Abstract
Objective
To compare the rates of depression in Alzheimer Disease (AD) determined using National Institute of Mental Health (NIMH) provisional criteria for depression in AD (NIMH-dAD) to those determined using other established depression assessment tools.
Design
Descriptive longitudinal cohort study.
Setting
The Alzheimer’s Disease Research Centers of California.
Participants
A cohort of 101 patients meeting NINDS-ADRDA criteria for possible/probable AD, intentionally selected to increase the frequency of depression at baseline.
Measurements
Depression was diagnosed at baseline and after 3 months using NIMH-dAD criteria and the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I Disorders. Depressive symptoms also were assessed with the Cornell Scale for Depression in Dementia (CSDD), the Geriatric Depression Scale (GDS), and the Neuropsychiatric Inventory Questionnaire.
Results
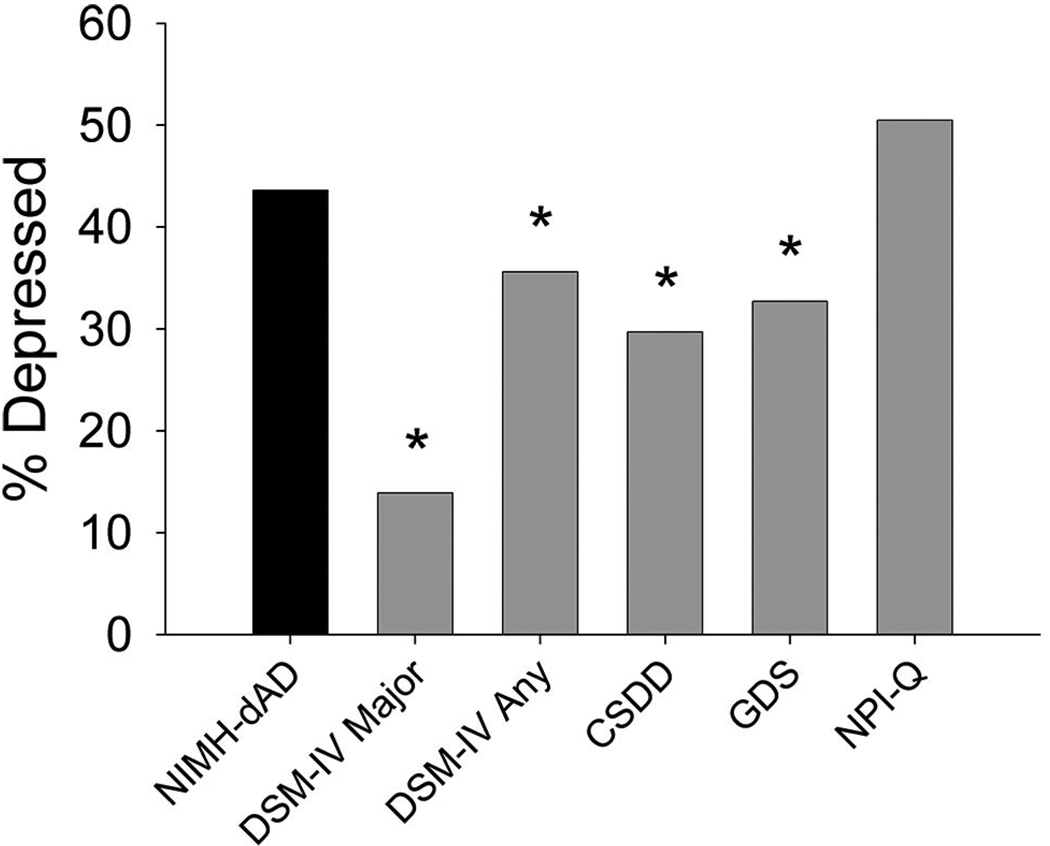
The baseline frequency of depression using NIMH-dAD criteria (44%) was higher than that obtained using DSM-IV criteria for major depression (14%; Z = −5.50, df = 101, p <0.001) and major or minor depression (36%; Z = −2.86, df = 101, p = 0.021) or using established cut-offs for the CSDD (30%; Z = −2.86, df = 101, p = 0.004) or GDS (33%; Z = −2.04, df = 101, p = 0.041). The NIMH-dAD criteria correctly identified all patients meeting DSM-IV criteria for major depression, and correlated well with DSM-IV criteria for major or minor depression (κ = 0.753, p <0.001), exhibiting 94% sensitivity and 85% specificity. The higher rates of depression found with NIMH-dAD criteria derived primarily from its less stringent requirements for the frequency and duration of symptoms. Remission rates at 3 months were similar across instruments.
Conclusions
The NIMH-dAD criteria identify a greater proportion of AD patients as depressed than several other established tools.
Depressive symptoms in Alzheimer disease (AD) are associated with significant morbidity and distress for both patients and their caregivers.1 Depression associated with AD may be amenable to treatment with both pharmacological and nonpharmacological therapies.2 However, the optimal methods for identifying individuals with clinically significant depression who are most likely to benefit from such interventions remain uncertain. Prior studies have reported that the rates of depression in AD range anywhere from 0%3 to 87%4 although most estimates fall between 30% and 50%.1
Some of the variability associated with these estimates may stem from differences between specific study populations.1 Another significant source of variability arises from differences in the tools used to assess for mood changes across studies.5,6 Many of the established diagnostic criteria and rating scales used for evaluating depression in research studies, including the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV),7 the Hamilton Scale for Depression,8 the Beck Depression Inventory,9 and the Geriatric Depression Scale (GDS)10 were designed for use in other patient populations. Some of these instruments may have poorer validity when used to evaluate patients with AD,11–15 as demented individuals may lack insight into their moods. Furthermore, depression in AD appears to be qualitatively different from depression in elderly nondemented populations. Depression in AD is notable for a higher frequency of motivational disturbances, such as fatigue, psychomotor slowing, and apathy, whereas geriatric depression in the absence of cognitive impairment is marked by a higher frequency of mood symptoms, such as depressed mood, anxiety, suicidality, and sleep and appetitive disturbances.16,17 Rating scales for depression and other neuropsychiatric symptoms have been created for use with demented patients, such as the Neuropsychiatric Inventory18 and the Cornell Scale for Depression in Dementia (CSDD).19 However, these tools were primarily designed to assess for the presence and severity of specific symptoms, and were not intended for diagnostic use, although some investigators have assessed their diagnostic validity.5,6,15,20,21
In 2001, the National Institute of Mental Health convened an expert panel that developed a provisional set of diagnostic criteria for depression in AD (NIMH-dAD; Table 1).22 These criteria were derived from DSM-IV criteria for major depression, with a few key distinctions. The number of symptoms required for a diagnosis of depression was decreased from five to three. The duration and frequency of depressive symptoms was also decreased; symptoms need only be present together within the same 2-week period, as compared with DSM-IV requirement that symptoms be present “most of the day, nearly every day” for at least 2 weeks.7 The decreased ability to think and concentrate was eliminated due to its expected poor specificity in this population. The criteria for anhedonia were modified to focus on decreased affect and pleasure associated with social and other activities. Social isolation / withdrawal and irritability were added as new symptoms. These changes were instituted in order to better reflect the clinical features of depression in AD.23
TABLE 1.
NIMH Provisional Diagnostic Criteria for Depression in Alzheimer Disease
|
Adapted from Olin et al., 2002. 22
A recent retrospective analysis extrapolated NIMH-dAD diagnoses from data collected using standardized patient and caregiver interviews from the Cambridge Examination for Mental Disorders of the Elderly (CAMDEX) in a university memory clinic sample.6 This implementation of NIMH-dAD criteria identified a higher prevalence of depression (27.4%) than several other instruments, including DSM-IV, CAMDEX, and International Classification of Diseases (ICD-10) criteria.
The utility of NIMH-dAD criteria has not yet, to our knowledge, been prospectively assessed. In the current study, we performed a comparison of diagnostic tools for depression in AD through the analysis of data from 101 participants recruited as part of a multicenter longitudinal study. The purpose of this study was to determine the frequency and rates of remission for depression diagnosed with NIMH-dAD criteria relative to diagnoses obtained using other established assessments for depression.
METHODS
Research Participants
Participants were drawn from a larger cohort recruited though the 10 Alzheimer’s Disease Research Centers of California for a descriptive longitudinal study of depression in AD. Written consent, approved by the Institutional Review Board of each center, was obtained from participants or their designated surrogates. Inclusion criteria included 1) diagnosis of possible or probable AD,24 2) Mini-Mental Status Examination (MMSE)25 score ≥10, 3) stable use of antidementia medications, other psychotropic medications, and / or any other medications that might affect the function of central nervous system for at least 1 month before study enrollment, and 4) the availability of a caregiver / study partner capable of providing collateral information. Exclusion criteria included the presence of any neurological, toxic, or metabolic syndromes that could affect cognition.
Initial recruitment goals included a total enrollment of 300 participants and a 2:1 ratio of depressed (DSM-IV major or minor depression7) to nondepressed participants. Ultimately, 183 individuals were enrolled and 38% met DSM-IV criteria for major or minor depression. Assessments with NIMH-dAD criteria were introduced to the protocol partway through the enrollment phase of the study. A total of 101 (of 183) participants received baseline assessments with NIMH-dAD criteria and are included in the analyses presented here.
Assessment Tools
Each participant was assessed for depression at baseline and 3 months later using two diagnostic instruments (NIMH-dAD, DSM-IV) and three rating scales (CSDD, GDS, Neuropsychiatric Inventory Questionnaire [NPI-Q]). The NIMH-dAD criteria were operationalized using a specifically designed structured interview derived from prior guidelines established by Rosenberg et al.26 that probes for the presence of target symptoms (Appendix B). The presence of DSM-IV major or minor depression was ascertained using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I).27 The CSDD19 was administered in a standardized fashion; scores ≥8 were considered indicative of depression.20 Diagnoses using NIMH-dAD criteria, SCID-I, and CSDD were based upon clinician impression after separate interviews with both the participant and their caregiver. The GDS10 was administered to each participant, and scores ≥10 were considered indicative of depression.28 The NPI-Q29 was administered to each patient’s caregiver, and the presence of dysphoric symptoms was considered indicative of depression.6 At the 3-month follow-up visit, remission rates for depressive symptoms identified at baseline were determined through the readministration of the same diagnostic instruments used at the initial visit.
Statistical Analyses
Statistical analyses were performed using SPSS 14.0 for Windows (SPSS Inc., Chicago). Demographic variables were compared between NIMH-dAD depressed and nondepressed participants using chi-square tests and independent sample t tests. Among participants with baseline diagnoses of depression using any of the assessments (NIMH-dAD, DSM-IV major depression, DSM-IV major or minor depression, CSDD, GDS, or NPI-Q), demographic variables and remission rates were compared using one-way analyses of variance or Kruskal-Wallis tests. In these analyses, subsets of depressed patients identified with each instrument were treated as independent groups.
Baseline rates of depression were determined for each diagnostic criteria or assessment tool and compared across all assessments using Friedman’s test.30 Post hoc comparisons between NIMH-dAD criteria and the other assessments were performed using the Wilcoxon signed-ranks test with subsequent Bonferroni correction for multiple comparisons. The concordance between diagnoses obtained using NIMH-dAD criteria and the other assessment tools was determined using Cohen’s kappa statistic.
The presence of the specific depressive symptoms specified by NIMH-dAD criteria was compared between depressed and nondepressed participants using chi-square tests with Bonferroni correction. The presence of significant associations between individual NIMH-dAD symptoms and a NIMH-dAD diagnosis of depression was investigated using a logistic regression analysis with 8 of the 10 symptoms entered as covariates. Symptoms of depressed mood and decreased positive affect or pleasure were excluded from this analysis, since NIMH-dAD criteria require that at least one of these symptoms be present for a diagnosis of depression.
RESULTS
At baseline, 44% of our highly selected cohort fulfilled NIMH-dAD criteria for depression. Demographic comparisons between depressed and nondepressed individuals (Table 2) indicated that those who were depressed were marginally more likely to be female and had marginally fewer years of formal education. There were no significant differences between the depressed and nondepressed groups in age or MMSE scores. Four participants (three depressed, one nondepressed) were excluded from the MMSE analysis due to miscoded data for one or more individual items. Demographic differences between the depressed and nondepressed groups did not survive Bonferroni correction (critical p = 0.0125).
TABLE 2.
Baseline Demographic Data for Depressed and Nondepressed Participants as Diagnosed With the NIMH-dAD Criteria
| Depressed | Not Depressed |
t/χ2 | p | |
|---|---|---|---|---|
| N | 44 | 57 | ||
| Age (SD) | 77.7 (7.7) | 77.3 (6.0) | 0.34 | 0.74 |
| % Male | 34% | 53% | 3.46 | 0.06 |
| Education (SD) | 13.3 (3.1) | 14.7 (3.8) | −1.86 | 0.07 |
| Antidepressant use | 55% | 19% | 13.62 | <0.001 |
| MMSE (SD) | 21.0 (5.3) | 21.2 (4.5) | −0.20 | 0.84 |
Notes: Age, education, and MMSE scores were analyzed with independent-samples t-tests (df = 99 for age and education; df = 95 for MMSE). Gender distribution and antidepressant use were analyzed with chi-square tests (df = 1,101).
Baseline rates of depression varied across assessment tools (Fig. 1; χ2 = 55.46, df = 5,100, p <0.001). Unadjusted comparisons indicated that the use of NIMH-dAD criteria resulted in higher rates of depression than the criteria for DSM-IV major depression (14%; Z = −5.5, df = 101, p <0.001) or DSM-IV criteria for major or minor depression (36%; Z = −2.31, df = 101, p = 0.021). The NIMH-dAD criteria also identified more patients as depressed than established cut-offs for the CSDD (30%; Z = −2.86, df = 101, p = 0.004) and the GDS (33%; Z = −2.04, df = 101, p = 0.041), but not the NPI-Q (50%; Z = −1.22, df = 100, p = 0.22). One participant, who did not meet NIMH-dAD criteria for depression, was missing baseline data for the NPI-Q dysphoria item and therefore was excluded from analyses involving this item. After Bonferroni correction (critical p = 0.01), NIMH-dAD criteria continued to produce significantly higher rates of depression than the criteria for DSM-IV major depression and the CSDD. Demographic comparisons between depressed patients identified with each assessment tool indicated that there were no significant differences in age, gender, education, antidepressant medication use, or MMSE scores between individuals identified with NIMH-dAD criteria versus the other assessments.
FIGURE 1. Baseline Rates of Depression as Determined With Different Assessment Tools.
NIMH-dAD: NIMH Provisional Diagnostic Criteria for Depression in Alzheimer Disease; DSM-IV Major: DSM-IV Major Depression; DSM-IV Any: DSM-IV Major or Minor Depression; CSDD: Cornell Scale for Depression in Dementia; GDS: Geriatric Depression Scale; NPI-Q: Neuropsychiatric Inventory Questionnaire. *p <0.05 versus NIMH-dAD (not corrected for multiple comparisons).
Investigation of the concordance between the different assessment tools indicated that diagnoses obtained with NIMH-dAD criteria most closely reflected those obtained with DSM-IV criteria for major or minor depression (Table 3). Relative to DSM-IV criteria for major depression, NIMH-dAD criteria exhibited sensitivity of 100%, specificity of 66%, positive predictive value (PPV) of 34%, and negative predictive value (NPV) of 100%. Relative to DSM-IV criteria for major or minor depression, NIMH-dAD criteria exhibited sensitivity of 94%, specificity of 85%, PPV of 77%, and NPV of 96%.
TABLE 3.
Concordance of Baseline Diagnoses With the NIMH-dAD Criteria With Diagnoses Obtained Using the Other Assessment Tools
| Assessment Tool | κ |
|---|---|
| DSM-IV Major Depression | 0.345 |
| DSM-IV Major or Minor Depression | 0.753 |
| Cornell Scale for Depression in Dementia | 0.499 |
| Geriatric Depression Scale | 0.399 |
| Neuropsychiatric Inventory Questionnaire | 0.342 |
Notes: All p values ≤0.001.
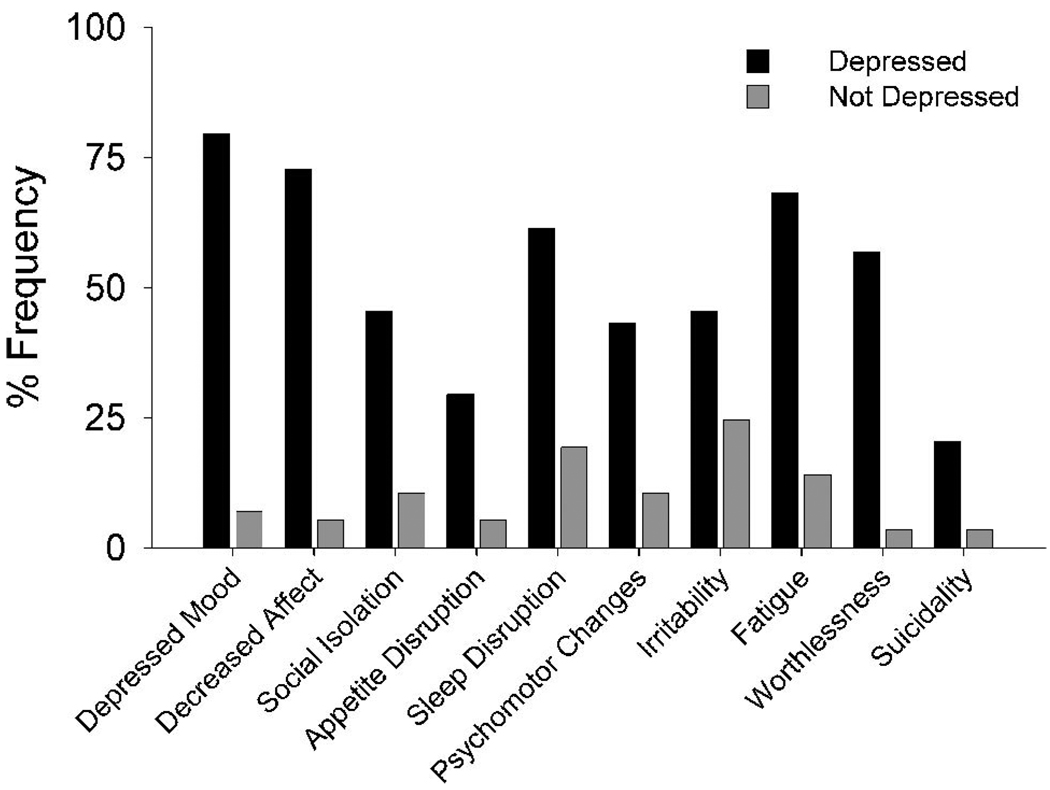
Analyses of the frequencies of the individual symptoms specified by NIMH-dAD criteria indicated that, as expected, depressed mood and decreased positive affect / pleasure were the most commonly reported symptoms among the depressed participants, since at least one of these two symptoms is required for a diagnosis of depression (Fig. 2). Conversely, appetitive disturbances and suicidality were the least commonly reported symptoms. Depressed individuals exhibited significantly more NIMH-dAD symptoms than nondepressed individuals (5.23 versus 1.04; t = 13.96, df = 99, p <0.001). Separate chi-square tests with Bonferroni correction (critical p = 0.005) indicated that all NIMH-dAD symptoms except for irritability (χ2 = 4.85, df = 1,101, p = 0.028) and suicidality (χ2 = 7.35, df = 1,101, p = 0.007) were significantly more common among depressed than nondepressed participants. Multivariate logistic regression analysis of the association between individual NIMH-dAD symptoms (excluding the two obligatory symptoms) and a diagnosis of depression yielded a Nagelkerke R2 of 0.71 and indicated that only psychomotor changes (β = 2.05, SE: 0.82; Wald χ2 = 6.27, odds ratio [OR] = 7.8, 95% confidence interval [CI]: 1.6–38.8; p = 0.012), fatigue (β = 2.83, SE: 0.82; Wald χ2 = 11.89, OR = 16.9, 95% CI: 3.4–84.2; p=0.001), and a sense of guilt / worthlessness (β = 3.46, SE: 1.05; Wald χ2 = 11.80, OR = 32.0, 95% CI: 4.1–252.4; p = 0.001) were independently predictive of depression. The model was not adjusted for baseline demographic variables or MMSE scores given the absence of significant differences between the depressed and nondepressed groups on these measures.
FIGURE 2. Baseline Frequencies of Individual NIMH-dAD Symptoms in Depressed and Nondepressed Participants.
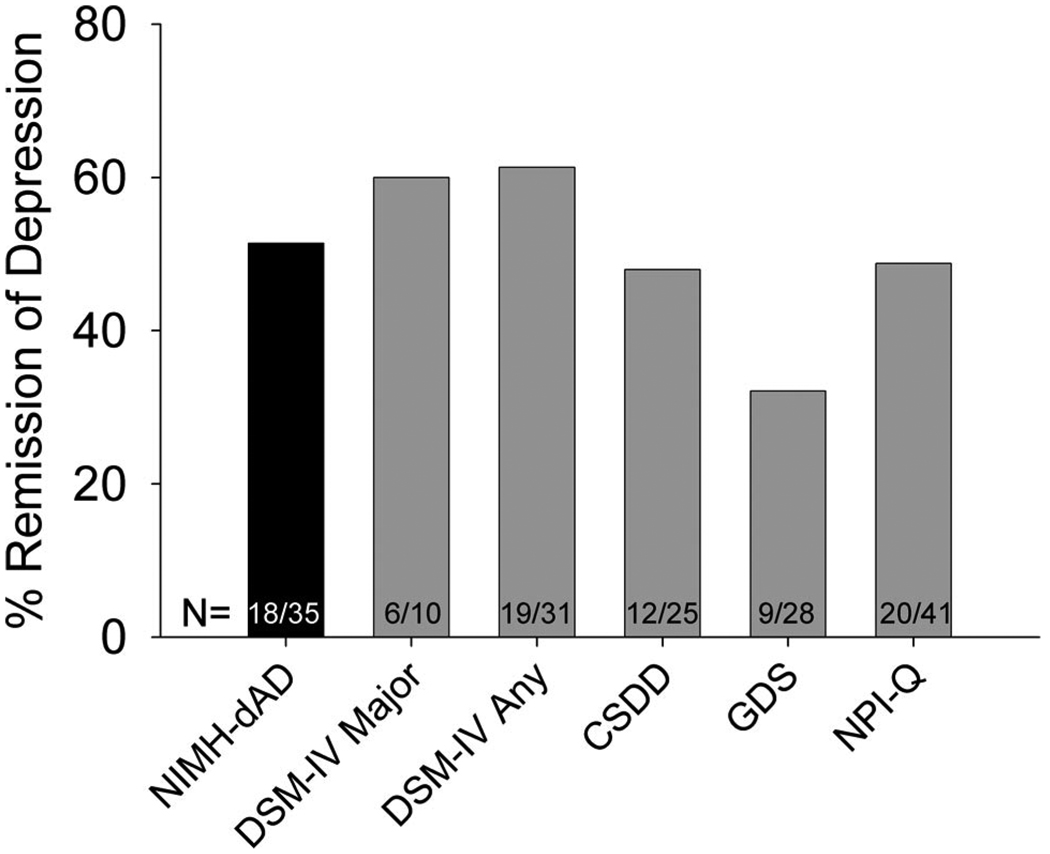
Ninety participants (89%) returned for follow-up assessment after a 3-month interval. Participants who were lost to follow-up had significantly fewer years of formal education (11.9 versus 14.3; t = 2.19, df = 99, p = 0.031) and marginally lower MMSE scores (18.4 versus 21.4; t = 1.76, df = 95, p = 0.081). At baseline, they were more likely to be diagnosed with depression using the criteria for DSM-IV major depression (36% versus 11%; χ2 = 5.26, df = 1,101, p = 0.022) or the NPI-Q (82% versus 47%; χ2 = 4.70, df = 1,100, p = 0.03) or to be receiving treatment with antidepressant medications (64% versus 31%; χ2 = 4.58, df = 1,101, p = 0.032) than participants that returned for follow-up. Remission rates at 3 months for depression diagnosed at baseline with the different assessments ranged from 32% to 61% (Fig. 3). There were no significant differences in remission rates between assessment tools (χ2 = 5.59, df = 6,170, p = 0.35). Five participants were missing data from at least one assessment tool at follow-up and were excluded from this analysis.
FIGURE 3. Remission Rates for Depression at 3 Months Among Participants Diagnosed With Depression at Baseline With Different Assessment Tools.
NIMH-dAD: NIMH Provisional Diagnostic Criteria for Depression in Alzheimer Disease; DSM-IV Major: DSM-IV Major Depression; DSM-IV Any: DSM-IV Major or Minor Depression; CSDD: Cornell Scale for Depression in Dementia; GDS: Geriatric Depression Scale; NPI-Q: Neuropsychiatric Inventory Questionnaire.
DISCUSSION
The results presented here indicate that NIMH-dAD criteria identify a greater proportion of AD patients as depressed than do several other established assessment tools for depression. Our data, which were obtained using a specifically designed structured interview for NIMH-dAD criteria, are consistent with the results from a previous study that interpolated NIMH-dAD diagnoses from data collected using a structured interview from another diagnostic instrument.6 Taken together, these findings support the hypothesis that NIMH-dAD criteria may more effectively distinguish AD patients with significant depressive symptoms.22
The subset of depressed participants identified with NIMH-dAD criteria was demographically similar to the subsets of depressed participants identified with the other assessments. Since NIMH-dAD criteria are based upon DSM-IV criteria for depression, it is perhaps not surprising that NIMH-dAD diagnoses of depression most closely reflected DSM-IV diagnoses of major or minor depression. The higher rates of depression identified using NIMH-dAD criteria relative to DSM-IV criteria for major or minor depression appear to arise primarily from the less stringent requirements for the frequency and duration of depressive symptoms. If the number of NIMH-dAD symptoms required for a diagnosis of depression were increased to ≥5 to match the number of symptoms required for a DSM-IV diagnosis of major depression, the frequency of depression in our cohort would decrease to 26%. These results suggest that the reduction in the number of symptoms required by NIMH-dAD criteria also contributes to higher rates of depression relative to DSM-IV criteria for major depression.
Notably, neither social isolation / withdrawal nor irritability, the two new symptoms that were introduced with NIMH-dAD criteria, was predictive of depression. These symptoms, although potentially more specific for the manifestations of depression in patients with AD, did not significantly influence the rates of depression in our study population. Indeed, the elimination of these symptoms changed the diagnoses for only two individuals, decreasing the frequency of depression only slightly, to 42%. In contrast, the symptoms that most strongly predicted a diagnosis of depression: psychomotor changes, fatigue, and a sense of guilt / worthlessness, were adapted directly from DSM-IV criteria for depression. Although the data collected using our structured interview do not distinguish between psychomotor agitation and retardation, these findings are consistent with previous work suggesting that psychomotor retardation and fatigue are seen with higher frequency in depressed AD patients than in nondepressed AD patients or in depressed elderly individuals without dementia.16,17
It has been suggested that the less stringent requirements for the frequency and duration of symptoms in NIMH-dAD criteria relative to DSM-IV may decrease the specificity for a diagnosis of depression, especially among patients with advanced dementia, resulting in an increased risk of overdiagnosis.31 Nevertheless, the concordance in our dataset between subsets of participants identified with NIMH-dAD criteria and the construct of DSM-IV major or minor depression was high. Since our study population was limited to patients with mild to moderate AD and excluded patients with MMSE scores <10, it remains unclear whether NIMH-dAD and DSM-IV criteria identify similar subsets of patients with severe AD as depressed. Similarly, since there is as yet no “gold standard” for the diagnosis of depression in AD, it is difficult to determine whether the higher rates of depression found with NIMH-dAD criteria represent a better reflection of the true prevalence of depressive disorders among demented individuals than the other assessments administered in this study.
The remission rate of baseline NIMH-dAD diagnoses of depression at the 3-month follow-up visit was 51%. Similar rates of remission were seen with the other assessment tools used in this study. It is possible that our calculations may have overestimated the true remission rates of depression in our study population, since participants who were lost to follow-up were more likely to be depressed or to be undergoing treatment with antidepressant medications. However, our follow-up rates were high (89%) and our findings are consistent with previous reports suggesting high rates of remission for depressive symptoms in AD.32,33
There are a few factors that may limit the interpretation of our results. The same interviewer typically administered all of the assessments. Thus, diagnoses with different instruments may not have been truly independent from one another, given the unblinded nature of the assessments. Although the cut-offs used to identify depression with CSDD, GDS, and NPI-Q have been cross-validated against DSM-III or DSM-IV,6,20,28 these tools were designed as screening tools for depression rather than diagnostic instruments.10,19,29 Our study population does not represent an epidemiological sample. Therefore, the rates of depression in our cohort may not be generalizable to other clinical- or community-based populations. Our participants were recruited for a longitudinal study of depression in AD, and our population was intentionally selected to increase the proportion of individuals with depressive symptoms at baseline. Nevertheless, since all of the participants were assessed with each of the diagnostic instruments, the relative rates of depression calculated with each assessment are likely to remain valid. Additionally, previous studies have suggested that caregivers of AD patients report significantly higher rates of depressive symptoms than patients themselves.34–36 Our implementation of NIMH-dAD criteria included caregiver input and may have overestimated the rates of depression in our cohort. However, given that caregiver input was also incorporated into diagnoses obtained with DSM-IV and CSDD criteria, the higher rate of depression seen with NIMH-dAD criteria relative to these other assessments is unlikely to be artifactually driven by caregiver responses. Finally, although remission rates for depression at 3 months did not differ between instruments, our data do not address whether depressive symptoms identified with individual assessment tools might differ in duration over intervals shorter than our follow-up period.
The NIMH-dAD criteria may be useful for determining which AD patients are likely to benefit from pharmacological and / or behavioral interventions for depressive symptoms. Given the higher rates of depression found with these criteria, it is tempting to conclude that they may spur clinicians to address depressive symptoms that might otherwise have been overlooked. However, the greater sensitivity of these criteria may potentially produce a higher rate of false positive diagnoses of depression and contribute to inappropriate polypharmacy, particularly since the efficacy of antidepressants for depression in AD remains uncertain.37 The ongoing Depression in Alzheimer’s Disease Study-2 (DIADS-2), which is designed to assess the safety and efficacy of sertraline in the treatment of depression in AD, has incorporated NIMH-dAD guidelines into its inclusion criteria. It is also powered to assess the validity of these criteria for identifying pharmacologically responsive depression in AD.37 This is an important question given the difficulty of establishing of diagnostic “gold-standard” for clinically significant depression in AD. The results from DIADS-2, when combined with the findings reported here and elsewhere,6 will help further determine the clinical utility of NIMH-dAD criteria.
Acknowledgments
This research was supported by the Alzheimer’s Disease Research Centers of California, the National Institute on Aging (P50 AG 16570), and the Sidell-Kagan Foundation.
APPENDIX A
The Alzheimer’s Disease Research Centers of California–Depression in Alzheimer’s Disease Investigators
|
APPENDIX B
Structured Interview Used to Assess for Depression as Specified by the NIMH Provisional Diagnostic Criteria for Depression in Alzheimer Disease
| The presence of each symptom should be assessed over the preceding 2 weeks | |||
|---|---|---|---|
| 1. “I am going to ask you some questions about (your/the participant’s) mood. Has there been a period of time when (you were/the participant was) feeling depressed or down most of the day? What was that like? Was this a change from the usual?” IF YES: “How long did it last? As long as 2 weeks?” | |||
| Clinically significant depressed mood: | Yes | No | Not Determined |
| 2. “What about losing interest in things (you/the participant) usually enjoy(s)? IF YES: “Was this a change from the previous level of functioning? How long did it last? As long as 2 weeks?” | |||
| Decreased positive affect or pleasure | Yes | No | Not Determined |
| 3. “Did (you/the participant) tend to withdraw from social contacts and (your/their) customary activities?” | |||
| Social isolation or withdrawal | Yes | No | Not Determined |
| 4. “(Have you/Has the participant) lost or gained any weight?” IF YES: “How much? (Were you/Was the participant) trying to lose weight?” IF NO: “How was (your/the participant’s) appetite? What about compared to (your/the participant’s) usual appetite? Did you have to force (yourself/the participant) to eat? Eat (more/less) than usual?” | |||
| Disruption in appetite | Yes | No | Not Determined |
| 5. How (have you/has the participant) been sleeping? Trouble falling asleep, waking frequently, trouble staying asleep, waking too early, or sleeping too much? How many hours a night compared to the usual? | |||
| Disruption in sleep | Yes | No | Not Determined |
| 6. (Have you/Has the participant) been so fidgety or restless that (you were/the participant was) unable to sit still?” IF YES: “Was it so bad that other people noticed it? What did they notice? Was it a change from (your/the participan t’s) typical behavior?” IF NO: “What about the opposite- talking or moving more slowly than is normal for (you/the participant)? Was it so bad that other people noticed it? What did they notice? Was it a change from (your/the participant’s) typical behavior?” | |||
| Psychomotor changes | Yes | No | Not Determined |
| 7. “Have (you/the participant) been feeling more irritable than usual?” | |||
| Irritability | Yes | No | Not Determined |
| 8. What has (your/the participant’s) energy been like? Tired all the time?” IF YES: “A change from (your/the participant’s) typical behavior?” | |||
| Fatigue or loss of energy | Yes | No | Not Determined |
| 9. “How (do you/does the participant) feel about (yourself/himself/herself)? Worthless?” IF YES: “Is this a change from (your/the participant’s) typical behavior?” | |||
| Feelings of worthlessness | Yes | No | Not Determined |
| 10. “Were things so bad that (you were/the participant was) thinking a lot about death or that (you/the participant) would be better off dead? What about thinking of hurting (yourself/himself/herself)? | |||
| Recurrent thoughts of death | Yes | No | Not Determined |
Footnotes
Presented in preliminary form at the 59th Annual Meeting of the American Academy of Neurology, April 28 – May 5, 2007, Boston.
References
- 1.Lee HB, Lyketsos CG. Depression in Alzheimer’s disease: heterogeneity and related issues. Biol Psychiatry. 2003;54:353–362. doi: 10.1016/s0006-3223(03)00543-2. [DOI] [PubMed] [Google Scholar]
- 2.Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52:243–252. doi: 10.1016/s0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- 3.Knesevich JW, Martin RL, Berg L, et al. Preliminary report on affective symptoms in the early stages of senile dementia of the Alzheimer type. Am J Psychiatry. 1983;140:233–235. doi: 10.1176/ajp.140.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Merriam AE, Aronson MK, Gaston P, et al. The psychiatric symptoms of Alzheimer’s disease. J Am Geriatr Soc. 1988;36:7–12. doi: 10.1111/j.1532-5415.1988.tb03427.x. [DOI] [PubMed] [Google Scholar]
- 5.Muller-Thomsen T, Arlt S, Mann U, et al. Detecting depression in Alzheimer’s disease: evaluation of four different scales. Arch Clin Neuropsychol. 2005;20:271–276. doi: 10.1016/j.acn.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Vilalta-Franch J, Garre-Olmo J, Lopez-Pousa S, et al. Comparison of different clinical diagnostic criteria for depression in Alzheimer disease. Am J Geriatr Psychiatry. 2006;14:589–597. doi: 10.1097/01.JGP.0000209396.15788.9d. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 4th Ed. Washington: APA; 1994. [Google Scholar]
- 8.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:53–63. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 10.Yesavage JA. Geriatric Depression Scale. Psychopharm Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 11.Feher EP, Larrabee GJ, Crook TH., III Factors attenuating the validity of the Geriatric Depression Scale in a dementia population. J Am Geriatr Soc. 1992;40:906–909. doi: 10.1111/j.1532-5415.1992.tb01988.x. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberg PA, Marcopulos BA, Steiner DA, et al. Comparison of the Hamilton Depression Rating Scale and the Geriatric Depression Scale: detection of depression in dementia patients. Psychol Rep. 1992;70:515–521. doi: 10.2466/pr0.1992.70.2.515. [DOI] [PubMed] [Google Scholar]
- 13.Gilley DW, Wilson RS. Criterion-related validity of the Geriatric Depression Scale in Alzheimer’s disease. J Clin Exp Neuropsychol. 1997;19:489–499. doi: 10.1080/01688639708403739. [DOI] [PubMed] [Google Scholar]
- 14.Wagle AC, Ho LW, Wagle SA, et al. Psychometric behaviour of BDI in Alzheimer’s disease patients with depression. Int J Geriatr Psychiatry. 2000;15:63–69. doi: 10.1002/(sici)1099-1166(200001)15:1<63::aid-gps78>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Korner A, Lauritzen L, Abelskov K, et al. The Geriatric Depression Scale and the Cornell Scale for Depression in Dementia. A validity study. Nord J Psychiatry. 2006;60:360–364. doi: 10.1080/08039480600937066. [DOI] [PubMed] [Google Scholar]
- 16.Chemerinski E, Petracca G, Sabe L, et al. The specificity of depressive symptoms in patients with Alzheimer’s disease. Am J Psychiatry. 2001;158:68–72. doi: 10.1176/appi.ajp.158.1.68. [DOI] [PubMed] [Google Scholar]
- 17.Janzing JG, Hooijer C, van ’t Hof MA, et al. Depression in subjects with and without dementia: a comparison using GMS-AGECAT. Int J Geriatr Psychiatry. 2002;17:1–5. doi: 10.1002/gps.526. [DOI] [PubMed] [Google Scholar]
- 18.Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 19.Alexopoulos GS, Abrams RC, Young RC, et al. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23:271–284. doi: 10.1016/0006-3223(88)90038-8. [DOI] [PubMed] [Google Scholar]
- 20.Vida S, Des Rosiers P, Carrier L, et al. Depression in Alzheimer’s disease: receiver operating characteristic analysis of the Cornell Scale for Depression in Dementia and the Hamilton Depression Scale. J Geriatr Psychiatry Neurol. 1994;7:159–162. doi: 10.1177/089198879400700306. [DOI] [PubMed] [Google Scholar]
- 21.Lam CK, Lim pp, Low BL, et al. Depression in dementia: a comparative and validation study of four brief scales in the elderly Chinese. Int J Geriatr Psychiatry. 2004;19:422–428. doi: 10.1002/gps.1098. [DOI] [PubMed] [Google Scholar]
- 22.Olin JT, Schneider LS, Katz IR, et al. Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry. 2002;10:125–128. [PubMed] [Google Scholar]
- 23.Olin JT, Katz IR, Meyers BS, et al. Provisional diagnostic criteria for depression of Alzheimer disease: rationale and background. Am J Geriatr Psychiatry. 2002;10:129–141. [PubMed] [Google Scholar]
- 24.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg PB, Onyike CU, Katz IR, et al. Clinical application of operationalized criteria for ‘Depression of Alzheimer’s Disease’. Int J Geriatr Psychiatry. 2005;20:119–127. doi: 10.1002/gps.1261. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 28.Lyness JM, Noel TK, Cox C, et al. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies–Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157:449–454. [PubMed] [Google Scholar]
- 29.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 30.Freidman M. The use of ranks to avoid the assumption of normality implicit in the analysis of variance. J Am Stat Assoc. 1937;32:675–701. [Google Scholar]
- 31.Starkstein SE, Jorge R, Mizrahi R, et al. The construct of minor and major depression in Alzheimer’s disease. Am J Psychiatry. 2005;162:2086–2093. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- 32.Devanand DP, Jacobs DM, Tang MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 33.Eustace A, Coen R, Walsh C, et al. A longitudinal evaluation of behavioural and psychological symptoms of probable Alzheimer’s disease. Int J Geriatr Psychiatry. 2002;17:968–973. doi: 10.1002/gps.736. [DOI] [PubMed] [Google Scholar]
- 34.Mackenzie TB, Robiner WN, Knopman DS. Differences between patient and family assessments of depression in Alzheimer’s disease. Am J Psychiatry. 1989;146:1174–1178. doi: 10.1176/ajp.146.9.1174. [DOI] [PubMed] [Google Scholar]
- 35.Burke WJ, Roccaforte WH, Wengel SP, et al. Disagreement in the reporting of depressive symptoms between patients with dementia of the Alzheimer type and their collateral sources. Am J Geriatr Psychiatry. 1998;6:308–319. [PubMed] [Google Scholar]
- 36.Snow AL, Kunik ME, Molinari VA, et al. Accuracy of self-reported depression in persons with dementia. J Am Geriatr Soc. 2005;53:389–396. doi: 10.1111/j.1532-5415.2005.53154.x. [DOI] [PubMed] [Google Scholar]
- 37.Martin BK, Frangakis CE, Rosenberg PB, et al. Design of Depression in Alzheimer’s Disease Study-2. Am J Geriatr Psychiatry. 2006;14:920–930. doi: 10.1097/01.JGP.0000240977.71305.ee. [DOI] [PubMed] [Google Scholar]