Abstract
The Ag receptors on αβ and γδ T cells differ not only in the nature of the ligands that they recognize but also in their signaling potential. We hypothesized that the differences in αβ- and γδTCR signal transduction were due to differences in the intracellular signaling pathways coupled to these two TCRs. To investigate this, we employed transcriptional profiling to identify genes encoding signaling molecules that are differentially expressed in mature αβ and γδ T cell populations. Unexpectedly, we found that B lymphoid kinase (Blk), a Src family kinase expressed primarily in B cells, is expressed in γδ T cells but not in αβ T cells. Analysis of Blk-deficient mice revealed that Blk is required for the development of IL-17-producing γδ T cells. Furthermore, Blk is expressed in lymphoid precursors and, in this capacity, plays a role in regulating thymus cellularity during ontogeny.
Introduction
The conservation, in all jawed vertebrates, of two T cell lineages suggests that αβ and γδ T cells have complementary and non-redundant roles in immunity. There is growing evidence to support this idea. First, αβ and γδ T cells have different antigen specificities, with γδ T cells recognizing native, unprocessed antigens and αβ T cells recognizing peptides in association with MHC molecules (1–3). Second, αβ and γδ T cells localize to different peripheral tissues. While most αβ T cells circulate through secondary lymphoid tissues, most γδ T cells reside in epithelial tissues, such as skin, intestine, lung, tongue, and female reproductive tract (1–3). Third, although αβ and γδ T cells share effector functions, epithelial resident γδ T cells display specialized roles in immunity, as evidenced by their ability to mediate epithelial cell homeostasis and wound healing (4–6). Fourth, αβ and γδ T cells respond at different stages during the host immune response, with γδ T cells often acquiring effector functions days before αβ T cells (7–10).
The ability of γδ T cells to manifest their unique functions and their rapid effector response during an immune response may be explained in part by the different signaling properties of the αβ- and γδTCRs. In a direct comparison of αβ- and γδTCR signal transduction, in assays that measure calcium mobilization and ERK activation, the γδTCR signaled with faster kinetics and greater magnitude than the αβTCR (11). Importantly, the enhanced signaling proficiency of the γδTCR affected the kinetics of T cell activation, as evidenced by the ability of stimulated γδ T cells to upregulate the expression of genes associated with T cell effector function faster than stimulated αβ T cells (12) and to undergo more rounds of proliferation than stimulated αβ T cells (11).
The molecular basis for the difference in αβ- and γδTCR signaling properties is currently unknown. One explanation for this difference is that the signaling pathways triggered by the γδTCR are distinct from those triggered by the αβTCR. To test this, we employed global gene expression profiling to identify signaling molecules that are differentially expressed between mature αβ and γδ T cells. Using this strategy, we discovered B lymphoid kinase (Blk), which encodes a B cell-specific member of the Src family of protein tyrosine kinases (SFKs) (13), to be preferentially expressed in γδ but not αβ T cells. Interestingly, protein expression studies showed that Blk is expressed in only a small subset of mature γδ T cells, indicating that Blk cannot be responsible for the enhanced signaling ability of the γδTCR per se. To determine the biological significance of Blk expression in γδ lineage cells, we analyzed γδ T cell development and function in Blk−/− mice and found that Blk is required for the development of IL-17-producing γδ T cells. In addition, we discovered that Blk, which is expressed in various lymphoid precursor populations, regulates thymus cellularity by controlling the number of early thymic progenitors and the proliferative capacity of immature thymocytes during ontogeny.
Materials and Methods
Mice
C57BL/6J (B6), B6.SJL-Ptprca Pep3b/BoyJ (B6-CD45.1+), B6;129S7-Fyntm1Sor/J (Fyn−/−), B6.129S2-Lcktm1Mak/J (Lck−/−) B6.129P2-Tcrbtm1Mom/J (TCRβ−/−), and B6.129S2-Tcratm1Mom/J (TCRα−/−) mice were all purchased from the Jackson Laboratory (Bar Harbor, ME). B6-Blktm1 (Blk−/−) mice (14) were provided by A. Tarakhovsky (Rockefeller University, New York, NY), B6-IL-23R-GFP knock-in mice (IL-23R-GFP.KI) (15) were provided by M. Oukka (Seattle Children’s Research Institute, Seattle, WA), and B6-Vγ6/Vδ1 γδTCR transgenic (γδTCR Tg; line 134) (16) mice were provided by P. Love (NIH, Bethesda, MD). All mice used in this study were bred and maintained in the Department of Laboratory Animal Resources at SUNY Upstate Medical University in accordance with the specifications of the Association for Assessment and Accreditation of Laboratory Animal Care. Mouse protocols were approved by the SUNY Upstate Medical University Committee on the Humane Use of Animals.
Abs and reagents
mAbs used for flow cytometric analysis and magnetic bead separation included anti-CD4 (RM4-5), anti-CD8α (53-6.7), anti-TCRγδ (UC7-13D5), anti-TCRβ (H57-597), anti-CD3 (145-2C11), anti-CD11b (M1/70), anti-CD19 (6D5), anti-CD25 (PC61), anti-CD44 (IM7), anti-NK1.1 (PK136), anti-CD45.1 (A20), anti-CD45.2 (104), anti-CD117 (2B8), anti-Ly6-G/Ly6-C (RB6-8C5), anti-I-Ab (AF6-120.1), anti-CCR6 (29-2L17) and anti-TER-119 (TER-119), which were purchased from BioLegend (San Diego, CA), eBioscience (San Diego, CA) and BD Pharmingen (San Jose, CA). mAbs against Vγ1 (2.11), Vγ4 (UC3-10A6) and Vγ5 (F536) were purified from their respective hybridoma supernatants using ImmunoPure® (A/G) IgG Purification kit (Pierce, Rockford, IL) and then biotinylated using Pierce’s Sulfo-NHS-LC-Biotin according to manufacturer’s instructions. PE-streptavidin was purchased from BioLegend. Abs used in intracellular flow cytometric assays were anti-Blk (Cell Signaling Technology, Danvers, MA), anti-Fyn (FYN-59; BioLegend), anti-Lck (3A5; Millipore, Billerica, MA), Ki-67 (B56; BD Pharmingen), anti-IL-17A (TC11-18H10.1; BioLegend), anti-IFNγ (XMG1.2; BD Pharmingen), anti-mouse IgG2b (R12-3; BioLegend) and donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA).
Purification of lymphocyte subsets
CD4+ αβ T cells, CD8+ αβ T cells, DN γδ T cells and DN γδ thymocytes were purified by negative selection using the magnetic bead separation system (Miltenyi, Auburn, CA) as previously described (11,12).
B cells were purified by positive selection using magnetic bead separation. Briefly, spleen cells from B6 mice were stained for 10 min with PE-conjugated anti-CD19 mAb, washed, and then incubated with anti-PE beads (Miltenyi) for 15 min, with all steps at 4°C. The purity of the resulting cell population was typically ≥95%.
Quantitative Western blot analysis
DN γδ thymocytes purified from γδTCR Tg mice were lysed in a buffer containing 20 mM Tris, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, protease inhibitors (Roche Laboratories, Indianapolis, IN), and 1% NP-40 Alternative (Calbiochem, Gibbstown, NJ). Serial dilutions of cleared lysates from a known number of DN γδ thymocytes were resolved by SDS-PAGE in parallel with serial dilutions of known amounts of recombinant His-tagged Blk or Lck (Millipore), transferred to a PVDF membrane, and immunoblotted with anti-Blk or Lck Abs. Abs used for Western blot analysis were anti-Blk (Santa Cruz Biotechnology, Santa Cruz, CA), anti-Lck (Millipore), and anti-β-actin (Sigma, St. Louis, MO). The volume (defined as intensity × mm2) of each band was determined using densitometric analysis. A standard curve was generated by plotting the concentration of Blk or Lck standard (in ng) versus the volume of each corresponding band. The concentration per cell was then estimated based on this standard curve.
Isolation of iIELs
iIELs were isolated as previously described (17).
Microarray analysis
The GeneChip® Mouse Genome 430 2.0 Array (Affymetrix, Santa Clara, CA) was used to screen for genes encoding signaling molecules whose gene expression was significantly different between DN γδ T cells and CD8+ αβ T cells. Total RNA from both T cell populations was extracted and processed for analysis as previously described (12). The labeled cRNA was hybridized to the GeneChip® Mouse Genome 430 2.0 Array (containing 45,000 probe sets that represent 39,000 transcripts and 34,000 mouse genes) at 45°C for 16 h with constant rotation (60 rpm), washed and then stained on a Fluidics Station 400 (Affymetrix) according to the EukGE-WS2v4 protocol. GeneChips were scanned using the Agilent G2500A Gene Array Scanner. After scanning, the Affymetrix GCOS Microarray Analysis Suite version 5.0 software was used to determine the presence (P) or absence (A) of a transcript, the differential change in gene expression, and the magnitude of the change in gene expression. The complete microarray data set can be found at the Gene Expression Omnibus (GEO) repository (http://www.ncbi.nlm.nih.gov/projects/geo/); accession number, GSE24281.
Flow cytometric analysis
Flow cytometric analysis for both surface and intracellular Ags was performed as previously described (18).
Real time RT-PCR analysis
RNA was extracted from B cells and T cell subsets using the RNeasy kit from Qiagen. cDNA was then synthesized using Invitrogen’s SuperScript® First-Strand Synthesis System. Quantitative real-time RT-PCR analysis was performed using a Bio-Rad iQ™5 Real-time PCR machine (Hercules, CA). All of the primer sets for the quantitative real-time RT-PCR analysis, which include Gapdh, Blk, and Lyn, were purchased from SABiosciences (Frederick, MD).
Mixed bone marrow chimeras
Bone marrow (BM) cells from 6 to 8-wk-old B6-CD45.2+ and Blk−/− mice (also CD45.2+) were mixed in a 1:1 ratio with BM cells from age-matched B6-CD45.1+ mice. 5 × 106 cells of this cell mixture were then injected i.v. into the tail vein of lethally irradiated (1100 rads) 6-wk-old B6-CD45.1+ mice. Three weeks post injection, thymocytes were harvested and stained with mAbs against various surface Ags as well as mAbs against CD45.1 and CD45.2 to determine the degree of chimerism.
In vitro stimulation of γδ T cells
γδ T cells from γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice were stimulated as previously described (18).
RESULTS
Blk is expressed in γδ T cells but not αβ T cells
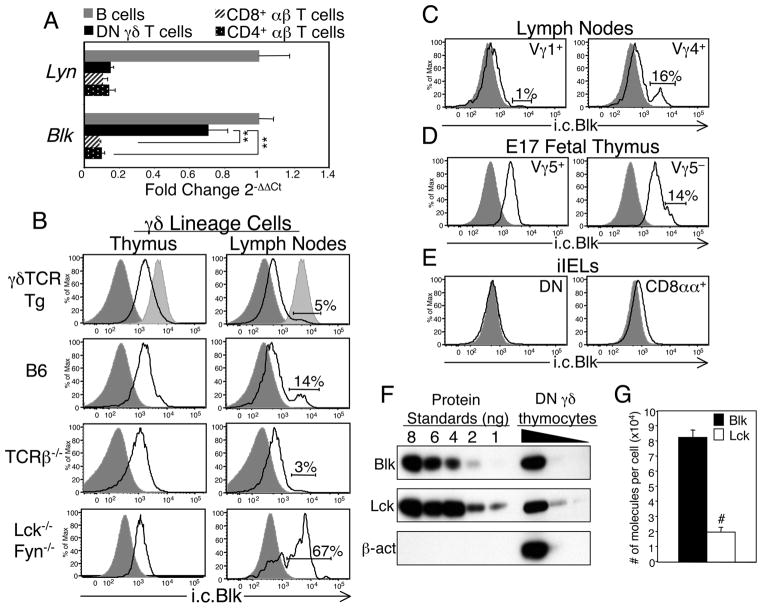
To identify signaling molecules that are differentially expressed between αβ and γδ T cells, we transcriptionally profiled mature CD4− CD8− (double negative; DN) γδ T cells and CD8+ αβ T cells. We reasoned that comparing the gene expression profiles of two T cell populations that share functional properties, such as the propensity to produce IFNγ following TCR activation (19), would highlight any qualitative or quantitative differences in their gene expression. To this end, we purified DN γδ T cells from the lymph nodes (LNs) of Vγ6/Vδ1 γδTCR transgenic (Tg) mice (hereafter referred to as γδ TCR Tg) and CD8+αβ T cells from the LNs of C57BL/6 (B6) mice, both by negative selection. Total RNA from the two T cell populations was extracted and then processed for microarray analysis. This approach identified many genes that are expressed at higher levels in DN γδ T cells than in CD8+ αβ T cells. Table I lists genes that exhibited the greatest difference in expression between DN γδ T cells and CD8+ αβ T cells. Some of these genes, such as Rgs1, Rgs2 and Sox13, have been previously reported to be preferentially expressed in γδ lineage cells (20,21). Since our goal was to identify signaling molecules that are differentially expressed in αβ and γδ T cells, we considered Blk to be the most interesting in this list of genes, as it encodes a kinase known to be involved in Ag receptor signal transduction (22–24). The differential expression of Blk was confirmed by performing real time RT-PCR analysis on not only purified DN γδ T cells and CD8+ αβ T cells but also purified CD4+ αβ T cells and B cells. Specifically, we observed a significant difference in the relative expression of Blk between γδ T cells and the two αβ T cell subsets but not between γδ T cells and B cells (Fig. 1A). Expression of Lyn, another SFK typically found in B cells, was absent in the purified γδ T cell population (Fig. 1A), indicating that the expression of Blk by γδ T cells was not due to B cell contamination.
Table I.
List of genes preferentially expressed in ex vivo DN γδ T cells compared to CD8+ αβ T cells.
| Gene | Symbol | Fold Changea |
|---|---|---|
| TCRg constant region | Tcrg-C | 9.8 |
| S100 calcium binding protein A6 | s100a6 | 6.8 |
| Ubiquitin specific peptidase 18 | Usp18 | 6.8 |
| S100 calcium binding protein A4 | s100a4 | 6.5 |
| Regulator of G-protein signaling 1 | Rgs1 | 6.2 |
| Tetraspanin 32 | Tspan32 | 5.6 |
| Placental protein 11 related | Pp11r | 5.5 |
| Insulin-like growth factor binding protein 4 | Igfbp4 | 5.4 |
| Caspase 1 | Casp1 | 5.1 |
| Lymphocyte antigen 6 complex, locus A | Ly6a | 5.1 |
| Niban protein | Niban | 4.9 |
| B lymphoid kinase | Blk | 4.9 |
| Leukocyte-associated Ig-like receptor 1 | Lair1 | 4.8 |
| Thyroid hormone receptor interactor 4 | Trip4 | 4.8 |
| Integrin beta 3 | Itgb3 | 4.7 |
| Deltex 1 homolog | Dtx1 | 4.7 |
| SRY-box containing gene 13 | Sox13 | 4.3 |
| Regulator of G-protein signaling 2 | Rgs2 | 3.9 |
Gene expression is shown as log2-fold change in expression in DN γδ T cells versus CD8+ αβ T cells.
FIGURE 1.
Blk is expressed in γδ lineage cells. A, Quantitative real-time RT-PCR analysis of the relative transcript levels of Blk and Lyn in DN γδ T cells purified from γδTCR Tg mice and in CD8+ αβ T cells, CD4+αβ T cells, and CD19+ B cells purified from B6 mice. Data were normalized to Gapdh transcript levels and are presented as fold change over B cells (set to 1). Bars represent mean ± SEM of at least 3 samples of each lymphocyte population. **p≤0.01. B, Black histograms show representative staining of the intracellular (i.c.) levels of Blk in gated populations of thymic and peripheral γδ lineage cells from γδTCR Tg, B6, TCRβ−/− and Lck−/−Fyn−/− mice. DP thymocytes (dark shaded histogram) and splenic B cells (light shaded histogram) are shown as negative and positive controls, respectively. C, Black histograms show representative staining of the i.c. levels of Blk in gated populations of Vγ1+ and Vγ4+γδ T cells from the LNs of B6 mice. DP thymocytes (dark shaded histogram) are shown as a negative control. D, Black histograms show representative staining of the i.c. levels of Blk in gated populations of Vγ5+ and Vγ5− thymocytes from B6 embryonic day 17 (E17) fetuses. DP thymocytes (dark shaded histogram) are shown as a negative control. E, Black histograms show representative staining of the i.c. levels of Blk in gated populations of DN and CD8αα+γδTCR+ iIELs from B6 mice. DP thymocytes (dark shaded histogram) are shown as a negative control. F, Serial dilutions of cleared lysates from a known number of DN γδ thymocytes were resolved by SDS-PAGE in parallel with serial dilutions of known amounts of recombinant His-tagged Blk or Lck, transferred to a PVDF membrane, and immunoblotted with anti-Blk, Lck or β-actin Abs. Data are representative of four independent experiments. G, Mean number of Blk and Lck molecules per DN γδ thymocytes. #p≤0.001.
Next, we developed an intracellular (i.c.) flow cytometric assay to examine both the pattern and level of Blk expression in various lymphocyte populations (Supplemental Fig. 1). Using this technique, we found, in both γδTCR Tg and B6 mice, that Blk is expressed in all γδ thymocytes but only in a small percentage of mature γδ T cells (Fig. 1B). Notably, the expression level of Blk in γδ thymocytes was lower than that in B cells, while its expression level in matureγδ T cells was comparable to that in B cells.
Many of the genes that are preferentially expressed in γδ lineage cells require a thymic microenvironment containing a quorum of lymphotoxin-producing CD4+CD8+ (double positive; DP) thymocytes for their proper expression (20,25). To determine whether Blk expression by γδ thymocytes is similarly regulated, we assayed γδ lineage cells from TCRβ−/− mice, which do not possess the appropriate DP thymocyte compartment to support the expression of the γδ-biased genes (25). As shown in Fig. 1B, we found that Blk is indeed expressed in TCRβ−/− γδ thymocytes and at levels comparable to those observed in B6 mice. These findings indicated that Blk expression in γδ lineage cells is not dependent on interactions with immature αβ lineage cells.
Unlike αβ T cell development, which is completely blocked in Lck−/−Fyn−/− mice, γδ T cell development is impaired but not abrogated (26). In fact, a small number of γδTCR+ cells are detected in secondary lymphoid tissues, small intestine, and epidermis of Lck−/−Fyn−/− mice (26,27). The differential requirements for SFKs in αβ and γδ T cell development may be explained by the expression of another SFK, namely Blk, in γδ lineage cells. To test this, we measured Blk expression levels in DN γδTCR+ thymocytes and LN cells from Lck−/−Fyn−/− mice. Significantly, Blk expression was detected not only in γδ thymocytes but also in two-thirds of theγδ T cells present in the LNs of Lck−/−Fyn−/− mice (Fig. 1B). These data suggested that γδ lineage cells that express Blk develop and survive in the absence of both Lck and Fyn.
Since murine γδ T cells can be divided into subsets based on their Vγ usage and their anatomical location (1–3), it was of interest to determine which γδ T cell subsets express Blk. In the LNs of B6 mice, only 14% of the γδ T cells expressed Blk (Fig. 1B). Phenotypic analysis of these Blk+ γδ T cells, using Vγ1- and Vγ4-specific mAbs, demonstrated that they are restricted to the Vγ4+γδ T cell subset (Fig. 1C). In fact, we noted that 50% of the Blk+ γδ T cells in B6 LNs were Vγ4+ (data not shown). Next, we assayed the γδ T cell populations that develop in the fetal thymus for Blk expression. We found that, although all fetal γδ thymocytes expressed Blk, the Vγ5− subset, the vast majority of which represent Vγ6+γδ T cells that home to lung, tongue, and female reproductive tract, contained a small population of Blkhi cells, while the Vγ5+ subset, which represents the skin-homing dendritic epidermal T cells (DETCs), did not (1–3) (Fig. 1D). Last, we performed a phenotypic analysis of the γδ T cells that reside in the small intestine (intestinal intraepithelial lymphocytes or iIELs) and detected minimal Blk expression in either the DN or CD8αα+γδ iIEL subset (Fig. 1E). Together, these data indicated that Blk expression in mature γd T cells is restricted to Vγ4+ and Vγ6+γδ T cell subsets.
If Blk were to contribute to the signaling proficiency of the γδTCR, then we would expect the expression level of Blk in γδ lineage cells to be comparable to that of the T cell-specific SFK Lck. To test this, we measured the protein concentrations of Blk and Lck in purified γδ thymocytes by quantitative Western blot analysis using His-tagged Blk and Lck as protein standards (Fig. 1F). We found that γδ thymocytes expressed approximately 4-fold more Blk (~80,000 molecules per cell) than Lck (~20,000 molecules per cell) (Fig. 1G). Because flow cytometric analysis showed that the peripheral Blk+ γδ T cell subset expresses 2.5-fold more Blk than γδ thymocytes (Fig. 1B), we estimate that these γδ T cells express ~200,000 molecules of Blk per cell. This amount is even more striking given that LN γδ T cells express significantly less Lck and Fyn than γδ thymocytes (18). These data indicated that Blk is the predominant SFK expressed in γδ thymocytes and in the mature Blk+ γδ T cell subset.
Blk is expressed in thymic precursors
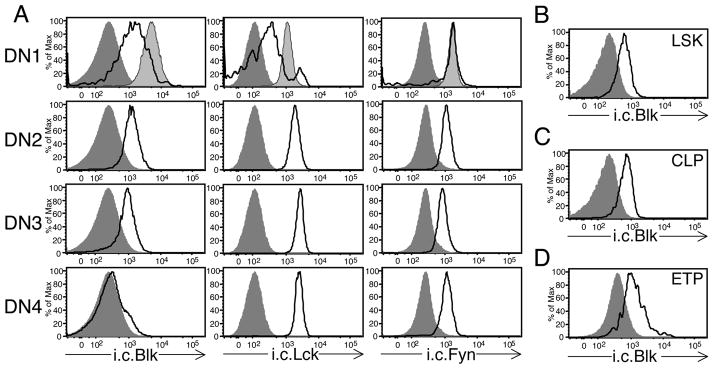
Given the relatively high expression levels of Blk in γδ thymocytes, we sought to determine whether its expression is induced or upregulated as a consequence of commitment to the γδ lineage. Using the i.c. flow cytometric assay, we found that Blk is expressed in immature DN thymocytes, which contain precursors with the potential to develop into αβ or γδ lineage cells (28), but at lower levels than those detected in γδ thymocytes (Fig. 1B and data not shown). When the immature DN thymocyte population was subdivided into four populations based on expression of CD44 and CD25 (29), we found that Blk is highly expressed in DN1 and DN2 thymocytes, slightly downregulated in DN3 thymocytes, and virtually absent in DN4 thymocytes (Fig. 2A). Notably, this expression pattern contrasted with those of Lck and Fyn, whose expression is maintained at relatively high levels throughout the DN4 stage (Fig. 2A). Because the divergence of αβ and γδ lineages has been shown to occur at the DN2 stage and, to a lesser extent, the DN3 stage (30) and because DN4 thymocytes represent immature αβ lineage cells transitioning to the DP stage (31), these findings demonstrated that Blk is expressed in thymic progenitors, and following αβ/γδ lineage choice, its expression is upregulated in γδ lineage cells but downregulated in αβ lineage cells.
FIGURE 2.
Blk is expressed in thymic and BM progenitor cells. A, Black histograms show representative staining of the i.c. levels of Blk, Lck, and Fyn in gated DN1 (Lin− CD25− CD44+), DN2 (Lin− CD25+ CD44+), DN3 (Lin− CD25+ CD44−), DN4 (Lin− CD25− CD44−) thymocyte populations from TCRα−/− mice. Lin− is defined as CD4− CD8− CD11b− TCRβ − TCRγδ− CD19− NK1.1− IAb− TER-119− Ly6-G/Ly6-C−. Dark shaded histograms represent negative staining controls (DP thymocytes for i.c. Blk staining, Lck−/− thymocytes for i.c. Lck staining, and Fyn−/− thymocytes for i.c. Fyn staining). Light shaded histograms represent positive staining controls (splenic B cells for i.c. Blk staining and mature CD4+ T cells for both i.c. Lck and Fyn staining). B, Black histograms show representative staining of the i.c. levels of Blk in Linv− Sca-1+ c-Kithi (LSK) progenitors from B6 BM. DP thymocytes (dark shaded histogram) are shown as a negative control. C, Black histograms show representative staining of the i.c. levels of Blk in Lin− Sca-1lo c-Kitlo cells or common lymphoid progenitors (CLPs) from B6 BM. DP thymocytes (dark shaded histogram) are shown as a negative control. D, Black histograms show representative staining of the i.c. levels of Blk in lin− CD25− CD44+ c-Kit+ thymocytes or early thymic progenitors (ETPs) from TCRα−/− thymus. DP thymocytes (dark shaded histogram) are shown as a negative control.
Blk-deficiency affects the early stages of T cell development
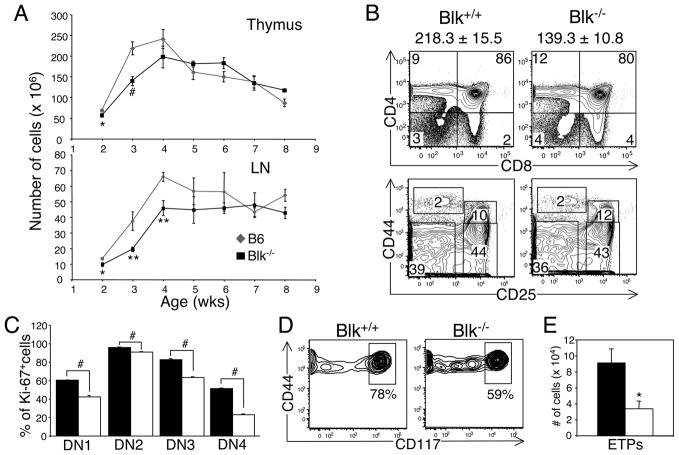
The expression of Blk at the earliest DN1 stage prompted us to evaluate Blk expression in defined precursor populations, such as early thymic progenitors (ETPs), common lymphoid progenitors (CLPs), and lineage-negative Sca-1+ c-Kit+ cells (LSKs). Strikingly, all of these precursor populations expressed Blk and at relatively high levels (Fig. 2B, 2C, 2D). To determine the significance of Blk expression in T cell progenitors, we analyzed T cell development in various aged Blk+/+ (B6) and Blk−/− mice. During ontogeny, we found that the cellularity of the Blk−/− thymus did not reach wild-type numbers until 4 wks of age, whereas the cellularity of Blk−/− LNs did not reach wild-type numbers until 5 wks of age (Fig. 3A). Since the most significant difference in thymus cellularity was observed between 3-wk-old Blk+/+ and Blk−/− mice, we chose this age to perform a more detailed analysis of T cell development.
FIGURE 3.
Effects of Blk-deficiency on T cell development. A, Cellularity of the thymus and LNs of Blk+/+ ( ) and Blk−/− (ν) mice plotted as a function of age. Mean cell number ± SEM from 3 to 10 mice are shown for each age. *p≤0.05, **p≤0.01, #p≤0.001. B, Phenotypic analysis of thymocytes from 3-wk-old Blk+/+ and Blk−/− mice. Top panel: Dot plots show representative CD4 versus CD8 staining profiles of total thymocytes. Bottom panel: Dot plots show representative CD44 versus CD25 staining on gated Lin− thymocytes, where Lin− is defined as in Fig. 2A. Numbers in quadrants represent percentage of cells in each quadrant. The mean thymus cell number ± SEM for each genotype are displayed above the two-color plots. C, Effect of Blk-deficiency at 3 wks of age on the proliferative capacity of the four DN subsets. Bars represent the mean percentage of Ki-67+ cells ± SEM from at least 3 mice per genotype. #p≤0.001. E, Dot plots show representative CD44 versus CD117 (c-Kit) staining profiles on gated Lin− CD25−CD44+ (DN1) thymocytes from 3-wk-old Blk+/+ and Blk−/− mice. Numbers represent percentages of ETPs. F, Comparison of the number of ETPs in 3-wk-old Blk+/+ and Blk−/− mice. The mean number of ETPs ± SEM from 3 mice are shown. *p≤0.05.
) and Blk−/− (ν) mice plotted as a function of age. Mean cell number ± SEM from 3 to 10 mice are shown for each age. *p≤0.05, **p≤0.01, #p≤0.001. B, Phenotypic analysis of thymocytes from 3-wk-old Blk+/+ and Blk−/− mice. Top panel: Dot plots show representative CD4 versus CD8 staining profiles of total thymocytes. Bottom panel: Dot plots show representative CD44 versus CD25 staining on gated Lin− thymocytes, where Lin− is defined as in Fig. 2A. Numbers in quadrants represent percentage of cells in each quadrant. The mean thymus cell number ± SEM for each genotype are displayed above the two-color plots. C, Effect of Blk-deficiency at 3 wks of age on the proliferative capacity of the four DN subsets. Bars represent the mean percentage of Ki-67+ cells ± SEM from at least 3 mice per genotype. #p≤0.001. E, Dot plots show representative CD44 versus CD117 (c-Kit) staining profiles on gated Lin− CD25−CD44+ (DN1) thymocytes from 3-wk-old Blk+/+ and Blk−/− mice. Numbers represent percentages of ETPs. F, Comparison of the number of ETPs in 3-wk-old Blk+/+ and Blk−/− mice. The mean number of ETPs ± SEM from 3 mice are shown. *p≤0.05.
Notably, we observed significant decreases in both the percentage and number of DP thymocytes in 3-wk-old Blk−/− mice compared to age-matched Blk+/+ mice (Fig. 3B and data not shown). This phenotype contrasts with the one observed at 6 wks of age, when T cell development has reached steady state levels, in that we did not detect any differences in the percentage and number of DN, DP and SP thymocytes between Blk+/+ and Blk−/− mice (data not shown). The reduction in the number of DP thymocytes in young Blk−/− mice was not to due to a defect in the maturation of the DN subsets, as no appreciable difference was observed between the two genotypes in the relative frequency of each DN subset (Fig. 3B). However, when we compared the proliferation status of the DN subsets in Blk+/+ and Blk−/− thymuses by measuring expression of the Ki-67 proliferation marker, we found that all Blk−/− DN subsets underwent significantly less proliferation than their wild-type counterparts (Fig. 3C). In addition, there was a significant reduction in the percentage and number of ETPs (c-Kithi DN1 thymocytes) in Blk−/− mice relative to Blk+/+ mice (Fig. 3D, 3E). It is interesting to note that we detected a significant population of cells in the Blk−/− DN1 subset that were c-Kitlo (Fig. 3D). The surface phenotype of these cells is similar to that of DN1c cells, which have been shown to have T lineage potential but to exhibit a low proliferative capacity (32). Together, these data demonstrated that Blk controls the frequency of the different thymic progenitors as well as the proliferative capacity of immature DN thymocytes.
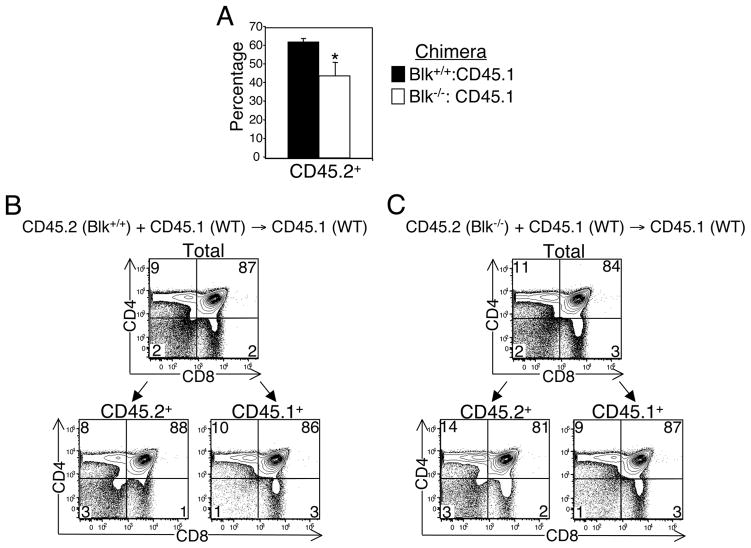
To determine whether the defects in the early stages of T cell development that were observed in young Blk−/− mice are due to the loss of Blk in thymic precursors, we generated BM chimeras, in which BM from Blk−/− (CD45.2+) or Blk+/+ (CD45.2+) mice were mixed in a 1:1 ratio with B6-CD45.1+ BM and then injected into lethally irradiated B6-CD45.1+ hosts. Three weeks after transplantation, we found that, in comparison to Blk+/+-origin precursors, Blk−/−-origin precursors did not compete as well against B6-CD45.1-origin precursors in repopulating the thymus (Fig. 4A). In addition, the CD4 versus CD8 staining profile of the Blk−/−-origin thymocytes in the chimeras mirrored that of 3-wk-old intact Blk−/− mice (Fig. 3B, 4C). Together, these findings indicated that the T cell developmental defects observed in Blk−/− mice are cell intrinsic.
FIGURE 4.
Defects in T cell development in Blk−/− mice are cell intrinsic. A, Degree of chimerism in the thymus of Blk+/+:CD45.1 and Blk−/−:CD45.1 mixed BM chimeras. Bars represent the percentages of CD45.2+ thymocytes ± SEM in Blk+/+:CD45.1 chimeras (n=4) and Blk−/−:CD45.1 chimeras (n=3). *p≤0.05. B, Phenotypic analysis of thymocytes from Blk+/+:CD45.1 mixed BM chimeras. Two-color plots show expression of CD4 versus CD8 on total and on gated CD45.2+ and CD45.1+ thymocytes. Numbers in quadrants of the two-color plots represent the percentage of cells in each quadrant. C, Phenotypic analysis of thymocytes from Blk−/−:CD45.1 mixed BM chimeras. Two-color plots show expression of CD4 versus CD8 on total and on gated CD45.2+ and CD45.1+ thymocytes. Numbers in quadrants of the two-color plots represent the percentage of cells in each quadrant.
Blk is not required for commitment to the γδ T cell lineage
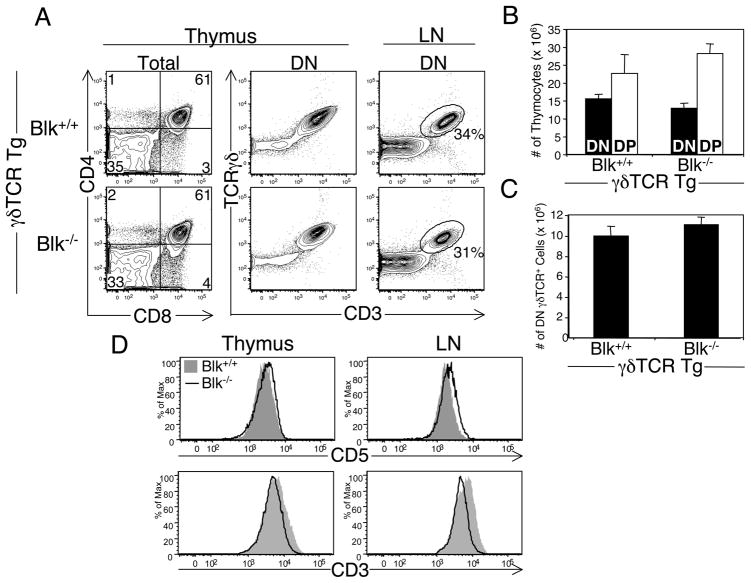
The differential expression of Blk between committed αβ and γδ lineage cells in the thymus suggested that it plays a role in γδ T cell commitment and/or development. To investigate this, we assessed γδ T cell development in various aged Blk+/+ and Blk−/− mice. At 3 wks of age, we found that the percentages of DN TCRγδ+ cells in the thymus and periphery are comparable between Blk+/+ and Blk−/− mice, but their absolute numbers are decreased due to the reduction in thymus and LN cellularity (Fig. 5A, 5C). By 6 wks of age, however, no differences were observed between Blk+/+ and Blk−/− mice in the percentage or number of thymic and LN γδ T cells (data not shown). Even though these data suggested that Blk is not required for commitment to the γδ lineage, we took another approach, by mating a γδTCR transgene onto the Blk−/− background, to determine whether fixing the specificity of the γδTCR uncovered a requirement for Blk in γδ lineage commitment. Using the γδTCR Tg system, we found that loss of Blk has no effect on αβ/γδ lineage choice nor on the generation of mature γδ T cells (Fig. 6A, 6B and 6C). Interestingly, despite the presence of wild-type numbers of thymic and peripheral γδ T cells in γδTCR Tg Blk−/− mice, the phenotype of these cells was altered compared to γδ T cells from γδTCR Tg Blk+/+ mice, in that their CD5 levels were significantly increased while their CD3 levels were significantly decreased (Fig. 6D). Because CD5 levels directly correlate with TCR signal strength (33), these results suggested that, in the absence of Blk, γδTCR signal strength is augmented.
FIGURE 5.
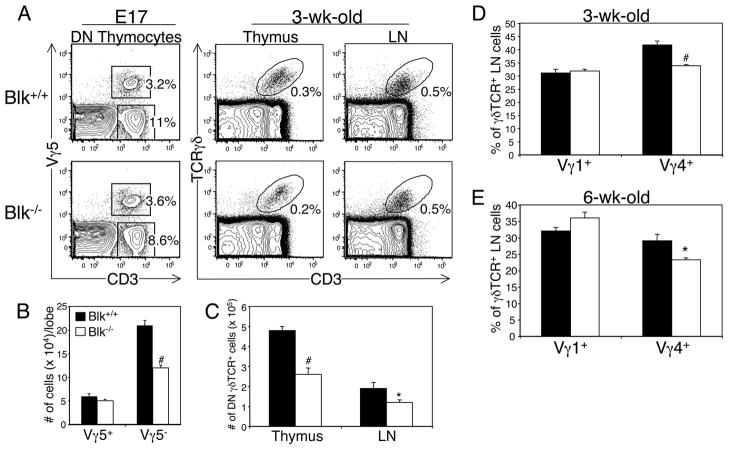
Effects of Blk-deficiency on γδ T cell development. A. Phenotypic analysis of γδ lineage cells in Blk+/+ and Blk−/− mice. Left panel: Dot plots show representative Vγ5 versus CD3 staining profiles on gated DN thymocytes from E17 Blk+/+ and Blk−/− fetuses. Right panel: Dot plots show representative TCRγδ versus CD3 staining on total thymocytes and LN cells from 3-wk-old Blk+/+ and Blk−/− mice. Numbers represent percentage of cells in each gate. B, Quantification of Vγ5+ and Vγ5− fetal thymocytes in E17 Blk+/+ and Blk−/− fetuses. Bars represent mean ± SEM of at least 8 thymic lobes per genotype. #p≤0.001. C, Quantification of DN TCRγδ+ cells in the thymus and LN of 3-wk-old Blk+/+ and Blk−/− mice. Bars represent mean ± SEM of 9 to 10 mice per genotype. *p≤0.05, #p≤0.001. D, Percentage of Vγ1+ and Vγ4+ cells in gated DN TCRγδ+ LN cells from 3-wk-old Blk+/+ and Blk−/− mice. Bars represent mean ± SEM of 5 mice per genotype. #p≤0.001. E, Percentage of Vγ1+ and Vγ4+ cells in gated DN TCRγδ+ LN cells from 6-wk-old Blk+/+ and Blk−/− mice. Bars represent mean ± SEM of 3 mice per genotype. *p≤0.05.
FIGURE 6.
Effects of Blk-deficiency on T cell development in γδTCR Tg mice. A, Phenotypic analysis of 4 to 6-wk-old γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice. Dot plots show representative CD4 versus CD8 staining profiles on total thymocytes. Numbers in quadrants of the two-color plots represent the percentage of cells in each quadrant. Adjacent dot plots show representative TCRγδ versus CD3 staining on DN thymocytes and LN cells. Numbers represent percentage of cells in each gate. B, Mean number ± SEM of DN (DN TCRγδ+; γδ lineage) and DP (αβ lineage) thymocytes in γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice. Data represent 5 mice per genotype. C, Mean number ± SEM of DN γδ T cells in the LNs of γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice. Data represent 5 mice per genotype. D, Comparison of CD5 and CD3 surface levels on DN TCRγδ+ cells in the thymus and LNs of γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice. Data are representative of 4 mice per genotype.
Blk is required for the development of IL-17-producing γδ T cell effectors
Although the total numbers of peripheral γδ T cells in Blk−/− and Blk+/+ mice were comparable, it is conceivable that the relative percentages of specific γδ T cell subsets, such as those expressing high levels of Blk, are reduced in Blk−/− mice. To test this, we examined the Vγ usage of DN γδTCR+ cells from the fetal thymus and adult LNs by flow cytometric analysis using various Vγ-specific mAbs. We found that the number of Vγ5− γδ thymocytes are significantly reduced in Blk−/− E17 fetuses compared to Blk+/+ E17 fetuses (Fig. 5A, 5B). Likewise, there were significantly fewer Vγ4+γδ T cells in the LNs of Blk−/− mice than in the LNs of Blk+/+ mice (Fig. 5D, 5E). Together, these findings demonstrated that Blk-deficiency results in a selective loss of cells within the Vγ4+ and Vγ6+γδ T cell subsets.
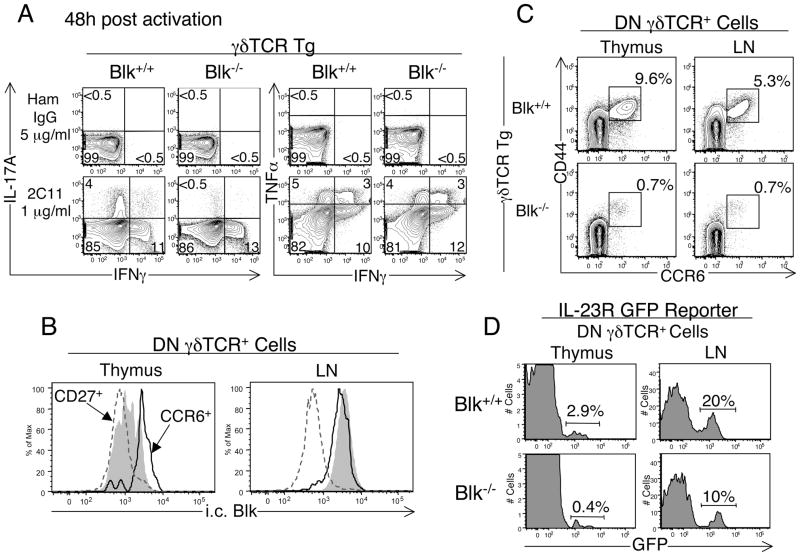
We reasoned that the changes in γδTCR repertoire observed in Blk−/− mice may affect γδ T cell effector function. Because γδ T cells from the Vγ6/Vδ1 γδTCR Tg mouse have the ability to acquire different effector fates (18), which match those reported for this γδ T subset following activation in vivo (34), we used our γδTCR Tg system to assess the effects of Blk-deficiency on γδ T cell effector function. To this end, we examined cytokine production by γδ T cells following TCR activation and found that the percentage of stimulated γδ T cells producing IL-17 is drastically reduced in γδTCR Tg Blk−/− mice compared to γδTCR Tg Blk+/+ mice (Fig. 7A). In contrast, the percentages of stimulated Blk−/− γδ T cells producing IFNγ and/or TNFα were comparable to those of stimulated Blk+/+ γδ T cells (Fig. 7A), suggesting that there is a selective loss of IL-17-producing γδ effectors in γδTCR Tg Blk−/− mice.
FIGURE 7.
Effects of Blk-deficiency on γδ T cell effector function. A, Comparison of IL-17 versus IFNγ production and IFNγ versus TNFα production by DN γδTCR+ LN cells from γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice. LN cells from the two genotypes were in vitro stimulated with 1 μg/ml of immobilized anti-CD3 mAb (2C11) or 5 μg/ml of immobilized hamster IgG. 48 h later, cells were harvested and cytokine production was assayed by i.c. flow cytometric analysis. Dot plots show representative i.c. staining for gated DN γδTCR+ cells. Numbers in the quadrants represent percentage of cells in that quadrant. Data shown are representative of 6 mice per genotype. B, Histogram showing representative i.c. Blk staining on gated CCR6+ (black histogram) and CD27+ (dashed dark gray histogram) DN γδTCR+ cells in the thymus and LN of Blk+/+ mice. Total γδ thymocytes (light shaded histogram) are shown as a staining control in the left histogram and B cells (light shaded histogram) are shown as a staining control in the right histogram. C, Dot plots showing representative CD44 versus CCR6 staining on DN γδTCR+ thymocytes and LN cells from γδTCR Tg Blk−/− (n=4) and γδTCR Tg Blk+/+ (n=6) mice. Numbers represent percentage of cells in each gate. D, Histograms showing representative IL-23R (GFP+) staining on DN γδTCR+ thymocytes and LN cells from Blk−/− IL-23R-GFP.KI (n=3) and Blk+/+ IL-23R-GFP.KI (n=5) mice. Numbers represent percentage of cells in each marker.
To investigate this further, we first determined whether Blk is differentially expressed in IL-17-producing and IFNγ-producing γδ T cells. It has recently been shown that expression of CCR6 and IL-23R marks γδ T cells of the IL-17 effector fate (15,35–37) and expression of CD27 and CD122 marks those of the IFNγ effector fate (38,39). Using our i.c. flow cytometric assay, we found that CCR6+ DN γδTCR+ cells, in both the thymus and LN, express relatively high levels of Blk, whereas thymic and peripheral CD27+ DN γδTCR+ cells express relatively low levels of Blk (Fig. 7B). These data demonstrated that Blk is preferentially expressed in IL-17-producing γδ T cells.
We next quantified potential IL-17-producing γδ effectors in γδTCR Tg Blk−/− mice and found a significant decrease (5 to 8-fold) in the number of CCR6+ γδ T cells in both the thymus and LN of γδTCR Tg Blk−/− mice relative to γδTCR Tg Blk+/+ mice (Fig. 7C). These findings indicated that, in the absence of Blk, Vγ6+ γδTCR Tg IL-17 effectors fail to develop. Moreover, because Vγ6+ fetal thymocytes in non-γδTCR Tg mice are monoclonal (3) and because we observed a loss in the number of Vγ5− fetal thymocytes in non-γδTCR Tg Blk−/− mice, our results suggested that Blk is required for the generation of Vγ6+ fetal thymocytes developing along the IL-17 effector fate pathway.
In wild-type mice, IL-17-producers are found primarily within the Vγ6+ and Vγ4+ γδ T cell subsets (40–42). Notably, while Vγ6+ T cells are only generated in the fetal thymus, Vγ4+γδ T cells are generated in the late fetal and adult thymus (3). Because of this difference in the timing of their development, we sought to determine whether loss of Blk affects the development of IL-17-producing γδ T cells that arise in the postnatal thymus. Analysis of 4-wk-old Blk+/+ and Blk−/− IL-23R-GFP.KI mice revealed a significant decrease in both the percentage and number of IL-23R+ γδ thymocytes (which are GFP+ in IL-23R-GFP.KI mice) in Blk−/− mice compared to Blk+/+ mice (Fig. 7D). Accordingly, significantly fewer IL-23R+ γδ T cells were detected in the LNs of Blk−/− IL-23R-GFP.KI mice than in the LNs of Blk+/+ IL-23R-GFP.KI mice (Fig. 7D). These findings indicated that the IL-17 γδ effectors that are generated postnatally require Blk for their development. However, because a small population of IL-23R+ γδ T cells can still be detected in Blk−/− mice, these results also suggested that there are differences between subsets of IL-17 γδ effectors in the molecular requirements for their development.
DISCUSSION
Many groups have used transcriptional profiling to gain a better understanding of the genetic programs involved in γδ T cell development and function. Likewise, we have used a genetic approach to identify signaling molecules that are expressed in γδ T cells but not αβ T cells as a means to elucidate the molecular basis for the enhanced signaling ability of the γδTCR. Global gene expression analysis identified Blk as a signaling molecule preferentially expressed in γδ T cells; however, subsequent analysis demonstrated that its expression in γδ T lineage cells does not provide a molecular mechanism by which the γδTCR can signal better than the αβTCR. Instead, this study revealed that Blk plays unanticipated roles in T lymphopoiesis and in the development of IL-17-producing γδ T cell effectors.
It is not surprising that we identified a gene involved in the generation of IL-17 producing γδ T cells, as our strategy was to compare the transcriptional profile of γδ T cells with that of CD8+αβ T cells, which are programmed to produce IFNγ during an immune response. What is surprising is that we identified Blk, an SFK whose expression was reported to be restricted to B lineage cells (13). It is interesting to note that, in humans, Blk is expressed in thymocytes, indicating that its expression is not limited to B cells (43). In this study, we show that murine Blk is expressed in progenitor populations from both the BM and thymus, in immature DN thymocytes, in γδ thymocytes, and in a small subset of mature γδ T cells that have the potential to produce IL-17. Therefore, Blk has a much broader expression pattern than previously reported.
Blk, because of its expression in T cell precursors, plays a role in controlling thymus cellularity. Remarkably, this requirement for Blk activity is most evident when T cell development has not reached equilibrium, such as during the postnatal period or during thymic reconstitution following BM transfer into irradiated recipients. Notably, in young (2 to 4-wk-old) Blk−/− mice, the decrease in thymus cellularity results in lower thymic output, as evidenced by the reduction in the number of mature T cells in Blk−/− mice compared to age-matched Blk+/+ mice. However, by 6 wks of age, we observed no difference between Blk+/+ and Blk−/− mice in the total numbers of thymocytes or mature T cells. As this is the age at which Blk−/− mice were initially analyzed (14), this may explain why these authors did not observe any defects in T cell development.
One way that Blk regulates thymus cellularity is by controlling proliferation. Notably, we found that, in the absence of Blk, all DN thymocytes exhibited reduced proliferation, with those in the DN4 subset displaying the greatest reduction. Remarkably, in wild-type mice, this is the only DN subset that does not express Blk. To explain this apparent paradox, we propose that Blk acts in a signaling pathway that not only induces proliferation but also regulates the cell’s fitness to respond to proliferative stimuli. Candidate receptor signaling pathways include those of IL-7R, c-Kit and Hedgehog, all of which have been shown to activate SFKs (44–47) and to be involved in thymocyte proliferation (48–51).
TCR signal strength plays a role in determining whether an immature thymocyte chooses the αβ or γδ lineage fate. Specifically, it has been shown, using γδTCR Tg mice, that a strong γδTCR signal favors commitment to the γδ lineage, while a weak γδTCR signal favors commitment to the αβ lineage (52,53). Paradoxically, although the thymic and peripheral γδ T cells in γδTCR Tg Blk−/− mice had all the earmarks of cells that have received a strong TCR signal, αβ/γδ lineage commitment was not affected, as evidenced by the comparable numbers ofαβ and γδ lineage cells in the thymuses of γδTCR Tg Blk−/− and γδTCR Tg Blk+/+ mice. This is in contrast with the results of previous studies, in which augmenting γδTCR signal strength consistently resulted in fewer αβ lineage cells (18,52,53). An explanation for these findings is that TCR-induced Blk activity is not required at the stage when αβ/γδ lineage commitment occurs but instead is required at a later developmental stage, after adoption of the γδ lineage fate.
The differential expression of Blk between γδ thymocytes destined to produce IL-17 and those destined to produce IFNγ suggests that CD27 (38) and/or strong TCR signaling (39,54), both of which are required for the development of IFNγ-effectors, downregulate Blk expression. Moreover, Blk expression is retained in mature IL-17 γδ effectors. This retention suggests that Blk enforces the IL-17 effector differentiation program that was established in the thymus, and may explain why cytokine production by the different γδ T cell effectors remains stable both in vitro and in vivo (38).
We also noted that Vγ6+γδ T cells with the potential to produce IL-17 are more dependent on Blk for their development then ones bearing other Vγ gene segments. Moreover, this difference in their requirement for Blk may reflect a divergence in their respective developmental pathways, and suggests that there may be differences in their surface phenotype and functional ability.
The mechanism(s) underlying the role of Blk in IL-17 effector cell generation are currently unknown. One possible mechanism is that Blk acts in a signaling pathway that initiates the genetic program for IL-17-producing γδ T cells. However, preliminary studies reveal that IL-23R+ γδ T cells from Blk−/− mice express Rorc and produce IL-17 following TCR stimulation (data not shown). Another mechanism by which Blk may mediate development of IL-17-producing γδ T cells is by regulating the γδTCR signaling threshold. In a recent study (39), it was shown that IL-17-producing γδ T cells develop in the absence of natural ligand, suggesting that TCR engagement is not required for their generation. However, under normal conditions, IL-17-producing γδ T cells do develop in the presence of ligand (39). Because it is unlikely that γδ thymocytes can avoid ligand encounter in a wild-type thymus, we propose that there are ligands with different affinities for the γδTCR and that these ligands are expressed at different levels within the thymus. Since Blk is a negative regulator of γδTCR signaling, it would have an active role in establishing the γδTCR signaling threshold in γδ thymocytes. In this capacity, Blk may function to test the affinity/avidity of γδTCR-ligand interactions, in order that signals delivered by low affinity/avidity interactions direct γδ thymocytes to the IL-17 effector fate and those delivered by high affinity/avidity interactions direct γδ thymocytes to the IFNγ effector fate. Studies are underway to investigate this possibility.
In summary, we have found that Blk is not only expressed in γδ T cells but is also required for the development of wild-type numbers of IL-17-producing γδ T cells. The finding that B cells and γδ T cells express Blk is quite intriguing, especially in light of the hypothesis that γδ T cells may be the primordial Ag receptor-bearing lymphocyte (55). Thus, the discovery of Blk expression in γδ lineage cells has broader implications in regards to the evolution of Ag receptor-coupled signal transduction.
Supplementary Material
Acknowledgments
We thank Jennifer Kieffer and Alyse Douglass for their excellent technical service, Karen Gentile and Dr. Frank Middleton from the SUNY Microarray Core facility for their service, and Drs. Mohamed Oukka, Paul Love and Alexander Tarakhovsky for mice. We also thank Dr. Paul Love for helpful advice and critical review of the manuscript.
This work was supported by the Hendricks Fund for Medical Research and NIH AI081068.
Abbreviations in this paper
- Blk
B lymphoid kinase
- SFK
Src family kinase
- i.c
intracellular
- DETC
dendritic epidermal T cell
- iIEL
intestinal intraepithelial lymphocyte
- ETP
early thymic progenitor
- CLP
common lymphoid progenitor
- LSK
lineage-negative Sca-1+ c-Kit+ cell
References
- 1.Chien YH, Jores R, Crowley MP. Recognition by γ/δ T cells. Annu Rev Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 2.Hayday AC. γδ cells: A right time and a right place for a conserved third way of protection. Annu Rev Immunol. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 3.Carding SR, Egan PJ. γδ T cells: Functional plasticity and heterogeneity. Nat Reviews Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chou K, Fuchs E, Havran WL, Boismenu R. Protection of the intestinal mucosa by intraepithelial γδ T cells. Proc Natl Acad Sci USA. 2002;99:1433–1443. doi: 10.1073/pnas.212290499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin γδ T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 6.Sharp LL, Jameson JM, Cauvi G, Havran WL. Dendritic epidermal T cells regulate skin homeostasis through local production of insulin-like growth factor 1. Nat Immunol. 2005;6:73–79. doi: 10.1038/ni1152. [DOI] [PubMed] [Google Scholar]
- 7.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J Exp Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;411:820–824. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Yang W, Pam M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. γδ T cells provide an early source of interferon γ in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girardi M, Glusac E, Filler RB, Roberts SJ, Propperova I, Lewis J, Tigelaar RE, Hayday AC. The distinct contributions of murine T cell receptor (TCR)γδ+ and TCRαβ+ T cells to different stages of chemically induced skin cancer. J Exp Med. 2003;198:747–755. doi: 10.1084/jem.20021282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes SM, Love PE. Distinct structure and signaling potential of the γδTCR complex. Immunity. 2002;16:827–838. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 12.Laird RM, Hayes SM. Profiling of the early transcriptional response of murine γδ T cells following TCR stimulation. Mol Immunol. 2009;46:2429–2438. doi: 10.1016/j.molimm.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 13.Dymecki SM, Niederhuber JE, Desiderio SV. Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells. Science. 1990;247:332–336. doi: 10.1126/science.2404338. [DOI] [PubMed] [Google Scholar]
- 14.Texido G, I, Su H, Mecklenbräuker I, Saijo K, Malek SN, Desiderio S, Rajewsky K, Tarakhovsky A. The B-cell-specific Src-family kinase Blk is dispensable for B-cell development and activation. Mol Cell Biol. 2000;20:1227–1233. doi: 10.1128/mcb.20.4.1227-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awasthi A, Riol-Blanco L, Jäger A, Korn T, Pot C, Galileos G, Bettelli E, Kuchroo VK, Oukka M. Cutting edge: IL-23 receptor GFP reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim GK, Olsson C, Augustin A. Commitment and maintenance of the αβ and γδ T cell lineages. J Immunol. 1995;154:5821–5831. [PubMed] [Google Scholar]
- 17.Laky K, Lefrançois L, Lingenheld EG, Ishikawa H, Lewis JM, Olson S, Suzuki K, Tigelaar RE, Puddington L. Enterocyte expression of interleukin 7 induces development of γδ T cells and Peyer’s patches. J Exp Med. 2000;191:1569–80. doi: 10.1084/jem.191.9.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laird RM, Hayes SM. Roles of the src tyrosine kinases Lck and Fyn in regulating γδTCR signal strength. PLoS ONE. 2010;5:e8899. doi: 10.1371/journal.pone.0008899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin Z, Chen CH, Szabo SJ, Glimcher LH, Ray A, Craft J. T-bet expression and failure of GATA-3 cross-regulation lead to default production of IFN-γ by γδ T cell. J Immunol. 2002;168:1566–1571. doi: 10.4049/jimmunol.168.4.1566. [DOI] [PubMed] [Google Scholar]
- 20.Pennington DJ, Silva-Santos B, Shires J, Theodoridis E, Pollitt C, Wise EL, Tigelaar RE, Owen MJ, Hayday AC. The inter-relatedness and interdependence of mouse T cell receptor γδ+ and αβ+ cells. Nat Immunol. 2003;4:991–998. doi: 10.1038/ni979. [DOI] [PubMed] [Google Scholar]
- 21.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, Held W, Chambers CA, Kang J. Regulation of γδ versus αβ T lymphocyte differentiation by the transcription factor Sox13. Science. 2007;315:230–233. doi: 10.1126/science.1135344. [DOI] [PubMed] [Google Scholar]
- 22.Burkhardt AL, Brunswick M, Bolen JB, Mond JJ. Anti-immunoglobulin stimulation of B lymphocytes activates src-related protein-tyrosine kinases. Proc Natl Acad Sci USA. 1991;88:7410–7418. doi: 10.1073/pnas.88.16.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saouaf SJ, Mahajan S, Rowley RB, Kut SA, Fargnoli J, Burkhardt AL, Tsukada S, Witte ON, Bolen JB. Temporal differences in the activation of three classes of non-transmembrane protein tyrosine kinases following B-cell antigen receptor surface engagement. Proc Natl Acad Sci USA. 1994;91:9524–9528. doi: 10.1073/pnas.91.20.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. doi: 10.1016/s0952-7915(97)80074-x. [DOI] [PubMed] [Google Scholar]
- 25.Silva-Santos B, Pennington DJ, Hayday AC. Lymphotoxin-mediated regulation of γδ cell differentiation by αβ T cell progenitors. Science. 2005;307:925–928. doi: 10.1126/science.1103978. [DOI] [PubMed] [Google Scholar]
- 26.van Oers NS, Lowin-Kropf B, Finlay D, Connolly K, Weiss A. αβ T cell development is abolished in mice lacking both Lck and Fyn protein tyrosine kinases. Immunity. 1996;5:429–436. doi: 10.1016/s1074-7613(00)80499-9. [DOI] [PubMed] [Google Scholar]
- 27.Page ST, van Oers NS, Perlmutter RM, Weiss A, Pullen AM. Differential contribution of Lck and Fyn protein tyrosine kinases to intraepithelial lymphocyte development. Eur J Immunol. 1997;27:554–562. doi: 10.1002/eji.1830270229. [DOI] [PubMed] [Google Scholar]
- 28.Petrie HT, Scollay R, Shortman K. Commitment to the T cell receptor-αβ or -γδ lineages can occur just prior to the onset of CD4 and CD8 expression among immature thymocytes. Eur J Immunol. 1992;22:2185–88. doi: 10.1002/eji.1830220836. [DOI] [PubMed] [Google Scholar]
- 29.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 30.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zúñiga-Pflücker JC. Stage-specific and differential notch dependency at the αβ and γδ T lineage bifurcation. Immunity. 2006;25:105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 31.Michie AM, Zúñiga-Pflücker JC. Regulation of thymocyte differentiation: pre-TCR signals and β-selection. Semin Immunol. 2002;14:311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 32.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zúñiga-Pflücker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamada S, Umemura M, Shiono T, Hara H, Kishihara K, Tanaka K, Mayuzumi H, Ohta T, Matsuzaki G. Importance of murine Vδ1 γδ T cells expressing interferon-γ and interleukin-17A in innate protection against Listeria monocytogenes infection. Immunology. 2008;125:170–177. doi: 10.1111/j.1365-2567.2008.02841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas JD, González FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, Forster R, Prinz I. CCR6 and NK1.1 distinguish between IL-17A and IFN-γ-producing γδ effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 36.Martin N, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 37.Sutton CE, Lalor SJ, Sweeney CM, Brerton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, Girardi M, Borst J, Hayday AC, Pennington DJ, Silva-Santos B. CD27 is a thymic determinant of the balance between interferon-γ- and interleukin 17-producing γδ T cell subsets. Nat Immunol. 2009;10:427–36. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen KD, Su X, Shin S, Li L, Youssef S, Yamasaki S, Steinman L, Saito T, Locksley RM, Davis MM, Baumgarth N, Chien YH. Thymic selection determines γδ T cell effector fate: antigen-naive cells make interleukin-17 and antigen-experienced cells make interferon γ. Immunity. 2008;29:90–100. doi: 10.1016/j.immuni.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O’Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing γδ T cells. J Immunol. 2007;179:5576–5583. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria Monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ γδ T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 43.Islam KB, Rabbani H, Larsson C, Sanders R, Smith CI. Molecular cloning, characterization and chromosomal localization of a human lymphoid tyrosine kinase related to murine Blk. J Immunol. 1995;154:1265–1272. [PubMed] [Google Scholar]
- 44.Venkitaraman AR, Cowling RJ. Interleukin 7 receptor functions by recruiting the tyrosine kinase p59fyn through a segment of its cytoplasmic tail. Proc Natl Acad Sci USA. 1992;89:12083–12087. doi: 10.1073/pnas.89.24.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Page TH, Lali FV, Foxwell BM. Interleukin-7 activates p56lck and p59fyn, two tyrosine kinases associated with the p90 interleukin-7 receptor in primary human T cells. Eur J Immunol. 1995;25:2956–2960. doi: 10.1002/eji.1830251036. [DOI] [PubMed] [Google Scholar]
- 46.Timokhina I, Kissel H, Stella G, Besmer P. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 1998;17:6250–6262. doi: 10.1093/emboj/17.21.6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yam PT, Langlois SD, Morin S, Charron F. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron. 2009;14:349–362. doi: 10.1016/j.neuron.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 50.Agosti V, Corbacioglu S, Ehlers I, Waskow C, Sommer G, Berrozpe G, Kissel H, Tucker CM, Manova K, Moore MA, Rodewald HR, Besmer P. Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. J Exp Med. 2004;199:867–878. doi: 10.1084/jem.20031983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shah DK, Hager-Theodorides AL, Outram SV, Ross SE, Varas A, Crompton T. Reduced thymocyte development in sonic hedgehog knockout embryos. J Immunol. 2004;172:2296–2306. doi: 10.4049/jimmunol.172.4.2296. [DOI] [PubMed] [Google Scholar]
- 52.Haks MC, Lefebvre JM, Lauritsen JP, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. Attenuation of γδTCR signaling efficiently diverts thymocytes to the αβ lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 53.Hayes SM, Li L, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 54.Kreslavsky T, Savage AK, Hobbs R, Gounari F, Bronson R, Pereira P, Pandolfi PP, Bendelac A, von Boehmer H. TCR-inducible PLZF transcription factor required for innate phenotype of a subset of γδ T cells with restricted TCR diversity. Proc Natl Acad Sci U S A. 2009;106:12453–12458. doi: 10.1073/pnas.0903895106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Flajnik MF. Churchill and the immune system of ectothermic vertebrates. Immunol Rev. 1998;166:5–14. doi: 10.1111/j.1600-065x.1998.tb01248.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.