Abstract
Objective:
To evaluate the utility of entheseal ultrasonography and serum COMP in the preclinical diagnosis of psoriatic arthritis.
Methods:
60 psoriatic patients were divided into: 30 patients with psoriasis (group I) and 30 patients with psoriatic arthritis as control (group II). They underwent independent clinical and ultrasonographic examination of both lower limbs at the calcaneal insertions of Achilles tendons. Psoriatic arthritis disease activity and severity was assessed by modified DAS28 and Steinbrockers scores. Serum levels of COMP were measured for all patients by ELISA.
Results:
On clinical examination, no entheseal abnormalities were detected in group I while they were present in 23.3% of group II with statistically significant difference between them (P < 0.001). Ultrasonographic entheseal abnormalities were detected in 33.3% of group I and in 46.7% of group II with no significant difference between them (P > 0.05). Serum COMP were significantly elevated in group I and II with no statistically significant difference between them (mean ± SD 5.9 ± 3 and 6.8 ± 12 respectively, P > 0.05). Entheseal ultrasound was more specific (67%) while serum COMP was more sensitive (87%) in the preclinical diagnosis of psoriatic arthritis. Serum COMP levels were significantly correlated with CRP in both groups and with DAS28 and Steinbrockers scores in group II (P < 0.01).
Conclusion:
Entheseal ultrasonography and serum COMP levels may be used complementary to each other for preclinical diagnosis of psoriatic arthritis. Serum COMP seems to be promising prognostic marker for psoriatic arthritis patients.
Keywords: psoriasis, psoriatic arthritis, entheseal ultrasound, cartilage oligomeric matrix protein
Introduction
Psoriasis is a chronic genetically determined and immunomediated inflammatory skin disease that affect approximately 2% of the population. In its typical form, psoriasis results in patches of thick, red skin covered with silvery scales.1
Psoriatic arthritis is an inflammatory rheumatic joint disease associated with psoriasis and commonly included among seronegative forms of spondyloarthropathy.2 It affects 2%–3% of the population, however its incidence has been rising over the last 30 years.3
In approximately 6%–39% of patients, psoriasis is present many years before the onset of arthritis.4
Psoriatic arthritis damages cartilage, synovium and bone of the joints causing pain, impairment and disability making early recognition and intervention important.5 Its marked entheseal involvement (the origin and insertion of ligaments, tendons and joint capsules) is a distinctive clinical aspect that helps to discriminate it from other conditions observed at their onset such as rheumatoid arthritis. It may be isolated to tendon insertions such as the Achilles tendon or plantar fascia or be more diffuse including multiple ligamentous attachments.6
Current methods for diagnosis of and monitoring the disease are only able to detect clinical manifestations of arthritis late in the process. However with the recent onset of successful treatments for inflammatory arthritis, it becomes important to identify factors that can predict the evolution of arthritis. This is especially critical in the early phases of the disease so that these treatments can started as soon as possible to slow down progression of the disease.7 Ultrasound has been increasingly used in rheumatology for assessing soft tissue involvement in patients with arthritis.8
Inspite of the high number of studies supporting its role and validity in the assessment of patients with rheumatoid arthritis and osteoarthritis, the potential role of ultrasound imaging in patients with psoriasis and psoriatic arthritis still waits to be adequately investigated including accuracy and reproducibility.9 Ultrasound particularly when coupled with power Doppler imaging can detect subclinical abnormalities of soft tissues, tendons and ligaments especially enthesitis, which has been postulated to be the hall mark and the initial site of joint inflammation in spondyloarthropathies.10
Another valuable approach to monitor arthritis would be by measuring biological markers of cartilage degradation and repair which reflect variations in joint remodeling. One such potential marker of arthritis is cartilage oligomeric matrix protein (COMP).7
COMP is a tissue specific pentameric glycoprotein and one component of the extracellular articular cartilage matrix and belongs to the thrombospondin family.11 It was first detected in the serum and synovial fluid of patients suffering from rheumatic disorders such as rheumatoid arthritis and osteoarthritis.12 However much less known about COMP production in synovial cells in other rheumatic diseases.13 Originally it is considered a marker of cartilage breakdown, but it has also been identified in tendons, ligaments and synovium.14 It has potential as a diagnostic and prognostic indicator and as a marker of the rheumatic disease severity and the effect of treatment.12,15
The aim of the present study was to evaluate the role of entheseal ultrasonography and serum COMP in the preclinical diagnosis of psoriatic arthritis.
Subjects and Methods
The present case control study included 60 psoriatic patients based on clinical diagnosis. They were recruited from Rheumatology, Dermatology Departments and Rheumatology outpatient clinic, Ain Shams University hospital. Their ages ranged from 23–48 years. They were divided into 2 groups; group I included 30 patients with psoriasis without any typical joint symptoms or signs, and group II included 30 patients with psoriatic arthritis (who were diagnosed according to the classification criteria for psoriatic arthritis CASPAR criteria)16 as control group. Both groups were age and sex matched and also matched as regard duration of skin disease and severity of skin affection as assessed by the psoriasis area and severity index (PASI) score.17 Patients with evidence of other rheumatic diseases, malignancies and infections were excluded from the study. An informed consent was obtained from each patient. The study was approved by Ain Shams Medical Ethics Committee.
All patients were subjected to full history taking and thorough clinical examination including dermatological and rheumatological examination to detect arthritis, clinical dactylitis (swelling of an entire digit) and enthesitis (swelling, pain, tenderness along the course of the Achilles tendon or at the level of the calcaneal insertion or its functional impairment). For psoriatic arthritis patient only the disease activity was assessed by Modified Disease Activity Score (DAS28).18
Radiological examination was done for all patients including:
Plain X-ray on hands, feets, spine and sacroiliac joints, then the psoriatic arthritis disease severity was assessed by Modified Steinbrockers scoring method.19
Real time musculoskeletal ultrasonography using ESAOTE MYLAB 70X VISION. (ESAOTE MEDICAL SYSTEM ITALY) with 9–11 MHz probe. An adequate amount of gel was used to obtain the best visualization of superficial soft tissues. Subjects were not allowed to assume nonsteroidal anti-inflammatory drugs three weeks before ultrasound examination. The examiner doctor was unaware of the clinical symptoms and signs of the patients. Longitudinal and transverse scans of Achilles tendons were obtained. Power Doppler sonography was used to detect the degree of vascularization as an indirect sign of inflammation. Early ultrasound signs of enthesitis are loss of normal fibrillar echogenicity, hypoechoic swelling of the tendon insertion, effusion, increase of blood flow detectable with power Doppler.20,21
Laboratory studies
Five milliliters of venous blood were collected from each patient, 2 ml on EDTA for ESR by Westergren method and 3 ml in a plane tube where serum was collected and stored at −70° for subsequent assay of COMP and C-reactive protein (CRP). Three milliliters of blood were withdrawn from 20 age and sex matched healthy lab workers for determination of normal COMP values.
COMP was assayed by enzyme immunoassay using Human Cartilage Oligomeric Matrix Protein ELISA Kit (Biovendon Research and Diagnostic Products. Im Neuenheimer Feld 583. D-69120 Heidelburg Germany). The mean ± SD of serum COMP among healthy lab workers was 0.9 ± 0.28 mg/mL.
CRP was assayed by latex using Omega Diagnostics Avitex (Omega diagnostics LTD. Omega house, Hillfoots business village, Alva FK12 5DQ, Scotland, United Kingdom).
Statistical analysis of data was done by IBM computer using SPSS (statistical program for social science version 12). Mean and standard deviation (SD) were used to describe quantitative variables and number and percentage for qualitative variables. Unpaired t-test was used to compare quantitative variables between both groups while Mann Whitney Willcoxon U test was used instead of t-test in non parametric data (SD > 50% mean). Chi-square test was used to compare qualitative variables between both groups. Spearman correlation test was used to rank different variables against each others positively or inversely. P-values less than 0.05 were accepted to be statistically significant. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of both entheseal ultrasound and serum COMP of both groups were calculated from the following equations: Sensitivity = % True positive cases/True positive cases + false negative cases, Specificity = % True negative cases/True negative cases + false positive cases, Positive predictive value = % True positive cases/True positive cases + false positive cases, Negative predictive value = % True negative cases/True positive cases + false negative cases and Accuracy = % True positive cases + true negative cases/Total number of cases.22
Results
The present study was carried out on 60 psoriatic patients divided into 2 groups: group I included 30 patients with psoriasis and group II included another 30 patients with psoriatic arthritis as control group. No statistically significant difference was detected between both groups as regard age, sex, duration and severity of skin disease (P > 0.05). While there was significant difference between them as regard clinical enthesitis and dactylitis and CRP (P < 0.05) (Table 1).
Table 1.
Comparison between group I and II patients as regard the main clinical features, ultrasonography and laboratory findings.
| Group I N = 30 | Group II N = 30 | P value | |
|---|---|---|---|
| Age (years) mean (SD) | 39 (5) | 40 (5.9) | 0.4 (NS)* |
| Gender (N & %) | |||
| Male | 17 (56.7%) | 12 (40%) | 0.3 (NS)# |
| Female | 13 (43.3%) | 18 (60%) | |
| Skin disease duration (years) mean (SD) | 7.7 (1.2) | 8.8 (3.0) | 0.31 (NS)* |
| Duration of arthritis (years) mean (SD) | – | 1.7 (0.7) | – |
| PASI score mean (SD) | 23 (4) | 19.5 (5.8) | 0.29 (NS)* |
| Clinical enthesitis and dactylitis (N & %) | Zero | 7 (23.3%) | 0.0005 (HS)# |
| DAS 28 score mean (SD) | – | 4.6 (1.8) | – |
| Steinbrockers score mean (SD) | – | 23.4 (10) | – |
| Ultrasonography of patients with entheseal abnormalities (N & %) | 10 (33.3%) | 14 (46.7%) | 0.19 (NS)# |
| Serum COMP (mg/ml) of patients with elevated levels | |||
| N & % | 22 (73.3%) | 26 (86.7%) | 0.20 (NS)# |
| Mean (SD) | 5.9 (3) | 6.8 (12) | 0.37 (NS)≠ |
| CRP (mg/ml) mean (SD) | 11.9 (4) | 24.5 (13) | 0.03 (S)≠ |
Unpaired t-test;
Chi-square test;
Mann Whitney test;
Abbreviations: PASI, psoriasis area and severity index; DAS 28, Disease activity score 28; COMP, cartilage oligomeric matrix protein; CRP, C-reactive protein.
Ultrasonographic findings
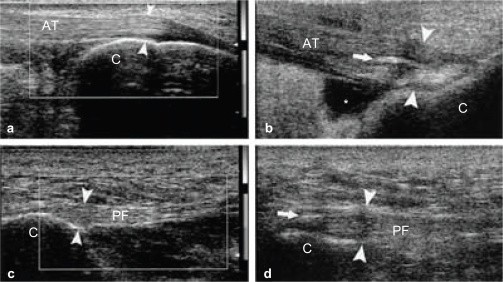
Achilles tendinitis was the predominant feature of enthesitis noted in the present study. Early ultrasound signs of enthesitis included loss of normal fibrillar echogenicity, hypoechoic swelling of the tendon insertion, effusion, increase of blood flow detectable with power Doppler, retrocalcaneal bursitis and plantar fasciitis. Achilles tendon and plantar fascia calcifications were also found in psoriatic arthritis patients (Fig. 1). No statistically significant difference was detected between psoriatic and psoriatic arthritis patients as regard these ultrasonographic findings (P > 0.05) (Table 1) and (Fig. 2).
Figure 1.
(A and C) Achilles tendon shows normal ultrasound appearance with thickness of about 3.5 mm at its insertion (normal less than 5.3 mm), no related fluid or abnormal vascularity seen. B) Achilles enthesopathy and retrocalcaneal bursitis, the Achilles insertional tract appears thickened (between arrowheads) and heterogeneously hypoechoic. A calcification (arrow) is evident within the distal portion of the tendon. The retrocalcaneal bursa is enlarged by fluid collection (asterisk). D) Plantar fasciitis, insertional tract is thickened (between arrowheads) and heterogeneously hypoechoic with thin linear calcification (arrow).
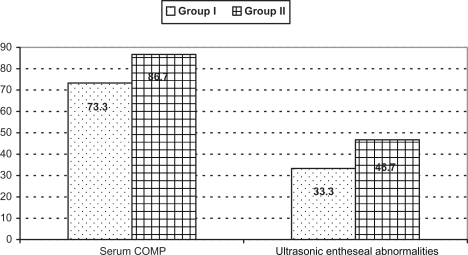
Figure 2.
Comparison between group I and II as regard percentages of patients who had elevated serum cartilage oligomeric matrix protein and ultrasound entheseal abnormalities.
Serum COMP was significantly elevated in group I and II patients with no statistically significant difference between them as regard its mean levels (Table 1 and Fig. 2). While there was statistically significant difference between both groups as regard CRP Table 1.
Serum COMP was more sensitive while entheseal ultrasonography was more specific in the preclinical diagnosis of psoriatic arthritis, both had the same accuracy (Table 2).
Table 2.
Sensitivity, specificity, PPV, NPV and accuracy of entheseal ultrasound and serum COMP in the preclinical diagnosis of psoriatic arthritis.
| Entheseal ultrasound | Serum COMP | |
|---|---|---|
| True positive cases (psoriatic arthritis patients with positive test) (N) | 14 | 26 |
| True negative cases (psoriatic patients with negative test) (N) | 20 | 8 |
| False positive cases (psoriatic patients with positive test) (N) | 10 | 22 |
| False negative cases (psoriatic arthritis patients with negative test) (N) | 16 | 4 |
| Sensitivity (%) | 47% | 87% |
| Specificity (%) | 67% | 27% |
| PPV (%) | 58.3% | 54% |
| NPV (%) | 55.6% | 66.7% |
| Accuracy (%) | 56.8% | 56.8% |
Abbreviations: PPV, Positive predictive value; NPV, Negative predictive value.
Serum COMP correlated significantly with CRP in both group I and II (r = 0.57, 0.86 respectively, P = 0.003) and with modified DAS28 and modified Steinbrockers scores in group II (r = 0.94 and 0.88 respectively, P = 0.0002). While no correlation was found between serum COMP and PASI score in both groups (r = 0.069 and 0.18 respectively, P = 0.09).
Discussion
Psoriatic arthritis occurs in up to one-third of patients with psoriasis.23 Usually the skin disease precedes the onset of psoriatic arthritis with two thirds of patients developing psoriasis before the onset of arthritis.24
Enthesopathic changes have been suggested as being the unifying feature of the clinical subtypes of psoriatic arthritis, and the disease can be considered an enthesis associated disorder rather than primary synovitic arthropathy.25
So we aimed in this work to evaluate the role of entheseal utrasonography and serum COMP in the preclinical diagnosis of psoriatic arthritis.
In the present study despite there was significant difference between psoriatic and psoriatic arthritis patients as regard clinical enthesitis, however ultrasonography showed almost the same entheseal abnormalities with no significant difference between them.
The Achilles tendons are among the most frequent sites of enthesopathic involvement in psoriatic arthritis. Ultrasound has shown that enthesitis can be asymptomatic and that some cases of Achilles tendinitis can go undiagnosed. It allows visualization of lesions less than 5 mm in diameter and identification of minimal amounts of fluid at the level of the tendon, peritendinous structures and serous bursae. The high specificity of ultrasound allow its use as a complementary procedure when clinical examination yields normal findings because the signs of involvement are mild and may be not accompanied by specific symptoms.26
Simone et al27 found sonographic evidence of involvement of the Achilles tendon in 35 of 59 psoriatic patients whereas clinical examination yielded 18 diagnosis of Achilles tendinitis. These results lent support to the hypothesis of frequent enthesopathic involvement in psoriatic arthritis.
Power Doppler ultrasonography provides information on the perfusion of the synovial tissue, tendons and entheses, so very slight changes in vascularity can be easily detected.10,21
In the present study, the sensitivity of ultrasound in the preclinical diagnosis of psoriatic arthritis was 47%, while its specificity was 67%. Weiner and Coworkers28 found that the sensitivity of ultrasound in the detection of overall joint pathology in painful and/or swollen joint in psoriatic arthritis was 71%, and in clinically unaffected joints was 17%, while its specificity ranged between 84% and 94% depending on the joint pathology.
The low cost and the acceptable specificity suggest that ultrasound is a useful imaging tool in early psoriatic arthritis. Ozçakar et al29 found that the percentage of asymptomatic enthesopathy detected by ultrasound in psoriatic patients was 56%, they strongly believed that ultrasound has an advantageous role in rendering the enthesopathy in psoriasis patients whether or not they are symptomatic.
COMP expressed primarily in cartilage, ligament and tendon.14 It is considered a marker of early cartilage breakdown in patients with psoriatic arthritis.30 It was also found elevated in the serum of patients with rheumatoid arthritis31 and osteoarthritis.12 In the present study serum COMP was significantly elevated in both psoriatic and psoriatic arthritis patients with no significant difference between them. The increased serum COMP levels in patients with psoriasis propose that patients with psoriatic skin lesions might have additional joint involvement.13
T cells and proinflammatory cytokines like tumor necrosis factor-α (TNF-α) play an essential role in the pathogenesis of psoriasis and psoriatic arthritis. They stimulate chondrocytes to release destructive proteases which lead to loss of proteoglycans, to destruction of collagen bundles and to the release of COMP.13 Despite the presence of previous studies on the utility of ultrasound and serum COMP in many rheumatic diseases, their combined validity, sensitivity and specificity in patients with psoriasis and psoriatic arthritis has not been previously reported.
In the present study the sensitivity of serum COMP in the preclinical diagnosis of psoriatic arthritis was 87% while its specificity was only 27%. The lack of specificity of serum COMP for cartilage may limit its use in assessing changes in joint damage in arthritis. The assay may need to be complemented by radiographic or MRI evaluation.7
In patients with symptoms and clinical signs of hip pathology but not radiographic evidence of osteoarthritis, a significant association was found between serum COMP and hip-related symptoms. This would support the use of serum COMP as a biomarker of hip joint pathology prior to radiographic findings.32 In the present study serum COMP levels have been demonstrated as an indicator for disease activity and severity in patients with psoriatic arthritis as it was significantly correlated with modified DAS28 and modified steinbrockers scores. Also significant correlation was found between serum COMP levels and laboratory disease activity in psoriatic patients. While no correlation was found between serum COMP and the extent of skin disease in both psoriatic and psoriatic arthritis patients. Skoumal et al13 couldn’t find any evidence that extended skin lesions of psoriasis have any influence on serum COMP and thus its elevated levels were due to on going joint inflammation. Patients with active psoriatic arthritis showed significantly elevated serum COMP levels compared to the patients with low clinical and laboratory disease activity.13 This reflects increased cartilage tumour in inflammatory diseases with high activity similar to findings in rheumatoid arthritis.33 Serum COMP levels were significantly high in rheumatoid arthritis patients positive for bone erosions on MRI compared with those who were negative for bone erosions, this may reflect joint damage that is dependent on the synovial inflammatory process in early rheumatoid arthritis.34
Conclusion
Entheseal ultrasonography and measurement of serum COMP may be used for all patients with psoriasis for preclinical diagnosis of psoriatic arthritis to get the benefit of the high sensitivity of serum COMP and the high specificity of ultrasound. In addition serum COMP seems to be promising marker of disease activity and severity in psoriatic arthritis patients. These findings might have an influence on the clinical decision in patients with psoriasis and psoriatic arthritis, and have implication for therapy since the new biologic drugs can delay or stop joint damage when given in time. Further prospective studies for patients with psoriasis without history of arthritis are needed to clarify the value of entheseal ultrasound and serum COMP in detecting their progression to psoriatic arthritis.
Abbreviations
- COMP
cartilage oligomeric matrix protein
- DAS28
Disease activity score 28
- ELISA
Enzyme linked immunosorbent assay.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis, section 1. Overview of psoriasis and guide lines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58(5):826–50. doi: 10.1016/j.jaad.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 2.Kane D, Pathare S. Early psoriatic arthritis. Rheum Dis Clin N Am. 2005;31:641–57. doi: 10.1016/j.rdc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM. Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: A population based study. J Rheumatol. 2009;36(2):361–7. doi: 10.3899/jrheum.080691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rozenblit M, Lebwohl M. New biologics for psoriasis and psoriatic arthritis. Dermatol Ther. 2009;22(1):56–60. doi: 10.1111/j.1529-8019.2008.01216.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruderman EM, Tambar S. Psoriatic arthritis: prevalence, diagnosis, and review of therapy for the dermatologist. Dermatol Clin. 2004;22(4):477–86. doi: 10.1016/S0733-8635(03)00127-X. [DOI] [PubMed] [Google Scholar]
- 6.Scarpa R, Cuocolo A, Peluso R, et al. Early psoriatic arthritis: the clinical spectrum. J Rheumatol. 2008;35:137–41. [PubMed] [Google Scholar]
- 7.Tseng S, Reddi AH, DiCesare PE. Cartilage oligomeric matrix protein (COMP): A biomarker of arthritis. Biomarker Insight. 2009;4:33–44. doi: 10.4137/bmi.s645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meenagh G, Filippucci E, Kane D, Taggart A, Grassi W. Ultrasonogrpahy in rheumatology: developing its potential in clinical practice and research. Rheumatology. 2007;46:3–5. doi: 10.1093/rheumatology/kel317. [DOI] [PubMed] [Google Scholar]
- 9.Filippucci E, De Angelis R, Salaffi F, Grassi W. Ultrasound, skin and joints in psoriatic arthritis. J Rheumatol. 2009;36(Suppl I):83-35-38. doi: 10.3899/jrheum.090220. [DOI] [PubMed] [Google Scholar]
- 10.Sturrock RD. Clinical utility of utlrasonography in spondyloarthropathies. Current Rheumatology Reports. 2009;11(5):317–20. doi: 10.1007/s11926-009-0045-x. [DOI] [PubMed] [Google Scholar]
- 11.Hedbom E, Antonsson P, Hjerpe A. Cartilge matrix proteins. An Acidic oligomeric protein (COMP) detected only in cartilage. J Biol Chem. 1992;267:6132–6. [PubMed] [Google Scholar]
- 12.Morozzi G, Fabbroni M, Bellisai F, Pucci G, Galeazzi M. Cartilage oligomeric matrix protein level in rheumatic diseases: potential use as a marker for measuring articular cartilage damage and/or the therapeutic therapy of treatments. Ann NY Scand Sci. 2007;1108:398–407. doi: 10.1196/annals.1422.041. [DOI] [PubMed] [Google Scholar]
- 13.Skoumal M, Haberhauer G, Fink A, et al. Increased serum levels of cartilage oligomeric matrix protein in patients with psoriasis vulgaris: A marker for unknown peripheral joint involvement? Clin Exp Rehumatol. 2008;26:1087–90. [PubMed] [Google Scholar]
- 14.Posey KL, Hecht JT. The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Current Drug Targets. 2008;9(10):869–77. doi: 10.2174/138945008785909293. [DOI] [PubMed] [Google Scholar]
- 15.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of higher levels of serum cartilage oligomeric matrix protein and N-telopeptide cross links with the development of radiographic hip osteoarthritis in elderly women. Arthritis Rheum. 2005;54:236–43. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 16.Taylor W, Gladman D, Helliwell P. Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arthritis Rheum. 2006;54:2665–73. doi: 10.1002/art.21972. [DOI] [PubMed] [Google Scholar]
- 17.Feldman SR, Krueger GG. Psoriasis assessment tools in clinical trials. Ann Rheum Dis. 2005;46(Suppl II):ii65–8. doi: 10.1136/ard.2004.031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mease PJ, Antoni CE, Gladman DD, Taylor WJ. Psoriatic arthritis assessment tools in clinical trials. Ann Rheum Dis. 2005;64(Suppl II):ii49–54. doi: 10.1136/ard.2004.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van der Heijde D, Sharp J, Wassenberg S, Gladman DD. Psoriatic arthritis imaging: a review of scoring methods. Ann Rheum Dis. 2005;64(Suppl II):ii61–4. doi: 10.1136/ard.2004.030809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Filippis LG, Caliri A, LoGullo R, et al. Ultrasonography in the early diagnosis of psoriasis-associated enthesopathy. Int J Tissue React. 2005;27(4):159–62. [PubMed] [Google Scholar]
- 21.Riente L, Sedie AD, Fillippucci E, et al. Ultrasound imaging for the rheumatologist IX. Ultrasound imaging in spondyloarthritis. Clin Exp Rheumatol. 2007;25:349–53. [PubMed] [Google Scholar]
- 22.Knapp RG, Miler MC. Clinical epidemiology and biostatistics. Harwal Publishing Company. 1992 [Google Scholar]
- 23.Atzeni F, Sarzi-Puttini P, Vena GA. Resistant cases of psoriatic arthritis: How to manage them. J Rheumatol. 2009;83(0):73–5. doi: 10.3899/jrheum.090232. [DOI] [PubMed] [Google Scholar]
- 24.Jones SM, Arma JB, Cohen MG, Lovell CR, Evison G, McHugh NJ. Psoriatic arthritis: outcome of disease subsets and relationship of joint disease to nail and skin disease. Br J Rheumatol. 1994;33(9):834–9. doi: 10.1093/rheumatology/33.9.834. [DOI] [PubMed] [Google Scholar]
- 25.McGonagle D, Conaghan PG, Emery P. Psoriatic arthritis: a unified concept twenty years on. Arthritis Rheum. 1999;42:1080–7. doi: 10.1002/1529-0131(199906)42:6<1080::AID-ANR2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 26.Galluzzo E, Lischi DM, Taglione E, Lombardini F, Pasero G, Perri G. Sonographic analysis of the ankle in patients with psoriatic arthritis. Scand J Rheumatol. 2000;29:52–5. doi: 10.1080/030097400750001806. [DOI] [PubMed] [Google Scholar]
- 27.Desimone C, Guerriero C, Giampietruzzi AR, Costantini M, Di-Gregorio F, Amerio P. Achilles tendinitis in psoriasis: clinical and sonographic findings. J Am Acad Dermatol. 2003;49:217–22. doi: 10.1067/s0190-9622(03)00904-6. [DOI] [PubMed] [Google Scholar]
- 28.Weiner SM, Jurenz S, Uhi M, et al. Ultrasonography in the assessment of peripheral joint involvement in psoriatic arthritis. Clin Rheumatol. 2008;27:983–9. doi: 10.1007/s10067-008-0835-y. [DOI] [PubMed] [Google Scholar]
- 29.Ozçakar L, Cetin A, Inanici F, Kaymak B, Gurer CK, Kolemen F. Ultrasonographical evaluation of the Achilles tendon in psoriasis patients. Int J Dermatol. 2005;44:930–2. doi: 10.1111/j.1365-4632.2004.02235.x. [DOI] [PubMed] [Google Scholar]
- 30.Jung YO, Do JH, Kang HJ, et al. Correlation of sonographic severity with biochemical markers of synovium and cartilage in knee osteoarthritis patients. Clin Exp Rehumatol. 2006;24:253–9. [PubMed] [Google Scholar]
- 31.Gomez-Barrena E, Lindross L, Ceponis A. Cartilage oligomeric matrix protein (COMP) is modified by intra-articular liposomal clodronate in an experimental model of arthritis. Clin Exp Rheumatol. 2006;24:622–8. [PubMed] [Google Scholar]
- 32.Dragomir AD, Kraus VB, Renner JB. Serum cartilage oligomeric matrix protein and clinical signs and symptoms of potential pre-radiographic hip and knee pathology. Osteoarthritis Cartilage. 2002;10:687–91. doi: 10.1053/joca.2002.0816. [DOI] [PubMed] [Google Scholar]
- 33.De Jong Z, Munneke M, Vilim V, et al. Value of serum cartilage oligomeric matrix protein as a prognostic marker of large joint damage in rheumatoid arthritis. Data from the RAPIT study. Rheumatology. 2008;47(6):868–71. doi: 10.1093/rheumatology/ken052. [DOI] [PubMed] [Google Scholar]
- 34.Fujikawa K, Kawakami A, Tamai M, et al. High serum cartilage oligomeric protein determines the subset of patients with early stage rheumatoid arthritis with high serum C-reactive protein, matrix metallo-proteinase-3, and MRI-proven bone erosion. J Rheumatol. 2009;36(6):1126–9. doi: 10.3899/jrheum.080926. [DOI] [PubMed] [Google Scholar]