Abstract
Background and Purpose
Frontotemporal lobar degeneration (FTLD) can be subdivided into those in which the abnormal protein is tau (FTLD-TAU), the TAR DNA binding protein 43 (FTLD-TDP) and the fused in sarcoma protein (FTLD-FUS). We have observed severe caudate atrophy at autopsy in FTLD-FUS, and hence we aimed to determine whether caudate atrophy on MRI is a feature that can distinguish FTLD-FUS from FTLD-TDP and FTLD-TAU.
Methods
From a cohort of 207 cases of FTLD we identified all cases of FTLD-FUS that had a volumetric antemortem head MRI (n=3). Caudate and frontal lobe volumes were measured in all three cases using atlas based parcellation and SPM5, and were compared to 10 randomly selected cases of FTLD-TDP and 10 randomly selected cases of FTLD-TAU. Total grey matter volumes were also calculated for all cases.
Results
The FTLD-FUS cases had significantly smaller caudate volumes (p=0.02) yet similar frontal lobe grey matter volumes (p=0.12) compared to FTLD-TDP and FTLD-TAU. Caudate volumes when corrected for total grey matter volume (p=0.01) or frontal lobe grey matter volume (p=0.01) were significantly smaller in FTLD-FUS than FTLD-TDP and FTLD-TAU, and showed no overlap with the other two groups.
Conclusions
Caudate atrophy on MRI appears to be significantly greater in FTLD-FUS compared with FTLD-TDP and FTLD-TAU suggesting that severe caudate atrophy may be a useful clinical feature to predict FTLD-FUS pathology.
Keywords: TDP-43, FTLD-TAU, FTLD-FUS, atlas based parcellation, caudate atrophy
INTRODUCTION
Frontotemporal lobar degeneration (FTLD) defines a group of patholological diseases that are all considered to be interrelated, as all can be associated with a clinical diagnosis of frontotemporal dementia [1]. For years, FTLD had been further classified on the basis of which pathological protein was identified at histology. At the highest level of sub-division, FTLD was divided into FTLD associated with the deposition of the protein tau (FTLD-TAU), and FTLD without tau, but having ubiquitin immunoreactive inclusions (FTLD-U) [1]. With the discovery that the ubiquitinated neuronal inclusions of most, although not all, cases of FTLD-U show immunoreactivity to the TAR DNA binding protein 43 (TDP-43) [2], it has been suggested to further subdivide FTLD-U into FTLD-U with and without TDP-43 immunoreactive inclusions. Hence, those with TDP-43 immunoreactivity became FTLD-TDP [3]. Recently, it has been demonstrated that the ubiquitinated protein in FTLD-U without TDP-43 is the fused in sarcoma (FUS) protein [4]. Therefore, FTLD-U without TDP-43 became FTLD-FUS, with FTLD-U now sub-divided into FTLD-TDP and FTLD-FUS (Figure 1).
Figure 1.
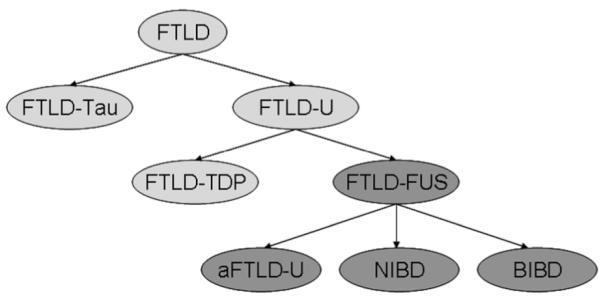
Diagram illustrating the subclassification of frontotemporal lobar degeneration (FTLD). FTLD-TAU = FTLD with tau immunoreactive inclusions; FTLD-U = FTLD with ubiquitin immunoreactive inclusions; FTLD-TDP = FTLD with TAR DNA binding protein 43 immunoreactive inclusions; FTLD-FUS = FTLD with fused in sarcoma immunoreactive inclusions; aFTLD-U = atypical FTLD-U (FTLD-U without TDP-43 immunoreactive inclusions); NIBD = neurofilament inclusion body disease (also known as NIFID, neuronal intermediate filament inclusion body disease); BIBD = basophilic inclusion body disease.
In our experience, one of the most characteristic features of FTLD-FUS is severe caudate atrophy, identified on gross and microscopic examination [5, 6], beyond what we typically see in FTLD-TDP and FTLD-TAU. We therefore set out to study antemortem caudate atrophy in FTLD-FUS compared to caudate atrophy in FTLD-TDP and FTLD-TAU. Based on our pathological observation, we hypothesize that caudate volume will be significantly smaller in FTLD-FUS than in FTLD-TDP and FTLD-TAU.
METHODS
Subjects
We reviewed the pathological database of the Mayo Clinic, Rochester, MN, to identify all cases of FTLD (n=207). Of these, 78 cases had been initially classified as FTLD-U. All 78 cases of FTLD-U had previously undergone TDP-43 immunohistochemistry of which 74 showed TDP-43 immunoreactivity and hence were classified as FTLD-TDP [3]. Of the remaining four (5%), one case had been previously been diagnosed as neurofilament inclusion body disease [6] and had neurofilament and alpha-internexin immunoreactive neuronal inclusions. All four FTLD-U cases without TDP-43 immunoreactivity underwent FUS immunohistochemistry for this study and all four were positive for FUS (Figure 2). Of these four cases, three also had at least one volumetric head MRI scan completed prior to death. As a comparison group to these three FTLD-FUS cases, we randomly selected 10 cases of FTLD-TDP, and 10 cases of FTLD-TAU, that also had at least one volumetric head MRI scan. The study was approved by the Mayo Clinic IRB.
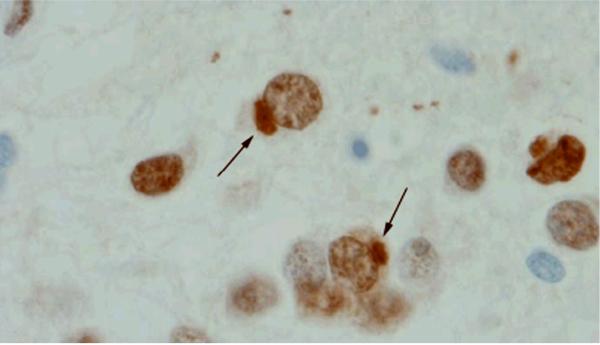
Figure 2.
FUS immunoreactive neuronal cytoplasmic inclusions (arrows) in the dentate granule cells of the hippocampus in a representative FTLD-FUS case (Case 1).
Pathological analysis
All 207 FTLD cases underwent standard pathological examination according to the recommendations of the Consortium to Establish a Registry for Alzheimer’s Disease [7] by one of two expert neuropathologists (DWD or JEP) as previously described [8]. Immunohistochemistry was performed including TDP-43 (1:3,000; ProteinTech Group, Chicago, IL), tau (clone AT8, 1:1000; Innogenetics, Alpharetta, GA) and FUS (1:100; Sigma–Aldrich, St Lois, MO). Final pathological diagnoses rendered were based on the most recent published criteria [9]. Pathological nomenclature throughout the manuscript is based on recently published consensus [3].
MRI analysis
All MRI studies were performed at 1.5 T with a standardized imaging protocol that included a coronal T1-weighted 3D volumetric spoiled gradient echo (SPGR) sequence with 124 contiguous partitions and 1.6 mm slice thickness (22 × 16.5 cm2 FOV, 25° flip angle). If more than one MRI scan was available, the first usable scan was utilized. An atlas-based parcellation technique was employed using SPM5 and the automated anatomic labeling (AAL) atlas [10] to generate caudate and total frontal grey matter volumes for all cases as previously described [11]. Left and right volumes were summed to give total caudate and frontal lobe volumes for each case. In addition, total grey matter volume was calculated for each patient from the grey matter segmentation. Total intracranial volume (TIV) was also calculated by propagating a template-drawn TIV mask to the subject space and then performing an erosion step to remove border voxels. All caudate, frontal and grey matter volumes were corrected for TIV [12] using the following formula: (volume/TIV)*100. We also assessed caudate volume as a proportion of total grey matter ((caudate/total grey matter)*100) and frontal grey matter volume ((caudate/frontal grey matter)*100), and assessed frontal volume as a proportion of total grey matter volume ((frontal grey matter/total grey matter)*100).
In order to validate the automated measurements of the caudate nucleus we performed manual measurements of the left caudate nucleus on nine cases (3 FTLD-FUS, 3 FTLD-TDP, and 3 FTLD-TAU). Measurements were performed blinded to pathological diagnosis and were based on a published validated protocol [13]. Volumes were traced in the axial plane and then further edited with reference to the coronal plane using ANALYZE (Mayo Biomedical Imaging Resource, Rochester, MN, USA).
Statistics
Differences in categorical variables between groups were assessed with Fishers Exact test. Differences in continuous variables between groups were assessed with Kruskal-Wallis test. Statistical analyses were performed utilizing the JMP computer software (JMP Software, version 6.0.0; SAS Institute Inc, Cary, NC) with α set at 0.05.
RESULTS
FTLD-FUS Case 1
A 66-year-old man presented for evaluation of behavioral disturbances over the past year. He had personality change whereby he became more placid, less argumentative and more easy-going. He also lacked a great deal of initiative. There was no family history of any similar behavioral disturbances. On initial neuropsychological testing he did reasonably well except for tests of executive function. Over the next four years of his illness, he developed language problems and eventually became mute. Behavioral disturbances persisted without any hypersexual behaviors although it was reported that he would eat anything placed in his mouth. Parkinsonism was a late feature and included tremor and increased muscle tone. He died at age 70 years old, five years after onset of his illness. This case had a pathological diagnosis of FTLD-U without TDP-43 immunoreactivity.
FTLD-FUS Case 2
A 51-year-old man presented for evaluation of a progressive change in cognition and behavior. He had a previous history of “spinal meningitis” at age 16 but had no obvious sequelae from this. His recent history began about a year ago when he began having difficulties keeping score when he and his wife played golf. He tended to laugh at inappropriate times, became less motivated to maintain hygiene, and was becoming more impatient and disinhibited. For example, he made inappropriate sexual comments about little girls. He began eating lots of sweets whereas before he was very meticulous in eating a good diet. There was no family history of any similar behavioral disturbances. Patient developed a rest tremor and intermittent semi-rhythmic masseter contractions after one year. Neuropsychological testing revealed inefficiencies in learning, memory and attention. He died at age 53, three years after onset. This case had a pathological diagnosis of FTLD-U without TDP-43 immunoreactivity.
FTLD-FUS Case 3
A 51-year-old woman with a one year history of behavioral changes and obsessive-compulsive like behaviors, such as not stepping on cracks. Her personal hygiene had declined; she became apathetic and more dependent. She had difficulty making decisions and had poor organization skills. There was no family history. Later she developed sweet cravings. Parkinsonism was a late feature. Detailed clinical information on this case has been previously published [6]. This case had been previously given a pathological diagnosis of neurofilament inclusion body disease [6], which has recently been shown to also have FUS immunoreactive inclusions and hence to be a type of FTLD-FUS [14].
FTLD-TDP Cases 1-10
Six of the 10 FTLD-TDP cases presented with behavioral and personality changes and had been given a clinical diagnosis of behavioral variant frontotemporal dementia, while the other four cases presented predominantly with language disturbances and had been diagnosed with progressive non-fluent aphasia or semantic dementia [15].
FTLD-TAU Cases 1-10
Four of the 10 FTLD-TDP cases presented with behavioral and personality changes and had been given a clinical diagnosis of behavioral variant frontotemporal dementia, while the other six cases presented predominantly with language disturbances and had been diagnosed with progressive non-fluent aphasia or semantic dementia [15]. Histological diagnoses of the FTLD-TAU cases included progressive supranuclear palsy (n=5), corticobasal degeneration (n=4), and Pick’s disease with Pick bodies (n=1).
Image analysis
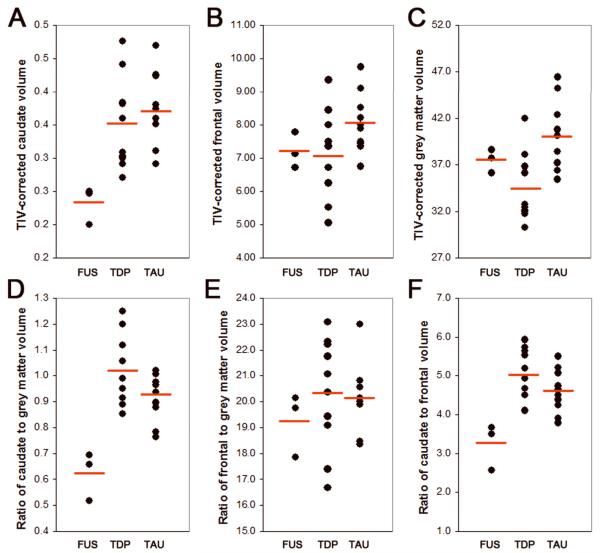
Demographics, caudate, frontal and total grey matter volumes are shown in Table 1. There was no significant difference between FTLD-FUS, FTLD-TDP and FTLD-TAU on demographics or measures of disease severity including the Mini-Mental State Examination [16] and Clinical Dementia Rating sum of boxes score [17]. There was an excellent correlation between automated and manual left caudate measurements (r=0.9, p=0.001). The FTLD-FUS cases had the smallest caudate volumes (Figure 3) which did not overlap (Figure 4A) with the caudate volumes of any of the FTLD-TDP or FTLD-TAU cases (p=0.02). Frontal lobe grey matter volumes (Figure 4B) were similar across both groups (p=0.12). The total grey matter volumes were largest in the FTLD-TAU group and smallest in the FTLD-TDP group (p=0.02) (Figure 4C). Caudate volume expressed as a proportion of total grey matter volume was lower in the FTLD-FUS cases (Figure 4D) compared to all FTLD-TDP and FTLD-TAU cases (p=0.01), whereas frontal grey matter volume expressed as a proportion of total grey matter volume (Figure 4E) was similar across all groups (p=0.46). Caudate volume expressed as a proportion of frontal grey matter volume was lower in FTLD-FUS (Figure 4F) compared to FTLD-TDP and FTLD-TAU (p=0.01), with no overlap observed between FTLD-FUS and the other groups.
Table 1.
Demographics and MRI volumes for FTLD-FUS and FTLD-TDP
| FTLD-FUS (n=3) |
FTLD-TDP (n=10) |
FTLD-TAU (n=10) |
p values |
|
|---|---|---|---|---|
| Sex (M:F) | 2:1 | 4:6 | 6:4 | 0.58 |
| Years of Education | 15 (12-18) | 13 (8-18) | 15 (8-20) | 0.43 |
| Age at onset (years) | 54.8 (49.5-65.0) | 64.0 (45.0-77.0) | 61.2 (40.6-76.0) | 0.30 |
| Age at death (years) | 59.1 (52.9-69.8) | 72.1 (56.3-83.1) | 68.6 (47.0-83.0) | 0.13 |
| Age at MRI scan | 57.0 (51.5-67.0) | 67.7 (53.3-79.5) | 64.7 (41.9-79.3) | 0.20 |
| Time from onset to scan (years) |
2.2 (1.5-3.0) | 3.7 (1.0-8.3) | 3.5 (1.3-6.1) | 0.47 |
| Time from scan to death (years) |
2.1 (1.4-2.8) | 4.4 (1.9-7.6) | 3.9 (0.6-6.7) | 0.12 |
| MMSE at scan/30 | 27 (26-28) | 23 (11-29) | 26 (22-30) | 0.51 |
| CDR-SB at scan | 5.0 (1.5-8.5) | 6.0 (0.5-18.0) | 2.6 (0-6.5) | 0.26 |
| Caudate volume* | 0.23 (0.20-0.25) | 0.35 (0.27-0.48) | 0.37 (0.29-0.47) | 0.02 |
| Frontal grey matter volume* |
7.2 (6.7-7.8) | 7.0 (5.1-9.4) | 8.1 (6.7-9.7) | 0.12 |
| Total grey matter volume* |
37.5 (36.2-38.6) | 34.4 (30.3-42.0) | 40.0 (35.4-46.5) | 0.02 |
| Caudate/total grey matter volume |
0.62 (0.52-0.69) | 1.02 (0.86-1.25) | 0.92 (0.76-1.02) | 0.01 |
| Frontal grey matter /total grey matter volume |
19.3 (17.9-20.1) | 20.3 (16.7-23.1) | 20.2 (18.4-23.0) | 0.46 |
| Caudate/frontal grey matter volume |
3.3 (2.6-3.7) | 5.0 (4.1-5.9) | 4.6 (3.8-5.5) | 0.01 |
Volumes are shown as a percentage of TIV, calculated using the following formula: (volume/TIV)100
Data shown as mean and range. CDR-SB = Clinical Dementia Rating sum of boxes; MMSE = Mini-Mental State Examination
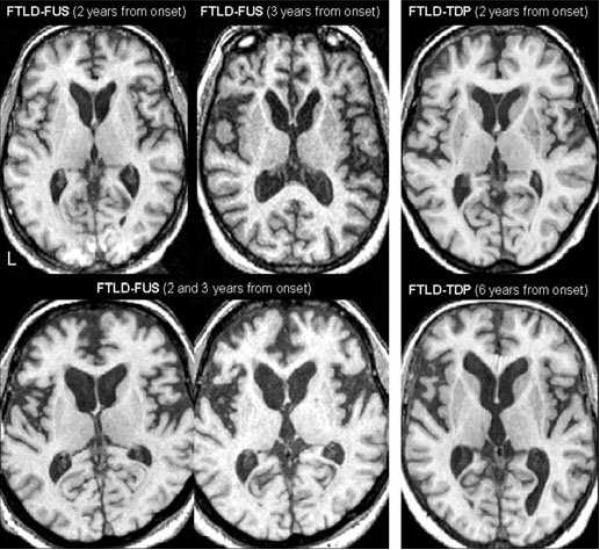
Figure 3.
Axial T1-weighted MRI scans show that the caudate in our three FTLD-FUS cases (left panel) are visually smaller than the caudate in two representative FTLD-TDP cases (right panel); in spite of the times from onset to MRI scan. Note one of the FTLD-TDP cases was six years from onset at the time of the MRI scan with relatively preserved caudate volume.
Figure 4.
Scatter plots demonstrating caudate (A), frontal grey matter (B), total grey matter (C), caudate/total grey matter (D), frontal grey matter/total grey matter (E), and caudate/frontal grey matter (F) volumes in FTLD-FUS cases compared to FTLD-TDP and FTLD-TAU cases. There was no overlap in caudate, caudate/total grey matter and caudate/frontal grey matter volumes between FTLD-FUS and the other groups, with smaller volumes observed in FTLD-FUS. The mean value for each group is shown using a red line.
DISCUSSION
In this study we found that caudate volume was significantly smaller in three cases of FTLD-FUS than in all 10 randomly selected FTLD-TDP and 10 randomly selected FTLD-TAU cases, in keeping with our hypothesis. This finding occurs in spite of the fact that the FTLD-FUS cases did not have more severe disease or longer time from onset to MRI scan than the FTLD-TDP and FTLD-TAU groups.
Three different pathological entities have now been recognized as FTLD-FUS, including the entities FTLD-U without TDP-43 immunoreactivity [5] (also known as atypical FTLD-U [18]), neurofilament inclusion body disease [6, 19] and basophilic inclusion body disease [20, 21]. In this study two of our cases of FTLD-FUS are FTLD-U without TDP-43 immunoreactivity and the third is a case of neurofilament inclusion body disease. The finding of severe caudate volume loss appears to be a feature of FTLD-FUS independent of whether the FTLD-FUS variant is atypical FTLD-U or neurofilament inclusion body disease. It remains to be determined whether basophilic inclusion body disease has similar severe caudate volume loss on MRI. Pathological findings in a previously published case of ours with basophilic inclusion body disease [5] would support the hypothesis that severe caudate atrophy is also a feature of this disease. Unfortunately we do not have any cases of basophilic inclusion body disease with antemortem volumetric head MRI.
All three FTLD-FUS cases presented with behavioral and personality changes and would meet criteria for behavioral variant frontotemporal dementia [15], similar to other reported cases of FTLD-FUS [5, 18, 22]. Of the 20 randomly selected cases of FTLD-TDP and FTLD-TAU there was an equal number of behavioral variant frontotemporal dementia and aphasia cases. The findings of this study would however have been identical had we limited our FTLD-TDP and FTLD-TAU cases to those with a clinical diagnosis of behavioral variant FTD since we observed no overlap in caudate volumes between FTLD-FUS and the other two groups.
The fact that the caudate volumes of the FTLD-FUS cases did not overlap with any of the caudate volumes of the other groups suggests that caudate volume may be a useful marker in predicting underlying FTLD-FUS pathology. We also assessed total grey matter volumes in each case to determine whether the caudate volume was a mere reflection of overall atrophy. Our FTLD-FUS cases did not have the smallest total grey matter volumes compared to the other groups. In fact, total grey matter volume was on average larger in FTLD-FUS than FTLD-TDP. Total grey matter volumes were the largest in FTLD-TAU which is not surprising since the majority of cases in this group had histological diagnoses of progressive supranuclear palsy, a disease which is not associated with widespread atrophy [23]. We divided caudate volume by total grey matter volume to correct for the effect of generalized atrophy and found a large difference in this ratio between FTLD-FUS and the other two groups, suggesting disproportionate involvement of the caudate in FTLD-FUS. In order to also determine whether disproportionate atrophy was specific to the caudate nuclei as opposed to occurring in parallel with frontal lobe atrophy, we also assessed frontal lobe volumes. There was no difference in frontal lobe grey matter volumes or frontal to total grey matter ratios across all groups. However, when correcting for frontal lobe volume the caudate was still significantly smaller in FTLD-FUS confirming that disproportionate atrophy is specific to the caudate nuclei in FTLD-FUS, i.e. caudate atrophy in FTLD-FUS is disproportionate to not only total grey matter loss but also disproportionate to frontal lobe grey matter loss.
Severe caudate atrophy is a feature of Huntington’s disease and neuroacanthocytosis which are both typically characterized by the presence of chorea [24]. Furthermore, in Huntington’s disease there is typically an autosomal dominant pattern of inheritance. None of our FTLD-FUS cases had a positive family history of dementia and none had chorea. Caudate atrophy in FTLD has also been associated with stereotypical movements [25]. Stereotypic movements were not reported in the FTLD-FUS cases, but may have been missed by the evaluating clinicians. Regardless, FTLD-FUS should be added to the differential diagnosis of diseases that may show early prominent caudate atrophy.
Based on the findings from this study we suggest that a diagnosis of FTLD-FUS be considered in any patient presenting at a relatively young age, i.e. under age 60 years old, with the behavioral variant frontotemporal dementia syndrome, with negative family history, absence of chorea, and head MRI scan demonstrating severe caudate atrophy, especially when the caudate atrophy is present early in the disease course.
Acknowledgments
FUNDING SOURCES KAJ is supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (K12/NICHD)-HD49078 and The Dana Foundation. Co-authors on this study are also supported by NIH grants P50-AG16574 and U01-AG06786, as well as the generous support of the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer’s Disease Research Program of the Mayo Foundation.
Footnotes
FINANCIAL DISCLOSURES The authors report no conflicts of interest.
REFERENCES
- [1].Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol. 2008;64:4–14. doi: 10.1002/ana.21426. [DOI] [PubMed] [Google Scholar]
- [2].Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- [3].Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR. A new subtype of frontotemporal lobar degeneration with FUS pathology. Brain. 2009;132:2922–2931. doi: 10.1093/brain/awp214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Josephs KA, Lin WL, Ahmed Z, Stroh DA, Graff-Radford NR, Dickson DW. Frontotemporal lobar degeneration with ubiquitin-positive, but TDP-43-negative inclusions. Acta Neuropathol. 2008;116:159–167. doi: 10.1007/s00401-008-0397-8. [DOI] [PubMed] [Google Scholar]
- [6].Josephs KA, Holton JL, Rossor MN, et al. Neurofilament inclusion body disease: a new proteinopathy? Brain. 2003;126:2291–2303. doi: 10.1093/brain/awg231. [DOI] [PubMed] [Google Scholar]
- [7].Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- [8].Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003;62:1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- [9].Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:5–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- [11].Whitwell JL, Przybelski SA, Weigand SD, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain. 2009;132:2932–2946. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR Am J Neuroradiol. 2001;22:1483–1489. [PMC free article] [PubMed] [Google Scholar]
- [13].Looi JC, Lindberg O, Liberg B, et al. Volumetrics of the caudate nucleus: reliability and validity of a new manual tracing protocol. Psychiatry Res. 2008;163:279–288. doi: 10.1016/j.pscychresns.2007.07.005. [DOI] [PubMed] [Google Scholar]
- [14].Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IR. Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease. Acta Neuropathol. 2009;118:605–616. doi: 10.1007/s00401-009-0581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- [16].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- [17].Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- [18].Mackenzie IR, Foti D, Woulfe J, Hurwitz TA. Atypical frontotemporal lobar degeneration with ubiquitin-positive, TDP-43-negative neuronal inclusions. Brain. 2008;131(Pt 5):1282–1293. doi: 10.1093/brain/awn061. [DOI] [PubMed] [Google Scholar]
- [19].Cairns NJ, Grossman M, Arnold SE, et al. Clinical and neuropathologic variation in neuronal intermediate filament inclusion disease. Neurology. 2004;63:1376–1384. doi: 10.1212/01.wnl.0000139809.16817.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kusaka H, Matsumoto S, Imai T. An adult-onset case of sporadic motor neuron disease with basophilic inclusions. Acta Neuropathol. 1990;80:660–665. doi: 10.1007/BF00307636. [DOI] [PubMed] [Google Scholar]
- [21].Munoz DG, Neumann M, Kusaka H, et al. FUS pathology in basophilic inclusion body disease. Acta Neuropathol. 2009 doi: 10.1007/s00401-009-0598-9. [DOI] [PubMed] [Google Scholar]
- [22].Roeber S, Mackenzie IR, Kretzschmar HA, Neumann M. TDP-43-negative FTLD-U is a significant new clinico-pathological subtype of FTLD. Acta Neuropathol. 2008;116:147–157. doi: 10.1007/s00401-008-0395-x. [DOI] [PubMed] [Google Scholar]
- [23].Josephs KA, Whitwell JL, Dickson DW, et al. Voxel-based morphometry in autopsy proven PSP and CBD. Neurobiol Aging. 2008;29:280–289. doi: 10.1016/j.neurobiolaging.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kutcher JS, Kahn MJ, Andersson HC, Foundas AL. Neuroacanthocytosis masquerading as Huntington’s disease: CT/MRI findings. J Neuroimaging. 1999;9:187–189. doi: 10.1111/jon199993187. [DOI] [PubMed] [Google Scholar]
- [25].Josephs KA, Whitwell JL, Jack CR., Jr. Anatomic correlates of stereotypies in frontotemporal lobar degeneration. Neurobiol Aging. 2008;29:1859–1863. doi: 10.1016/j.neurobiolaging.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]