Abstract
Objective:
To detect the early preclinical alterations in cardiac autonomic control as well as altered cardiac function in systemic sclerosis (SSc) patients and their relevance to the clinical features of the disease using noninvasive methods.
Methods:
30 SSc patients and 15 healthy controls matched for age and sex underwent clinical examination, serological analysis, and echocardiographic assessment including Doppler flow imaging to evaluate cardiac function, and 24-hour Holter monitoring analyzed for arrhythmia and heart rate variability (HRV) in the time and frequency domains.
Results:
The trans-mitral Doppler of early to atrial wave (E/A) ratio was reversed in five patients (16.6%) and the tricuspid E/A ratio was reversed in 10 patients (33.3%). Holter analysis for SSc patients revealed an increased prevalence of premature ventricular contractions (PVC) ≥ 10/h (P = 0.02), supra-ventricular tachycardias (SVTs) (P = 0.2), and total PVC count (P = 0.0000). Highly significant (P = 0.000) impairment in all HRV parameters was demonstrated in the SSc patients. Total skin thickness score (TSS), Raynaud’s phenomenon and anti-scleroderma 70 (anti-SCL70) showed significant positive correlations with all arrhythmia parameters, while showing a significant negative correlation with the impaired ventricular diastolic function and various HRV parameters. No correlation was found between arrhythmia and HRV parameters and disease duration, disease type, or presence of anti-centromere antibodies.
Conclusion:
Low heart rate variability, increased TSS and the presence of anti-SCL70 are correlated with preclinical cardiac involvement in SSc patients and may predict the likelihood of malignant arrhythmia and sudden cardiac death. Therefore, noninvasive HRV evaluation before clinical cardiac involvement in these patients might be beneficial when added to the clinical and laboratory assessments in detecting high-risk patients, and may allow for implementation of preventive measures and initiation of appropriate therapy early in the course of the disease.
Keywords: autonomic dysfunction, heart rate variability, systemic sclerosis
Introduction
Systemic sclerosis (SSc), a multi-systemic disorder of connective tissue, is characterized by widespread vascular abnormalities and fibrosis of the skin and visceral organs.1,2 Two major SSc subtypes are known, and these are defined according to the extent of skin involvement: limited cutaneous SSc (LcSSc) and diffuse cutaneous SSc (DcSSc). Numerous autoantibodies can be detected in the sera of SSc patients. Three of them are specific for SSc: anti-centromere antibody (ACA) in LcSSc, anti-scleroderma 70 (anti-SCL70) in DcSSc and anti RNA polymerase III in diffuse SSc with renal involvement.3
Cardiac involvement is one of the most frequent visceral complications that can affect the overall prognosis of the disease.4 More interestingly, heart involvement can occur independently from other typical complications of SSc,5 and it can be clinically occult.2
Clinically evident cardiac involvement is recognized as a poor prognostic indicator. Cardio-myopathy with ventricular diastolic dysfunction and rhythmic disturbances are the most important forms, since they are associated with a very poor prognosis.6 The disturbed rhythms may be life threatening, with sudden cardiac death reported in some cases of SSc.7
Increasing evidence strongly suggests that cardiac involvement is related to recurrent focal ischemic injury causing irreversible myocardial fibrosis.8 The underlying mechanism appears to be micro-circulatory impairment with abnormal vaso-reactivity, the so-called myocardial Raynaud’s phenomenon, which is caused by abnormal autonomic nervous control of the heart.9
Autonomic dysfunction is extremely common in SSc, starting early in the disease process and might precede the development of fibrosis.10 There is a clinical and experimental evidence of a link between the propensity for life threatening arrhythmias and sympatho-vagal imbalance.9
The diagnosis of altered cardiac function may sometimes be late or difficult because of the discrepancies between the clinical manifestations and actual cardiac involvement. For this reason, early detection is valuable for optimal treatment and for implementation of preventive measures in the early stages of the disease.9 The current challenge is to detect the early preclinical cardiac function alterations and to identify SSc patients at risk of arrhythmic complications using simple, noninvasive diagnostic procedures.
Heart rate variability (HRV) measurements are easy to perform and noninvasive, and have good reproducibility if used under standardized conditions.11,12
Changes in heart rate, and subsequently HRV, may be measured by a number of techniques. Since changes in heart rate and HRV are autonomically mediated, these measurements will reflect autonomic nervous control of the heart. In addition, it has been shown that information about the general health of the heart, including the likelihood of malignant ventricular arrhythmias and prognostic information about survival with various cardiac diseases, may be gleaned from an analysis of HRV.13–15
Since a low HRV is associated with an increased risk for arrhythmic complications, including malignant ventricular arrhythmias and sudden death, assessment of patients with SSc for decreased HRV using an ambulatory Holter echocardiogram (ECG) recording might establish a method of detecting altered autonomic control of the heart and predicting the resultant adverse outcome.15
Echocardiography assessing the left and right ventricular systolic and diastolic functions of the heart is a valuable, safe, noninvasive and reproducible method for early detection of preclinical changes in cardiac function in SSc patients.16
The aim of the present study was to detect early preclinical changes in the cardiac autonomic control as well as altered cardiac functions in SSc patients, and their relevance to the clinical features of the disease using noninvasive methods, with the goal of possibly implementing preventive measures and initiating therapy early in the course of the disease.
Subjects and Methods
The case control study included 30 SSc patients fulfilling the American College of Rheumatology classification criteria for SSc.17 They were recruited from the Internal Medicine and Rheumatology Departments and the Rheumatology Outpatients clinic, Ain Shams University Hospital. They were recruited according to the following inclusion criteria: Age: ≤60 years, absence of heart failure on clinical examination, normal cardiac silhouette on chest radiography, and normal left ventricular systolic function on echocardiogram.
We excluded patients with diabetes mellitus or other systemic diseases that could possibly cause autonomic dysfunction, myositis, arterial hypertension and/or renal involvement, atrial fibrillation, atrial flutter or any other ectopic atrial rhythms that precluded HRV analysis, sick sinus syndrome, high grade atrioventricular (AV) block or pacemaker rhythm, valvular heart disease and Holter recordings lasting less than 16 hours or with technical deficiencies resulting in unreliable analysis.
Fifteen healthy subjects matched for age and sex without known systemic, immunological or cardiovascular diseases and without any evidence or cause of autonomic neuropathies, including idiopathic Raynaud’s phenomenon, served as a control group.
Informed consent was obtained from each subject. The study was approved by the Ain Shams Medical Ethics Committee.
SSc patients were classified into two subtypes according to the criteria proposed by LeRoy et al19 into limited cutaneous SSc (LcSSc) and diffuse cutaneous SSc (DcSSc) groups.
All patients were subjected to the following:
Full history taking with special emphasis on disease onset, course and duration; symptoms of cardiac rhythm disturbances (e.g. palpitations, dizziness and syncope); symptoms of left ventricular diastolic dysfunctions (exertional dyspnea and dyspnea at rest)
A thorough clinical examination with special emphasis on the extent and degree of skin involvement using a modified Rodnan skin score18 where cutaneous thickness was assessed using a 0–3 point scale in 17 body surface areas (face, anterior chest, abdomen, and separate right and left upper arms, forearms, hands, thighs, fingers, legs and feet) with a maximum score of 51 points. The rating scale consisted of 0 = normal, 1 = thickened, 2 = thickened and unable to pinch, and 3 = thickened and unable to move. The examination also checked the presence of telangiectasis, cutaneous calcinosis, cutaneous ulcerations, Raynaud’s phenomenon, arthralgia or arthritis, and a chest examination to detect interstitial pulmonary fibrosis
Barium meal to detect esophageal dysmotility
Chest radiography to exclude patients with an abnormal cardiac silhouette on chest radiography and to detect the presence of basal fibrosis in cases of interstitial pulmonary fibrosis
12-lead surface electrocardiogram to exclude patients with atrial fibrillation, atrial flutter, ectopic atrial rhythm, sick sinus syndrome, high-grade atrioventricular (AV) block or pacemaker rhythm.
- Trans-thoracic echocardiography: M-mode, 2D and color Doppler flow imaging with pulsed and continuous wave spectral analysis of trans-valvular flow were performed on all patients to assess:
- Segmental wall motion abnormalities,
- Left ventricular systolic function by M-mode using fractional shortening (FS) as an ejection phase index,20
- Transvalvular Doppler flow analysis was used to assess left and right ventricular diastolic function, where inversion of the early to atrial wave (E/A) wave ratio (E/A ratio < 1) of the trans-mitral flow was considered as an index of left ventricular diastolic dysfunction,20 and inversion of the E/A wave ratio of the trans-tricuspid flow was considered as an index of right ventricular diastolic dysfunction.1,21
24 hour ambulatory Holter monitoring: All patients underwent 24 hours Holter monitoring using either two- or three-channel real-time tape recorders (HILLMED Holter Premier IV recorder, HILLMED Corporation, Miami, USA) and monitoring of the bipolar leads CM5 and CM3 and/or modified augmented vector foot (aVF).
Holter tapes were analyzed using the Holter Premier IV device (HILLMED). The total number of ventricular premature beats, presence of premature ventricular contractions (PVCs) ≥ 10/hour and episodes of non-sustained ventricular tachycardia (defined as ≥3 consecutive ventricular premature beats with a rate ≥100 beats/minute) were obtained for each patient. This monitoring was also carried out for the control group to determine the normal values of various HRV in the normal population.
Heart rate variability analysis
The 24-hour HRV was assessed in both time domain and frequency domain after full revision of the electrocardiogram and editing of beats when indicated.
Time domain HRV variables included:
Mean RR interval (the interval between two adjacent QRS complexes).
Standard deviation of all normal RR intervals (SDNN).
Standard deviation of mean normal RR intervals for all five-minute segments (SDANN).
Mean of the standard deviation of all normal RR intervals for all five-minute segments (the SDNN index or SDNNi).
Root mean square of successive differences = the square root of the mean of the summation of squares of differences between adjacent normal RR intervals (rMSSD).
Percentage of differences between adjacent normal RR intervals greater than 50 ms (pNN50).22,23
Most time domain parameters reflect the overall autonomic modulation of the heart but provide no information regarding sympathetic and parasympathetic activity individually,24 except for pNN50 and rMSSD,12,22 which have been reported to reflect mostly parasympathetic activity.
Frequency domain
HRV was assessed by the auto regression parametric method with a spectral resolution of 0.0005 Hz using the Premiere IV HILLMED Holter analysis package. The amplitudes of the following variables were obtained:
Total power (TP) = variance of all RR intervals (0–0.500 Hz),
Very low frequency (VLF) = power in the VLF range (0.003–0.04 Hz),
Low frequency (LF) = power in the low frequency range (0.04–0.15 Hz),
High frequency (HF) = power in HF range (0.15–0.4 Hz),
Spectral analysis of HRV can partially separate parasympathetic from sympathetic effects on the heart.24
HF power represents a pure vagal efferent signal that is modulated by ventilation (respiratory sinus arrhythmia).25,26 VLF and LF variables are thought to be related to the sympatho-vagal baroreflex control of arterial pressure and are currently interpreted as an index of sympathetic activity; the LF/HF ratio can provide information on the state of the sympatho-vagal balance.27 High values for this ratio suggest a predominance of sympathetic nervous activity relative to vagal activity.26
Laboratory studies
Six milliliters of venous blood were collected from each subject. Two milliliters were combined with EDTA for a complete blood count (CBC) using the Coulter counter T660, and the erythrocyte sedimentation rate (ESR) following the Westergren method. Serum was collected from the other 4 ml in a plane tube and stored at −70 °C for subsequent assay of the fasting plasma glucose level and kidney function tests (serum creatinine and blood urea nitrogen) using the CX5 system (Beckman instruments Inc, Minnesota, USA), antinuclear antibodies (ANA) were detected by indirect immunoflorescence using the Kallestad kit (Bio-Rad Laboratories, South Africa), anti-centromere antibodies (ACA) were detected using the ELISA technique (anticentromere B, ORGENTEC, Diagnostika GmbH, Mainz, Germany), and serum anti-topoisomerase-1 (anti-SCL70) antibodies were detected using the ELISA technique (QUANTA lite, Inova Diagnostics, San Diego, USA).
Statistical analysis
Statistical analysis of the data was performed on an IBM computer using SPSS (Version 12). The mean and standard deviation (SD) were used to describe quantitative variables. Unpaired t-tests were used to compare quantitative variables between both groups while the Mann—Whitney—Willcoxon u-test was used instead of the t-test in non-parametric data (SD > 50% mean). Spearman’s correlation test was used to rank different variables against each others positively or inversely. A P-value less than 0.05 was accepted as being statistically significant.28
Results
This case control study was carried out on 30 SSc patients and 15 controls.
The control group were not diabetic (normal fasting plasma glucose levels), having neither cause nor evidence of autonomic neuropathies, and having no organic or structural heart disease. Their demographic, clinical, laboratory and Holter data are shown in Table 1.
Table 1.
Demographic, laboratory and Holter data of control group.
| Parameter | Range | Mean | SD |
|---|---|---|---|
| Age (years) | 28–46 | 36.1 | 7.8 |
| FBS (mg/dL) | 65–99 | 82.5 | 10.1 |
| Serum creatinine (mg/dL) | 0.6–1.2 | 0.9 | 0.18 |
| Total PVCs (beats per minute) | 0.0–39 | 12.0 | 11.4 |
| Mean RR (ms) | 660–1133 | 812.8 | 126.7 |
| SDN’N (ms) | 115–191 | 147.1 | 18.8 |
| SDANN (ms) | 107–183 | 136.3 | 18.8 |
| SDNNi (ms) | 36–75 | 61.2 | 12.0 |
| rMSSD (ms) | 20–90 | 39.1 | 17.9 |
| pNN50 (%) | 2–27 | 10.3 | 7.9 |
| TP (ms2) | 979–5132 | 3150 | 1379 |
| VLF (ms2) | 615–3745 | 2171 | 985 |
| LF (ms2) | 236–1348 | 740 | 363 |
| HF (ms2) | 92–464 | 210 | 98 |
| LF/HF | 2.0–7.1 | 3.5 | 1.5 |
Abbreviations: FBS, fasting blood sugar; PVC, premature ventricular contractions; SDNN, standard deviation of all normal RR intervals in the entire 24-hr ECG recording; SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24-hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals (the interval between two successive normal waves); VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF (ms2).
The 24-hour ambulatory Holter monitoring was recorded and analyzed. None of the control group had PVC ≥ 10/h, none had SVT and total PVCs counts were measured for all. Holter data were analyzed for HRV parameters in both the time and frequency domains (Table 1).
The SSc patients consisted of 25 females (83%) and 5 males (17%). Their demographic clinical, laboratory and Holter data re shown in Table 2.
Table 2.
Demographic, clinical, laboratory and Holter data of systemic sclerosis patients.
| Parameter | Range | Mean | SD |
|---|---|---|---|
| Age (years) | 25–50 | 36.8 | 6.5 |
| Disease duration (years) | 1–7 | 4.4 | 1.8 |
| TSS (points) | 3–35 | 14.1 | 7.2 |
| FBS (mg/dL) | 59–98 | 76.8 | 8.9 |
| Serum creatinine (mg/dL) | 0.6–1.3 | 0.9 | 0.26 |
| Left ventricular FS (%) | 26–38 | 32.1 | 4.3 |
| Total PVCs (beats per minute) | 0–14468 | 798.7 | 2654 |
| Mean RR (ms) | 527–1018 | 685.5 | 113.5 |
| SDNN (ms) | 19–69 | 41.7 | 11.3 |
| SDANN (ms) | 6–58 | 33.3 | 11.3 |
| SDNNi (ms) | 10–41 | 22.3 | 7 |
| rMSSD (ms) | 9–44 | 21.2 | 8.3 |
| pNN50 (%) | 0–30 | 4.7 | 5.7 |
| TP (ms2) | 86–1859 | 502.7 | 383.5 |
| VLF (ms2) | 16–1123 | 252.9 | 235.8 |
| LF (ms2) | 10–483 | 124.9 | 95.6 |
| HF (ms2) | 6.7–280 | 87.6 | 62.1 |
| LF/HF | 0.87–2.2 | 1.4 | 0.3 |
Abbreviations: TSS, total skin thickness score; FBS, fasting blood sugar; FS, fractional shortening; PVCs, premature ventricular contractions; SDNN, standard deviation of all normal RR intervals in the entire 24-hr ECG recording; SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24-hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals; VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF(ms2).
Diffuse SSc (DcSSc) was diagnosed in 16 patients (53%). Raynaud’s phenomenon was present in 24 patients (80%), 4 patients (13%) had calcinosis, 6 patients (20%) had telangictasis, 5 patients (17%) had arthralgia, 12 patients (40%) had digital ischemic changes and 15 patients (50%) had sclerodactly. Esophageal dysmotility was found in 13 patients (43%) and basal pulmonary fibrosis detected by chest radiography was detected in seven patients (23%).
ANA was positive in 28 patients (93%), six patients (20%) had positive ACA, and in 15 patients (50%), the anti-SCL70 titer was positive. None of the patients was diabetic (i.e. they had normal fasting plasma glucose levels) and had no renal involvement in any with normal serum creatinine (Table 2).
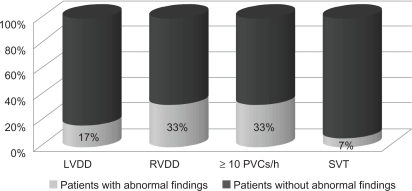
Transthoracic echocardiography: all patients showed normal systolic function of both left and right ventricles with no segmental wall motion abnormalities at rest. The mean overall left ventricular FS was 32.1 ± 4.3%. Five patients (17%) had left ventricular diastolic dysfunction (relaxation type) with a reversed E/A ratio of trans-mitral color flow Doppler waves, while 10 patients (33%) had right ventricular diastolic dysfunction (relaxation type) with reversed E/A ratio of trans-tricuspid color flow Doppler waves (Fig. 1).
Figure 1.
Echo and ECG Holter data of systemic sclerosis patients.
Abbreviations: LVDD, left ventricular diastolic diameter; RVDD, right ventricular diastolic diameter.
24 hour ambulatory Holter monitoring: 10 patients (33%) had ≥10 PVCs/h, and SVT was present in 2 patients (7%) (Fig. 1). Total PVC count and HRV parameters are shown in Table 2.
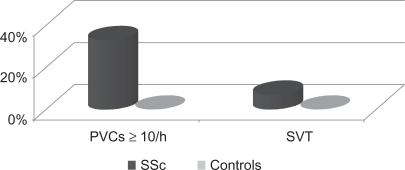
On comparing the results of Holter monitoring of arrhythmia and HRV parameters in patients with SSc and the control group, we found a significant increase in arrhythmia (PVCs ≥ l0/h; total PVC count and presence of SVT). Within the SSc patients, 33% had ≥10 PVCs/h and 7% had SVT compared to none in control group (P = 0.02 and 0.2 respectively) (Fig. 2). The total PVC count increased from (12.0 ± 11.4 beats per minute) in the control group to (798.75 ± 2654.2 beats per minute) (P = 0.0001) in the SSc patients.
Figure 2.
Arrhythmia in systemic sclerosis patients and controls.
The parameters of the time and frequency domains of HRV were significantly decreased (with the exception of LF/HF which was significantly increased) in SSc patients compared to the controls (Table 3).
Table 3.
Changes in the time and frequency domains of HRV in SSc patients and controls.
| Parameter |
SSc patients |
Controls |
P | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| SDANN (ms) | 99.3 | 11.3 | 136.3* | 18.8 | 0.0003 |
| SDNNi (ms) | 42.3 | 7 | 61.2* | 12 | 0.0001 |
| rMSSD (ms) | 12.2 | 8.3 | 35.1** | 17.9 | 0.0003 |
| pNN50 (%) | 4.7 | 6 | 10.3** | 7.9 | 0.0002 |
| TP (ms2) | 512.8 | 383.5 | 2640.6** | 1379 | 0.0001 |
| VLF (ms2) | 322.8 | 235.8 | 2171.1** | 984.9 | 0.0001 |
| LF (ms2) | 124.9 | 95.6 | 240** | 163 | 0.0004 |
| HF (ms2) | 57.6 | 62.1 | 210.5** | 97.9 | 0.0002 |
| LF/HF | 2.2 | 1.5 | 1.1** | 0.03 | 0.0001 |
Abbreviations: SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24 hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals; VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF (ms2).
t-test,
Mann—Whitney—Willcoxon u-test.
No fixed correlation was found between the various HRV parameters or arrhythmia and disease duration, disease subtypes (limited or diffuse SSc) or presence of ACA (Table 4).
Table 4.
Correlation between various HRV parameters and disease duration, disease subtypes and presence of ACA.
| Parameter |
SSc duration |
DcSSc |
LcSSc |
ACA |
||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | R | P | |
| Mean RR (ms) | −0.34 | 0.010 | −0.35 | 0.008 | −0.25 | 0.19 | −0.29 | 0.03 |
| SDNN (ms) | −0.08 | 0.560 | −0.20 | 0.14 | −0.06 | 0.76 | −0.15 | 0.26 |
| SDANN (ms) | −0.15 | 0.26 | −0.20 | 0.14 | −0.21 | 0.27 | −0.14 | 0.30 |
| SDNNi (ms) | 0.004 | 0.97 | −0.15 | 0.26 | 0.24 | 0.21 | −0.02 | 0.92 |
| rMSSD (ms) | 0.11 | 0.42 | 0.07 | 0.58 | 0.13 | 0.39 | 0.01 | 0.95 |
| pNN50 (%) | 0.16 | 0.25 | 0.13 | 0.35 | 0.11 | 0.55 | 0.09 | 0.60 |
| TP (ms2) | −0.10 | 0.47 | −0.26 | 0.05 | 0.09 | 0.62 | 0.05 | 0.77 |
| VLF (ms2) | −0.06 | 0.75 | −0.21 | 0.11 | 0.17 | 0.38 | 0.08 | 0.65 |
| LF (ms2) | −0.11 | 0.41 | −0.26 | 0.05 | 0.02 | 0.90 | −0.28 | 0.04 |
| HF (ms2) | −0.13 | 0.34 | −0.16 | 0.23 | 0.05 | 0.78 | −0.07 | 0.59 |
| LF/HF | 0.08 | 0.57 | −0.31 | 0.02 | −0.08 | 0.66 | 0.009 | 0.99 |
| PVCs ≥ 10/h | −0.29 | 0.03 | 0.004 | 0.97 | 0.14 | 0.30 | 0.07 | 0.58 |
| Total PVCs | 0.09 | 0.60 | −0.15 | 0.26 | −0.21 | 0.11 | −0.11 | 0.42 |
| SVT | −0.06 | 0.76 | 0.29 | 0.03 | −0.08 | 0.560 | 0.13 | 0.35 |
Abbreviations: SSc, systemic sclerosis; DcSSc, diffuse cutaneous SSc; LcSSc, limited cutaneous SSc; ACA, anti-centromere antibody; SDNN, standard deviation of all normal RR intervals in the entire 24-hr ECG recording; SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24 hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals; VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF (ms2); PVC, premature ventricular contractions; SVT, supravetricular tachycardia.
A significant negative correlation was found between various HRV parameters (with the exception of the LF/HF ratio, which showed a positive correlation) and TSS, presence of Raynaud’s phenomenon and detection of anti-SCL70. On the other hand, a significant positive correlation was found between various arrhythmia parameters and TSS, presence of Raynaud’s phenomenon and detection of anti-SCL70 (Table 5).
Table 5.
Correlation between HRV parameters and arrhythmia, and TSS, Raynaud’s phenomenon and presence of anti-SCL70 in SSc patients.
| Parameter |
TSS |
Raynaud’s phenomenon |
Anti-SCL70 |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Mean RR (ms) | −0.29 | 0.030 | −0.35 | 0.008 | −0.31 | 0.022 |
| SDNN (ms) | −0.30 | 0.025 | −0.65 | 0.0003 | −0.35 | 0.008 |
| SDANN (ms) | −0.81 | 0.0002 | −0.33 | 0.020 | −0.29 | 0.030 |
| SDNNi (ms) | −0.65 | 0.0003 | −0.68 | 0.0003 | −0.68 | 0.0003 |
| rMSSD (ms) | −0.69 | 0.0003 | −0.53 | 0.0004 | −0.49 | 0.0005 |
| pNN50 (%) | −0.49 | 0.0005 | −0.39 | 0.005 | −0.45 | 0.001 |
| TP (ms2) | −0.58 | 0.0004 | −0.62 | 0.0003 | −0.60 | 0.0003 |
| VLF (ms2) | −0.48 | 0.0005 | −0.51 | 0.0004 | −0.49 | 0.0005 |
| LF (ms2) | −0.34 | 0.009 | −0.31 | 0.025 | −0.27 | 0.040 |
| HF (ms2)″ | −0.49 | 0.0005 | −0.52 | 0.0004 | −0.53 | 0.0004 |
| LF/HF | 0.36 | 0.007 | 0.42 | 0.003 | 0.52 | 0.0004 |
| PVCs ≥ 10/h | 0.53 | 0.0004 | 0.49 | 0.0005 | 0.57 | 0.0004 |
| Total PVCs | 0.68 | 0.0003 | 0.39 | 0.005 | 0.78 | 0.0002 |
| SVT | 0.31 | 0.022 | 0.48 | 0.0005 | 0.33 | 0.020 |
Abbreviations: TSS, total skin thickness score; anti-SCL70, serum anti-topoisomerase-1; SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24 hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals; VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF (ms2); PVC, premature ventricular contractions; SVT, supravetricular tachycardia.
A significant negative correlation was found between various HRV parameters (with the exception of the LF/HF ratio, which showed a positive correlation) and various arrhythmia parameters (Table 6).
Table 6.
Correlation between HRV parameters and arrhythmia parameters in systemic sclerosis patients.
| Parameter |
PVCS ≥ 10/h |
Total PVCs |
SVT |
|||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| Mean RR (ms) | −0.36 | 0.007 | −0.49 | 0.0005 | −0.42 | 0.003 |
| SDNN (ms) | −0.28 | 0.033 | −0.37 | 0.007 | −0.29 | 0.030 |
| SDANN (ms) | −0.40 | 0.005 | −0.39 | 0.005 | −0.33 | 0.010 |
| SDNNi (ms) | −0.31 | 0.025 | −0.50 | 0.0004 | −0.39 | 0.005 |
| rMSSD (ms) | −0.52 | 0.0004 | −0.60 | 0.0003 | −0.47 | 0.0005 |
| pNN50 (%) | −0.61 | 0.0003 | −0.55 | 0.0004 | −0.49 | 0.0005 |
| TP (ms2)″ | −0.66 | 0.0003 | −0.70 | 0.0002 | −0.70 | 0.0002 |
| VLF (ms2) | −0.38 | 0.006 | −0.29 | 0.030 | −0.28 | 0.033 |
| LF (ms2) | −0.26 | 0.050 | −0.36 | 0.008 | −0.40 | 0.005 |
| HF (ms2) | −0.88 | 0.0002 | −0.51 | 0.0004 | −0.55 | 0.0004 |
| LF/HF | 0.54 | 0.0004 | 0.72 | 0.0002 | 0.81 | 0.0002 |
Abbreviations: PVC, premature ventricular contractions; SVT, supravetricular tachycardia, SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24 hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals; VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF (ms2).
A significant positive correlation was found between the presence of left and/or right ventricular diastolic dysfunction and both TSS and anti-SCL70. A significant negative correlation was found between the presence of left and/or right ventricular diastolic dysfunction and various HRV parameters (with the exception of the LF/HF ratio, which showed positive correlation). A non-significant correlation was found between the presence of left and/or right ventricular diastolic dysfunction and detection of ACA (Table 7).
Table 7.
Correlation between the presence of ventricular diastolic dysfunction and TSS, the presence of anti-SCL70 and ACA in SSc patients.
| Parameter |
LVDD |
RVDD |
||
|---|---|---|---|---|
| r | P | r | P | |
| TSS | 0.29 | 0.030 | 0.63 | 0.0003 |
| +ve anti-SCL70 | 0.31 | 0.022 | 0.57 | 0.0004 |
| +ve ACA | 0.19 | 0.390 | 0.21 | 0.065 |
| Mean RR (ms) | −0.30 | 0.025 | −0.36 | 0.007 |
| SDNN (ms) | −0.35 | 0.008 | −0.29 | 0.030 |
| SDANN (ms) | −0.28 | 0.033 | −0.40 | 0.005 |
| SDNNi (ms) | −0.26 | 0.050 | −0.34 | 0.010 |
| rMSSD (ms) | −0.53 | 0.0004 | −0.68 | 0.0003 |
| pNN50 (%) | −0.49 | 0.0005 | −0.78 | 0.0002 |
| TP (ms2) | −0.66 | 0.0003 | −0.82 | 0.0002 |
| VLF (ms2) | −0.33 | 0.020 | −0.35 | 0.009 |
| LF (ms2) | −0.28 | 0.033 | −0.26 | 0.050 |
| HF (ms2) | −0.55 | 0.0004 | −0.79 | 0.0002 |
| LF/HF | 0.51 | 0.0004 | 0.85 | 0.0002 |
Abbreviations: TSS, total skin thickness score; anti SCL70, serum anti-topoisomerase-1; ACA, anti-centromere antibody, SDANN, standard deviation of the mean of normal RR intervals for each 5-min period of the 24 hr ECG recording; SDNNi, mean of the standard deviation of all normal RR intervals for all 5-min segments of a 24-hr ECG recording; rMSSD, root mean square successive difference between adjacent normal RR intervals over the entire 24-hr ECG recording; pNN50, percent of difference between adjacent normal RR intervals greater than 50 ms calculated over the entire 24-hr ECG recording; TP, variance of all NN intervals; VLF, power in the very low frequency range; LF, power in the low frequency range; HF, power in the high frequency range; LF/HF, ratio of LF (ms2) to HF (ms2); LVDD, left ventricular diastolic dysfunction; RVDD, right ventricular diastolic dysfunction.
Discussion
Systemic sclerosis is a multi-systemic connective tissue autoimmune disorder. It is characterized by widespread vascular abnormalities and fibrosis. Altered cardiac functions and rhythm disturbances are considered important prognostic indicators, since they are associated with very poor prognosis.29
The underlying mechanism for cardiac involvement appears to be micro-circulatory impairment with abnormal vaso-reactivity, the so-called myocardial Raynaud’s phenomenon, which is caused by abnormal autonomic nervous control of the heart; the resultant dysrhythmia is considered one of the hallmarks of sclerodermal heart involvement.9,30
Autonomic dysfunction is extremely common in SSc, starting early in the disease and possibly preceding the development of fibrosis.10
Compelling evidence links sudden cardiac death and life threatening ventricular arrhythmia to autonomic nervous system dysfunction. Increased sympathetic activity appears to be pro-arrhythmic, whereas β-blocker therapy and enhanced parasympathetic tone counteract this arrhythmogenic effect. Abnormalities in the baseline parasympathetic tone (represented by reduced HRV) and in the ability to reflexively activate vagal tone (Baroreflex sensitivity) leave patients at a high risk for developing ventricular tachycardia and sudden cardiac death.9,31
The noninvasive evaluation of HRV using 24-hour ambulatory Holter ECG recording as a reflection of the autonomic control of the cardiovascular system might establish a reliable method for detecting altered autonomic control of the heart and predicting any resultant adverse outcome. Echocardiography is a valuable, safe, noninvasive and reproducible method for detection of early changes in preclinical cardiac function.4,16
This study was designed to detect the early preclinical alterations in cardiac autonomic control as well as altered cardiac functions in SSc patients, and their relevance to the clinical features of the disease using noninvasive methods, with the goal of possibly implementing preventive measures and initiating therapy early in the course of the disease.
In the present study, we excluded patients over the age of 60 years, those with clinical or radiological heart failure, diabetic patients, patients with renal impairment, and patients with any kind or cause of autonomic dysfunction to eliminate other causes for arrhythmia and/or impaired HRV other than SSc.32
The mean disease duration in our study was 4.4 years. This relatively short disease duration, together with the tight selection criteria, resulted in a decreased prevalence of patients with visceral involvement in our study in contrast to other nonselected studies that were conducted on SSc patients. In our study, 17% of our patients had LVDD, 33% had RVDD, 33% had ≥0 PVCs/h, and 7% had SVT. However, in Giunta et al33 26% of their SSc patients had LVDD and 40% had RVDD. In our patients, 23% had pulmonary fibrosis in contrast to 48% in D’Andrea et al.1
Dimitroulas et al34 found impaired left and right ventricular diastolic function in SSc patients expressed by the inverted ratio of early peak to late peak trans-mitral flow (E/A) and trans-tricuspid velocity, and increased left atrial diameter as detected by tissue Doppler ECG compared with controls. They concluded that depressed cardiac function is common even in asymptomatic patients with SSc.
Eighty percent of our SSc patients had Raynaud’s phenomenon and 43% had esophageal dysmotility. This high prevalence for both phenomena is attributed to the hypothesis that Raynaud’s phenomenon might represent the expression of autonomic dysfunction in the microcirculation.9 Esophageal motility disorders have been associated with cardiac autonomic neuropathy. This autonomic dysfunction occurs early in the disease, even before the development of fibrosis, or any other visceral complication or manifestation.30,35,36
One-third of our SSc patients had arrhythmia in the form of PVC ≥ 10/h (33%) and SVTs (7%) in comparison to none of the controls. In addition, the number of total PVCs increased significantly in our patients compared to the control group.
In a study by Bielous et al.37 Holter monitoring of SSc patients revealed a large number of PVC and decreased parameters in the time and frequency domains relative to the controls.
These findings reflect a higher incidence of arrhythmic complications and a greater propensity for sudden death in patients with SSc that can be explained by the severe impairment in autonomic control of the heart in SSc patients.16 This impairment in the autonomic control of the heart is evidenced by the significantly impaired HRV parameters both in the time and frequency domain analysis in our patients compared to the control group. Bienias et al16 found in his study that all estimated time and frequency domain values of HRV parameters in SSc patients were significantly lower than in the controls.
The maximum impairments in HRV in our SSc patients were found in the parameters that reflect the parasympathetic activity rather than those reflecting sympathetic or total autonomic control, in spite of the finding that all HRV parameters were reduced. In the time domain analysis, rMSSD and pNN50 were more affected than SDNN, SDANN and SDNNi; in the frequency domain analysis, the HF power was affected more than LF power, resulting in increase in the LF/HF ratio in spite of the reduction in the values of both LF and HF in SSc patients compared to the control group.
It is important to note that HRV measures fluctuations in autonomic inputs to the heart rather than the mean level of autonomic inputs. Thus, both autonomic withdrawal and a saturating (high) level of sympathetic input lead to diminished HRV.38
Kawase et al39 who demonstrated increased sympathetic nerve activity and decreased parasympathetic nerve activity throughout the day in SSc patients. In addition, Pancera et al40 found that HRV was reduced in patients with SSc in comparison to control subjects. On the contrary, Morelli et al41 analyzed HRV in the time domain and showed no significant difference in any variable between SSc patients and the controls. However, in the same study, analysis of the frequency domain showed a reduction in HRV parameters in SSc patients in contrast to the controls. On the other hand, Morelli et al demonstrated an increase in the total PVC count, the prevalence of frequent ventricular ectopy (PVCs ≥ 10/h) and SVT episodes in SSc patients compared to the controls.
To the best of our knowledge, few studies have evaluated the correlation of altered HRV and/or abnormal cardiac function and the subtypes, laboratory findings or clinical manifestation of SSc.
In the present study, we could not demonstrate any significant fixed correlation between thevarious HRV parameters or arrhythmia and disease duration, disease subtype or the presence of ACA in the sera of patients. These findings supported our hypothesis that an alteration in HRV and arrhythmia can occur early in the disease and in both subtypes allowing them to be used as markers for early assessment of altered cardiac function in all SSc patients.16
On the contrary, Hertmosillo et al42 found that patients with crest syndrome (LcSSc) had significantly decreased parasympathetic heart rate control compared to patients with the diffuse type. Their study had many important limitations, including the short time used for HRV analysis (only a few minutes) and the non-selectivity of the study population regarding the cardiovascular characterization during patient recruitment.
Our study demonstrated a significant negative correlation between various HRV parameters denoting autonomic dysfunction and worsening of skin sclerosis (represented by an increased TSS score), presence of microcirculatory autonomic dysfunction (represented by Raynaud’s phenomenon) and the presence of anti-SCL70 antibodies in the sera of SSc patients. There was significant positive correlation between the LF/HF ratio (representing sympatho-vagal imbalance with sympathetic predominance) and TSS, Raynaud’s phenomenon and the presence of anti-SCL70 antibodies. This sypmatho-vagal imbalance with sympathetic predominance provides an explanation for the positive correlation demonstrated between various arrhythmia parameters and TSS, Raynaud’s phenomenon and anti-SCL70 antibodies.
The correlation of sympatho-vagal imbalance with the increased propensity for arrhythmic complications in our SSc patients was well evident in the significant negative correlation between HRV parameters and the various arrhythmia parameters. It was also supported by the highly significant positive correlation demonstrated between arrhythmia and the LF/HF ratio in the frequency domain HRV analysis.
Ferri et al5 found a correlation between the modulation of sinus node activity with a higher relative risk of death in older patients with circulating anti-SCL70 antibodies.
In the present study, we found a significant positive correlation between subclinical deterioration of cardiac function in SSc patients (detected by the presence of LVDD and/or RVDD by trans-valvular Doppler assessment), worsening of skin sclerosis and presence of anti-SCL70 antibodies in the sera of SSc patients, but not with the detection of ACA antibodies.
D’Andrea et al1,43 found an independent negative correlation between right ventricular early myocardial (Em) peak velocity, Rodnan skin score and anti-SCL70 antibodies. Nakajima et al44 found a significant positive correlation between ventricular diastolic dysfunction and worsening of skin sclerosis.
However, Lindqvist et al21 and Giunta et al33 found no correlation between right ventricular diastolic dysfunction and skin involvement. In both studies, patient recruitment represented an important limitation; both studies were conducted on SSc patients with no exclusion criteria. In addition, Lindqvist et al21 had only two patients (10%) with DcSSc and 18 patients with LcSSc.
Our study demonstrated a significant negative correlation between subclinical deterioration of cardiac function in SSc patients and various HRV parameters, denoting increased sympathetic predominance in these patients.
Malliani et al27 and Appel et al45 observed a consistently reduced HRV in patients with cardiac dysfunction characterized by signs of sympathetic activation such as faster heart rates and high levels of circulating catecholamines. A relation between changes in HRV and the extent of left ventricular dysfunction was reported by Casolo et al.46
Further prospective studies may be necessary to assess the effect of altered autonomic control of the heart with sypmpathetic predominance on morbidity and mortality in SSc patients.
Conclusion
Abnormal autonomic nervous control of the heart, increased TSS, and the presence of anti-SCL70 correlate with subclinical cardiac involvement in SSc patients, and may predict the likelihood of malignant arrhythmia and sudden cardiac death. Therefore, noninvasive HRV evaluation, undertaken before clinical cardiac involvement, in these patients might be beneficial when added to the clinical and laboratory assessments in detecting high-risk patients, and may also allow for implementation of preventive measures and initiation of appropriate therapy early during the course of the disease to prevent the deleterious effects on the prognosis of SSc patients.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.D’Andrea A, Bellissimo S, Scotto di Uccio F, et al. Associations of right ventricular myocardial function with skin and pulmonary involvement in asymptomatic patients with systemic sclerosis. Ital Heart J. 2004;5:831–9. [PubMed] [Google Scholar]
- 2.Geyer M, Muller-Ladner U.The pathogenesis of systemic sclerosis Clin Rev Allergy Immunol 201020 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 3.Mouthon L, Garcia De La Pena-Lefebvre P, Chanseaud Y, Tamby MC, Boissier MC, Guillevin L. [Pathogenesis of systemic scleroderma: immunological aspects] (in French) Ann Med Interne (Paris) 2002;153:167–78. [PubMed] [Google Scholar]
- 4.Poanta L, Dadu R, Tiboc C, Rednic S, Dumitrascu D. Systolic and diastolic function in patients with systemic sclerosis. Eur J Intern Med. 2009;20:378–82. doi: 10.1016/j.ejim.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Ferri C, Emdin M, Giuggioli D, et al. Autonomic dysfunction in systemic sclerosis: time and frequency domain 24 hour heart rate variability analysis. Br J Rheumatol. 1997;36:669–76. doi: 10.1093/rheumatology/36.6.669. [DOI] [PubMed] [Google Scholar]
- 6.Marasini B, Massarotti M, Cossutta R. Scleroderma heart disease. Int J Immunopathol Pharmacol. 2005;18:609–14. doi: 10.1177/039463200501800401. [DOI] [PubMed] [Google Scholar]
- 7.Seferovic PM, Ristic AD, Maksimovic R, et al. Cardiac arrhythmias and conduction disturbances in autoimmune rheumatic diseases. Rheumatology (Oxford) 2006;45(Suppl 4):iv39–42. doi: 10.1093/rheumatology/kel315. [DOI] [PubMed] [Google Scholar]
- 8.Kahan A, Coghlan G, McLaughlin V. Cardiac complications of systemic sclerosis. Rheumatology (Oxford) 2009;48(Suppl 3):iii45–8. doi: 10.1093/rheumatology/kep110. [DOI] [PubMed] [Google Scholar]
- 9.Ferri C, Giuggioli D, Sebastiani M, Colaci M, Emdin M. Heart involvement and systemic sclerosis. Lupus. 2005;14:702–7. doi: 10.1191/0961203305lu2204oa. [DOI] [PubMed] [Google Scholar]
- 10.Cozzolino D, Naclerio C, Iengo R, D’Angelo S, Cuomo G, Valentini G. Cardiac autonomic dysfunction precedes the development of fibrosis in patients with systemic sclerosis. Rheumatology (Oxford) 2002;41:586–8. doi: 10.1093/rheumatology/41.5.586. [DOI] [PubMed] [Google Scholar]
- 11.Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1991;28:201–16. doi: 10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- 12.Kleiger RE, Bigger JT, Bosner MS, et al. Stability over time of variables-measuring heart rate variability in normal subjects. Am J Cardiol. 1991;68:626–30. doi: 10.1016/0002-9149(91)90355-o. [DOI] [PubMed] [Google Scholar]
- 13.Cripps TR, Malik M, Farrell TG, Camm AJ. Prognostic value of reduced heart rate variability after myocardial infarction: clinical evaluation of a new analysis method. Br Heart J. 1991;65:14–9. doi: 10.1136/hrt.65.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farrell TG, Paul V, Cripps TR, Malik M, Bennett ED, Ward D, Camm AJ. Baroreflex sensitivity and electrophysiological correlates in patients after acute myocardial infarction. Circulation. 1991;83:945–52. doi: 10.1161/01.cir.83.3.945. [DOI] [PubMed] [Google Scholar]
- 15.Wozniak J, Dabrowski R, Luczak D, et al. Evaluation of heart rhythm variability and arrhythmia in children with systemic and localized scleroderma. J Rheumatol. 2009;36:191–6. doi: 10.3899/jrheum.080021. [DOI] [PubMed] [Google Scholar]
- 16.Bienias P, Ciurzynski M, Glinska-Wielochowska M, et al. Heart rate turbulence assessment in systemic sclerosis: the role for the detection of cardiac autonomic nervous system dysfunction. Rheumatology (Oxford) 2010;49:355–60. doi: 10.1093/rheumatology/kep394. [DOI] [PubMed] [Google Scholar]
- 17.Masi A, Rodnan G, Medsger T. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association diagnostic and therapeutic criteria committee. Arthritis Rheum. 1980;23:581–90. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 18.Clements P, Lachenbruch P, Siebold J, et al. Inter- and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–5. [PubMed] [Google Scholar]
- 19.LeRoy EC, Black C, Fleischmajer R, et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J Rheumatol. 1988;15:202–5. [PubMed] [Google Scholar]
- 20.Cheitlin MD, Armstrong WF, Aurigemma GP, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/ASE) committee to update the 1997 guidelines for the clinical application of echocardiography) J Am Soc Echocardiogr. 2003;16:1091–110. doi: 10.1016/S0894-7317(03)00685-0. [DOI] [PubMed] [Google Scholar]
- 21.Lindqvist P, Caidahl K, Neuman-Andersen G, et al. Disturbed right ventricular diastolic function in patients with systemic sclerosis: a Doppler tissue imaging study. Chest. 2005;128:755–63. doi: 10.1378/chest.128.2.755. [DOI] [PubMed] [Google Scholar]
- 22.Task Force of the European Society of Cardiology and the North American Society of pacing and Electrophysiology Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 23.Lanza GA, Guido V, Galeazzi MM, et al. Prognostic role of heart rate variability in patients with a recent acute myocardial infarction. Am J Cardiol. 1998;82:1323–8. doi: 10.1016/s0002-9149(98)00635-3. [DOI] [PubMed] [Google Scholar]
- 24.Fei L. Effect of pharmacological intervention on heart rate variability: animal experiments and clinical observation. In: Malik M, Cam AJ, editors. Heart Rate Variability. Armonk, NY: Futura; 1995. pp. 275–91. [Google Scholar]
- 25.Akselrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 26.Pagani M, Lombardi F, Guzzetti S, et al. Power spectral density of heart rate variability as an index of sympatho-vagal interaction in normal and hypertensive subjects. J Hypertens Suppl. 1984;2:S383–5. [PubMed] [Google Scholar]
- 27.Malliani A, Pagani M, Lombardi F, Cerutti S. Cardiovascular neural regulation explored in the frequency domain. Circulation. 1991;84:482–92. doi: 10.1161/01.cir.84.2.482. [DOI] [PubMed] [Google Scholar]
- 28.Knapp RG, Miller MC.Clinical Epidemiology and BiostatisticsNational Medical Series. Baltimore: Williams & Wilkins; 1992;ch., 3, 31–45. [Google Scholar]
- 29.Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: Evolving concept and diagnostic methodologies. Arch Cardiovasc Dis. 2010;103:46–52. doi: 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Muller-Ladner U, Distler O, Ibba-Manneschi L, Neumann E, Gay S. Mechanisms of vascular damage in systemic sclerosis. Autoimmunity. 2009;42:587–95. doi: 10.1080/08916930903002487. [DOI] [PubMed] [Google Scholar]
- 31.Barron HV, Lesh MD. Autonomic nervous system and sudden cardiac death. J Am Coll Cardiol. 1996;27:1053–60. doi: 10.1016/0735-1097(95)00615-X. [DOI] [PubMed] [Google Scholar]
- 32.Klein AL, Leung DY, Murray RD, Urban LH, Bailey KR, Tajik AJ. Effects of age and physiologic variables on right ventricular filling dynamics in normal subjects. Am J Cardiol. 1999;84:440–8. doi: 10.1016/s0002-9149(99)00330-6. [DOI] [PubMed] [Google Scholar]
- 33.Giunta A, Tirri E, Maione S, Cangianiello S, et al. Right ventricular diastolic abnormalities in systemic sclerosis. Relation to left ventricular involvement and pulmonary hypertension. Ann Rheum Dis. 2000;59:94–8. doi: 10.1136/ard.59.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimitroulas T, Giannakoulas G, Papadopoulou K, et al. Early detection of cardiac involvement in systemic sclerosis assessed by tissue-Doppler echocardiography: relationship with neurohormonal activation and endothelial dysfunction J Rheumatol 20101(Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 35.Iovino P, Valentini G, Ciacci C, et al. Proximal stomach function in systemic sclerosis: relationship with autonomic nerve function. Dig Dis Sci. 2001;46:723–30. doi: 10.1023/a:1010779729184. [DOI] [PubMed] [Google Scholar]
- 36.Stacher G, Merio R, Budka C, Schneider C, Smolen J, Tappeiner G. Cardiovascular autonomic function, autoantibodies and esophageal motor activity in patients with systemic sclerosis and mixed connective tissue disease. J Rheumatol. 2000;27:692–97. [PubMed] [Google Scholar]
- 37.Bielous-Wilk A, Poreba M, Staniszewska-Marszalek E, et al. Electrocardiographic evaluation in patients with systemic scleroderma and without clinically evident heart disease. Ann Noninvasive Electrocardiol. 2009;14:251–7. doi: 10.1111/j.1542-474X.2009.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malik M, Camm AJ. Heart rate variability and clinical cardiology. Br Heart J. 1994;71:3–6. doi: 10.1136/hrt.71.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawase H, Maeda M, Horai T, Wada H, Kitajima Y, Seishima M. [Autonomic nerve function in patients with systemic scleroderma using heart rate variability analysis] (in Japanese) Rinsho Byori. 2006;54:325–8. [PubMed] [Google Scholar]
- 40.Pancera P, Sansone S, Presciuttini B, et al. Autonomic nervous system dysfunction in sclerodermic and primary Raynaud’s phenomenon. Clin Sci (Lond) 1999;96:49–57. [PubMed] [Google Scholar]
- 41.Morelli S, Piccirillo G, Fimognari F, et al. Twenty-four hour heart period variability in systemic sclerosis. J Rheumatol. 1996;23:643–5. [PubMed] [Google Scholar]
- 42.Hermosillo AG, Ortiz R, Dabague J, Casanova JM, Martinez-Lavin M. Autonomic dysfunction in diffuse scleroderma vs. crest: an assessment by computerized heart rate variability. J Rheumatol. 1994;21:1849–54. [PubMed] [Google Scholar]
- 43.D’Andrea A, Stisi S, Bellissimo S, et al. Early impairment of myocardial function in systemic sclerosis: non-invasive assessment by Doppler myocardial and strain rate imaging. Eur J Echocardiogr. 2005;6:407–18. doi: 10.1016/j.euje.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Nakajima K, Taki J, Kawano M, et al. Diastolic dysfunction in patients with systemic sclerosis detected by gated myocardial perfusion spect: an early sign of cardiac involvement. J Nucl Med. 2001;42:183–8. [PubMed] [Google Scholar]
- 45.Appel ML, Berger RD, Saul JP, Smith JM, Cohen RJ. Beat to beat variability in cardiovascular variables: noise or music? J Am Coll Cardiol. 1989;14:1139–48. doi: 10.1016/0735-1097(89)90408-7. [DOI] [PubMed] [Google Scholar]
- 46.Casolo G, Balli E, Taddei T, Amuhasi J, Gori C. Decreased spontaneous heart rate variability in congestive heart failure. Am J Cardiol. 1989;64:1162–7. doi: 10.1016/0002-9149(89)90871-0. [DOI] [PubMed] [Google Scholar]