nature of what is being tested has not been subjected to external scrutiny. Likewise, basic scientists in this field should remember that although measurement of CCP reactive antibodies may be useful as RA biomarkers, the anti-CCP2 and anti-CCP3 tests do not necessarily measure true auto-antibodies in RA patients, since the peptides used in these commercial anti-CCP assays may not contain bona fide auto-antigens.4
Antibodies to citrullinated vimentin are referred to as anti-Sa, after the index patient, Savoie.1 The Sa antigen is the starting point for the development of an enzyme-linked immunosorbent assay (ELISA) to detect mutated citrullinated vimentin (anti-MCV), which was developed few years later. Vossenaar et al5 demonstrated that, antibodies in anti-Sa positive RA sera bind to citrullinated vimentin, indicating that anti-Sa belongs to a group of antibodies against citrullinated proteins. Anti-Sa reactivity has been shown to consist at least partially—of anti-MCV. However, the patterns of reactivity for anti-Sa and anti-vimentin are not identical to that of anti-CCP antibodies. Thus it should be of interest to investigate whether analysis of immunoreactivity with citrullinated vimentin adds to the information concerning the diagnosis and prognosis of RA gained from the anti-CCP assay or not.6
A recently developed ELISA for the quantification of anti-MCV antibodies was used to assess its clinical and predictive values in RA patients.
Despite the fact that any joint can be affected in RA patients, axial joints involvement seems to be a taboo issue in RA. Most literatures were concerned with the peripheral joints disease. Apophesyeal, costovertebral and discovertebral joints can be affected in RA.7 Narvaez et al8 reported that the cervical spine is the next region to be affected after the metacarpophalengial joints in RA patients.
Axial joints affection in RA has its definitive radiological characteristics that help to differentiate it from that of seronegative spondyloarthritis (SpA). In RA, the cervical spine is the most frequently affected region of the spine followed by the dorsal and the lumbar regions [descending marsh in contrary to the ascending marsh in SpA]. No boney calcinosis, ankylosis, new bone formation, osteophytes or syndesmophytes; the hallmark of SpA. There are apophyseal joints erosions, disc space narrowing, endplate sclerosis, multiple vertebral subluxations [malalignment] at the atlantoaxial, sub-axial and lumbar regions. Osteoporosis is a common finding in RA spine in contrary to new bone formation, calcification and bamboo spine in SpA. Degenerative disk disease may supervene and contribute to the severity of cervical spine involvement and to the development of dislocation.9
Aim of the study
Our aim was to investigate the diagnostic and prognostic value of anti-MCV and its correlation—if any—with disease activity, peripheral and axial skeleton affection in RA patients.
Patients and Methods
Study population
A total of 123 patients with different rheumatic diseases were enrolled in a prospective two-year study: 64 RA patients and 59 patients with other rheumatic diseases as controls; (29 with systemic lupus erythematosus (SLE), 8 with spondyloarthritis (SpA), 6 with undifferentiated connective tissue disease, 6 with 1ry antiphospholipid syndrome and 10 patients with gouty arthritis).
All RA patients fulfiled the traditional American College of Rheumatology ACR criteria10 and the new rheumatoid arthritis diagnostic criteria recently released by ACR/Europian league against Rheumatism-EULAR panel.11 Patients were either attending the outpatients’ clinic or they were inpatients in the Rheumatology Department, Ain Shams University Hospital, between January 2007 and July 2009; they were followed up for two years with occasional admissions (disease flares or complications).
All patients gave informed consent to participate in the study, which was approved by the Ain Shams Medical ethics committee. Patients underwent; clinical assessment of disease activity by full history taking general and local examinations, measurement of the 28-joint count of tender and swollen joint’ with calculation of the disease activity score (DAS-28) for each RA patient by DAS-28 score calculator.12
DAS-28 ≤ 3.2 = inactive disease.
DAS-28 > 3.2 ≤ 5.1 = moderate disease activity.
DAS-28 > 5.1 = severe disease activity.
The response to therapy and remissions were determined according to the EULAR-28 response criteria (DAS-28 ≤ 2.6).13
Clinical data were recorded for each patient: Swollen joint count (SJC), tender joint count (TJC), duration of morning stiffness, fever and systemic upset symptoms. At baseline, 52 RA patients (81%) were treated by different combinations of disease modifying anti-rheumatic drugs (DMARDs), with or without corticosteroids, in absence of any information about their serological status regarding anti-MCV reactivity.
Laboratory method
Samples and analysis
After an overnight fast, 5 mL of venous blood was withdrawn from each patient; 2 mL was put in a test tube with EDTA anticoagulant for complete blood count (CBC) on a Sysmex KN21 (Beckman Instrument Incorporation, California, USA). Erythrocytes sedimentation rate (ESR) was measured by Westergreen method as cited in clinical laboratory methods. The other 3 mL was put in a clean plain test tube; after centrifugation, the separated serum was stored at—20 °C until use. C-reactive protein (CRP) was tested by the Latex method, following standard clinical laboratory methods. Rheumatoid factor (RF) titer was assayed according to (Orgentec Diagnostika GmbH Germany).14 Anti-MCV IgG immunoglobins’ assay was performed at the study endpoint- by the kits and the standards of ORGENTEC Diagnostika (GmbH Germany).1
Radiological method
Plain X-ray films were performed on the small joints of the hands, wrists, feet, cervical and lumbosacral regions in all RA patients at baseline (n = 64) and after two years (n = 52). These films were examined by expert radiologists. The simplified erosion narrowing score (SEN score) method was used in assessing the peripheral joints (narrowing/erosion) at 28 joint regions and a score was given for each patient. Radiological progression was defined as an increase in the SEN score from the baseline to endpoint that was greater than the median value for each patient.15
Magnetic resonance imaging (MRI) scanning was performed at the MRI unit of the Delta Radiology center on the cervical and lumbo-sacral regions to 22 RA patients who were found to be symptomatic; [neck pain, backache, stiffness, limitation of movement, nerve root compression, sciatica with motor and/or sensory dysfunctions] and for those suspected to have atlantoaxial subluxation by plain X-ray films.
The MRI scans involved antroposterior and lateral projections for the cervical and lumbar spine. Scans were performed on the cervical region from C1 to C7 and on the lumbar region from L1 to L5 vertebral bodies, using the Siemens machine (Somatom model) set to an 0.23 open system using a phased array cervical coil and spinal coil. In some cases, we included extension and flexion to the cervical region, and in other scans were undertaken following intravenous contrast material administration by manual injection at a dose of 0.2 mL/kg.
Statistical method
The data was analyzed on an IBM computer using SPSS (version 12). Quantitative variables were described as mean, standard deviation (SD) and range. Qualitative variables were described as number and percentage. The Chi-square test was used to compare qualitative variables between groups. The Kruskall-Wallis test was used instead of ANOVA in non-parametric data (SD > 50% mean). Spearman’s correlation test was used to rank different variables against each other. Unpaired student’s t-test was used to compare quantitative variables in parametric data (SD < 50% mean). Receiver operator characteristic curve (ROC) was drawn to find out the best cut-off value of anti-MCV in diagnosing RA and to test for its statistical efficacy. P-value > 0.05 was considered insignificant, P < 0.05 was significant and P < 0.01 was highly significant.
Results
After anti-MCV testing, RA patients were classified into anti-MCV positive group (n = 51) and anti-MCV negative group (n = 13) for statistical comparison.
According to Student’s t-test; there was significantly higher ESR, CRP, DAS-28 and SEN scores in anti-MCV positive than negative patients, at 95% CI in spite of non-significant differences in the RF titers between both groups. The Chi-square test revealed a significantly higher incidence of axial skeleton affection in anti-MCV positive group (Table 3).
Table 3.
Comparison between anti-MCV positive and negative RA patients at study endpoint.
| Anti-MCV +ve (n = 51) mean ± SD | Anti-MCV −ve (n = 13) mean ± SD | 2-tailed significance (P<) | |
|---|---|---|---|
| Age (years) | 42.32 ± 11.57 | 40.12 ± 11.5 | 0.992 |
| DD (years) | 5.91 ± 3.25 | 4.61 ± 2.32 | 0.453 |
| ESR (1st h) | 48.77 ± 11.25 | 28.46 ± 10.54 | 0.001* |
| CRP (mg%) | 21.80 ± 29.83 | 9.0 ± 3.05 | 0.035* |
| DAS-28 | 4.24 ± 1.14 | 2.82 ± 0.35 | 0.05* |
| VAS/100 mm | 61.45 ± 11.40 | 55.76 ± 9.54 | 0.901 |
| SEN score | 20.22 ± 8.85 | 9.46 ± 2.78 | 0.05* |
| Flares per year (n =) | 4.15 ± 1.32 | 2.31 ± 0.05 | 0.05* |
| RF titer (U/L) | 59.91 ± 155.7 | 41.69 ± 73.45 | 0.422 |
| Anti-MCV titer (U/L) | 153.62 ± 240.4 | 19.00 ± 5.59 | 0.000+ |
| Axial joints/Chi-square test | 16/51 (31%) | 1/13 (7%) | 0.044* |
Significant by welch statistics due to absence of variance homogeneity.
On comparing baseline and endpoint data of anti-MCV positive patients by the Student’s t-test, there was statistically non-significant improvement in inflammatory markers of disease activity (CRP, DAS-28 and VAS). Although most patients were on regular combinations of disease modifying anti-rheumatic drugs (DMARD) therapy. Importantly, significantly higher SEN scores were seen after two years in anti-MCV positive patients, indicating significant radiological progression (Table 4).
Table 4.
Comparison between baseline and endpoint data of anti-MCV positive RA patients.
|
Anti-MCV positive (n = 51) |
|||
|---|---|---|---|
| Baseline mean ± SD | Endpoint mean ± SD | Sig. (2-tailed) (P<) | |
| ESR (1st h) | 52.57 ± 13.07 | 48.77 ± 11.25 | 0.03* |
| CRP (mg%) | 27.57 ± 6.38 | 21.80 ± 29.83 | 0.106 |
| DAS-28 | 5.24 ± 1.29 | 4.24 ± 1.14 | 0.350 |
| VAS/100 mm | 65.76 ± 12.37 | 61.45 ± 11.40 | 0.781 |
| SEN score | 14.45 ± 7.97 | 20.22 ± 8.85 | 0.05* |
| RF-titer (U/L) | 64.39 ± 113.7 | 59.91 ± 155.7 | 0.322 |
The Student’s t-test showed significant improvement in DAS-28 and the RF titer in anti-MCV negative RA patients after two years (P = 0.003 and 0.023, respectively), with non-significant progression in radiological damage to the peripheral joints (SEN score, ESR or CRP) (Table 5).
Table 5.
Comparison between baseline and endpoint data of anti-MCV negative RA patients.
|
Anti-MCV negative (n = 13) |
|||
|---|---|---|---|
| Baseline (mean ± SD) | Endpoint (mean ± SD) | Sig. (2-tailed) (P<) | |
| ESR (1st h) | 33.15 ± 12.80 | 28.46 ± 10.54 | 0.012 |
| CRP (mg%) | 10.53 ± 2.53 | 9.00 ± 3.05 | 0.284 |
| DAS-28 | 3.80 ± 0.40 | 2.82 ± 0.35 | 0.003* |
| VAS/100 mm | 60.76 ± 7.65 | 55.76 ± 9.54 | 0.351 |
| SEN score | 7.69 ± 3.06 | 8.46 ± 2.78 | 0.063 |
| RF titer (U/L) | 57.34 ± 56.76 | 41.69 ± 73.45 | 0.023* |
Spearman’s correlation test ranks the anti-MCV titer against different variables. The anti-MCV titer was found to be positively correlated with markers of disease activity: (DAS-28, CRP, VAS, SEN score and with axial joint involvement by MRI). However, it was not correlated either with ESR or RF titer (Table 6).
Table 6.
Correlations of anti-MCV titer with different disease parameters.
|
Anti-MCV titer (U/L) |
||
|---|---|---|
| Spearman’s r | Sig. (2-tailed) (P <) | |
| ESR (1st h) | 0.331 | 0.367 |
| CRP (mg/dl) | 0.651 | 0.021* |
| RF titer (U/L) | 0.460 | 0.212 |
| DAS-28 | 0.802 | 0.001* |
| VAS/100 mm | 0.847 | 0.005* |
| SEN score | 0.821 | 0.003* |
| Axial joints by MRI | 0.637 | 0.042* |
Multivariate analysis at 95% CI was done to study the influence of different disease parameters on the radiological outcome of RA patients at two years. It was found to be mostly affected by CRP and anti-MCV titers (Table 7).
Table 7.
Multivariate analysis for the radiological outcome at 24 months.
| Regression coefficient (β) | “t” | Significance P < 0.05 | |
|---|---|---|---|
| Age (years) | −0.031 | −0.366 | 0.716 |
| Sex | 0.124 | 1.280 | 0.208 |
| DD (years) | −0.42 | −0.456 | 0.651 |
| ESR (1st h) | −0.093 | −0.522 | 0.605 |
| CRP (mg%) | −0.132 | −1.184 | 0.034* |
| RF titer (U/L) | 0.158 | 1.635 | 0.227 |
| DAS-28 | 0.216 | 0.962 | 0.342 |
| VAS/100 mm | 0.290 | 1.276 | 0.210 |
| Anti-MCV titer (U/L) | 0.634 | 6.389 | 0.001* |
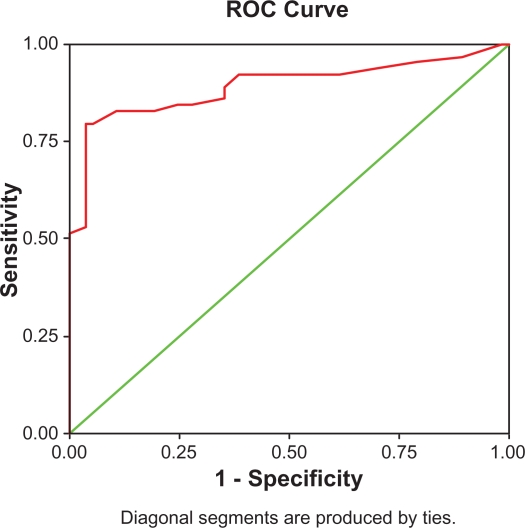
The best cut-off value for the anti-MCV test was found at 20.5 U/L. The area under the curve was 0.893 at the 95% CI, conferring an odds ratio of 32.5. Sensitivity was 79.6% and specificity was 96.6% in diagnosing RA patients (Table 8).
Table 8.
Diagnostic effectiveness of the anti-MCV test.
| Anti-MCV titer best cut-off value = 20.5 U/L | |
|---|---|
| Sensitivity | 79.6% |
| Specificity | 96.6%* |
| PPV | 96.22%, 95% CI = 87.24% |
| NPV | 81.42%, 95%, CI = 87.24% |
| Accuracy | 87.80% |
| Positive DLR | 23.50 (+ve odds ratio) |
| Negative DLR | 0.21 (−ve odds ratio) |
| Prevalence | 52.03% |
| Pre-test odds/probability | 1.08 |
| post-test odds +ve | 25.49 |
| TPFN ratio | 3.9231 |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; DLR, diagnostic likelihood ratio (odds ratio); TPF N, the proportion of true positives to false negatives for the test condition; Pre-test odds/probability, the odds/probability of the condition (prevalence) in the whole community.16
Out of the 29 SLE patients; four were tested positive for the anti-MCV test (13.7%), as did one of the eight SpA patients and two of the six undifferentiated CT disease patients, but none of the gouty arthritis patients.
MRI results of the cervical spine
Seventeen of the 22 (77%) RA patients were found to have different forms of cervical spine lesions, three (13%) were found to have atlantoaxial subluxation due to typical destruction of the atlantoaxial complex by pannus tissue. The bone segment of the cervical spine showed destruction of the apophesyeal joints with narrowed disc spaces in five patients (22%). Minor indentation of the dura opposite the disc spaces was found in 11/22 (50%); this was a frequent finding as a consequence of disc bulging and/or osteophyte formation of the cervical bodies due to superadded degenerative disc disease. Increased signal intensity in the spinal cord was found in 4/22 (18%) patients, which was adjusted to the sites of compression suggested by edema and inflammation of the cord (mylopathy). Two cases (9%) showed superficial and deep enhancements of the pannus.
MRI results of the lumbar spine
Grade 0 = normal findings (14/22, 63%). Grade I = shows signal intensity changes at the vertebral end plates (8/22, 36%). Grade II shows irregular margins of the vertebral bodies with change of the signal intensity (4/22, 18%). Grade III = collapse of the intervertebral discs or the vertebral bodies ± vertebral subluxation (two cases, 9%), there was collapse of L3/4 vertebral bodies and vertebral subluxation spondylolithesis of L4 over L5 vertebrae with narrowed L4/L5 disc space.
Discussion
Since the switch in paradigm in treatment of RA from retarding the start of specific therapy to early aggressive and sometimes biological therapy, the development of the disease can now be effectively controlled or even be halted.17 In this context, it is of a particular importance to identify a sensitive diagnostic marker which permits early definitive diagnosis of RA.
Recently, a new diagnostic criteria for RA has been developed by the EULAR/ACR panel. Thist included for the first time—the anti-citrullinated peptide antibodies (ACPA) as serological marker for the diagnosis of RA. In spite of the improvement that has been achieved in the diagnosis of RA by including these antibodies in the diagnostic criteria yet, the low sensitivity of the test (40%–50%) in most published cohorts indicates that a negative anti-CCP test does not exclude the disease. The sensitivity of anti-CCP2 ranged from 25 to 58%.Very low sensitivity of anti-CCP2 in some ethnic groups has also been reported. Accordingly, ethnical variations should be considered in evaluating the value of any anti-citrullinated protein antibodies test currently available in the market.18–23
In the present study, (123) Caucasian patients with different rheumatic diseases, were enrolled in a prospective study. All were tested by the newly developed ELISA for the presence of anti-MCV: Out of 64 RA patients, 51 (79.6%) tested positive for anti-MCV (mean ± SD, 153.62 ± 240.4), as did 4/29 SLE patients (13.7%), 1/8 SpA patients (12.5%) and 2/6 (33%) undifferentiated connective tissue disease patients. None of the anti-phospholipid syndrome or gouty arthritis patients tested positive for the anti-MCV antibodies. The area under the curve was 0.893 at the 95% CI, conferring an odds ratio of 23.5 in diagnosing RA with a negative odds ratio of 0.21. Anti-MCV sensitivity and specificity were 79.6% and 96.6%, respectively, at cut-off value 20.5 U/L. In agreement with our results; Egerer et al24 who studied anti-MCV antibodies in 1151 RA patients, found that anti-MCV antibodies have the same specificity as anti-CCP antibodies but with much better sensitivity (82% versus 72%). Similarly, Mathsson et al25 and Gross et al17 concluded that the anti-MCV assay extends the diagnostic spectrum for RA with higher sensitivity and prognostic value concerning radiological progression than anti-CCP antibodies (71% versus 58%, respectively).
In this study, a positive anti-MCV test in a non-rheumatoid arthritis patients; did not absolutely contradict the specificity of the test, as most of these patients had rheumatoid-like inflammatory polyarthritis: Jaccaud’s arthropathy (JA) was present in the four SLE patients who tested positive for anti-MCV. Patients with undifferentiated connective tissue disease who were found to have positive anti-MCV tests fulfilled the ACR criteria for RA during follow-up. However, no explanation could be given for the positive anti-MCV test in one patient with ankylosing spondylitis. In agreement with our results, Galvao et al26 found that a positive anti-MCV test was found in 10.4% of SLE patients (five cases), all of which were found to have Jaccaud’s arthropathy (JA). By contrast, anti-CCP was found in six SLE patients, with no difference between those with or without JA (P > 0.05).
In the present study, anti-MCV positive RA patients were found to have a more aggressive disease course (P < 0.05) with a higher number of flares per year (4.15 vs. 2.3) and poor disease control (a non-significant improvement in DAS-28 in spite of good complaint on combined DMARD therapy) than anti-MCV negative patients. Additionally, they were found to have progressive radiological damage with significant progression in SEN scores over time than the anti-MCV negative patients (20.22 vs. 9.46), who were found to have a more benign disease course with significantly lower DAS-28 scores, and significant improvement in SJC, TJC and ESR with DMARD therapy. Significant positive correlations have been found between an anti-MCV titer withDAS-28, SEN scores, VAS and CRP, but not with ESR or RF titer.
Innala et al27 concluded that anti-MCV titer was significantly correlated with DAS-28, SJC and ESR. In a three-year follow-up study of 427 RA patients, Keskin et al28 found that patients with active RA were found to have higher anti-MCV titers than patients with inactive disease, while, the anti-CCP titer failed to show this correlation (a non-significant difference in anti-CCP titer between patients with active or inactive disease). Evidence from diagnostic case-control studies suggests that the anti-MCV test may be used as an alternative to the anti-CCP test.6
In our study, multivariate analysis for the radiological outcome at 24 months revealed that anti-MCV titer and CRP were the most implicated in radiological damage of both peripheral and axial joints. In concordance with our results, Syversen et al29 studied the correlation between the anti-MCV titer and radiological damage in a 10-year follow-up study on 238 RA patients; however, they studied the correlation only with peripheral joints, assessed by the modified sharp score of Van Der Hijde.30 They concluded that a positive anti-MCV titer increased the odds of radiographic progression by 7.3 (95% CI: 3.2–16.5) compared to odds of 5.7 for positive anti-CCP test. Anti-MCV positivity was associated with an average increase in total sharp score. Interestingly, they found that a positive anti-MCV test was associated with boney erosions rather than joint space narrowing (cartilage damage). However, positive anti-CCP was associated with joint space narrowing.
Few reports were found regarding the axial joint affection in RA, as most were concerned with the study of the peripheral joints. In the present study—as a novelty out of 22 RA patients who underwent MRI scans on the cervical and lumbar regions, 17 (77%) were found to have cervical joint damage, ranging from apophesyeal joints erosion and disc space narrowing to atlantoaxial subluxation. Involvement of the lumbo-sacral regions was significantly less frequently observed (8/22, 36%; P < 0.05); this involvement ranged from irregular margins of the vertebral bodies with changed signal intensity to collapsed intervertebral discs, disc space narrowing and lumbar vertebral subluxation.
In agreement with Sakai et al9 we have found that axial joint involvement in RA patients was significantly associated with aggressive peripheral joints erosive disease and high disease activity scores, but they didn’t test or correlate this with the anti-MCV titer as we did. Importantly, in our study, all RA patients with lumbo-sacral joint affection were found to have cervical joint involvement, but the reverse was not true.
Narvaez et al8 studied the correlation between cervical spine involvement by MRI and neurological manifestations in 41 RA patients with symptomatic cervical joint affection. They concluded that cervical spine MRI appears to be a valuable method to identify RA patients at risk of developing neurological dysfunctions. Kauppi et al31 evaluated the five-year incidence of cervical spine involvement in patients with early RA (n = 199),—in agreement with our findings- they concluded that higher RADAS-28 scores and poor health assessment questionnaire results were significantly associated with cervical spine involvement and atlantoaxial subluxation. They added that RA patients on single DMARD therapy and with poor disease control were found to be more liable to cervical joint affection than those on combined DMARDs therapy and tight disease control.
Conclusion and recommendations
Anti-MCV antibodies can be regarded as a promising diagnostic and prognostic marker in RA patients with high sensitivity and specificity. Anti-MCV testing would help in early definitive diagnosis of RA and may identify a subset of RA patients with aggressive early erosive disease with high disease activity scores and poor disease control. Testing for these antibodies early in the disease can guide the choice of initial therapy, reserving aggressive regimens for those with positive anti-MCV titers and high disease activity scores who are suspected to have an aggressive early erosive disease course, in attempts to improve RA disease control and outcome. The axial skeleton can be affected in RA patients, especially the cervical region. MRI scanning of the spine is a sensitive method for detecting early axial joints affection in RA patients.
Figure 1.
ROC (red line) assessing the validity of the anti-MCV test in diagnosing RA. The area under the curve was 0.893 at the 95% CI.
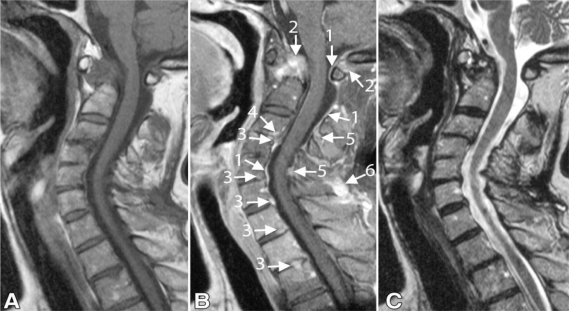
Figure 2.
Sagittal fast spin-echo MRI of the cervical spine in an RA patient (a 51-year-old woman who had suffered RA for 31 years) with superficial and deep enhancement. A) (T1-weighted) and C- (T2-weighted) images show; stenoses at levels C1–2 and C3–4, mostly caused by pannus and subluxation at level C1–2 and by discopathy and ligamentum flavum hypertrophy. B) (gadolinium-enhanced T1-weighted image) shows; superficial enhancement lining the cerebrospinal fluid (arrow 1) and enhancement involving deeper structures. Deep enhancing tissue is recognized as bone and pannus tissue on C1–2 (arrow 2), as a disc on anterior levels (arrow 3), level C3–4 (arrow 4). Ligamentum flavum and interspinal ligaments are enhanced at the posterior levels (arrow 5). Deep enhancement coincides mostly with narrowing of the spinal canal at these levels. Note enhancement of the ligamentum nuchae (arrow 6).
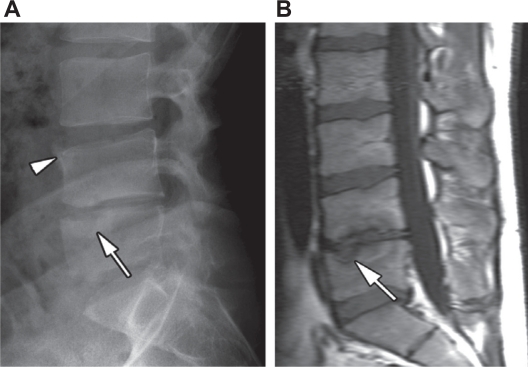
Figure 3.
A) Lateral radiograph of the lumbar spine in a male RA patient shows height reduction and narrowing of the intervertebral disk spaces, sclerosis of the endplates at L4–5 and erosion (arrow) of the superior endplate of L5. B) Sagittal T1-weighted fast spin-echo MRI reveals erosive defects of the inferior endplate (arrow) of L4 and superior endplate of L5, as well as signal loss in the surrounding bone marrow.
Table 1.
Methodology of the MRI scans on the cervical and lumbar spine.
|
Cervical spine: sagittal fast spin echo |
Lumbar spine: sagittal fast spin echo |
|||||
|---|---|---|---|---|---|---|
| T1 W1 | T2 W1 | Axial gradient echo | T1 W1 | T2 W1 | Axial T2 W1 | |
| RT | 550 | 3900 | 95 | 600 | 4000 | 4730 |
| ET | 12 | 127 | 47 | 30 | 127 | 117 |
| SL | 4 mm | 4.5 mm | 4.5 mm | 4 mm | 4 mm | 5 mm |
| FOV | 280 × 280 | 220 × 220 | 220 × 220 | 320 × 320 | 320 × 320 | 240 × 240 |
Abbreviations: RT, repetition time; ET, echo time; SL, slice thickness; FOV, field of view; Unit = matrix size.
Table 2.
Demographic, laboratory and clinical data of all RA patients (n = 64) at study baseline (two years ago).
| Mean ± SD | Range | |
|---|---|---|
| Age (years) | 41.58 ± 11.44 | (18–55) |
| Sex | (45♀, 19♂) | – |
| DD (years) | 5.58 ± 3.7 | (3.0–15) |
| ESR (1st h) | 43.27 ± 14.25 | (30–95) |
| CRP (mg%) | 25.62 ± 27.4 | (4.0–45) |
| DAS-28 | 4.18 ± 1.73 | (1.8–6.3) |
| VAS/100 mm | 51.25 ± 20.09 | (30–100) |
| SEN score | 17.31 ± 9.06 | (10–55) |
| RF titer (U/mL) | 106.02 ± 143.12 | (12–305) |
| *Anti-MCV titer (U/mL) | 114.45 ± 214.57 | (11–329) |
Abbreviations: DD, disease duration; VAS, visual analogue scale.
Anti-MCV testing was performed for all RA patients only at the study endpoint.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Bang H, Egerer K, Gauliard A, et al. Mutation and citrullination modifies vimentin to a novel autoantigen for rheumatoid arthritis. Arthritis Rheum. 2007;56(8):2503–11. doi: 10.1002/art.22817. [DOI] [PubMed] [Google Scholar]
- 2.Van Gaalen F, Ioan-Facsinay A, Huizinga TW, Toes RE. The devil in the details: the emerging role of anticitrulline autoimmunity in rheumatoid arthritis. J Immunol. 2005;175:5575–80. doi: 10.4049/jimmunol.175.9.5575. [DOI] [PubMed] [Google Scholar]
- 3.Greiner A, Plischke H, Kellner H, Gruber R. Association of anti-cyclic citrullinated peptide antibodies, anti-citrullin antibodies, and IgM and IgA rheumatoid factors with serological parameters of disease activity in rheumatoid arthritis. Ann NY Acad Sci. 2005;1050:295–303. doi: 10.1196/annals.1313.031. [DOI] [PubMed] [Google Scholar]
- 4.Levesque MC, Zhou Z, Moreland LW. Anti-cyclic citrullinated peptides testing for the diagnosis of rheumatoid arthritis and the quest for improved sensitivity and predictive value [editorial] Arthritis Rheum. 2009;60:2211–5. doi: 10.1002/art.24720. [DOI] [PubMed] [Google Scholar]
- 5.Vossenaar ER, Despres N, Lapointe E, et al. Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther. 2004;6:R142–50. doi: 10.1186/ar1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luime J, Colin E, Hazes J, Lubberts E. Does anti-mutated citrullinated vimentin have additional value as a serological marker in the diagnostic and prognostic investigations of patients with rheumatoid arthritis? A systemic review. Ann Rheum Dis. 2010;69:337–44. doi: 10.1136/ard.2008.103283. [DOI] [PubMed] [Google Scholar]
- 7.Hirohashi N, Sakai T, Sairyo K, et al. Lumbar radiculopathy caused by extradural rheumatoid nodules. Case report. J Neurosurg Spine. 2007;7(3):352–6. doi: 10.3171/SPI-07/09/352. [DOI] [PubMed] [Google Scholar]
- 8.Narvaez JA, Narvaez J, Serrallonga M, et al. Cervical spine involvement in rheumatoid arthritis: Correlation between neurological manifestations and magnetic resonance imaging findings. Rheumatology (Oxford) 2008;47(12):1814–9. doi: 10.1093/rheumatology/ken314. [DOI] [PubMed] [Google Scholar]
- 9.Sakai T, Sairyo K, Hamada D, et al. Radiological features of lumbar spinal lesions in patients with rheumatoid arthritis with special reference to the changes around intervertebral discs. The Spine Journal. 2008. pp. 605–11. [DOI] [PubMed]
- 10.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987, revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1987;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 11.Mitchel L.Zoler: New Rheumatoid Arthritis Criteria Released by ACR/EULAR Panel [Monday, October 26, 2009—Elsevier Global Medical News]. [Google Scholar]
- 12.Van Gestel AM, Haagsma CJ, van Riel PL. Validation of rheumatoid arthritis improvement criteria that include simplified joint counts. Arthritis Rheum. 1998;41:1845–50. doi: 10.1002/1529-0131(199810)41:10<1845::AID-ART17>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Van Gestel AM, Prevoo ML, van ’t Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European League Against Rheumatism (EULAR) response criteria for rheumatoid arthritis. Arthritis Rheum. 1996. pp. 34–40. [DOI] [PubMed]
- 14.Nardella FA, Dayer MJ, Roelke M, Krane MS, Mannik M. Self associating IgG rheumatoid factors stimulate monocytes to release prostaglandins and mononuclear cell factor that stimulates collagenase and prostaglandin production by synovial cells. Rheumatology International. 1983;3:183–6. doi: 10.1007/BF00541598. [DOI] [PubMed] [Google Scholar]
- 15.Dias EM, Lukas C, Landewe R, Fatenejad S, van der Heijde D. Reliability and sensitivity to change of the simple erosion narrowing score compared with the Sharp—van der Heijde method for scoring radiographs in rheumatoid arthritis. Ann Rheum Dis. 2007. pp. 375–9. [DOI] [PubMed]
- 16.Hassey A, Gerrett D, Wilson A, David JR. A survey of validity and utility of electronic patient records in a general practice. 322. BMJ; 2001. pp. 1401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross WL, Moosig F, Lamprecht P. Anticitrullinated protein/peptide antibodies in rheumatoid arthritis. Dtsch Arztebl Int. 2009;106(10):157–8. doi: 10.3238/arztebl.2009.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schellekens GA, Visser H, de Jong BA, et al. The diagnostic properties of rheumatoid arthritis antibodies recognizing a cyclic citrullinated peptide. Arthritis Rheum. 2000;43:155–63. doi: 10.1002/1529-0131(200001)43:1<155::AID-ANR20>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Jansen AL, van der Horst-Bruinsma I, van Schaardenburg D, van de Stadt RJ, de Koning MH, Dijkmans BA. Rheumatoid factor and antibodies to cyclic citrullinated peptide differentiate rheumatoid arthritis from undifferentiated polyarthritis in patients with early arthritis. J Rheumatol. 2002;29:2074–6. [PubMed] [Google Scholar]
- 20.Saraux A, Berthelot JM, Devauchelle V, et al. Value of antibodies to citrulline-containing peptides for diagnosing early rheumatoid arthritis. J Rheumatol. 2003;30:2535–9. [PubMed] [Google Scholar]
- 21.Van Gaalen FA, Linn-Rasker SP, van Venrooij WJ, et al. Autoantibodies to cyclic citrullinated peptides predict progression to rheumatoid arthritis in patients with undifferentiated arthritis: A prospective cohort study. Arthritis Rheum. 2004;50:709–15. doi: 10.1002/art.20044. [DOI] [PubMed] [Google Scholar]
- 22.Vittecoq O, Incaurgarat B, Jouen-Beades F, et al. Autoantibodies recognizing citrullinated rat filaggrin in an ELISA using citrullinated and non-citrullinated recombinant proteins as antigens are highly diagnostic for rheumatoid arthritis. Clin Exp Immunol. 2004;135:173–80. doi: 10.1111/j.1365-2249.2004.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niewold TB, Harrison MJ, Paget SA. Anti-CCP antibody testing as a diagnostic and prognostic tool in rheumatoid arthritis. QJM. 2007;100(4):193–201. doi: 10.1093/qjmed/hcm015. [DOI] [PubMed] [Google Scholar]
- 24.Egerer K, Feist E, Burmester GR. The serological diagnosis of rheumatoid arthritis: Antibodies to citrullinated antigens. Dtsch Arztebl Int. 2009;106(10):159–63. doi: 10.3238/arztebl.2009.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathsson L, Mullazehi M, Wick MC, et al. Antibodies against citrullinated vimentin in rheumatoid arthritis: Higher sensitivity and extended prognostic value concerning future radiographic progression as compared with antibodies against cyclic citrullinated peptides. Arthritis Rheum. 2008;58:36–45. doi: 10.1002/art.23188. [DOI] [PubMed] [Google Scholar]
- 26.Galvao V, Atta A, Sousa M, et al. Profile of antibodies in Jaccaud’s arthropathy. Joint Bone Spine. 2009;76(4):356–60. doi: 10.1016/j.jbspin.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Innala L, Kokkonen H, Eriksson C, Jidell E, Berglin E, Dahlqvst SR. Antibodies against mutated citrullinated vimentin are a better predictor of disease activity at 24 months in early rheumatoid arthritis than antibodies against cyclic citrullinated peptides. J Rheumatol. 2008;35:1002–8. [PubMed] [Google Scholar]
- 28.Keskin G, Inal A, Keskin D, et al. Diagnostic utility of anti-cyclic citrullinated peptide and anti-modified citrullinated vimentin antibodies in rheumatoid arthritis. Protein Pept Lett. 2008;15(3):314–7. doi: 10.2174/092986608783744153. [DOI] [PubMed] [Google Scholar]
- 29.Syversen SW, Goll GL, van der Heijde D, et al. Prediction of radiographic progression in rheumatoid arthritis and the role of antibodies against mutated citrullinated vimentin: Results from a 10-year prospective study. Ann Rheum Dis. 2010;69:345–51. doi: 10.1136/ard.2009.113092. [DOI] [PubMed] [Google Scholar]
- 30.Van der Heijde D, Dankert T, Nieman F, Rau R, Boers M. Reliability and sensitivity to change of a simplification of the sharp/van der heijde radiological assessment in rheumatoid arthritis. Rheumatology (Oxford) 1999;38:941–7. doi: 10.1093/rheumatology/38.10.941. [DOI] [PubMed] [Google Scholar]
- 31.Kauppi MJ, Neva MH, Laiho K, et al. Rheumatoid atlantoaxial subluxation can be prevented by intensive use of traditional disease modifying antirheumatic drugs. J Rheumatol. 2009;36:273–8. doi: 10.3899/jrheum.080429. [DOI] [PubMed] [Google Scholar]