Abstract
OATP1B1 and 1B3 are related transporters mediating uptake of numerous compounds into hepatocytes. A putative model of OATP1B3 with a “Positive Binding Pocket” containing conserved positively charged amino acids was predicted (Meier-Abt et al., J Membr Biol 208:213–227, 2005). Based on this model, we tested the hypothesis that these positive amino acids are important for OATP1B1 function. We made mutants and measured surface expression and uptake of estradiol-17β-glucuronide, estrone-3-sulfate and bromosulfophthalein in HEK293 cells. Two of the mutants had low surface expression levels: R181K at 10 % and R580A at 30 % of wild-type OATP1B1. A lysine at position 580 (R580K) rescued the expression of R580A. Mutations of several amino acids resulted in substrate dependent effects. The largest changes were seen for estradiol-17β-glucuronide while estrone-3-sulfate and bromosulfophthalein transport was less affected. The wild-type OATP1B1 Km value for estradiol-17β-glucuronide of 5.35 ± 0.54 μM was increased by R57A to 30.5 ± 3.64 μM and decreased by R580K to 0.52 ± 0.18 μM. For estrone-3-sulfate the wild-type high affinity Km value of 0.55 ± 0.12 μM was increased by K361R to 1.8 ± 0.47 μM and decreased by R580K to 0.1 ± 0.04 μM. In addition, R580K also reduced the Vmax values for all three substrates to less than 25% of wild-type OATP1B1. Mutations at the intracellular K90, H92 and R93 mainly affected Vmax values for estradiol-17β-glucuronide uptake. In conclusion, the conserved amino acids R57, K361 and R580 seem to be part of the substrate binding sites and/or translocation pathways in OATP1B1.
Keywords: Organic anion transporting polypeptides, liver, transporter, site-directed mutagenesis
INTRODUCTION
OATP1B1 is a transport protein expressed at the basolateral membrane of human hepatocytes (Abe et al., 1999; Abe et al., 2001; Hsiang et al., 1999; KönigKönig et al., 2000). It belongs to the organic anion transporting polypeptide superfamily (OATP, gene symbol SLCO) (Hagenbuch & Meier, 2004) and mediates the sodium-independent transport of a wide range of structurally unrelated amphipathic organic compounds (Hagenbuch & Gui, 2008; KönigKönig et al., 2006). Due to its broad substrate spectrum, OATP1B1 is important for hepatic clearance of many drugs and other xenobiotics. In order to explain and prevent potential adverse drug interactions that occur at the transporter level a thorough understanding of the transport mechanisms of OATP1B1 is essential.
Several functional studies have suggested that there are multiple substrate recognition sites or translocation pathways for the different OATP1B1 substrates (Gui et al., 2008; Noe et al., 2007; Tamai et al., 2001). Two recent studies identified transmembrane domain (TM) 8 and 9 (Miyagawa et al., 2009), and 10 (Gui & Hagenbuch, 2009) to be important for OATP1B1-mediated substrate transport. Furthermore, several amino acids within TM10 were identified to be important for substrate binding (L545) or protein structure/folding (F546, L550, S554) (Gui & Hagenbuch, 2009).
Because so far none of the OATPs has been crystallized, comparative modeling has been used to predict the structure of OATPs. Based on their 12 transmembrane domain topology (Wang et al., 2008) common to members of the major facilitator superfamily (MFS), the known crystal structures of two bacterial transporters in the MFS were used as templates to generate a 3D structural model for OATP1B3 (Meier-Abt et al., 2005). Based on this model, Meier-Abt et al. (2005) proposed a putative binding pocket within an aqueous pore that contains several positively charged amino acid residues conserved throughout the OATP1 family. In a recent study with OATP1B3, K41 in TM1 and R580 in TM11 were confirmed to be important for bromosulfophthalein (BSP) transport (Glaeser et al., 2010). However, the roles of the positively charged amino acids within the putative binding pocket for OATP1B1-mediated transport are still unknown. Therefore, in this study, we performed site-directed mutagenesis of these conserved positively charged amino acids and characterized the mutant proteins with respect to surface expression and transport function. We determined the significance of the extracellular R57 and K361, of the transmembrane R181 (TM4) and R580 (TM11), and of the intracellular K90, H92 and R93 for OATP1B1-mediated transport of the model substrates estradiol-17β-glucuronide, estrone-3-sulfate and BSP.
MATERIALS AND METHODS
Materials
Radiolabeled [3H]-estradiol-17β-glucuronide (46.9 Ci/mmol) and [3H]-estrone-3-sulfate (57.3 Ci/mmol) were purchased from Perkin Elmer (Boston, MA). Radiolabeled [3H]-sulfobromophthalein (14.5 Ci/mmol) was purchased from International Isotope Clearing House (Leawood, KS). Unlabeled chemicals were obtained from Sigma-Aldrich (St. Louis, MO). The anti-OATP1B1 K23 antibody was kindly provided by Dr. Bruno Stieger, University Hospital, Zurich, Switzerland.
Site-directed mutagenesis
Human OATP1B1*1b was subcloned into the pcDNA5/FRT vector (Invitrogen, Carlsbad, CA) and is considered wild-type as compared to the introduced mutations. Single amino acid mutations were introduced by site-directed mutagenesis using the QuickChange® system (Stratagene, La Jolla, CA) following the manufacturer’s instructions. Primers used in the mutagenesis reactions are listed in Table 1. Plasmid DNA was prepared using HiSpeed Plasmid Midi or Maxi Kits (Qiagen, Germantown, MD), and both strands of all constructs were sequenced to confirm the presence of the designed mutations and the absence of additional spontaneous mutations.
Table 1.
Primers for site-directed mutagenesis
| Mutation | Primers (5′-3′) |
|---|---|
| R181A |
forward: GTGTTCATGGGTAATATGCTTGCTGGAATAGGGGAGACTCCC reverse: GGGAGTCTCCCCTATTCCAGCAAGCATATTACCCATGAACAC |
| R181K |
forward: GTGTTCATGGGTAATATGCTTAAAGGAATAGGGGAGACTCCCATAG reverse: CTATGGGAGTCTCCCCTATTCCTTTAAGCATATTACCCATGAACAC |
| R181H |
forward: CATGGGTAATATGCTTCATGGAATAGGGGAGACTCCC reverse: GGGAGTCTCCCCTATTCCATGAAGCATATTACCCATG |
| R580A |
forward: CCACTCAATGGTTATAGCAGCACTAGGAGGAATTCTAGC reverse: GCTAGAATTCCTCCTAGTGCTGCTATAACCATTGAGTGG |
| R580K |
forward: CCACTCAATGGTTATAAAAGCACTAGGAGGAATTCTAGCTCC reverse: GGAGCTAGAATTCCTCCTAGTGCTTTTATAACCATTGAGTGG |
| R580H |
forward: CCACTCAATGGTTATACATGCACTAGGAGGAATTCTAGCTCC reverse: GGAGCTAGAATTCCTCCTAGTGCATGTATAACCATTGAGTGG |
| R57A |
forward: GTTCCATCATTCATATAGAAGCGAGATTTGAGATATCCTCTTCTCTTG reverse: CAAGAGAAGAGGATATCTCAAATCTCGCTTCTATATGAATGATGGAAC |
| R57K |
forward: GAAAAGAGATTTGAGATATCCTCTTCTC reverse: AAATCTCTTTTCTATATGAATGATGGAAC |
| K361A |
forward: GGTGCTTTTACTTATGTCTTCGCATACGTAGAGCAACAGTATGG reverse: CCATACTGTTGCTCTACGTATGCGAAGACATAAGTAAAAGCACC |
| K361R |
forward: TTCAGATACGTAGAGCAACAGTATGG reverse: GTATCTGAAGACATAAGTAAAAGCACC |
| K90A |
forward: GTGAGTTACTTTGGATCCGCACTACATAGACCAAAGTTAATTGG reverse: CCAATTAACTTTGGTCTATGTAGTGCGGATCCAAAGTAACTCAC |
| K90R |
forward: GTGAGTTACTTTGGATCCCGACTACATAGACCAAAGTTAATTGG reverse: CCAATTAACTTTGGTCTATGTAGTCGGGATCCAAAGTAACTCAC |
| H92A |
forward: GTTACTTTGGATCCAAACTAGCTAGACCAAAGTTAATTGGAATCGG reverse: CCGATTCCAATTAACTTTGGTCTAGCTAGTTTGGATCCAAAGTAAC |
| H92K |
forward: GAGTTACTTTGGATCCAAACTAAAGAGACCAAAGTTAATTGGAATCGG reverse: CCGATTCCAATTAACTTTGGTCTCTTTAGTTTGGATCCAAAGTAACTC |
| H92R |
forward: CTTTGGATCCAAACTACGTAGACCAAAGTTAATTGGAATCGG reverse: CCGATTCCAATTAACTTTGGTCTACGTAGTTTGGATCCAAAG |
| R93A |
forward: CTTTGGATCCAAACTACATGCACCAAAGTTAATTGGAATCGGTTG reverse: CAACCGATTCCAATTAACTTTGGTGCATGTAGTTTGGATCCAAAG |
| R93K |
forward: CTTTGGATCCAAACTACATAAACCAAAGTTAATTGGAATCGGTTG reverse: CAACCGATTCCAATTAACTTTGGTTTATGTAGTTTGGATCCAAAG |
Protein expression in HEK293 cells
Human embryonic kidney (HEK293) cells were grown at 37°C in a humidified 5% CO2 atmosphere in Dulbecco’s Modified Eagle Medium High Glucose (Invitrogen, Carlsbad, CA), supplemented with 10% FBS (Hyclone, Logan, UT), 100 U/ml penicillin and 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA). Twenty-four hours before transfection, HEK293 cells were harvested by trypsinization and re-plated at 250,000 cells/well in 24-well plates (coated with 0.1 mg/ml poly-D-lysine). The transfection mixture consisted of 0.8 μg of plasmid DNA and 2 μl Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA). Transfected cells were incubated at 37°C for 48 hours before use.
Surface biotinylation
The method described here is a modification of the one published by Ho et al., 2004. Briefly, forty eight hours after transfection, HEK293 cells were washed with ice-cold phosphate-buffered saline Ca2+/Mg2+ (PBS-CM; 138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.4) and then treated with 1 mg/ml membrane-impermeable biotinylating agent sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate (sulfo-NHS-SS-biotin; Pierce, Rockford, IL) at 4°C for 1 hour. Subsequently, cells were washed three times with ice cold PBS-CM containing 100 mM glycine to remove the remaining labeling reagent. Then, cells were lysed in 300 μl lysis buffer (150 mM NaCl, 10 mM Tris·HCl, 1 mM EDTA, 1% Triton X-100 and 0.1% SDS, pH 7.5) containing protease inhibitors (Complete, Roche Applied Science, Indianapolis, IN) at 4°C with constant agitation for 1 hour. Following centrifugation at 10,000 × g (4°C) for 2 minutes, 50& mu;l of NeutrAvidin (Pierce) beads were added to 250 μl of cell lysate supernatant and incubated at room temperature for 1 hour with constant agitation. Beads were then washed 4 times with lysis buffer, and the biotinylated proteins were recovered from the beads by incubating with 2xLaemmli buffer with 50 mM DTT for 30 minutes at room temperature.
SDS-PAGE and Western blotting
Forty micro liters of surface proteins were separated on 8% SDS-PAGE minigels at 150V for 1 hour and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membrane was blocked for 1 hour with 5% non fat dry milk in PBS at room temperature, followed by overnight incubation with anti-OATP1B1 antibody (1:2500 dilution) at 4°C. After washing with 0.1% PBS/Tween-20, the membrane was incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Pierce) for 1 hour at room temperature in 2.5% milk in PBS (1:10,000 dilution). Following extensive washing with 0.1% PBS/Tween-20, the secondary antibody was detected using the ECL kit (Amersham™, Buckinghamshire, UK). Protein loading was normalized using the plasma membrane marker Na+/K+ ATPase α subunit antibody (Abcam, Cambridge, MA) (1:5000 dilution). Horseradish peroxidase-conjugated goat anti-mouse IgG (Pierce) was used as secondary antibody (1:10,000 dilution). Immunoblots were developed using the ECL plus kit (Amersham™). The intensity of protein bands was quantified using ImageJ (http://rsbweb.nih.gov/ij/). Band intensities at 5–6 different exposure times were quantified to ensure that the signal was within the linear range of the film.
Transport assay
After washing the cells three times with prewarmed (37°C) uptake buffer modified from Cui et al. (Cui et al., 2001) (142 mM NaCl, 5 mM KCl, 1 mM KH2PO4, 1.2 mM MgSO4, 1.5 mM CaCl2, 5 mM glucose, and 12.5 mM HEPES, pH 7.4), 200 μl uptake buffer containing radiolabeled substrates with sufficient unlabeled compound to achieve the indicated concentrations were added to initiate transport. After incubating for the indicated time periods, transport was terminated by four washes with ice-cold uptake buffer. Cells were lysed with 300 μl 1% Triton X-100 at room temperature for 30 minutes. Two hundred micro liters of cell lysate were transferred to 24-well scintillation plates (Perkin Elmer, Shelton, CT) and radioactivity was measured after adding Optiphase Supermix scintillation cocktail (Perkin Elmer) in a MicroBeta liquid scintillation counter. The remaining 100 μl cell lysate were used to determine the protein concentration using the BCA™ Protein Assay (Pierce). All transport activities in transiently transfected HEK293 cells expressing wild type or mutated OATP1B1 were measured within the initial linear time range and corrected by total protein concentration.
Kinetic analysis
Kinetics for wild type OATP1B1 and mutants were all determined within the initial linear time range. Transport of radiolabeled estradiol-17β-glucuronide was measured from 1 μM to 50 μM for 1 min; transport of radiolabeled estrone-3-sulfate was measured from 0.1 μM to 2 μM for 30 sec and transport of radiolabeled sulfobromophthalein was measured from 0.05 μM to 3 μM for 1 min. Transporter-specific uptake was obtained by subtracting the uptake into empty vector-transfected cells from the uptake into OATP1B1-transfected cells. Michaelis-Menten type nonlinear curve fitting was carried out to obtain estimates of the maximal uptake rate (Vmax) and the apparent affinity constant (Km) (Graphpad Prism, GraphPad Software Inc., La Jolla, CA).
Statistical analysis
Statistical significance was calculated using the two-tailed unpaired Student’s t-test and a p value of < 0.05 was considered significant.
RESULTS AND DISCUSSION
Functional characterizations of wild type OATP1B1 transiently transfected in HEK293 cells
Because OATP1B1 is a multispecific transporter (Hagenbuch & Gui, 2008) and because for certain substrates multiple substrate binding sites have been suggested (Hagenbuch & Gui, 2008; Noe et al., 2007; Tamai et al., 2001), we established normal OATP1B1 function by characterizing uptake of the three model substrates [3H]-estradiol-17β-glucuronide, [3H]-estrone-3-sulfate and [3H]-bromosulfophthalein (BSP) in transiently transfected HEK293 cells. OATP1B1-mediated uptake of estradiol-17β-glucuronide was linear at both low (1 μM) and high (50 μM) substrate concentration for at least 1 min. Kinetic experiments performed at 1 min revealed a Km value of 5.35 ± 0.54 μM, a value well within the range of published values for estradiol-17β-glucuronide reported with other expression systems (Cui et al., 2001; Gui et al., 2008; Hirano et al., 2004; König et al., 2000; Tamai et al., 2001). Similar as estradiol-17β-glucuronide, transport of estrone-3-sulfate by HEK293 cells transiently transfected with wild type OATP1B1 was linear at least over 30 sec at 0.1, 1 and 50 μM and therefore kinetics were performed at 30 sec. Although two binding sites were identified for OATP1B1 mediated estrone-3-sulfate transport (Gui & Hagenbuch, 2009; Noe et al., 2007; Tamai et al., 2001), we only investigated the high affinity site and could confirm that the Km of 0.55 ± 0.12 μM was comparable to previously published values (Gui & Hagenbuch, 2009; Hirano et al., 2004; Noe et al., 2007). Uptake of the other high affinity substrate of OATP1B1, BSP (Cui et al., 2001; Kullak-Ublick et al., 2001) was linear over at least 1 min both at low (0.02 μM) and high (3 μM) concentrations. Therefore, concentration dependent uptake of BSP was measured at 1 min and the Km value of 0.46 ± 0.04 μM was in the same range as values previously published (Cui et al., 2001; Kullak-Ublick et al., 2001). Taken together, these results demonstrated that our transient expression system with HEK293 cells was suitable to characterize uptake mediated by OATP1B1 and its mutants.
Expression of OATP1B1 Mutants in HEK293 cells
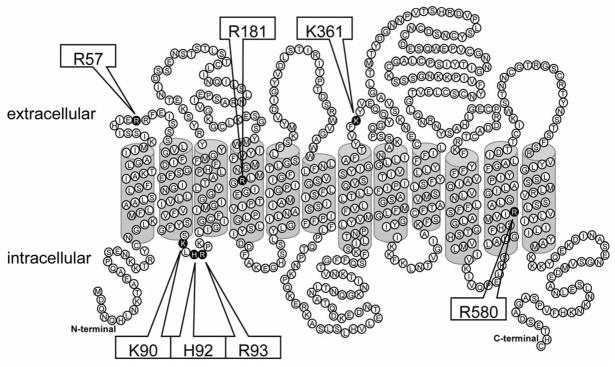
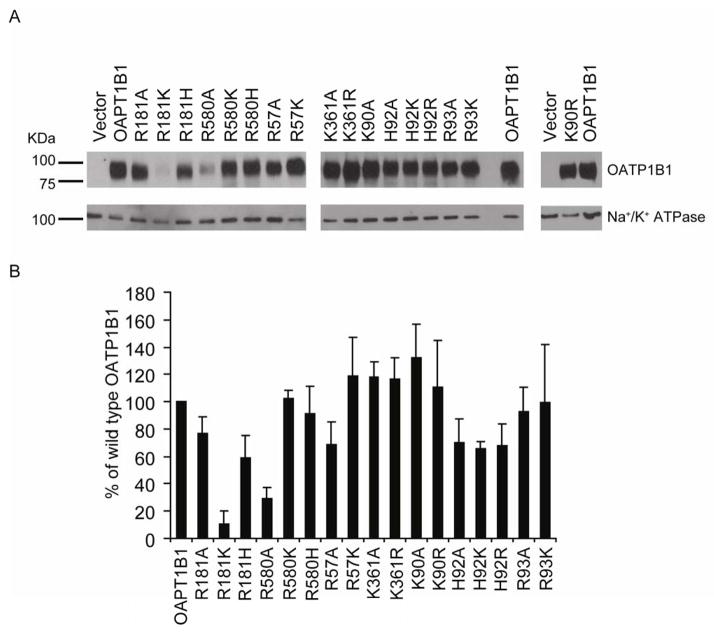
To determine the functional effects of the individual conserved positively charged amino acids facing the putative binding pocket (Meier-Abt et al., 2005), we performed site-directed mutagenesis and changed amino acid residues at the seven positions indicated in Figure 1; R57 and K361 at the predicted extracellular side, R181 and R580 in predicted TM 4 and 11, and K90, H92 and R93 at the predicted intracellular side of OATP1B1 were individually replaced with alanine and other charged amino acids such as lysine, arginine or histidine. Both wild type and mutated OATP1B1 were then transiently expressed in HEK293 cells. Membrane proteins were purified using surface biotinylation, and western blot analysis was performed using an anti-OATP1B1 antibody targeted to the cytoplasmic C-terminal end. Thus, none of these mutations would affect the antibody recognition site and differences on the western blots would reflect different amounts of OATP1B1 at the plasma membrane of HEK293 cells. Na+/K+ ATPase, a membrane protein naturally expressed in all HEK293 cells, was used as loading control for surface proteins. As demonstrated in Figure 2A, all OATP1B1 constructs were detectable at the cell surface, two of them (R181K and R580A) at strongly reduced levels. We quantified western blots from 3 such individual and independent experiments and the data are shown in Figure 2B. It can be seen that R181K and R580A were only expressed at 10 and 30 % of the value of wild-type OATP1B1 at the cell surface, respectively. Total protein expression of these two mutants compared to wild-type OATP1B1 was also very low (data not shown), suggesting that there were problems with protein synthesis and/or folding rather than only problems with the translocation to the plasma membrane. Surface expression levels of R181A, R181H, R57A, H92A, H92K and H92R were between 60% and 80% of the surface expression of wild type OATP1B1. Expression of all the other mutants at the surface of HEK293 cells was comparable to wild type OATP1B1. None of the mutations affected HEK293 cell growth rate and therefore, the low surface expression can be explained by impaired protein synthesis, folding, stability or trafficking to the cell surface. Because the reduced surface expression of R57A and R580A could be rescued by re-introducing a positive R57K and R580K, we conclude that a positive charge at positions 57 and 580 could be important for normal synthesis and trafficking of OATP1B1 to the cell surface or for its normal anchoring in the cell membrane. Surface expression levels of R181A and R181H were 77% and 59% of wild-type OATP1B1, respectively. However, R181K surface expression was only 10% of wild-type and the total expression level of this mutation was also drastically reduced (data not shown), indicating that a lysine at this position is not compatible with normal protein expression. This conclusion is supported by amino acid sequence alignments with over 50 OATPs/Oatps that demonstrate that at this position an arginine is absolutely conserved in the OATP1 family. OATPs/Oatps in other families have different amino acids at this position, including alanines or histidine but none of the amino acid residues is a lysine. Thus, we conclude that a lysine at position 181, although it is positively charged like the normally found arginine, does not lead to normal surface expression of the protein. Expression levels of all mutants except for the very low expressed R181K and R580A correlated well with the different exposure times of the films. Therefore, the surface corrected Vmax values for R580A might be overcorrected.
Figure 1. Predicted topological model of human OATP1B1 with mutation sites.
The topological structure of OATP1B1 was predicted by TMPred (http://www.ch.embnet.org/software/TMPRED_form.html). Amino acids mutated in this study are indicated in black and the positions are listed in the boxes pointing to these residues.
Figure 2. Surface expressions of OATP1B1 mutants in HEK293 cells.
(A) Representative Western blots of surface-biotinylated proteins detected with a polyclonal anti-OATP1B1 antibody (75 kDa). HEK293 cells transfected with empty vector (Vector) were used as negative control. The same blot was also probed with an antibody against the plasma membrane marker Na+/K+-ATPase α subunit (103 kDa) as a surface protein loading control. (B) Quantification of surface expression normalized to the loading control Na+/K+-ATPase. The relative intensities are presented as a percentage of wild type OATP1B1 (OATP1B1). Band intensities at 5–6 different exposure times were quantified using Image J to ensure that the signal was within the linear range of the film. Each bar is mean ± standard deviation (SD) of three independent experiments.
Initial uptake rates of OATP1B1 mutants
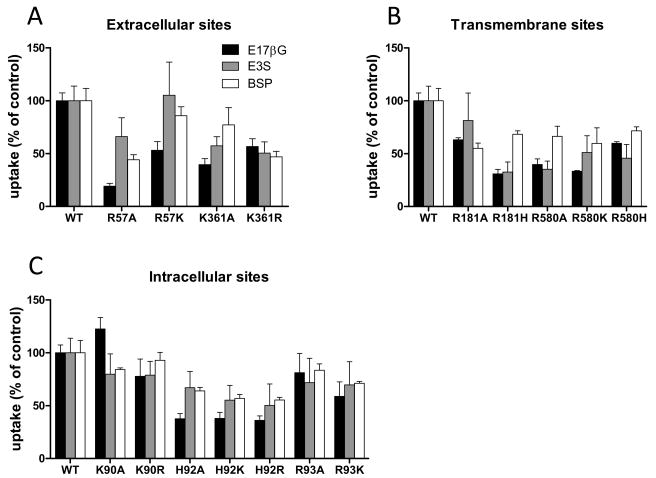
In order to screen all sixteen mutants for their effects on transport of the three OATP-model substrates, we measured uptake of 1 μM estradiol-17β-glucuronide, 0.1 μM estrone-3-sulfate and 0.05 μM BSP under initial linear rate conditions. The results are summarized in Figure 3. The extracellular R57 when mutated to an alanine or lysine exhibited substrate specific effects with uptake of estradiol-17β-glucuronide being the most and estrone-3-sulfate the least compromised. BSP uptake rates were in between the rates of estradiol-17β-glucuronide and estrone-3-sulfate (Fig. 3A). The other extracellular residue, K361 showed less substrate specific effects. In particular, K361R exhibited only about 50 % of the uptake rates for all three substrates as compared to wild-type OATP1B1 (Fig. 3A). For the two transmembrane residues uptake rates for estradiol-17β-glucuronide and estrone-3-sulfate were in general lower than the ones for BSP (Fig. 3B), except for R181A and R580H which had similar rates for all three different substrates. For the intracellular residues (Fig. 3C) mutations at K90 had no affect on any of the three substrates tested. At H92 all mutations resulted in a stronger decrease of estradiol-17β-glucuronide transport rates as compared to estrone-3-sulfate and BSP (Fig. 3C). It is important to emphasize that all these experiments were performed at a single time point and at a single substrate concentration within the initial linear range. Although such experiments can yield important information about changes in the transport activities of the tested proteins, kinetic analyses are needed to determine whether the reduced transport function is due to changes in the level of expression (Fig. 2), the apparent affinity and/or the translocation rate across the membrane. Therefore we performed kinetics with all three substrates and all sixteen mutants.
Figure 3. Uptake rates of estradiol-17β-glucuronide (E17βG), estrone-3-sulfate (E3S) and sulfobromophthalein (BSP) mediated by wild-type OATP1B1 and its mutants.
Uptake of 1 μM [3H]-E17βG (black bar) and 0.05 μM [3H]-BSP (white bar) was measured at 37°C for 1 minute; uptake of 0.1 μM [3H]-E3S (gray bar) was measured at 37°C for 30 seconds with HEK293 cells that were transfected with empty vector, OATP1B1 wild-type, or extracellular (A), transmembrane (B), and intracellular (C) mutants. The results were normalized for total protein expression in each well and presented as percentage of wild type OATP1B1 (WT). Each bar is the mean ± standard error (SE) of 5–6 independent experiments with triplicate determinations in each experiment.
Kinetic characterization of mutant OATPs
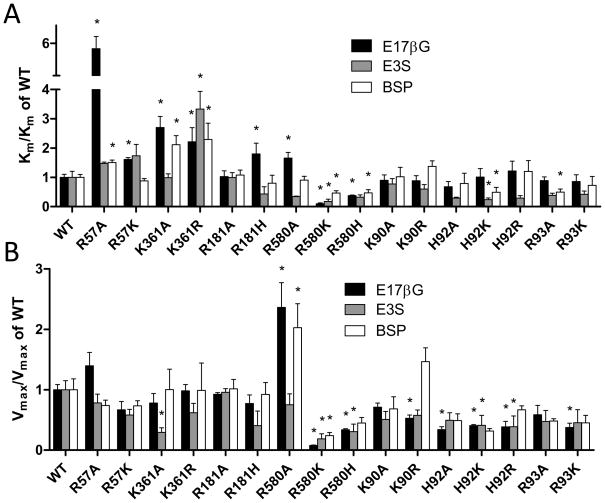
Kinetic parameters for the three model substrates estradiol-17β-glucuronide, estrone-3-sulfate and BSP are summarized for the extracellular mutants in Table 2, for the transmembrane mutants in Table 3, and for the intracellular mutants in Table 4. In order to better visualize the effects we calculated and compare the relative Km and Vmax values of all mutants in Figure 4. We discovered that there were some mutants that displayed almost no effects on Km (R181A, K90A, K90R, H92A, H92R and R93K) or Vmax values (R57A, R57K, K361R, R181A, R181H, K90A and R93A) for all three substrates tested, and some mutants that showed substrate-independent decreases in Km (R580K and R580H) or Vmax values (R580K, R580H, H92A, H92K, H92R and R93K). However, seven of the sixteen mutants showed substrate dependent effects on the Km values (R57A, R57K, K361A, R181H, R580A, H92K and R93A) and three of the mutants a substrate dependent effect on the Vmax values (K361A, R580A and K90R). Among these mutants that exhibited substrate dependent effects, two (the extracellular K361A and the transmembrane R580A) affected both Km and Vmax in a substrate dependent way.
Table 2.
Kinetic parameters of wild-type OATP1B1 and extracelluar mutants
| Substrate | Mutant | Km (μM) | Vmax (pmol/mg/min) |
|---|---|---|---|
| E17βG | WT | 5.35 ± 0.54 | 228 ± 19.8 |
| R57A | 30.5 ± 3.64 * | 319 ± 50.6 | |
| R57K | 8.62 ± 0.33 * | 152 ± 31.3 | |
| K361A | 14.5 ± 2.02 * | 178 ± 36.0 | |
| K361R | 11.9 ± 2.57 * | 224 ± 24.3 | |
| E3S | WT | 0.55 ± 0.12 | 38.6 ± 5.81 |
| R57A | 0.81 ± 0.03 | 30.2 ± 5.53 | |
| R57K | 0.96 ± 0.21 | 22.4 ± 3.64 | |
| K361A | 0.54 ± 0.07 | 11.34 ± 2.95 * | |
| K361R | 1.83 ± 0.33 * | 23.9 ± 5.90 | |
| BSP | WT | 0.46 ± 0.04 | 52.2 ± 9.42 |
| R57A | 0.69 ± 0.04 * | 38.5 ± 4.69 | |
| R57K | 0.40 ± 0.04 | 38.3 ± 4.34 | |
| K361A | 0.97 ± 0.14 * | 52.4 ± 17.6 | |
| K361R | 1.05 ± 0.15 * | 51.7 ± 23.7 |
Kinetic parameters of wild type and mutated OATP1B1-mediated E17βG (estradiol-17β-glucuronide), E3S (estrone-3-sulfate) and BSP (bromosulfophthalein) uptake were measured under initial linear rate conditions, corrected for surface expression and presented as mean ± SE of 3 to 13 independent experiments. Uptake of E17βG (1 μM to 50 μM) and BSP (0.05 μM to 3 μM) was measured at 37°C for 1 minute; uptake of E3S (0.05 μM to 2 μM) was measured at 37°C for 30 seconds;
p < 0.05.
Table 3.
Kinetic parameters of wild-type OATP1B1 and transmembrane mutants
| Substrate | Mutant | Km (μM) | Vmax (pmol/mg/min) |
|---|---|---|---|
| E17βG | WT | 5.35 ± 0.54 | 228 ± 19.8 |
| R181A | 5.48 ± 1.07 | 211 ± 6.71 | |
| R181H | 9.65 ± 1.95 * | 176 ± 32.5 | |
| R580A | 8.86 ± 1.04 * | 539 ± 93.2 * | |
| R580K | 0.52 ± 0.18 * | 17.8 ± 1.55 * | |
| R580H | 2.10 ± 0.12 * | 77.1 ± 3.88 * | |
| E3S | WT | 0.55 ± 0.12 | 38.6 ± 5.81 |
| R181A | 0.54 ± 0.09 | 37.0 ± 2.28 | |
| R181H | 0.24 ± 0.14 | 15.7 ± 9.27 | |
| R580A | 0.19 ± 0.01 | 29.0 ± 6.93 | |
| R580K | 0.10 ± 0.04 * | 7.27 ± 3.26 * | |
| R580H | 0.18 ± 0.04 | 11.8 ± 4.88 * | |
| BSP | WT | 0.46 ± 0.04 | 52.2 ± 9.42 |
| R181A | 0.49 ± 0.08 | 53.0 ± 8.01 | |
| R181H | 0.37 ± 0.12 | 48.1 ± 10.4 | |
| R580A | 0.42 ± 0.06 | 106 ± 20.7 * | |
| R580K | 0.21 ± 0.04 * | 12.5 ± 2.67 * | |
| R580H | 0.22 ± 0.05 * | 23.4 ± 4.78 |
Kinetics experiments were done under the same conditions as described in Table 2.
Table 4.
Kinetic parameters of wild-type OATP1B1 and intracelluar mutants
| Substrate | Mutant | Km (μM) | Vmax (pmol/mg/min) |
|---|---|---|---|
| E17βG | WT | 5.35 ± 0.54 | 228 ± 19.8 |
| K90A | 4.82 ± 0.96 | 162 ± 15.6 | |
| K90R | 4.72 ± 0.94 | 120 ± 12.4 * | |
| H92A | 3.63 ± 0.97 | 77.5 ± 11.2 * | |
| H92K | 5.41 ± 1.57 | 92.5 ± 4.12 * | |
| H92R | 6.53 ± 1.79 | 87.9 ± 20.8 * | |
| R93A | 4.76 ± 0.65 | 134 ± 35.3 | |
| R93K | 4.58 ± 1.23 | 85.5 ± 16.4 * | |
| E3S | WT | 0.55 ± 0.12 | 38.6 ± 5.81 |
| K90A | 0.43 ± 0.10 | 19.6 ± 5.09 | |
| K90R | 0.33 ± 0.08 | 22.3 ± 3.41 | |
| H92A | 0.17 ± 0.02 | 19.1 ± 4.77 | |
| H92K | 0.13 ± 0.03 * | 15.8 ± 6.37 * | |
| H92R | 0.16 ± 0.05 | 15.0 ± 6.86 * | |
| R93A | 0.21 ± 0.04 | 18.3 ± 7.00 | |
| R93K | 0.23 ± 0.06 | 17.5 ± 8.37 | |
| BSP | WT | 0.46 ± 0.04 | 52.2 ± 9.42 |
| K90A | 0.47 ± 0.15 | 35.6 ± 10.5 | |
| K90R | 0.63 ± 0.09 | 76.4 ± 11.9 | |
| H92A | 0.36 ± 0.16 | 25.5 ± 5.75 | |
| H92K | 0.22 ± 0.08 * | 16.5 ± 2.11 | |
| H92R | 0.55 ± 0.17 | 34.8 ± 3.48 | |
| R93A | 0.23 ± 0.05 * | 25.1 ± 2.05 | |
| R93K | 0.33 ± 0.14 | 23.6 ± 6.45 |
Kinetics experiments were done under the same conditions as described in Table 2.
Figure 4. Apparent Km and Vmax values relative to wild-type OATP1B1.
(A) Km and (B) Vmax values summarized in Tables 2, 3, and 4 were normalized to the values obtained with OATP1B1.
The extracellular mutants of R57 and K361
Kinetics for R57 and K361 that are predicted at the extracellular side of OATP1B1 are shown in Table 2. The major effects of mutations at R57 as compared to wild-type OATP1B1 were on the apparent affinities for the different substrates. Replacing the arginine by an alanine increased the Km value for estradiol-17β-glucuronide by 5.7 fold and the value for BSP by 1.5 fold (Table 2). Replacing the arginine by the positively charged lysine resulted in a Km value that was now only 1.6 fold higher for estradiol-17β-glucuronide and not significantly different for BSP (Table 2). These results suggest that a positive charge at position 57 is favorable for normal estradiol-17β-glucuronide and BSP binding or transport and changing this amino acid residue to an alanine reduces transport significantly, especially at low substrate concentrations.
The Vmax values for R57A and R57K were similar to wild-type OATP1B1 for estradiol-17β-glucuronide, estrone-3-sulfate and BSP (Table 2). Although expression of R57A at the surface was reduced by 30% and expression of R57K was increased to 120% (Fig. 2) these different expression levels did not affect Vmax values significantly for all the three different substrates. Therefore, R57 may somehow be involved in substrate recognition or translocation especially for estradiol-17β-glucuronide and BSP. Overall, a positive charge at position 57 seems to be preferable for normal OATP1B1 expression and function and this conclusion is supported by the fact that in all 11 human OATPs there is a positive charge at the position corresponding to R57 of OATP1B1 (arginine: OATP1A2, OATP1B1, OATP1B3, OATP1C1, OATP3A1, OATP4A1, and OATP5A1; lysine: OATP2A1, OATP2B1, OATP4C1, and OATP6A1).
We also noted substrate dependent differences for mutations at position K361. An alanine at position 361 increased the apparent Km values for estradiol-17β-glucuronide and BSP but not for estron-3-sulfte. However, the Vmax value for estrone-3-sulfate was 3 times lower, explaining the reduced uptake seen in Figure 3A. An arginine at position 361 increased the apparent Km values for all three substrates to 2.2 to 3.3 fold but did not affect the Vmax values. Contrary to R57, the charge conserved mutation K361R did not improve the substrate binding affinity compared to the K361A mutation (Table 2). Therefore, the conserved lysine rather than simply the positive charge at this position seems to be important for OATP1B1 function. This conclusion is further supported by the fact that at position 361, lysine is conserved in all human OATPs except for OATP6A1 for which so far no functional data are available.
The transmembrane mutants R181 and R580
Because the expression of R181K was very low, we could not characterize its transport function in detail. Kinetics for R181 and R580 are summarized in Table 3. Replacing arginine at position 181 with an alanine did not affect the apparent Km or Vmax values for all three substrates tested. For R181H we found a 1.8 fold increase in the Km value for estradiol-17β-glucuronide but no significant changes for the other two substrates. Similarly, the maximal transport rates for the three substrates were not significantly different from wild-type OAT1B1 (Table 3). Thus, mutating R181 to a histidine decreased the affinity for estradiol-17β-glucuronide presumably because the aromatic imidazole ring of histidine at this position is less favorable for the binding or translocation of estradiol-17β-glucuronide. This conclusion is supported by the observation that the arginine at position 181 is only conserved within the OATP1 family and a histidine is only found in members of the OATP4A1 family, transporters that are much less effective in mediating uptake of estradiol-17β-glucuronide (Tamai et al., 2000).
At position R580, a residue that has been shown to be important for BSP transport in OATP1B3 (Glaeser et al., 2010), Km and Vmax values for the three different substrates were affected in different ways. For the alanine replacement, the Km value for estradiol-17β-glucuronide increased 1.7 fold, decreased for estrone-3-sulfate to 35% (p value = 0.069) did not change for BSP (Table 3). A similar pattern was seen for the effect on Vmax values. A 2.4 and 2 fold increase of the Vmax values was seen for estradiol-17β-glucuronide and BSP, respectively. The Vmax for estrone-3-sulfate was not changed. Replacing the arginine at position 580 by a lysine or histidine increased the apparent affinities (reduced the Km values) for all three substrates (p value = 0.065 for estrone-3-sulfate) with a parallel decrease in the respective maximal transport rates (Table 3). Thus, the increased apparent affinities might have resulted in the decreased maximal transport rates. Such decreased maximal transport rates, corrected for surface expression, indicates that the turnover number or translocation rate is decreased, possibly due to a higher affinity of the substrate to the transporter and therefore a less efficient release at the intracellular side. In order to investigate this further we will have to isolate membrane vesicles and perform detailed kinetic experiments with exactly controlled substrate concentrations on both sides of the membrane. Such experiments are not possible with whole cells and were therefore not included in these studies.. Therefore, R580 might be involved in either directly facilitating the substrate translocation or maintaining a correct conformation which is required for the translocation process. Previous studies with rat Oatp2a1 have shown that replacing R560, the arginine at the corresponding position of R580 in TM11 with the neutral asparagine abolished transport activity. However, substitution with the positively charged lysine resulted in a comparable Km but a greatly decreased Vmax value (Chan et al., 2002), indicating that arginine might be important in substrate translocation. Furthermore, the recent publication by Glaeser et al. 2010 has shown that R580K and R580G mutations in OATP1B3 also decreased the Vmax values for BSP transport while their Km values were slightly increased. In addition to the consistent observations in several OATPs/Oatps, the importance of an arginine at position 580 is also supported by the absolute conservation of this arginine residue at this position within all eleven human OATPs.
The intracellular mutants K90, H92 and R93
Mutations of the three intracellular residues K90, H92 and R93 had little substrate dependent effects. Besides decreased Km values for estrone-3-sulfate (H92K) and BSP (H92K and R93A) the major effects were seen on the Vmax values for estradiol-17β-glucuronide and estrone -3-sulfate (Table 4). The maximal transport rates for estradiol-17β-glucuronide were reduced for all the intracellular mutants although two were not statistical significant (K90A, p value = 0.14 and R93A, p value = 0.53). For estrone-3-sulfate transport R93K and H92R showed a significantly reduced Vmax value but all other mutants had also reduced Vmax values (p values between 0.068 and 0.10) while for BSP uptake only the Vmax for H92K was reduced (p value = 0.061). Thus, these intracellular positively charged amino acids seem to be more important for normal translocation of estradiol-17β-glucuronide and estrone-3-sulfate than for BSP. Being part of a short intracellular loop they might interact as some kind of gate for the transport of certain substrates.
The major findings of this study are 1) that some of the positively charged amino acids within the predicted binding pocket indeed are important for normal OATP1B1-mediated transport of estradiol-17β-glucuronide, estrone-3-sulfate and BSP, and 2) that several of the tested mutations affected OATP1B1 function in a substrate dependent way. Such substrate dependent effects have also been observed when naturally occurring polymorphisms were investigated. OATP1B1*1b (388A>G, N130D) and OATP1B1*5 (521T>C, V174A) are the two most frequently observed polymorphisms (König et al., 2006; Nozawa et al., 2002; Tirona et al., 2001). In vitro, N130D does not affect surface expression of the protein or transport of estradiol-17β-glucuronide and estrone-3-sulfate (Iwai et al., 2004; Kameyama et al., 2005; Tirona et al., 2001) but it reduces uptake of rifampicin and taurocholate (Michalski et al., 2002; Tirona et al., 2003) and increases uptake of bromosulfophthalein (BSP) (Michalski et al., 2002). In vivo, N130D correlates with lower serum levels of ezetimibe, pravastatin and tacrolimus (Elens et al., 2007; Maeda et al., 2006; Oswald et al., 2008) and with an increased oral clearance of torasemide (Vormfelde et al., 2008). Thus, compared to wild-type OATP1B1, OATP1B1*1b has unchanged or increased or decreased transport rates depending on the substrate that is transported. The polymorphism V174A alone or together with N130D (OATP1B1*15) decreases clearance and increases plasma concentration of pravastain, simvastatin, ezetimibe, SN-38 and bilirubin (Han et al., 2008; Ieiri et al., 2004; Neuvonen et al.,, 2008; Niemi et al., 2004; Nishizato et al., 2003; Nozawa et al., 2005; Oswald et al., 2008; Pasanen et al., 2008; van der Deure et al., 2008; Zhang et al., 2007). In vitro studies using transiently transfected cell lines suggest that the decreased transport activity of OATP1B1*15 may be due to decreased protein expression on the cell surface (Kameyama et al., 2005) or due to decreased turnover number of the mutated transporter (Iwai et al., 2004).
In conclusion, for proteins with unknown structure and transport mechanisms, comparative modeling combined with the analysis of conserved amino acid residues is a powerful tool to identify candidate residues that may play important roles in structure and/or function of a transporter. Site-directed mutagenesis provided useful structural and mechanistic information for the bacterial transporters in the major facilitator superfamily, lactose permease (Abramson et al., 2003), glycerol-3-phosphate transporter (Huang et al., 2003) and multidrug transporter EmrD (Yin et al., 2006) prior to their crystallization. Because of the mainly anionic nature of OATP1B1 substrates, in this study we used site-directed mutagenesis to investigate the roles of several conserved positively charged amino acids facing the putative binding pocket of OATP1B1. We have demonstrated the important role of the positive charge at R580 for surface expression. In addition, substrate dependent effects were seen for the extracellular mutants at R57 and K361 mainly on the Km values, for the transmembrane R181 and R580 on both, the Km and Vmax values, and for the intracellular K90, H92 and R93 mainly on the Vmax values, suggesting the possibility of multiple binding sites for the different substrates. These findings provided experimental validations for the prediction of the computer model and are important steps towards elucidating the structure-function relationship of OATP1B1.
Acknowledgments
We thank Patrick A. Courtney for his help with some of the mutations.
FUNDING
This work was supported by the National Institutes of Health grant numbers RR021940 and GM077336 and by an unrestricted grant from the 3M Company.
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, Matsuno S, Yawo H. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–63. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, Nunoki K, Sato E, Kakyo M, Nishio T, Sugita J, Asano N, Tanemoto M, Seki M, Date F, Ono K, Kondo Y, Shiiba K, Suzuki M, Ohtani H, Shimosegawa T, Iinuma K, Nagura H, Ito S, Matsuno S. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120:1689–99. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S. Structure and mechanism of the lactose permease of Escherichia coli. Science. 2003;301:610–5. doi: 10.1126/science.1088196. [DOI] [PubMed] [Google Scholar]
- Chan BS, Bao Y, Schuster VL. Role of conserved transmembrane cationic amino acids in the prostaglandin transporter PGT. Biochemistry. 2002;41:9215–21. doi: 10.1021/bi0203031. [DOI] [PubMed] [Google Scholar]
- Cui Y, König J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J Biol Chem. 2001;276:9626–30. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Elens L, Capron A, Kerckhove VV, Lerut J, Mourad M, Lison D, Wallemacq P, Haufroid V. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17:873–83. doi: 10.1097/FPC.0b013e3282e9a533. [DOI] [PubMed] [Google Scholar]
- Glaeser H, Mandery K, Sticht H, Fromm MF, König J. Relevance of conserved lysine and arginine residues in transmembrane helices for the transport activity of organic anion transporting polypeptide 1B3. Br J Pharmacol. 2010 doi: 10.1111/j.1476-5381.2009.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Hagenbuch B. Role of transmembrane domain 10 for the function of organic anion transporting polypeptide 1B1. Protein Sci. 2009;18:2298–306. doi: 10.1002/pro.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, Hagenbuch B. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur J Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008;38:778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–65. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Kim HT, Lee JS. Influence of the organic anion-transporting polypeptide 1B1 (OATP1B1) polymorphisms on irinotecan-pharmacokinetics and clinical outcome of patients with advanced non-small cell lung cancer. Lung Cancer. 2008;59:69–75. doi: 10.1016/j.lungcan.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J Pharmacol Exp Ther. 2004;311:139–46. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Ho RH, Leake BF, Roberts RL, Lee W, Kim RB. Ethnicity-dependent polymorphism in Na+-taurocholate cotransporting polypeptide (SLC10A1) reveals a domain critical for bile acid substrate recognition. J Biol Chem. 2004;279:7213–22. doi: 10.1074/jbc.M305782200. [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J Biol Chem. 1999;274:37161–8. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Huang Y, Lemieux MJ, Song J, Auer M, Wang DN. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301:616–20. doi: 10.1126/science.1087619. [DOI] [PubMed] [Google Scholar]
- Ieiri I, Suzuki H, Kimura M, Takane H, Nishizato Y, Irie S, Urae A, Kawabata K, Higuchi S, Otsubo K, Sugiyama Y. Influence of common variants in the pharmacokinetic genes (OATP-C, UGT1A1, and MRP2) on serum bilirubin levels in healthy subjects. Hepatol Res. 2004;30:91–95. doi: 10.1016/j.hepres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Iwai M, Suzuki H, Ieiri I, Otsubo K, Sugiyama Y. Functional analysis of single nucleotide polymorphisms of hepatic organic anion transporter OATP1B1 (OATP-C) Pharmacogenetics. 2004;14:749–57. doi: 10.1097/00008571-200411000-00006. [DOI] [PubMed] [Google Scholar]
- Kameyama Y, Yamashita K, Kobayashi K, Hosokawa M, Chiba K. Functional characterization of SLCO1B1 (OATP-C) variants, SLCO1B1*5, SLCO1B1*15 and SLCO1B1*15+C1007G, by using transient expression systems of HeLa and HEK293 cells. Pharmacogenet Genomics. 2005;15:513–22. doi: 10.1097/01.fpc.0000170913.73780.5f. [DOI] [PubMed] [Google Scholar]
- König J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278:G156–64. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- König J, Seithel A, Gradhand U, Fromm MF. Pharmacogenomics of human OATP transporters. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:432–43. doi: 10.1007/s00210-006-0040-y. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–33. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, Hirano M, Watanabe T, Kitamura Y, Kusuhara H, Sugiyama Y. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther. 2006;79:427–39. doi: 10.1016/j.clpt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J Membr Biol. 2005;208:213–27. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- Michalski C, Cui Y, Nies AT, Nuessler AK, Neuhaus P, Zanger UM, Klein K, Eichelbaum M, Keppler D, König J. A naturally occurring mutation in the SLC21A6 gene causing impaired membrane localization of the hepatocyte uptake transporter. J Biol Chem. 2002;277:43058–63. doi: 10.1074/jbc.M207735200. [DOI] [PubMed] [Google Scholar]
- Miyagawa M, Maeda K, Aoyama A, Sugiyama Y. The eighth and ninth transmembrane domains in organic anion transporting polypeptide 1B1 affect the transport kinetics of estrone-3-sulfate and estradiol-17beta-D-glucuronide. J Pharmacol Exp Ther. 2009;329:551–7. doi: 10.1124/jpet.108.148411. [DOI] [PubMed] [Google Scholar]
- Neuvonen PJ, Backman JT, Niemi M. Pharmacokinetic comparison of the potential over-the-counter statins simvastatin, lovastatin, fluvastatin and pravastatin. Clin Pharmacokinet. 2008;47:463–74. doi: 10.2165/00003088-200847070-00003. [DOI] [PubMed] [Google Scholar]
- Niemi M, Schaeffeler E, Lang T, Fromm MF, Neuvonen M, Kyrklund C, Backman JT, Kerb R, Schwab M, Neuvonen PJ, Eichelbaum M, Kivisto KT. High plasma pravastatin concentrations are associated with single nucleotide polymorphisms and haplotypes of organic anion transporting polypeptide-C (OATP-C, SLCO1B1) Pharmacogenetics. 2004;14:429–40. doi: 10.1097/01.fpc.0000114750.08559.32. [DOI] [PubMed] [Google Scholar]
- Nishizato Y, Ieiri I, Suzuki H, Kimura M, Kawabata K, Hirota T, Takane H, Irie S, Kusuhara H, Urasaki Y, Urae A, Higuchi S, Otsubo K, Sugiyama Y. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin Pharmacol Ther. 2003;73:554–65. doi: 10.1016/S0009-9236(03)00060-2. [DOI] [PubMed] [Google Scholar]
- Noe J, Portmann R, Brun ME, Funk C. Substrate-dependent drug-drug interactions between gemfibrozil, fluvastatin and other organic anion-transporting peptide (OATP) substrates on OATP1B1, OATP2B1, and OATP1B3. Drug Metab Dispos. 2007;35:1308–14. doi: 10.1124/dmd.106.012930. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Minami H, Sugiura S, Tsuji A, Tamai I. Role of organic anion transporter OATP1B1 (OATP-C) in hepatic uptake of irinotecan and its active metabolite, 7-ethyl-10-hydroxycamptothecin: in vitro evidence and effect of single nucleotide polymorphisms. Drug Metab Dispos. 2005;33:434–9. doi: 10.1124/dmd.104.001909. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A, Yokoi T. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J Pharmacol Exp Ther. 2002;302:804–13. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- Oswald S, König J, Lutjohann D, Giessmann T, Kroemer HK, Rimmbach C, Rosskopf D, Fromm MF, Siegmund W. Disposition of ezetimibe is influenced by polymorphisms of the hepatic uptake carrier OATP1B1. Pharmacogenet Genomics. 2008;18:559–68. doi: 10.1097/FPC.0b013e3282fe9a2c. [DOI] [PubMed] [Google Scholar]
- Pasanen MK, Miettinen TA, Gylling H, Neuvonen PJ, Niemi M. Polymorphism of the hepatic influx transporter organic anion transporting polypeptide 1B1 is associated with increased cholesterol synthesis rate. Pharmacogenet Genomics. 2008;18:921–6. doi: 10.1097/FPC.0b013e32830c1b5f. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem Biophys Res Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm Res. 2001;18:1262–9. doi: 10.1023/a:1013077609227. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J Biol Chem. 2001;276:35669–75. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther. 2003;304:223–8. doi: 10.1124/jpet.102.043026. [DOI] [PubMed] [Google Scholar]
- van der Deure WM, Friesema EC, de Jong FJ, de Rijke YB, de Jong FH, Uitterlinden AG, Breteler MM, Peeters RP, Visser TJ. Organic anion transporter 1B1: an important factor in hepatic thyroid hormone and estrogen transport and metabolism. Endocrinology. 2008;149:4695–701. doi: 10.1210/en.2008-0169. [DOI] [PubMed] [Google Scholar]
- Vormfelde SV, Toliat MR, Schirmer M, Meineke I, Nurnberg P, Brockmoller J. The polymorphisms Asn130Asp and Val174Ala in OATP1B1 and the CYP2C9 allele *3 independently affect torsemide pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;83:815–7. doi: 10.1038/sj.clpt.6100404. [DOI] [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1052–9. doi: 10.1152/ajpgi.00584.2007. [DOI] [PubMed] [Google Scholar]
- Yin Y, He X, Szewczyk P, Nguyen T, Chang G. Structure of the multidrug transporter EmrD from Escherichia coli. Science. 2006;312:741–4. doi: 10.1126/science.1125629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, He YJ, Gan Z, Fan L, Li Q, Wang A, Liu ZQ, Deng S, Huang YF, Xu LY, Zhou HH. OATP1B1 polymorphism is a major determinant of serum bilirubin level but not associated with rifampicin-mediated bilirubin elevation. Clin Exp Pharmacol Physiol. 2007;34:1240–4. doi: 10.1111/j.1440-1681.2007.04798.x. [DOI] [PubMed] [Google Scholar]