Abstract
OBJECTIVE
We sought to identify risk factors for congenital microcephaly in extremely low gestational age newborns.
STUDY DESIGN
Demographic, clinical, and placental characteristics of 1445 infants born before the 28th week were gathered and evaluated for their relationship with congenital microcephaly.
RESULTS
Almost 10% of newborns (n = 138), rather than the expected 2.2%, had microcephaly defined as a head circumference >2 SD below the median. In multivariable models, microcephaly was associated with nonwhite race, severe intrauterine growth restriction, delivery for preeclampsia, placental infarction, and being female. The risk factors for a head circumference between <1 and >2 SD below the median were similar to those of microcephaly.
CONCLUSION
Characteristics associated with fetal growth restriction and preeclampsia are among the strongest correlates of microcephaly among children born at extremely low gestational ages. The elevated risk of a small head among nonwhites and females might reflect the lack of appropriate head circumference standards.
Keywords: extreme prematurity, head size, intrauterine growth restriction, microcephaly
Microcephaly in the newborn is characterized by disproportionately small head circumference for a given gestational age (GA). A variety of antenatal exposures contribute to the risk of congenital microcephaly, including genetic and chromosomal anomalies, infectious exposures, drug or chemical exposure, phenylketonuria, and high levels of ionizing radiation.1 However, congenital microcephaly is frequently observed in pregnancies that were free of these insults and can be associated with antenatal complications such as severe intra-uterine growth restriction (IUGR), suggesting that a disordered intrauterine environment may also be an antecedent.2
Congenital microcephaly predicts reduced brain growth, particularly reduction in forebrain development.3–5 and reduced intellectual ability in the adult.6,7 Among term-born infants with a head circumference 2 SD below the mean, 50% will have reduced cognitive attainment. This rises to 82% among those whose head circumference is >3 SD below the mean.8,9 This progression in developmental limitation with reduced head size suggests that microcephaly and its later associations exist along a continuum of dysfunction. Investigations into the antecedents of microcephaly will therefore need to be aware that imposing a break point in the definition of microcephaly is to some degree artificial.2 Given the potential adverse developmental outcomes in these infants, we sought to identify the antenatal antecedents of congenital microcephaly in extremely low GA newborns (ELGAN).
Materials and Methods
The ELGAN study was designed to identify characteristics and exposures that increase the risk of structural and functional neurologic disorders in ELGAN. During the years 2002 through 2004, women delivering >23.0 but <28.0 weeks’ gestation at 1 of 14 participating institutions in 11 cities in 5 states were asked to enroll in the study. The enrollment and consent processes were approved by the individual institutional review boards.
Mothers were approached for consent either upon antenatal admission or shortly after delivery, depending on clinical circumstance and institutional preference. Only nonanomalous and infants free of obvious chromosomal abnormality were enrolled. Ultimately, 1249 mothers of 1506 infants consented to participate. Approximately 260 women were either missed or did not consent.
Demographic and pregnancy variables
After delivery, a trained research nurse interviewed each mother in her native language using a structured data collection form and following procedures documented in a manual. The mother’s report of her own characteristics and exposures, as well as the sequence of events leading to preterm delivery were taken as truth, even when her medical record provided discrepant information.
Shortly after the mother’s discharge, the research nurse reviewed the maternal chart using a second structured data collection form. The medical record was reviewed for information about events following admission.
The clinical circumstances that led to each maternal admission and ultimately to each preterm delivery were operationally defined using both data from the maternal interview and data abstracted from the medical record.10
Newborn variables
The GA estimates were based on a hierarchy of the quality of available information. Most desirable were estimates based on the dates of embryo retrieval or intrauterine insemination or last menstrual period with confirming fetal ultrasound before the 14th week (62%). When these were not available, reliance was placed sequentially on a fetal ultrasound at ≥14 weeks (29%), last menstrual period without fetal ultrasound (7%), and GA recorded in the log of the neonatal intensive care unit (1%).
The birthweight Z-score is the number of SD the infant’s birthweight is above or below the median weight of infants at the same GA in a standard data set.11
The head circumference was measured as the largest possible occipital-frontal circumference. Measurements were rounded to the closest 0.1 cm. All head circumferences are presented as Z-scores because newborns were assessed at different GA at birth (23–27 weeks). These Z-scores were based on standards in the Oxford, United Kingdom, dataset.11
Placentas were biopsied under sterile conditions. In all, 82% of the samples were obtained within 1 hour of delivery. The microbiologic procedures are described in detail elsewhere.12,13 Placentas were examined grossly in keeping with the guidelines of the 1991 College of American Pathologists Conference.14 Procedures and definitions of histologic terms are presented elsewhere.15
Data analysis
We evaluated the generalized null hypothesis that the risk of a small head circumference is not associated with maternal demographic characteristics, pregnancy exposures and characteristics, characteristics of the newborn, or characteristics of the placenta. We adjusted for GA using groups of weeks (23–24, 25–26, 27). This procedure does just as well as adjusting for each week of gestation and results in fewer groups.
Several definitions for microcephaly have been proposed.16 We define microcephaly as a head circumference Z-score of<−2 (ie, >2 SD below the GA-specific median).2,17 We use the term “minicephalic” to indicate the larger set of children with head circumference Z-scores ≥−2 but<−1. By and large, what applies to the infants with microcephaly also applies to those with minicephaly. To minimize repetition of this statement, we reserve comments for the situations when this generalization does not apply. For the ease of reading the “Results” section, we use the term “small head circumference” to encompass both the microcephaly and minicephaly groups.
Because our 2 small head circumference Z-score groups are mutually exclusive and each is appropriately compared to the same referent group (those with head circumference Z-score ≥−1), we created multinomial logistic regression models to identify the contribution of relevant characteristics and exposures to the risk of each head circumference outcome.18,19 We began by including all variables that appeared to be related to microcephaly. Using manual backward selection, we then sequentially dropped the least significant variable and assessed the model with the remaining variables. A model was complete when all of the variables that remained were associated with the outcome at a level of P < .1. The dropped variables were individually reintroduced to see if they now contributed to the complete model. The contribution of each antecedent to the model is presented as a risk ratio with 95% confidence intervals.
Results
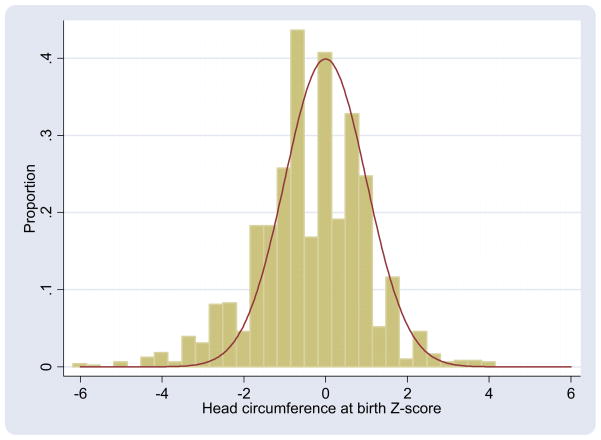
In a normal distribution, 2.2% of measurements are >2 SD below the mean. In our sample of ELGAN, almost 10% of infants (138/1445) were microcephalic at birth. In all, 22% (317/1445) were minicephalic when only 13.8% is expected. A notable portion of the head circumference Z-score distribution in our sample lies to the left of the expected normal distribution (Figure). In all, 31% (138 + 317/1445) of the ELGAN sample had a small head circumference Z-score.
Figure. Observed and expected distributions of birth head circumference Z-scores.
Vertical bars represent narrow groupings of head circumference Z-scores in extremely low gestational age newborns study sample. Expected normal distribution of Z-scores (red line; mean = 0, SD = 1) is overlaid.
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
Maternal education, marital status, (financial) self-support, and receipt of public insurance were not associated with microcephaly (Table 1). Microcephaly was more common among women who identified themselves as nonwhite. Mothers with an advanced maternal age (>35 years) tended to have babies at reduced risk of microcephaly. Higher prepregnancy body mass index was associated with higher risk for both microcephaly and minicephaly.
TABLE 1.
Distribution of birth head circumference Z-score groups in relation to social and demographic characteristics of mother
| Maternal characteristic | Head circumference Z-scorea | n | ||
|---|---|---|---|---|
| <−2 | ≥−2 to <−1 | |||
| Racial identity | White | 7 | 18 | 821 |
| Black | 12 | 27 | 419 | |
| Other | 13 | 28 | 181 | |
| Hispanic | Yes | 10 | 23 | 175 |
| No | 9 | 21 | 1258 | |
| Age, y | <21 | 11 | 26 | 206 |
| 21–35 | 11 | 22 | 930 | |
| >35 | 7 | 18 | 245 | |
| Education, y | <12 | 9 | 23 | 235 |
| 12 (HS) | 11 | 26 | 378 | |
| >12 to <16 | 11 | 21 | 332 | |
| 16 (College) | 9 | 18 | 234 | |
| >16 | 6 | 16 | 171 | |
| Marital status | Single | 10 | 27 | 639 |
| Not single | 9 | 18 | 806 | |
| Self-supportb | Yes | 9 | 22 | 891 |
| No | 10 | 22 | 181 | |
| Medicaidb | Yes | 10 | 26 | 578 |
| No | 9 | 19 | 803 | |
| Prepregnancy BMI | <18.5 | 8 | 19 | 106 |
| ≥18.5 to <25 | 8 | 20 | 650 | |
| ≥25 to <30 | 11 | 25 | 279 | |
| ≥30 | 12 | 24 | 312 | |
| Maximum no. of infants | 138 | 317 | 1445 | |
These are row percents. They do not total to 100% because group with larger Z-scores is not shown.
BMI, body mass index; HS, high school.
External standard is Oxford, United Kingdom, dataset;11
Infants may be in >1 category.
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
Maternal smoking, whether before or during pregnancy, was not associated with microcephaly (Table 2). Similarly, self-reported vaginal bleeding, antepartum fever, vaginitis, or urinary tract infections were not associated with an increased risk of a microcephalic newborn. While multigravidity was not associated with microcephaly, mothers with a birth interval of >2 years were at higher risk of having a microcephalic baby. The use of conception assistance was associated with a modestly reduced risk of having a microcephalic, but not a minicephalic baby. Maternal ingestion of a nonsteroidal antiinflammatory drug was associated with a reduced risk of microcephaly, but not of minicephaly.
TABLE 2.
Distribution of children with small head circumference growth in strata defined by pregnancy characteristics and exposures during pregnancy
| Exposures and characteristics | Head circumference Z-scorea | n | ||
|---|---|---|---|---|
| <−2 | ≥−2 to <−1 | |||
| Smoking prepregnancyb | Yes | 8 | 23 | 359 |
| No | 10 | 21 | 1012 | |
| Smoking during pregnancyb | Yes | 8 | 25 | 205 |
| No | 10 | 21 | 1166 | |
| Years since last pregnancy | <1 | 7 | 23 | 163 |
| 1–2 | 8 | 17 | 235 | |
| ≥2 | 11 | 23 | 417 | |
| Conception assistance | Yes | 7 | 17 | 271 |
| No | 10 | 23 | 1096 | |
| Vaginal bleeding | ||||
| ≤12 wkb | Yes | 8 | 21 | 522 |
| No | 10 | 23 | 844 | |
| >12 wkb | Yes | 7 | 21 | 392 |
| No | 11 | 22 | 974 | |
| Illnesses this pregnancy | ||||
| Feverb | Yes | 10 | 25 | 83 |
| No | 10 | 22 | 1282 | |
| Vaginal/cervical infection | Yes | 7 | 24 | 187 |
| No | 10 | 22 | 1179 | |
| UTIb | Yes | 10 | 25 | 219 |
| No | 10 | 21 | 1147 | |
| Medications | ||||
| Anyb | Yes | 10 | 22 | 159 |
| No | 6 | 23 | 1206 | |
| Aspirinb | Yes | 8 | 26 | 80 |
| No | 10 | 22 | 1280 | |
| NSAIDb | Yes | 4 | 27 | 99 |
| No | 10 | 21 | 1260 | |
| Acetaminophenb | Yes | 11 | 21 | 697 |
| No | 8 | 22 | 662 | |
| Antibioticb | Yes | 10 | 24 | 430 |
| No | 9 | 21 | 930 | |
| Maximum no. of infants | 138 | 317 | 1445 | |
These are row percents.
NSAID, nonsteroidal antiinflammatory drug; UTI, urinary tract infection.
External standard is Oxford, United Kingdom, dataset;11
infants may be in >1 category.
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
Children whose mother presented in labor or with abruption were at lowest risk of microcephaly while those delivered for maternal or fetal indications were at a notably increased risk of microcephaly (Table 3). This increase was much less prominent for a minicephaly. Correlates of delivery for preeclampsia and fetal indications, including receipt of magnesium for seizure prophylaxis, and of no labor, were also strongly associated with an increased risk of a small head. Among infants delivered after preterm premature membrane rupture, the latency interval between rupture and delivery was unrelated to the risk of having either microcephaly or minicephaly. Receipt of a complete course of antenatal corticosteroid (regardless of whether betamethasone or dexamethasone) was not associated with an increased risk of microcephaly. Girls were at greater risk than boys of having a small head circumference. The risk of microcephaly was lowest in the youngest GA groups, and regardless of GA, most elevated in those with the lowest birthweight Z-scores (Table 4).
TABLE 3.
Distribution of head circumference groups in strata defined by delivery or newborn characteristics
| Characteristics of delivery | Head circumference Z-scorea | n | ||
|---|---|---|---|---|
| <−2 | ≥−2 to <−1 | |||
| Antenatal steroid course | Complete | 10 | 25 | 903 |
| Partial | 8 | 17 | 378 | |
| None | 9 | 16 | 159 | |
| Magnesium | No | 12 | 22 | 481 |
| Tocolysis | 5 | 19 | 776 | |
| For preeclampsia | 23 | 33 | 178 | |
| Cesarean delivery | Yes | 12 | 21 | 929 |
| No | 5 | 24 | 516 | |
| Pregnancy complication | Preterm labor | 3 | 19 | 640 |
| pPROM | 8 | 26 | 305 | |
| PE | 29 | 34 | 191 | |
| Abruption | 4 | 22 | 150 | |
| Cervical insufficiency | 6 | 8 | 87 | |
| Fetal indication | 35 | 21 | 72 | |
| Duration of labor, h | 0 | 23 | 25 | 371 |
| >0 to ≤12 | 7 | 14 | 339 | |
| >12 | 4 | 24 | 735 | |
| Duration of membrane rupture, h | <1 | 11 | 21 | 850 |
| 1–24 | 7 | 17 | 235 | |
| >24–48 | 11 | 14 | 64 | |
| >48–72 | 5 | 18 | 38 | |
| >72 | 6 | 31 | 258 | |
| No. of fetuses | 1 | 10 | 25 | 978 |
| ≥2 | 8 | 16 | 467 | |
| Sex | Male | 7 | 19 | 773 |
| Female | 13 | 25 | 672 | |
| Gestational age, wk | 23–24 | 4 | 20 | 388 |
| 24–25 | 13 | 22 | 632 | |
| 27 | 10 | 23 | 425 | |
| Birthweight, g | ≤750 | 20 | 29 | 628 |
| 751–1000 | 2 | 21 | 568 | |
| >1000 | 1 | 6 | 249 | |
| Birthweight Z-scorea | <−2 | 76 | 19 | 103 |
| ≥−2 to<−1 | 20 | 55 | 202 | |
| ≥−1 | 2 | 16 | 1140 | |
| Maximum no. of infants | 138 | 317 | 1445 | |
These are row percents.
pPROM, preterm premature rupture of membranes.
External standard is Oxford, United Kingdom, dataset.11
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
TABLE 4.
Risk ratios (and 95% confidence intervals) of each head circumference entity associated with each placental organism or group of organisms
| Microorganism | Head circumference Z-score | |||
|---|---|---|---|---|
| Vaginal delivery | Cesarean section delivery | |||
| <−2 | ≥2 to <−1 | <−2 | ≥−2 to <−1 | |
| Any aerobe | 2.0 (0.8–5.0) | 1.4 (0.9–2.3) | 0.6 (0.3–1.02) | 0.9 (0.4–1.4) |
| Any anaerobe | 1.1 (0.5–2.8) | 1.3 (0.8–2.0) | 0.7 (0.4–1.3) | 1.1 (0.7–1.7) |
| Any Mycoplasma | 1.0 (0.3–3.7) | 1.9 (1.1–3.1) | 0.6 (0.2–1.6) | 1.5 (0.8–2.6) |
| No. of species | ||||
| 1 | 3.0 (0.8–11) | 1.5 (0.8–2.8) | 1.1 (0.7–1.7) | 0.8 (0.6–1.3) |
| ≥2 | 2.7 (0.8–9.0) | 1.9 (1.1–3.1) | 0.1 (0.03–0.5) | 1.0 (0.7–1.7) |
| Lactobacillus species | d | 0.7 (0.4–1.5) | 0.2 (0.03–1.6) | 0.5 (0.2–1.4) |
| Propionibacterium species | d | 0.6 (0.2–1.7) | 2.4 (1.2–4.8) | 1.7 (0.9–3.1) |
| Mycoplasma speciesa | 1.2 (0.3–5.4) | 2.2 (1.2–4.2) | d | 1.9 (0.9–3.9) |
| Skin organismsb | 1.6 (0.6–4.0) | 1.1 (0.7–1.7) | 1.9 (1.1–3.3) | 1.2 (0.7–1.9) |
| Vaginal organismsc | 0.9 (0.3–2.5) | 1.3 (0.8–2.0) | 0.3 (0.1–0.98) | 0.6 (0.3–1.2) |
Data are presented separately for placentas delivered vaginally (first 2 data columns) and for sample of placentas delivered by cesarean section (last 2 data columns). Only adjustment is for gestational age (23–24, 25–26, and 27 weeks).
Non-Ureaplasma;
Corynebacterium species, Propionibacterium species, Staphylococcus species;
Prevotella bivia, Lactobacillus species, Peptostreptococcus magnus, Gardnerella vaginalis;
Empty cells prohibit calculation of risk ratio.
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
Among infants delivered vaginally, those whose placenta harbored an organism (or multiple organisms) were almost 3 times more likely than others to be microcephalic, although these increased risks were not statistically significant (Table 5). On the other hand, the recovery of multiple organisms, especially Mycoplasma, was associated with statistically significant increased risks of minicephaly.
TABLE 5.
Risk ratios (and 95% confidence intervals) of each head circumference entity associated with each histologic characteristic
| Histologic lesion | Head circumference Z-score | |||
|---|---|---|---|---|
| Vaginal delivery | Cesarean section delivery | |||
| <−2 | ≥2 to <−1 | <−2 | ≥−2 to <−1 | |
| Inflammation chorionic platea | 1.0 (0.4–2.8) | 1.6 (0.99–2.5) | 0.3 (0.1–0.8) | 1.0 (0.6–1.7) |
| Inflammation chorion/deciduab | 3.5 (1.3–9.9) | 2.4 (1.5–3.9) | 0.2 (0.1–0.5) | 0.7 (0.5–1.03) |
| Neutrophil infiltration fetal stem vessels | 1.1 (0.4–2.8) | 2.4 (1.5–3.7) | 0.2 (0.1–0.6) | 0.9 (0.6–1.4) |
| Umbilical cord vasculitisc | 2.4 (0.96–5.9) | 1.9 (1.2–3.2) | 0.3 (0.1–0.9) | 1.1 (0.6–1.7) |
| Thrombosis of fetal stem vessels | 0.7 (0.1–5.2) | 1.1 (0.5–2.4) | 3.0 (1.4–6.4) | 1.2 (0.5–2.8) |
| Infarct | 0.6 (0.1–2.8) | 1.2 (0.6–2.1) | 4.1 (2.6–6.6) | 2.7 (1.7–4.0) |
| Increased syncytial knots | d | 1.2 (0.6–2.4) | 4.1 (2.6–6.4) | 2.1 (1.5–3.2) |
| Decidual hemorrhage/fibrin deposition | 1.1 (0.4–2.8) | 0.9 (0.5–1.5) | 1.4 (0.8–2.7) | 1.5 (0.9–2.4) |
Data are presented separately for placentas delivered vaginally (first 2 data columns) and for sample of placentas delivered by cesarean section (last 2 data columns). Only adjustment is for gestational age (23–24, 25–26, and 27 wk).
Stage 3 and severity 3;
Grades 3 and 4;
Grades 3, 4, and 5;
Empty cells prohibit calculation of risk ratio.
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
Among infants delivered by cesarean section, and therefore at lower risk of having their placenta contaminated by passage through the vagina, recovery of ≥2 organisms, and the recovery of normal vaginal flora were associated with a significantly reduced risk of microcephaly. On the other hand, recovery of Pro-pionibacterium species and other skin organisms was associated with increased risk of microcephaly.
Among infants delivered vaginally, those whose placenta had inflammation of the chorion/decidua were at increased risk of a small head (Table 6). The increased risk associated with umbilical cord vasculitis did not quite achieve statistical significance. On the other hand, the risk of minicephaly was increased among infants whose fetal stem and umbilical cord vessels were inflamed.
TABLE 6.
| Variables | Head circumference Z-score | |
|---|---|---|
| <−2 | ≥2 to <−1 | |
| Birthweight Z-score<−2 | 14 (7.7–27) | 3.6 (1.9–6.8) |
| Maternal or fetal indication | 3.8 (2.0–7.3) | 2.4 (1.6–3.6) |
| Female | 2.3 (1.3–4.0) | 1.7 (1.2–2.2) |
| Placenta infarct | 2.0 (1.03–3.7) | 1.6 (1.1–2.4) |
| Nonwhite race | 1.9 (1.1–3.5) | 1.5 (1.03–2.0) |
| Unmarried | 1.9 (1.00–3.5) | 1.7 (1.2–2.5) |
| Vaginal organism | 1.4 (0.6–3.3) | 1.4 (0.9–2.0) |
| Tobacco smokea | 1.2 (0.7–2.2) | 1.1 (0.8–1.5) |
| Any antenatal corticosteroid | 1.1 (0.5–2.9) | 1.7 (0.99–2.9) |
| Gestational age 25–26 wk | 1.1 (0.6–2.1) | 1.2 (0.8–1.7) |
| Gestational age 23–24 wk | 0.5 (0.2–1.1) | 1.2 (0.8–1.8) |
| NSAIDs | 0.3 (0.1–1.3) | 1.0 (0.6–1.8) |
Odds ratios (point estimates and 95% confidence intervals) for each small head circumference entity associated with each of antecedents and characteristics listed on left in models of that contain all other variables in this table.
NSAIDs, nonsteroidal antiinflammatory drugs.
During pregnancy and exposure to smoke of others.
McElrath. Factors associated with small head circumference at birth among ELGAN. Am J Obstet Gynecol 2010.
In contrast, among infants delivered by cesarean section, umbilical cord vasculitis was associated with reduced risk of microcephaly. Placenta lesions in this subsample associated with increased risk of microcephaly included thrombosis of fetal stem vessels, infarct, and increased syncytial knots. Infarct and increased syncytial knots were also associated with increased risks of minicephaly.
In an attempt to identify if the risk of a small head required both organism recovery and a histologic response, we created multivariable models with a variable for organism recovery, a variable for the histologic characteristic of interest, and an interaction term for these 2 variables. No interaction term was statistically significant for models of the risk of microcephaly (data not shown). Interaction terms for organism recovery and decidual hemorrhage/fibrin deposition in the cesarean section sample conveyed information about reduced risk of minicephaly.
Multivariate table
Multinomial regression is displayed in Table 6. By and large, what is seen for microcephaly is also seen for minicephaly, but usually with a less elevated risk ratio. The only exception was an almost statistically significant increased risk of minicephaly, but not microcephaly among those exposed to any antenatal corticosteroid. The variables that contributed significantly to the risk of a small head even in the presence of potentially confounding variables were mother’s self-identification as nonwhite, a birthweight Z-score<−2, delivery for a maternal or fetal indication, a mother who was not married, and being female. Although not achieving statistical significance, low GA was associated with a reduced risk of microcephaly.
Comment
Recent query of the PubMed database with the key words “microcephaly” and “premature infant” indicates that this is the first exploration of the antecedents of congenital microcephaly in a sample of ELGAN. Our 2 main findings are that microcephaly occurs much more commonly than expected among infants born remote from term, and that microcephaly is especially common among ELGAN with severe IUGR.
Preterm delivery in this population tends to be associated with disorders attributed either to placenta hypoperfusion or to intrauterine inflammation.10 The first group includes deliveries for severe maternal disease (eg, preeclampsia) or for nonreassuring fetal conditions (eg, IUGR). The second group includes deliveries for indications such as preterm labor, preterm premature membrane rupture, or cervical insufficiency. The hypothesis that impaired placenta perfusion impairs brain growth is supported by our findings that both microcephaly and minicephaly are associated with fetal/maternal indications for delivery, very low birthweight Z-score, as well as placental infarction–a condition associated with placental dysfunction. Additional support comes from observations that a small head circumference is associated with other characteristics of a pregnancy complicated by presumed placenta hypoperfusion (delivery without labor, cesarean delivery, maternal receipt of magnesium for seizure prophylaxis, increased syncytial knots in the placenta).10 Finally, some of our observations are without precedent. We are not sure why increased birth interval should be associated with small head size. Noting that conception assistance was also associated with both microcephaly and minicephaly, this might be a relationship with infertility. However, we invite additional inquiry into this association.
In contrast, conditions associated with spontaneous delivery, such as early GA interval and recovery of vaginal flora from the placenta,10 were not associated with a small head circumference. In addition, the risks of a small head circumference were reduced among newborns with characteristics associated with intrauterine inflammation and spontaneous delivery, such as the recovery of organisms from the placenta and histologic inflammation of the placenta.
Our observations are not without precedent. Others have observed that IUGR is a common consequence of placental insufficiency.20 Both human and animal studies have suggested that placental in-sufficiency is associated with a reduction in brain volume.21,22 In the rodent, experimental simulation of placental in-sufficiency by ligation of the uterine arteries is associated with both reduced birth- and brainweight.21,23 While the growth-restricted fetus attempts to compensate for the substrate limitations associated with placental insufficiency by preferentially perfusing the central nervous system,24–26 our results suggest that this compensation is often insufficient to maintain normal head growth. Indeed, so-called brain-sparing appears not to achieve all that is desired.27,28 Yet in our sample of extremely low GA neonates, microcephaly at birth did not predict motor and cognitive impairment at 24 months postterm equivalent. In contrast, children who are microcephalic at 24 months are more likely than their peers to have motor and cognitive impairments.29 While a more extensive treatment of the technical and methodological issues involved in microcephaly research are available elsewhere,2 some specific issues deserve comment here. First is the definition of reduced head growth. Our findings support the hypothesis that antecedents of the most marked reductions in head circumference also contribute to less severe reductions. For example, observations often seen in association with a microcephaly (Z-score<−2) were also seen in association with a minicephaly (Z-scores<−2 to<−1). We encourage the view that reduced head circumference is a continuum, and not the discrete categories we accept and construct.
The next methodological issue involves the choice of an external standard. We have advocated for external standards appropriate to the sample under study.2 Unfortunately, our choice of an external standard does not have a large representation of ELGAN. What makes our external standard attractive, however, is the exclusion of children delivered for maternal and fetal indications.
Our standard was further limited with regard to race-specific classifications. The mothers of 29% of the newborns in our sample identified themselves as black. Blacks tend to have both lower birthweights and head circumferences than whites. Unfortunately, we could not find a good external head circumference standard for births <28 weeks that separates blacks from whites. Similarly, the external standard we used did not separate male from female babies in providing median and SD for head circumference at each week of gestation. This deficiency might account for our finding that girls are at higher risk than boys of a small head circumference. We therefore caution that the race- and gender-specific differences in the risks of microcephaly we observe may be related to the characteristics of our standard.
Congenital microcephaly is often a component of syndromes of malformations.1 We did not identify any such syndromes in our sample. A relatively high proportion of children had such minor anomalies as inguinal hernia, but these anomalies were not appreciably overrepresented among the infants with a small head circumference.
In conclusion, characteristics associated with fetal growth restriction and maternal and fetal indications for delivery are among the strongest correlates of reduced head circumference at birth among children born at extremely low GA. These disorders are themselves associated with presumed suboptimal placentation and/or placenta hypoperfusion leading to the inference that the associations we observed might contribute to impaired brain growth. Our observations that black and female ELGAN are more likely than others to be classified as microcephalic and minicephalic might be consequences of our not having appropriate sex and race standards.
Acknowledgments
The ELGAN project was supported by National Institutes of Health, National Institute of Neurologic Diseases and Stroke (U01 NS 400069-01). Dr McElrath was supported by the Women’s Reproductive Health Research program, National Institutes of Child Health and Development (K12 HDO1255).
Footnotes
Reprints not available from the authors.
References
- 1.Ross J. Microcephaly handbook of clinical neurology. Vol. 30. Amsterdam: Elsevier; 1993. [Google Scholar]
- 2.Leviton A, Holmes LB, Allred EN, Vargas J. Methodologic issues in epidemiologic studies of congenital microcephaly. Early Hum Dev. 2002;69:91–105. doi: 10.1016/s0378-3782(02)00065-8. [DOI] [PubMed] [Google Scholar]
- 3.Davies H, Kirman BH. Microcephaly. Arch Dis Child. 1962;37:623–7. doi: 10.1136/adc.37.196.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinlin M, Zurrer M, Martin E, Boesch C, Largo RH, Boltshauser E. Contribution of magnetic resonance imaging in the evaluation of microcephaly. Neuropediatrics. 1991;22:184–9. doi: 10.1055/s-2008-1071438. [DOI] [PubMed] [Google Scholar]
- 5.Persutte WH. Microcephaly–no small deal. Ultrasound Obstet Gynecol. 1998;11:317–8. doi: 10.1046/j.1469-0705.1998.11050317.x. [DOI] [PubMed] [Google Scholar]
- 6.Gale CR, O’Callaghan FJ, Godfrey KM, Law CM, Martyn CN. Critical periods of brain growth and cognitive function in children. Brain. 2004;127:321–9. doi: 10.1093/brain/awh034. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Horbar JD, Malloy MH, Tyson JE, Wright L. Very low birthweight outcomes of the National Institute of Child Health and Human Developmental Network. Pediatrics. 1991;87:587–97. [PubMed] [Google Scholar]
- 8.Avery GB, Meneses L, Lodge A. The clinical significance of ”measurement microcephaly. Am J Dis Child. 1972;123:214–7. doi: 10.1001/archpedi.1972.02110090084008. [DOI] [PubMed] [Google Scholar]
- 9.Martin HP. Microcephaly and mental retardation. Am J Dis Child. 1970;119:128–31. doi: 10.1001/archpedi.1970.02100050130007. [DOI] [PubMed] [Google Scholar]
- 10.McElrath TF, Hecht JL, Dammann O, et al. Pregnancy disorders that lead to delivery before the 28th week of gestation: an epidemiologic approach to classification. Am J Epidemiol. 2008;168:980–9. doi: 10.1093/aje/kwn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 12.Onderdonk AB, Delaney ML, DuBois AM, Allred EN, Leviton A. Detection of bacteria in placental tissues obtained from extremely low gestational age neonates. Am J Obstet Gynecol. 2008;198:110.e1–7. doi: 10.1016/j.ajog.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199:52.e1–10. doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driscoll SG, Langston C. College of American Pathologists Conference XIX on the examination of the placenta: report of the working group on methods for placental examination. Arch Pathol Lab Med. 1991;115:704–8. [PubMed] [Google Scholar]
- 15.Hecht JL, Onderdonk A, Delaney M, et al. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol. 2008;11:15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 16.Raymond GV, Holmes LB. Head circumferences standards in neonates. J Child Neurol. 1994;9:63–6. doi: 10.1177/088307389400900116. [DOI] [PubMed] [Google Scholar]
- 17.Nellhaus G. Head circumference from birth to eighteen years. Practical composite international and interracial graphs. Pediatrics. 1968;41:106–14. [PubMed] [Google Scholar]
- 18.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 19.Dubin N, Pasternack BS. Risk assessment for case-control subgroups by polychotomous logistic regression. Am J Epidemiol. 1986;123:1101–17. doi: 10.1093/oxfordjournals.aje.a114338. [DOI] [PubMed] [Google Scholar]
- 20.Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. Eur J Obstet Gynecol Reprod Biol. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- 21.Mallard C, Loeliger M, Copolov D, Rees S. Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea-pig following intrauterine growth-restriction. Neuroscience. 2000;100:327–33. doi: 10.1016/s0306-4522(00)00271-2. [DOI] [PubMed] [Google Scholar]
- 22.Rees S, Inder T. Fetal and neonatal origins of altered brain development. Early Hum Dev. 2005;81:753–61. doi: 10.1016/j.earlhumdev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Tashima L, Nakata M, Anno K, Sugino N, Kato H. Prenatal influence of ischemia-hypoxia-induced intrauterine growth retardation on brain development and behavioral activity in rats. Biol Neonate. 2001;80:81–7. doi: 10.1159/000047125. [DOI] [PubMed] [Google Scholar]
- 24.Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111:1031–41. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- 25.Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. Am J Obstet Gynecol. 1974;120:817–24. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- 26.Sheldon RE, Peeters LL, Jones MD, Jr, Makowski EL, Meschia G. Redistribution of cardiac output and oxygen delivery in the hypoxemic fetal lamb. Am J Obstet Gynecol. 1979;135:1071–8. doi: 10.1016/0002-9378(79)90739-7. [DOI] [PubMed] [Google Scholar]
- 27.Scherjon S, Briet J, Oosting H, Kok J. The discrepancy between maturation of visual-evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain-sparing. Pediatrics. 2000;105:385–91. doi: 10.1542/peds.105.2.385. [DOI] [PubMed] [Google Scholar]
- 28.Roza SJ, Steegers EA, Verburg BO, et al. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am J Epidemiol. 2008;168:1145–52. doi: 10.1093/aje/kwn233. [DOI] [PubMed] [Google Scholar]
- 29.Kuban KCK, Allred EN, O’Shea TM, et al. Developmental correlates of head circumference at birth and at two years in a cohort of extremely low gestational age newborns. J Pediatr. 2009;155:344–9. doi: 10.1016/j.jpeds.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]