Abstract
In the past decade there has been a dramatic increase in the number of Americans considered obese. Over this same period, the number of individuals diagnosed with diabetes has increased by over 40%. Interestingly, in a great number of cases individuals considered obese develop diabetes later on. Although a link between obesity and diabetes has been suggested, conclusive scientific evidence is thus far just beginning to emerge. The present pilot study is designed to identify a possible link between obesity and diabetes. The plasma proteome is a desirable biological sample due to their accessibility and representative complexity due, in part, to the wide dynamic range of protein concentrations, which lead to the discovery of new protein markers. Here we present the results for the specific depletion of 14 high-abundant proteins from the plasma samples of obese and diabetic patients. Comparative proteomic profiling of plasma from individuals with either diabetes or obesity and individuals with both obesity and diabetes revealed SERPINE 1 as a possible candidate protein of interest, which might be a link between obesity and diabetes.
Keywords: Angiotensinogen, Diabetes, Obesity, SERPINE 1
Introduction
Over the past decade obesity has become a major public health problem in most industrialized nations (Pischon et al., 2007). Obesity is defined as an individual with a body mass index (BMI) ≥ 30.0 kg/m2. BMI, also called Quetelet Index, is the ratio of body weight (in kg) to body height (in m) squared (Expert-Panel, 1998). BMI has been demonstrated to correlate well with fat mass, morbidity and mortality, and reflects obesity-related disease risk in a wide range of populations. However, BMI as an indication of obesity status is less accurate in, 1) older people (over 65 yrs of age) as elderly tend to have a higher body fat composition (Rivlin, 2007), 2) Asian populations where the current BMI cut-off points for obesity is too high (Choo, 2002), and 3) abdominal obesity in men is associated with a higher morbidity than gluteofemoral obesity characteristically seen in women (Expert-Panel, 1998).
Obesity is a complex disease and is a known risk factor for a variety of chronic diseases including dyslipidemia, hypertension, coronary heart disease and type 2 diabetes. It has been estimated that the costs associated with the management of obesity and obesity-related diseases account for about 5% of total healthcare expenditures in most industrialized nations (Thompson and Wolf, 2001). Body fat or adipose tissue was once thought to be a passive fuel storage component. However, it is now recognized as an endocrine organ with the ability to effectively communicate with the brain and peripheral tissues via secretion of bioactive mediators to regulate appetite and metabolism (Kershaw and Flier, 2004). A variety of adipose-derived factors have been suggested to contribute to systemic insulin resistance including adipose-derived free fatty acid, which contribute to insulin resistance in liver and muscle in obesity (Boden and Shulman, 2002), and adipose secreted proteins which modulates glucose metabolism and insulin function, like leptin, adiponectin and resistin, tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), angiotensinogen, serum amyloid A and α1-acid glycoprotein [for review see (Lazar, 2005)].
The pathogenesis of type 2 diabetes is complicated, involving multiple genetic, metabolic and environmental factors. However, one of the earliest detectable abnormalities in the development of type 2 diabetes is insulin resistance in the skeletal muscle (Narayan et al., 2003). This is characterized by dysfunction in insulin-mediated signaling, gene expression, utilization of glucose, glycogen synthesis, and the accumulation of intramyocellular triglycerides (O’Rahilly et al., 2003; Zimmet and Thomas, 2003). However, type 2 diabetes only results when insulin producing pancreatic β-cells fail to compensate for the increased metabolic demands associated with insulin resistance (Unger, 2003). To date, the exact mechanisms by which β-cells fail is still incompletely understood (Farooqi et al., 2001; Halsall et al., 2001). What is known is that the prevalence of type 2 diabetes is reaching epidemic proportions and presents a severe health burden worldwide (Mokdad et al., 2003). For many years a link between obesity and type 2 diabetes has been assumed but not proven. Obese individuals have a greater than 10-fold increased risk of developing type 2 diabetes as compared to normal weight individuals (Must et al., 1999; Field et al., 2001). Stumvoll and colleagues demonstrated that type 2 diabetes develops due to an interaction between insulin resistance and beta cell failure (Stumvoll et al., 2005). These authors implicated several other factors including glucose toxicity, lipotoxicity and obesity-derived cytokines in the development of type 2 diabetes (Stumvoll et al., 2005).
In this pilot study, we use a proteomic approach to uncover important candidate proteins that might link obesity with diabetes. Depletion of high-abundant proteins in plasma has become an accepted technique because high abundant protein components interfere with the identification and characterization of important low-abundant proteins by limiting the dynamic range for mass spectral and electrophoretic analyses. It is estimated that there are greater than 106 different proteins in human plasma. In addition, the 30 most abundant proteins comprise approximately 99% of the total protein mass. By depleting 14 high-abundant proteins, it is possible to remove approximately 94% of the total protein mass, which results in an improved dynamic range for proteomic analysis. The detection of low-abundant proteins due to the masking effects of high-abundant proteins is important and improves the loading capacity on liquid chromatography/mass spectrometry (LC-MS), resulting in the simplification of a complex system and enabling the detection of low-level proteins. Comparative proteomic profiling of immunodepleted plasma from healthy volunteers and individuals with diabetes only and obesity only and individuals with both obesity and diabetes by LC-MS/MS revealed apolipoproteins, angiotensinogen, complement components, ceruloplasmin, fibronectin, inter-alpha inhibitors, and hemopexin as possible candidate proteins of interest which might link obesity and diabetes. Taken together, our data demonstrates a first step in understanding a link between diabetes and obesity and suggest that further studies using larger numbers of individuals will provide critical information, which can then be used for early detection.
Materials and Methods
Sample collection and patient inclusion criteria
Since variation in HSP levels are known to be present between various ethnic groups, genders, and ages, we chose to focus our study population on patients closely matched on these factors to ensure that a meaningful difference in HSP level might be discovered. Based on the most common group of patients seen in our clinic setting, we chose to focus on female Caucasians aged 40–50 years in this pilot study. Additionally, patients were excluded from this pilot project if they were pregnant, a cigarette smoker or had certain conditions such as cardiovascular disease, cancer, or an autoimmune disease, given that several medical conditions are also known to be associated with variation in HSP levels. Therefore, evaluation of gender and ethnicity in biomarker discovery was not a priority in this initial pilot phase. Information on duration of diabetes, blood pressure and medications were not collected for the same reasons given above.
Six volunteer adult female Caucasian patients aged between 40–47 years old were identified with study inclusion criteria via Electronic Medical Record (EMR) search. Patients were divided into four groups on the basis of body mass index (BMI) and fasting blood glucose (FBG). Group A were obese patients presenting with BMI >25, FBG <100; group B were patients with diabetic symptoms with BMI >25, FBG 110–125; group C were patients with both obesity and diabetic symptoms with BMI >25, FBG >125; and group D were the controls with BMI <25 and FBG <100. Plasma was separated from drawn blood, aliquoted and kept at −80°C.
Immunodepletion of human plasma using multi-affinity reverse spin (MARS) column
The Multiple Affinity Removal System (MARS) Human 14 (4.6 mm id × 100 mm) and Buffers A and B were obtained from Agilent Technologies, Inc. (Wilmington, DE). The newly developed affinity column is an extension and improvement on the Agilent Multiple Affinity Removal System described and evaluated previously (Chromy et al., 2004; Bjorhall et al., 2005). Based on affinity-purified polyclonal antibody binders, the Human 14 column also contains low molecular weight antibody ligands for the depletion of several proteins. Before injection onto a MARS column, the human plasma was diluted 4X with Buffer A. The samples were transferred to a 0.22-µm spin filter and centrifuged for 1 min at 16,000 × g to remove particulates. Diluted plasma was prepared just prior to use and stored at 4°C until injected. The MARS column was equilibrated with 2 ml binding buffer and the sample was loaded at a low flow rate of 0.5 ml/min for 2.5 min. The flow rate was then set at 1 ml/min for the remaining run. The plasma sample was mixed with binding buffer and loaded onto the column slowly and incubated at room temperature for 15 min. The MARS fractionates the plasma into two fractions: an unbound (flow-through) fraction consisting of low abundant proteins and a bound protein fraction consisting of high abundant proteins, which needed to be depleted. After the incubation period, unbound proteins were centrifuged and collected directly into a spin concentrator 5 kDa molecular weight filter (Agilent). The bound fraction was eluted with 3ml of elution buffer into another spin concentrator filter. Each depletion run cycle took 30min of total run time. The column was washed with an additional 3 ml of MARS binding buffer and this wash was discarded for reuse. Both fractions (unbound and bound) were concentrated down to 100 µl. Both depleted (flow-through fraction) and abundant plasma proteins (bound fraction) were collected and stored at −80°C until further analysis. The total amount of bound (high abundant proteins) and unbound low abundant proteins present in the samples was determined using a Bradford protein assay.
Plasma sample preparation
To analyze the specificity of the immunodepletion, the bound and flow through fractions were resolved by Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The immunodepleted plasma sample was further desalted for LC-MS/MS analysis using 10K microcon and the pellet was resuspended in 100 µl of 50 mM ammonium bicarbonate buffer and cysteine residues were reduced with 10 mM dithiothretiol (DTT) incubating at 65°C for 45 min. After cooling to room temperature, the sulfhydryls were alkylated with 55 mM iodoacetamide (IAA) for 30 min at room temperature in the dark. Finally, the reduced and alkylated sample was digested with 20 µl of 20 ng/ml trypsin (Promega, Madison, WI) at 37°C overnight. Tryptic peptides were completely dried in a SpeedVac and reconstituted with 50 µl of 0.1% trifluro acetic acid (TFA).
HPLC-Chip/MS analysis
Peptides (1µl) were injected onto an LC-MS system consisting of an 1100 Series liquid chromatograph, HPLC-Chip Cube MS interface and 1100 Series LC/MSD Trap XCT Ultra ion trap mass spectrometer (Agilent Technologies). The system was equipped with an HPLC-Chip (Agilent Technologies) that incorporated a 40-nl enrichment column and a 43-mm × 75-µm analytical column packed with Zorbax 300SB-C18 5-µm particles. Peptides were loaded onto the enrichment column with 97% solvent A (water with 0.1% formic acid). They were then eluted with a gradient from 3% B (acetonitrile with 0.1% formic acid) to 45% B in 25 min, followed by a steep gradient to 90% B in 5min at a flow rate of 0.3 µl/min. The total runtime, including column reconditioning, was 35 minutes. The column effluent was directly coupled to an LC/MSD Trap XCT Ultra ion trap mass spectrometer from Agilent Technologies via a HPLC-Chip Cube nanospray source operated at ~1900 volts in ultra-ultra mode. The gain control was set to 500,000 with a maximum accumulation time of 150 milliseconds. Collision-induced dissociation (CID) was triggered on the six most abundant, not singly charged peptide ions in the m/z range of 450–1500. Precursors were set in an exclusion list for 1min after two MS/MS spectra.
Data analysis and statistics
CID data was searched against the SwissProt all species database, using the Agilent Spectrum Mill Server software (Rev A.03.03.) installed on a HP Intel® Xeon (TM) dual processor server. Peak lists were created with the Spectrum Mill Data Extractor program with the following attributed: scans with the same precursor ± 1.4 m/z were merged within a time frame of ± 15 seconds. Precursor ions needed to have a minimum signal to noise value of 25. Charges up to a maximum of 7 were assigned to the precursor ion and the 12C peak was determined by the Data Extractor. The SwissProt database was searched for tryptic peptides with a mass tolerance of ± 2.5 Da for the precursor ions and a tolerance of ± 0.7 Da for the fragment ions. Two missed cleavages were allowed. A Spectrum Mill autovalidation was performed first in the protein details followed by peptide mode using default values [Minimum scores, minimum scored peak intensity (SPI), forward minus reversed score threshold, and rank 1 minus rank 2 score threshold]. All protein hits found in a distinct database search by Spectrum Mill were non-redundant.
Bioinformatics
The bioinformatics analysis was performed with Ingenuity Pathway Analysis (IPA). Ingenuity Pathways Analysis is an all-in-one software application to identify the biological mechanisms, pathways and functions most relevant to experimental datasets or genes of interest and thus is very effective for understanding protein-protein interactions within the context of metabolic or signaling pathways, understanding how proteins operate and form pathways by manually abstracting and curating a large fraction of the biomedical literature according to a very strict curation process, followed by storing the data in a highly structured manner.
Results
Patient information
This pilot study was undertaken to find important candidate proteins that might link obesity with diabetes. Six volunteer adult female Caucasian patients aged 40–47, were identified with study inclusion criteria via an EMR search. Patients were divided into four groups on the basis of body mass index (BMI) and fasting blood glucose (FBG). This resulted in group A, two obese patients presenting with a BMI>25 and FBG<100. Group B, one patient presenting with diabetic symptoms with a BMI >25 and FBG 110–125. Group C, one patient presenting with obese and diabetic symptoms with a BMI>25 and FBG>125. Group D, two patient controls presenting with a BMI<25 and FBG <100 (Table 1).
Table 1.
Patient’s clinical data.
| Number | Patient ID | Age (Years) | Sex | Race | Blood glucose |
BMI |
|---|---|---|---|---|---|---|
| 1 | 5040-7-D | 47 | F | W | 83 | <25 |
| 2 | 5040-8-D | 47 | F | W | 85 | <25 |
| 3 | 5040-3-B | 46 | F | W | 102 | 33 |
| 4 | 5040-2-A | 42 | F | W | 98 | 33.5 |
| 5 | 5040-1-A | 40 | F | W | 83 | 42.5 |
| 6 | 5040-5-C | 41 | F | W | 100 | 32 |
W = Caucasian; F = female; BMI = Body mass index
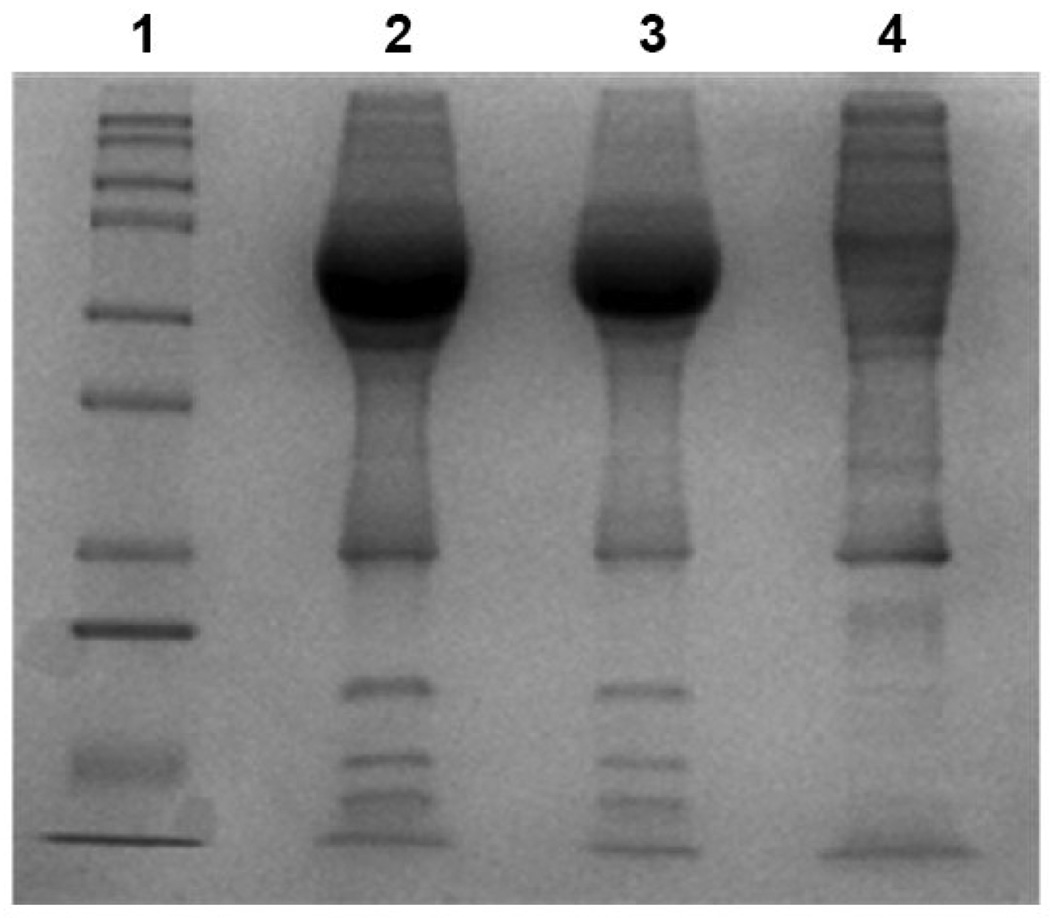
Initial studies were performed to deplete plasma of most abundant proteins using Multi Affinity Reverse Spin (MARS) column, and then run on a sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). We demonstrated that highly abundant proteins were retained in the MARS column in a bound fraction and the low abundant proteins were retained in the unbound or flow through fraction (Figure 1). Using 1D gel electrophoretic analysis we demonstrated that beginning with crude plasma (lane 2), the immunodepletion column were able to successfully deplete high-abundant protein (lane 3) and reveal the flow-through fraction (lane 4), and the appearance of the low-abundant proteins bands previously not seen in the crude sample lanes (lane 2). The flow-through fraction was trypsin digested and run for mass spectrometry analysis by LC-MS/MS. The MS data files were extracted and run for bioinformatics on Ingenuity Pathway Analysis.
Figure 1.
Immunodepletion of patient plasma. The depletion of highly abundant proteins was achieved by Multi Affinity Reverse Spin (MARS) Column in a bound fraction and the low abundant proteins were retained in the unbound or flow through fraction. After the incubation period, unbound proteins were centrifuged and collected directly into a spin concentrator 5 kDa molecular weight filter (Agilent). The bound fraction was eluted with elution buffer into another spin concentrator filter. The total amount of protein in depleted (flow-through fraction) and abundant plasma proteins (bound fraction) was determined using a Bradford protein assay and run on a SDS-PAGE. Lane 1: Protein molecular weight marker; lane 2: Crude human plasma; lane 3: bound fraction and lane 4: flow through fraction after immunodepletion. Results are a representative experiment from three independently performed experiments with similar results.
Differential expression of unique proteins in obesity group
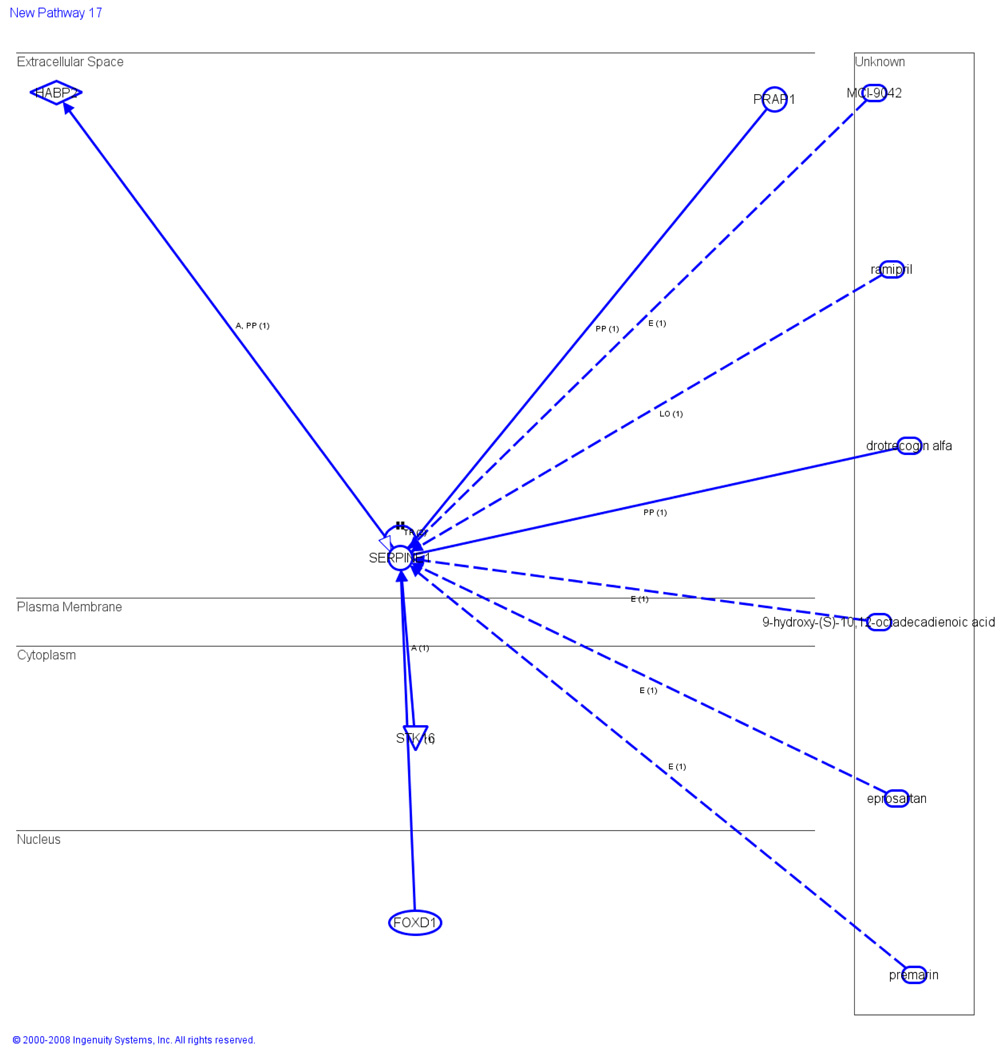
To determine the protein profiles of obese patients, differentially expressed probe sets in flow through fraction were run in LC-MS/MS. We demonstrated that the expression of Complement Component 4A increased by 5-fold in group A (obese) and B (diabetic symptoms) and increased by 4-fold in group C (patients with both obese and diabetic symptoms) as compared to control (Table 2). The expression of beta 2-glycoprotein 1-apolipoprotein H was extremely high in group A (15-fold), group C (12-fold) and group B (7-fold) (Table 2). The expression of inter-alpha trypsin inhibitor family heavy chain-related protein was significantly higher in group A (8-fold), B (6-fold) and C (6-fold) as compared to group D (Table 2). FOXD1, a transcription regulator, found in the cell nucleus directly acts on SERPINE1 (found in the extracellular space), which then activates the peptidase HABP2 and translocate to the kinase STK16 (Figure 2). A number of drugs used in obese patients, including drotrecogin alfa was shown to directly bind to SERPINE1, whereas 9-hydroxy-(S)-10, 12-octadecadiencic acid indirectly acts on SERPINE1. Eprosartan, premarin and ramipril, drugs used in both obesity and hypertension, were shown to indirectly act on SERPINE1 (Figure 2). Interestingly, PRPA1, a protein known to play an important role in cancer was also shown to directly act on SERPINE1 (Figure 2).
Table 2.
Identification of candidate biomarkers obesity and diabetes1.
| Protein Name2 | Protein MW (Da)3 | Protein pI4 |
MS/MS Search Score5 |
Distinct Peptides6 | Spectral Intensity (×107) for respective patients ID7 |
|||
|---|---|---|---|---|---|---|---|---|
| 1-A | 3-B | 5-C | 8-D | |||||
| Apolipoprotein B precursor | 515533.1 | 6.58 | 640.05 | 49 | 5.96 | 16.8 | 17.2 | 4.20 |
| Complement component 4A | 192788.6 | 6.72 | 298.16 | 21 | 22.3 | 25.5 | 21.0 | 4.99 |
| Ceruloplasmin | 123002.7 | 5.46 | 281.99 | 19 | 35.1 | 27.7 | 51.3 | 9.77 |
| Fibronectin 1 | 272337.8 | 5.3 | 210.73 | 16 | 4.83 | 6.28 | 11.9 | 1.71 |
| Inter-alpha-trypsin inhibitor family heavy chain-related protein | 103385.9 | 6.51 | 153.9 | 11 | 40.6 | 33.2 | 29.9 | 4.53 |
| Inter-alpha inhibitor H1 | 101366.7 | 6.43 | 143.71 | 10 | 8.85 | 13.5 | 9.96 | 3.81 |
| Hemopexin | 51676.7 | 6.55 | 140.64 | 9 | 39.3 | 61.8 | 48.2 | 18.6 |
| Apolipoprotein A-1 preproprotein | 30778 | 5.56 | 117.16 | 7 | 3.33 | 39.3 | 27.9 | 11.7 |
| Beta-2-glycoprotein 1 apolipoprotein H | 38312.5 | 8.34 | 101.84 | 7 | 87.9 | 41.2 | 70.6 | 6.05 |
| Alpha-1B-glycoprotein precursor | 54272.8 | 5.58 | 100.81 | 7 | 26.9 | 32.3 | 34.1 | 7.33 |
| Angiotensinogen precursor variant | 53778.2 | 5.97 | 87.81 | 6 | 36.9 | 36.8 | 71.6 | 6.26 |
LC-MS/MS Spectrum Mill database-search for plasma samples obtained from control obese and/or diabetic patients.
List of the protein.
Theoretical MW (Da) of the identified protein from the database.
Theoretical isolelectric points (pI) of the identified protein from the database.
MS/MS search score is computed by scoring function that measure the degree of similarity between the experimental spectrum and the theoretical fragmentation patterns of the candidate peptides.
Number of the distinct peptides of the protein after fragmentation and is based on the homology of the protein with the known protein in the database.
The intensity fold increase of the spectra in the sample is calculated by the factor 1×107.
Figure 2.
Canonical pathways for obesity. Schematic representation of networking in which SERPINE1 is found to be the key molecule in the extracellular space and the other members are linked with SERPINE1 by dotted and solid lines. FOXD1 gene might be the transcription factor (in the nucleus) activated the SERPINE1 protein synthesis. Data is the sum of three independently performed experiments.
Differential expression of unique proteins in diabetic group
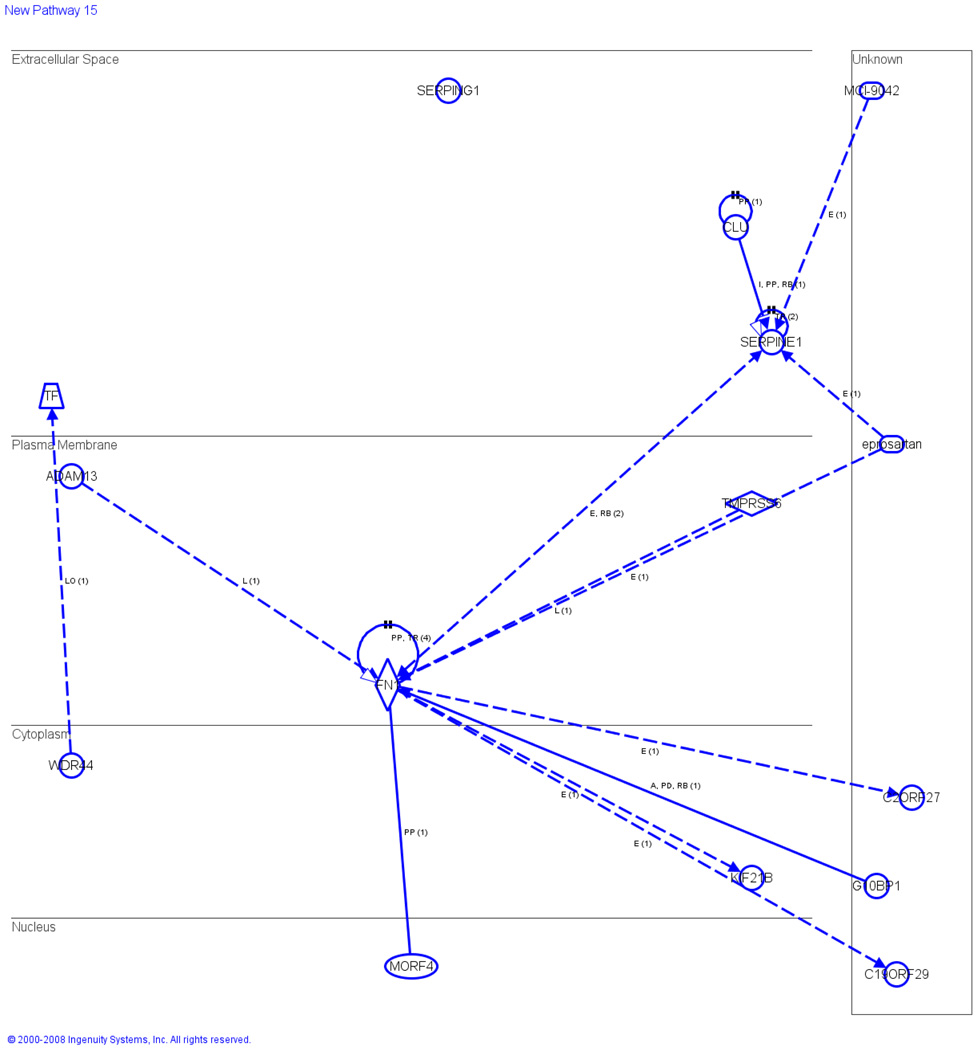
We next determined protein linkage networks important in patients with diabetes, and showed that apolipoprotein B (ApoB) precursor was highly expressed in group B (diabetic symptoms) and with group C (obese and diabetic symptoms), as compared to controls (group D) (Table 2). In comparison to the control group D, the expression of Apolipoprotein A-1 preproprotein was 3-fold higher in group B (diabetic sympotoms), 2-fold in the individual from group C (obese and diabetic symptoms), whereas bottom expression (4-fold) in group A (obese) than the control (Table 2). Hemopexin was 3-fold higher in group B and C and 2-fold higher in group A (Table 2). The expression of inter-alpha globulin inhibitor was 4-fold higher in group B (Table 2). We demonstrated that in diabetic patients, the protein CLL found in the extracellular space directly acts on SERPINE1 (Figure 3). SERPINE1 in turn indirectly acts on the enzyme FN1, found in the plasma membrane, which binds to the transcription regulators MORF4, and known to indirectly act on a protein known to play an important role in diabetes, C19ORF29. In group B (diabetic symptoms) it was shown that the diabetic drugs MCL-9042 and eprostartan indirectly acts on SERPINE1 (Figure 3).
Figure 3.
Canonical pathways for diabetes. Schematic representation of networking in which SERPINE1 is found to be the key molecule in the extracellular space induced by FN 1 (plasma membrane) and CLL (extracellular space) and the other members are linked with SERPINE1 by dotted and solid lines. The expression of SERPINE1 is induced indirectly through FN which is induced by MORF 4 (in the nucleus). SERPING1 is not interconnected with any pathway. Data is the sum of three independently performed experiments.
Differential expression of unique proteins in individual with both obese and diabetic symptoms
Our next step was to determine differentially expressed proteins in individuals with both obese and diabetic symptoms. We demonstrated that angiotensinogen (AGT) precursor variant was 11-fold higher in individuals with both obese and diabetic symptoms as compared to individuals with only obesity or diabetes symptoms (Table 2). Ceruloplasmin showed 5-fold higher expression in group C (obese and diabetic symptoms), 4-fold higher expression in group A (obese) and 3-fold higher expression in group B (diabetic symptoms), as compared to group D (controls) (Table 2). The expression of fibronectin was 7-fold higher in group C (obese and diabetic symptoms) and 4- and 3-fold higher in group B (diabetic symptoms) and A (obese), respectively, as compared to group D (control) (Table 2). Alpha-1B-glycoprotein precursor protein expression was 5-fold higher in group C (obese and diabetic symptoms), 4-fold in both group B (diabetic symptoms) and A (obese) (Table 2).
Linkage of obesity and diabetes with cardiovascular disease pathway
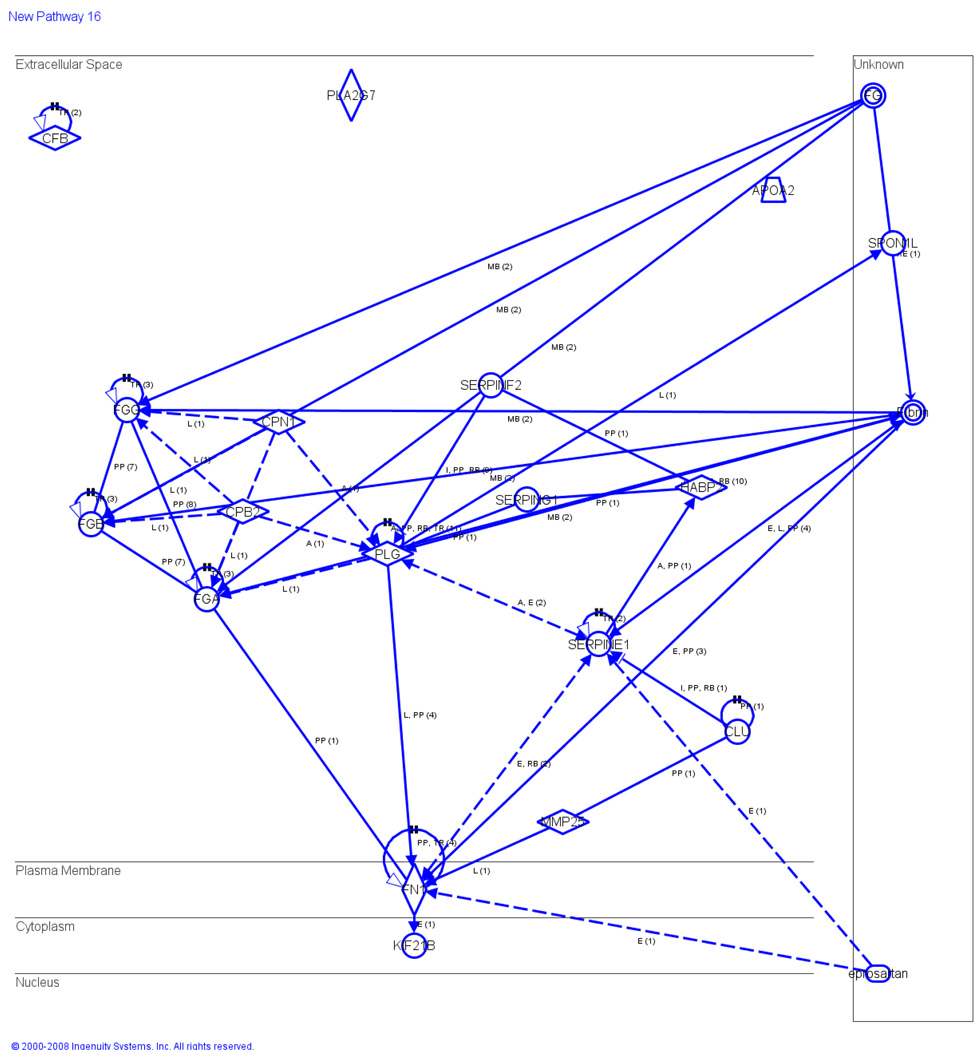
The determination of proteins important in the cardiovascular pathway revealed that the enzyme, FN1, and the peptidase, PLG, both known to play an important role in cardiovascular disease indirectly acts on SERPINE1. Fibrin was found to directly act on both SERPINE1 and FN1. Epipsartan, was also shown to indirectly act on SERPINE1 in the cardiovascular disease pathway (Figure 4). Interestingly, in the cardiovascular disease pathway, CLL inhibits and acts on SERPINE1 (Figure 4). Peptidases, known to play an important role in cardiovascular diseases including CPB2 and CPN1 were shown to indirectly act on SERPINE1 through PLG (Figure 4).
Figure 4.
Canonical pathways for cardiovascular disease. Schematic representation of networking in which SERPINE1 and SERPINF2 are found to be the key molecules in the extracellular space and the other members are linked with SERPINE1 by dotted and solid lines. The cardiovascular pathway is integrated by obesity and diabetes pathways. KF2B (cytoplasm) induces FN 1 (part of diabetes pathway) to induce SERPINE1 (part of both obesity and diabetes pathway) (extracellular space). Also, FN 1 induces SERPINF2 and SERPING 1 through PLG (extracellular space). SERPINE1 is induced by CLL (extracellular space) and indirectly by FN1 and PLG. However, all SERPINE1, SERPING1 and SERPINF2 interconnected with each other. Although SERPING1 interconnection was not clear in the diabetes pathway. Data is the sum of three independently performed experiments.
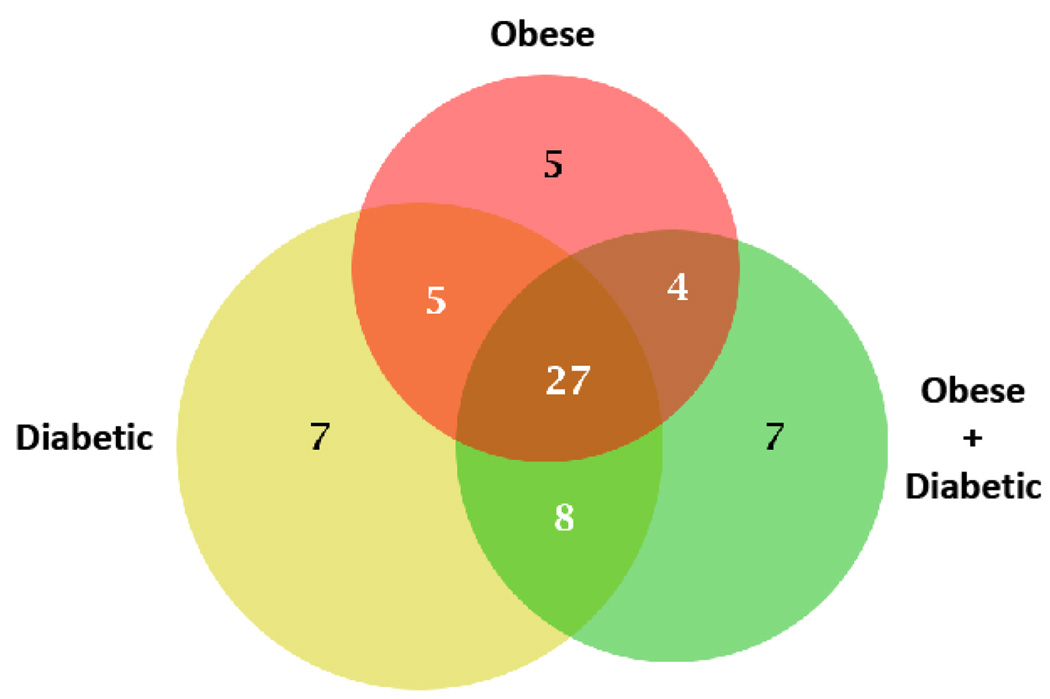
The Venn diagram demonstrates the number of overlapping proteins in obese, diabetic and individuals with both obese and diabetic symptoms (Figure 5). We demonstrated that there are 27 proteins overlapping between all three groups, with 4 proteins overlapping between group A (obese) and C (obese and diabetic symptoms), 5 proteins overlapping between group A (obese) and B (diabetic symptoms) and 8 proteins overlapping between group B (diabetic symptoms) and C (obese and diabetic symptoms) (Figure 5).
Figure 5.
Venn diagram illustrating protein expression in obese and diabetic patients. The immunodepleted fractions from all of the groups were run on MS after trypsin digestion. The MS data files were extracted and run for bioinformatics on ProteinCenter. The number of differentially expressed proteins in obese patients (5 proteins), diabetic patients (7 proteins), patients with both obesity and diabetes (7 proteins), and common in all groups (27 proteins). Data is the sum of three independently performed experiments.
Discussion
Human blood plasma is the most complex derived proteome. The attractiveness of plasma for disease diagnosis lies in two characteristics: the ease with which it can be obtained and the fact that it comprehensively samples the human phenotype (the bodily state at a particular point in time) (Robinson et al., 2009). In this pilot study we have used plasma from individuals to determine a possible link between obesity and diabetes.
In this study, we have performed a moderately high throughput platform for proteomic analysis of human plasma using the MARS system. We investigated the combination of MARS with LC-MS/MS to study the unbound fraction consisting of low abundant proteins. This fraction is conceptually less complex and thus it is an attractive sample for LC-MS/MS analysis. We applied this approach to a sample set collected from female patients with obesity and its associated complications such as diabetes and hypertension, and compared their plasma proteome profile with that of a healthy (control) group. We also used a sample pooling technique to first catalog as many as possible proteins in the disease groups in a high throughput manner.
The multiple affinity removal column for the depletion of 14 high-abundant proteins (Human 14) was based on a Human 6 multiple affinity removal column. In addition to the affinity resins for the depletion of HSA, transferrin, haptoglobin, immunoglobulins like IgG, IgA, and α1-antitrypsin (Level I proteins), the column contains binders for the depletion of fibrinogen, α2-macroglobulin, α1-acid glycoprotein, complement C3, IgM, apolipoprotein AI, apolipoprotein AII, and transthyretin (prealbumin) – (Level II proteins). Based on affinity-purified polyclonal antibody binders, the Human 14 column also contains low molecular weight antibody binders for the depletion of several proteins, which were selected on the basis of specific and efficient target protein binding and their ability to function under specified loading and elution conditions. To determine the overall depletion with MARS Human 14, the flow through fractions were analyzed on SDS-PAGE for total protein content and compared to the starting plasma crude sample data (Figure 1). The results show that with human plasma there is approximately 92% depletion of total protein content in the flow through fraction in the plasma sample (Figure 1).
We demonstrated that angiotensinogen precursor variant (AGT), also known as SERPINE1, was highly expressed in the individual with both obese and diabetic symptoms in group C (Table 2). AGT the protein encoded by this gene, pre-angiotensinogen or angiotensinogen precursor, the resulting product, angiotensin I, is then cleaved by angiotensin converting enzyme (ACE) to generate the physiologically active enzyme angiotensin II. The protein is involved in maintaining blood pressure and in the pathogenesis of essential hypertension and preeclampsia. Mutations in this gene are associated with susceptibility to essential hypertension, and can cause renal tubular dysgenesis (RTD), a severe disorder of renal tubular development (Lacoste et al., 2006). RTD is an autosomal recessive severe disorder of renal tubular development characterized by persistent fetal anuria and perinatal death, probably due to pulmonary hypoplasia from early-onset oligohydramnios (the Potter phenotype) (Morris et al., 2004). Defects in AGT are associated with susceptibility to essential hypertension. Hypertension also occurs in 5–7% of all pregnancies where it is a leading cause of maternal, fetal and neonatal morbidity and mortality (Berg et al., 1996). An 8-week long supplementation of conjugated linoleic acid (CLA) enhanced the effect of ramipril (found in the obesity pathway; Figure 2), on blood pressure reduction in treated obese hypertensive patients (Zhao et al., 2009). Angiotensin receptor type 1 blockers (ARBs) like eprosartan found in the obesity, diabetes and cardiovascular pathways (Figure 2, Figure 3 and Figure 4) is used to treat hypertension and heart failure (Weiss et al., 2010).
Interestingly, the major functions for the candidate proteins we identified showed strong linkage with the cardiovascular disease pathways (Figure 4). Importantly, we demonstrated that SERPINE1 is the key protein found to be associated with the network pathway analysis of obesity, diabetes and cardiovascular pathway analysis (Figure 2, Figure 3 and Figure 4). Serpins are a group of proteins with the ability to inhibit chymotrypsin-like serine proteases (serine protease inhibitors). SERPINE1 (also known as plasminogen activator inhibitor 1 (PA1), plasminogen activator inhibitor type 1 (PAI1), serine (or cysteine) proteinase inhibitor, serpin peptidase inhibitor clade E, SERPINE1), inhibits both tissue type palsminogen activator (tPA) and urokinase type plasminogen activator (uPA) and are associated with an increased risk of thromboembolic disease. In vitro experiments are currently underway to validate the potential role of SERPINE1 as a candidate protein that links obesity and diabetes in our laboratory preparation.
Apolipoprotein B (ApoB) was found to be highly expressed in individuals diagnosed with diabetes only and both diabetes and obesity, but not in obese (Table 2). ApoB is the main apolipoprotein of chylomicrons and low-density lipoproteins (LDL) and occurs as two main isoforms, apoB-48 (synthesized in the gut) and apoB-100 (synthesized in the liver). Mutations in this gene or its regulatory region cause hypobetalipoproteinemia, normotriglyceridemic hypobetalipoproteinemia, and hypercholesterolemia due to ligand-defective apoB, and grossly affect plasma cholesterol and apoB levels (Farese et al., 1995; Benn et al., 2007; McQueen et al., 2008). Another type of multifunctional apolipoprotein is Apolipoprotein H (Apo-H), previously known as beta-2 glycoprotein, which binds to cardiolipin was highly expressed in group C in our study (Table 2). The activity of Apo-H appears to involve the binding of agglutenating, negatively charged compounds, and inhibits agglutenation by the contact activation of the intrinsic blood coagulation pathway (Schousboe, 1985). Apo-H causes a reduction of the prothrombinase binding sites on platelets and reduces the activation caused by collagen when thrombin is present at physiological serum concentrations of Apo-H suggesting a regulatory role of Apo-H in coagulation (Matsuda et al., 1995). Lipoprotein associated phospholipase A2 (Lp-PLA2) modulates low-density lipoprotein (LDL) oxidation and were found to be the mediators of atherosclerosis in patients diagnosed with diabetes (Wootton et al., 2006).
In our study, group C, ceruloplasmin (151kDa) was highly expressed (Table 2) and known as ferroxidase or iron (II): oxygen oxidoreductase and is the major copper-carrying protein in the blood, and in addition plays a role in iron metabolism. Ceruloplasmin exhibits a copper-dependent oxidase activity, which is associated with possible oxidation of Fe2+ (ferrous iron) into Fe3+ (ferric iron), therefore assisting in its transport in the plasma in association with transferrin, which can only carry iron in the ferric state. Mutations in the ceruloplasmin gene can lead to the rare genetic human disease aceruloplasminemia, characterized by iron overload in the brain, liver, pancreas, and retina (Scheinberg and Gitlin, 1952; Gitlin, 1998; Lutsenko et al., 2008).
Fibronectin is a high molecular weight (~440kDa) extracellular matrix glycoprotein that binds to membrane-spanning receptor proteins called integrins was observed with high expression in group C of our study (Table 2). In addition to integrins, fibronectin also binds extracellular matrix components such as collagen, fibrin and heparan sulfate proteoglycans (e.g. syndecans). It is reported that linkage disequilibrium (LD) structure at the fibrinogen gene cluster including fibrinogen-beta (FGB), FGA, and FGG (found in the cardiovascular pathway in Figure 4), factor VII (F7), and tissue plasminogen activator (PLAT) (Kathiresan et al., 2006). In women, PLG levels were higher in individuals with diagnosed ischemic heart disease (IHD) and increased with triglycerides (TG) and glucose level, however, in men, PLG levels decreased with advancing age, decreased with the percentage of body fat, increased with total cholesterol (T-CH), low density lipoprotein cholesterol (LDL-CH) and high density lipoprotein cholesterol (HDL-CH) levels, and were directly correlated with two different PA indices (Kostka et al., 2009). Fibrinogen gamma and alpha (FGG and FGA) gene haplotypes (chromosome 4q28) are associated with fibrin network structure, and thereby with rigidity of the fibrin clot and sensitivity of the fibrin clot to the fibrinolytic system and may influence risk of cardiovascular disease (Kardys et al., 2007).
Similar to our results of hemopexin is expressed in the diabetic group in our study (Table 2), haptoglobin (Hp) was found only in individuals diagnosed with both obesity and diabetes. Hp is a protein found in the blood and is known to bind free hemoglobin, which is released from red blood cells. Mutations in this gene and/or its regulatory regions cause ahaptoglobinemia or hypohaptoglobinemia. 9-hydroxy-(S)-10, 12 octadecadienoic acid (9-HODE) found in the obesity pathway (Figure 2) is a reactive oxygen species generated by mononuclear cells (MNC) and polymorphonuclear leukocytes (PMN) and is associated with lipid peroxidation in obese patients (Dandona et al., 2001).
Conclusion and Perspectives
In conclusion, our ultimate goal of using this technique is to perform rapid screening of a large number of clinical samples, and to identify the proteins that change in their relative abundance due to a particular disease. However, in this work, we focus mainly on a proof-of-concept proteomics discovery study to investigate the usefulness of the depletion approach for the clinical proteomics. This small sample set (N = 5 per set) is not meant to be statistically significant in terms of biomarker discovery, but rather it is a step ahead to explore the some candidate protein profiles associated with significant changes in blood plasma proteins of obese and diabetic patients.
Acknowledgements
The authors thank the Functional Proteomics Core Facility (Scott & White Hospital) for expert technical assistance. This work was supported in part by a Research Advancement Award from Scott & White Memorial Hospital and Clinic (to P. K.), and the US National Institutes of Health grant RO1CA91889, institutional support from Scott & White Memorial Hospital and Clinic, Texas A&M Health Science Center College of Medicine, the Central Texas Veterans Health Administration and an Endowment from the Cain Foundation (to A. A.).
Abbreviations
- ACE
Angiotensin Converting Enzyme
- AGT
Angiotensinogen
- ApoB
Apolipoprotein B
- BMI
Body Mass Index
- CID
Collision-Induced Dissociation
- DTT
Dithiothretiol
- EMR
Electronic Medical Record
- FBG
Fasting Blood Glucose
- HP
Haptoglobin
- HPLC
High-Performance Liquid Chromatography
- IAA
Iodoacetamide
- IgG
Immunoglobulin
- IL-6
Interleukin-6
- IPA
Ingenuity Pathway Analysis
- LC-MS/MS
Liquid Chromatography- Mass Spectrometry
- LDL
Low-Density Lipoproteins
- MARS
Multiple Affinity Removal System
- MS
Mass Spectrometry
- RTD
Renal Tubular Dysgenesis
- SDS-PAGE
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis
- TFA
Trifluro Acetic acid
- TNF-α
Tumor Necrosis Factor-α
References
- 1.Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol. 2007;27:661–670. doi: 10.1161/01.ATV.0000255580.73689.8e. [DOI] [PubMed] [Google Scholar]
- 2.Berg CJ, Atrash HK, Koonin LM, Tucker M. Pregnancy-related mortality in the United States, 1987–1990. Obstet Gynecol. 1996;88:161–167. doi: 10.1016/0029-7844(96)00135-4. [DOI] [PubMed] [Google Scholar]
- 3.Bjorhall K, Miliotis T, Davidsson P. Comparison of different depletion strategies for improved resolution in proteomic analysis of human serum samples. Proteomics. 2005;5:307–317. doi: 10.1002/pmic.200400900. [DOI] [PubMed] [Google Scholar]
- 4.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32:14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 5.Choo V. WHO reassesses appropriate body-mass index for Asian populations. Lancet. 2002;360:235. doi: 10.1016/S0140-6736(02)09512-0. [DOI] [PubMed] [Google Scholar]
- 6.Chromy BA, Gonzales AD, Perkins J, Choi MW, Corzett MH, et al. Proteomic analysis of human serum by two-dimensional differential gel electrophoresis after depletion of high-abundant proteins. J Proteome Res. 2004;3:1120–1127. doi: 10.1021/pr049921p. [DOI] [PubMed] [Google Scholar]
- 7.Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–362. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 8.Expert-Panel. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med. 1998;158:1855–1867. doi: 10.1001/archinte.158.17.1855. [DOI] [PubMed] [Google Scholar]
- 9.Farese RV, Jr, Ruland SL, Flynn LM, Stokowski RP, Young SG. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc Natl Acad Sci USA. 1995;92:1774–1778. doi: 10.1073/pnas.92.5.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqi IS, Keogh JM, Kamath S, Jones S, Gibson WT, et al. Partial leptin deficiency and human adiposity. Nature. 2001;414:34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 11.Field AE, Coakley EH, Must A, Spadano JL, Laird N, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 12.Gitlin JD. Aceruloplasminemia. Pediatr Res. 1998;44:271–276. doi: 10.1203/00006450-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Halsall DJ, Luan J, Saker P, Huxtable S, Farooqi IS, et al. Uncoupling protein 3 genetic variants in human obesity: the c-55t promoter polymorphism is negatively correlated with body mass index in a UK Caucasian population. Int J Obes Relat Metab Disord. 2001;25:472–477. doi: 10.1038/sj.ijo.0801584. [DOI] [PubMed] [Google Scholar]
- 14.Kardys I, Uitterlinden AG, Hofman A, Witteman JC, de Maat MP. Fibrinogen gene haplotypes in relation to risk of coronary events and coronary and extracoronary atherosclerosis: the Rotterdam Study. Thromb Haemost. 2007;97:288–295. [PubMed] [Google Scholar]
- 15.Kathiresan S, Yang Q, Larson MG, Camargo AL, Tofler GH, et al. Common genetic variation in five thrombosis genes and relations to plasma hemostatic protein level and cardiovascular disease risk. Arterioscler Thromb Vasc Biol. 2006;26:1405–1412. doi: 10.1161/01.ATV.0000222011.13026.25. [DOI] [PubMed] [Google Scholar]
- 16.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 17.Kostka T, Para J, Kostka B. Cardiovascular diseases (CVD) risk factors, physical activity (PA) and plasma plasminogen (Plg) in a random sample of community-dwelling elderly. Arch Gerontol Geriatr. 2009;48:300–305. doi: 10.1016/j.archger.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Lacoste M, Cai Y, Guicharnaud L, Mounier F, Dumez Y, et al. Renal tubular dysgenesis, a not uncommon autosomal recessive disorder leading to oligohydramnios: Role of the Renin-Angiotensin system. J Am Soc Nephrol. 2006;17:2253–2263. doi: 10.1681/ASN.2005121303. [DOI] [PubMed] [Google Scholar]
- 19.Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 20.Lutsenko S, Gupta A, Burkhead JL, Zuzel V. Cellular multitasking: the dual role of human Cu-ATPases in cofactor delivery and intracellular copper balance. Arch Biochem Biophys. 2008;476:22–32. doi: 10.1016/j.abb.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda J, Gohchi K, Kawasugi K, Gotoh M, Saitoh N, et al. Inhibitory activity of anti-beta 2-glycoprotein I antibody on factor Va degradation by activated-protein C and its cofactor protein S. Am J Hematol. 1995;49:89–91. doi: 10.1002/ajh.2830490116. [DOI] [PubMed] [Google Scholar]
- 22.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 23.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Morris S, Akima S, Dahlstrom JE, Ellwood D, Kent A, et al. Renal tubular dysgenesis and neonatal hemochromatosis without pulmonary hypoplasia. Pediatr Nephrol. 2004;19:341–344. doi: 10.1007/s00467-003-1319-6. [DOI] [PubMed] [Google Scholar]
- 25.Must A, Spadano J, Coakley EH, Field AE, Colditz G, et al. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–1529. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 26.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–1890. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 27.O’Rahilly S, Farooqi IS, Yeo GS, Challis BG. Minireview: human obesity-lessons from monogenic disorders. Endocrinology. 2003;144:3757–3764. doi: 10.1210/en.2003-0373. [DOI] [PubMed] [Google Scholar]
- 28.Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, et al. Obesity, inflammation, and periodontal disease. J Dent Res. 2007;86:400–409. doi: 10.1177/154405910708600503. [DOI] [PubMed] [Google Scholar]
- 29.Rivlin RS. Keeping the young-elderly healthy: is it too late to improve our health through nutrition? Am J Clin Nutr. 2007;86:1572S–1576S. doi: 10.1093/ajcn/86.5.1572S. [DOI] [PubMed] [Google Scholar]
- 30.Robinson JM, Vandre DD, Ackerman WE., 4th Placental proteomics: a shortcut to biological insight. Placenta. 2009;30:S83–S89. doi: 10.1016/j.placenta.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheinberg IH, Gitlin D. Deficiency of ceruloplasmin in patients with hepatolenticular degeneration (Wilson’s disease) Science. 1952;116:484–485. doi: 10.1126/science.116.3018.484. [DOI] [PubMed] [Google Scholar]
- 32.Schousboe I. beta 2-Glycoprotein I: a plasma inhibitor of the contact activation of the intrinsic blood coagulation pathway. Blood. 1985;66:1086–1091. [PubMed] [Google Scholar]
- 33.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365:1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 34.Thompson D, Wolf AM. The medical-care cost burden of obesity. Obes Rev. 2001;2:189–197. doi: 10.1046/j.1467-789x.2001.00037.x. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH. Lipid overload and overflow: metabolic trauma and the metabolic syndrome. Trends Endocrinol Metab. 2003;14:398–403. doi: 10.1016/j.tem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 36.Weiss J, Sauer A, Divac N, Herzog M, Schwedhelm E, et al. Interaction of angiotensin receptor type 1 blockers with ATP-binding cassette transporters. Biopharm Drug Dispos. 2010;31:150–161. doi: 10.1002/bdd.699. [DOI] [PubMed] [Google Scholar]
- 37.Wootton PT, Stephens JW, Hurel SJ, Durand H, Cooper J, et al. Lp-PLA2 activity and PLA2G7 A379V genotype in patients with diabetes mellitus. Atherosclerosis. 2006;189:149–156. doi: 10.1016/j.atherosclerosis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhao WS, Zhai JJ, Wang YH, Xie PS, Yin XJ, et al. Conjugated linoleic acid supplementation enhances antihypertensive effect of ramipril in Chinese patients with obesity-related hypertension. Am J Hypertens. 2009;22:680–686. doi: 10.1038/ajh.2009.56. [DOI] [PubMed] [Google Scholar]
- 39.Zimmet P, Thomas CR. Genotype, obesity and cardiovascular disease--has technical and social advancement outstripped evolution? J Intern Med. 2003;254:114–125. doi: 10.1046/j.1365-2796.2003.01170.x. [DOI] [PubMed] [Google Scholar]