Abstract
Calcium phosphate cement (CPC) has in situ-setting ability and bioactivity, but the brittleness and low strength limit CPC to only non-load-bearing bone repairs. Human umbilical cord mesenchymal stem cells (hUCMSCs) can be harvested without an invasive procedure required for the commonly studied bone marrow MSCs. However, little has been reported on hUCMSC delivery via bioactive scaffolds for bone tissue engineering. The objectives of this study were to develop CPC scaffolds with improved resistance to fatigue and fracture, and to investigate hUCMSC delivery for bone tissue engineering. In fast fracture, CPC with 15% chitosan and 20% polyglactin fibers (CPC–chitosan–fiber scaffold) had flexural strength of 26 MPa, higher than 10 MPa for CPC control (p < 0.05). In cyclic loading, CPC–chitosan–fiber specimens that survived 2 × 106 cycles had the maximum stress of 10 MPa, compared to 5 MPa of CPC control. CPC–chitosan–fiber specimens that failed after multiple cycles had a mean stress-to-failure of 9 MPa, compared to 5.8 MPa for CPC control (p < 0.05). hUCMSCs showed excellent viability when seeded on CPC and CPC–chitosan–fiber scaffolds. The percentage of live cells reached 96–99%. Cell density was about 300 cells/mm2 at day 1; it proliferated to 700 cells/mm2 at day 4. Wst-1 assay showed that the stronger CPC–chitosan–fiber scaffold had hUCMSC viability that matched the CPC control (p > 0.1). In summary, this study showed that chitosan and polyglactin fibers substantially increased the fatigue resistance of CPC, and that hUCMSCs had excellent proliferation and viability on the scaffolds.
Keywords: Calcium phosphate cement, Fatigue, Load-bearing, Bioactive scaffolds, Human umbilical cord stem cells, Bone regeneration
1. Introduction
Six million bone fractures occurred each year in the United States [1]. Musculoskeletal conditions cost the U. S. $215 billion in 1995 [1,2]. These numbers are predicted to increase dramatically because of an aging population [3]. The introduction of stem cells into the clinical setting opens new horizons [4–8]. Embryonic stem cells are pluripotent, able to become over 200 types of cells in the body. Adult mesenchymal (or stromal) stem cells (MSCs) derived from the bone marrow are multipotent, able to differentiate into bone tissue, neural tissue, cartilage, muscle, and fat [9,10]. MSCs can be harvested from the patient’s bone marrow, expanded in culture, induced to differentiate and combined with a scaffold to repair bone defects.
Recently, stem cells have been derived from the Wharton’s Jelly in umbilical cords [11–16]. These cells appear to bear multipotent stem cell characteristics, and can differentiate into adipocytes, osteoblasts, chondrocytes, neurons, and endothelial cells. Previous studies have termed them “human umbilical cord stroma-derived stem cells” [15] and “human mesenchymal stem cells derived from umbilical cord” [13]. The present study refers to them as “human umbilical cord mesenchymal stem cells”, or hUCMSCs. The use of hUCMSCs has major advantages: (1) Umbilical cords are a medical waste discarded after birth, hence they can be collected at a low-cost; (2) because numerous babies are born each year, hUCMSCs are an inexhaustible stem cell source; (3) they can be collected without an invasive procedure required for bone marrow-derived MSCs; (4) they can be collected without the ethical controversies of embryonic stem cells (hESCs); (5) hUCMSCs are a primitive MSC population that express certain hESC markers and exhibit high plasticity and developmental flexibility; (6) hUCMSCs have greater expansion capability and are more potent than bone marrow MSCs [12]; (7) hUCMSCs appear to cause no immunorejection and are not tumorigenic [12]. These advantages make hUCMSCs a highly desirable stem cell source for tissue regeneration. However, despite of its high promise, little has been published on hUCMSC delivery via bioactive scaffolds for bone tissue engineering.
The development of suitable scaffolds will constitute a centerpiece for bone tissue engineering. The structure needs to be maintained to define the shape of the regenerated tissue. Mechanical properties are of crucial importance for the regeneration of load-bearing tissues such as bone, to withstand stresses to avoid scaffold fracture. Bio-inert implants can induce an undesirable fibrous capsule in vivo, while bioactive implants with bone-like calcium phosphate (CaP) minerals beneficially bond to native bone. This is because CaP minerals provide a preferred substrate for cell attachment and support the proliferation and expression of osteoblast phenotype [17,18]. Hence, hydroxyapatite (HA) and other bioactive CaP scaffolds are important for bone repair [19–25]. However, for sintered HA and other bioactive ceramics to fit into a bone cavity, the surgeon needs to machine the graft to the desired shape or carve the surgical site around the implant. This leads to increases in bone loss, trauma, and surgical time [3].
In contrast, calcium phosphate cements can be molded and set in situ to provide intimate adaptation to bone defects [26–33]. The first calcium phosphate cement was comprised of a mixture of tetracalcium phosphate [TTCP: Ca4(PO4)2O] and dicalcium phosphate anhydrous (DCPA: CaHPO4), and was referred to as CPC [34]. The CPC powder can be mixed with an aqueous liquid to form a paste that can be sculpted during surgery to conform to the defects in hard tissues. The paste self-hardens to form a resorbable hydroxyapatite implant [35–37]. Due to its excellent bioactivity and ability to be replaced by new bone, CPC was approved in 1996 by the Food and Drug Administration (FDA) for repairing craniofacial defects in humans, thus becoming the first CPC for clinical use [36]. However, because it is brittle and weak, the use of CPC was “limited to the reconstruction of non-stress-bearing bone” [35], and “none of the indications include significant stress-bearing applications” [36]. Recent studies used resorbable fibers to provide the needed early-strength to CPC and then to create macropores after fiber dissolution [38–40]. These previous studies measured mechanical properties using single-load, fast fracture methods. However, implants in vivo are subjected to repeated loadings. A literature search revealed no publication on the fatigue properties of CPC.
Accordingly, the objective of this study was to investigate the fatigue behavior of fiber-reinforced CPC, and the seeding of hUCMSCs on CPC-based scaffolds for bone tissue engineering. Two hypotheses were tested: (1) Fiber reinforcement will substantially increase the resistance of CPC to cyclic fatigue and fracture; (2) Fiber-reinforced CPC will support hUCMSC attachment and proliferation, and will not adversely affect cell viability.
2. Materials and methods
2.1. Fabrication of absorbable fiber-reinforced CPC scaffold
The TTCP powder was synthesized from a solid-state reaction between equimolar amounts of DCPA and CaCO3 (J. T. Baker, Phillipsburg, NJ), which were mixed and heated at 1500 °C for 6 h in a furnace (Model 51333, Lindberg, Watertown, WI). The heated mixture was quenched to room temperature, ground in a blender and sieved to obtain TTCP particles with sizes of approximately 1–80 μm, with a median of 17 μm. DCPA was ground for 24 h to obtain particle sizes of 0.4–3.0 μm, with a median of 1.0 μm. TTCP and DCPA powders were mixed in a blender at a molar ratio of 1:1 to form the CPC powder.
Chitosan and its derivatives are natural biopolymers that are biocompatible, biodegradable and osteoconductive [41]. Chitosan has been shown to strengthen and toughen CPC [42,43], resist the washout of CPC paste in physiological solution, and accelerate CPC setting [39]. Chitosan lactate (referred to as chitosan; VANSON, Redmond, WA) was mixed with water at chitosan/(chitosan +water) mass fractions of: 0%, 5%, 10%, and 15%, to form four CPC liquids. Chitosan fractions ≥ 20% were not used because the paste became relatively dry.
An absorbable fiber (Vicryl, polyglactin 910, Ethicon, Somerville, NJ) was used because it is clinically used as sutures, and it possessed a relatively high strength [38,39]. This suture consisted of individual fibers braided into a bundle with a bundle diameter of 322 μm, provided strength for about four weeks, and then dissolved and created long macropores in CPC [38,39]. As in previous studies, the suture fiber was cut to a length of 8 mm [39,40]. The CPC powder was mixed with a liquid at a powder:liquid mass ratio of 3:1 to form a paste. The polyglactin fibers were mixed into the CPC paste randomly to form a paste, which was placed into a rectangular mold of 3 mm × 4 mm × 25 mm [38]. A fiber volume fraction of 20% was used to obtain a CPC–chitosan–fiber paste that was readily mixed and not dry. The fiber volume fraction was equal to the volume of fibers divided by the volume of the entire specimen. The paste in the mold was set in a humidor with 100% relative humidity for 4 h at 37 °C. Then the specimens were demolded and immersed in a physiological solution (1.15 mmol/L Ca, 1.2 mmol/L P, 133 mmol/L NaCl, 50 mmol/L Hepes, buffered to 7.4 pH) at 37 °C for 20 h prior to testing [42].
Three experiments were performed: (1) A fast fracture test in which the materials were fractured in a single-load; (2) a fatigue test; and (3) seeding of hUCMSCs on CPC.
2.2. Fast fracture testing
Five materials were tested: CPC control (0% chitosan), CPC with 5% chitosan, CPC with 10% chitosan, CPC with 15% chitosan, and CPC with 15% chitosan and 20% polyglactin fibers. CPC control was also referred to as the FDA-approved CPC, because that material consisted of the same TTCP-DCPA mixture with no chitosan or fibers [36].
A three-point flexural test [44] with a span of 20 mm was used to fracture the specimens at a crosshead speed of 1 mm/min on a Universal Testing Machine (5500R, MTS, Cary, NC). Flexural strength was calculated by S = 3 FmaxL/(2bh2), where Fmax is the maximum load on the load-displacement curve, L is span, b is specimen width and h is thickness. Elastic modulus was calculated by E = (F/c) (L3/[4bh3]), where load F divided by the corresponding displacement c is the slope of the load-displacement curve in the linear elastic region.
2.3. Fatigue testing
The fast fracture test showed that the CPC with 15% chitosan and 20% fibers had the highest strength. Hence, this material was used for the fatigue test and referred to as “CPC–chitosan–fiber”. Two materials were tested: CPC–chitosan–fiber, and CPC control.
Fatigue testing was conducted in cyclic four-point flexure using a universal testing system (Model 3200, Enduratec, Bose, Eden Prairie, MN). The loading arrangement consisted of a conventional 1/3 span with distance between the two interior and two exterior supports being 6 mm and 18 mm, respectively. The flexure apparatus conformed to a scaled version of ASTM D790 [44]. Cyclic loading was performed at room temperature (22 °C) in the physiological solution using load-control actuation, at a frequency of 5 Hz and a minimum to maximum stress ratio of 0.1. It took more than 3 days to complete 2 ×106 cycles for one specimen. The evaluation was started using a maximum cyclic stress of 90% of the single-load strength. Successive specimens were then tested using a maximum cyclic load decreased in increment of 1 MPa, according to a staircase fatigue method [45,46].
2.4. hUCMSC seeding on CPC scaffolds
hUCMSCs were purchased from ScienCell Research laboratories (human umbilical cord mesenchymal stem cells #7530, Carlsbad, CA). They were obtained from the umbilical cord of a healthy baby born by normal term delivery. The hUCMSCs were harvested as described previously [47]. The use of hUCMSCs was approved by the University of Maryland Baltimore. Cells were cultured in a low-glucose Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (PS) (Invitrogen, Carlsbad, CA). hUCMSCs were plated in flasks at 6,000 cells/cm2 (passage 1) and the medium was changed every two days. At 80–90% confluence, hUCMSCs were detached by trypsin (Invitrogen) and passaged at 6,000 cells/cm2. Passage 5 hUCMSCs were used for all experiments.
Two materials were seeded with hUCMSCs: CPC control; and CPC–chitosan–fiber. Following a previous study [48], 150,000 cells were diluted into 2 mL of media and added to each well containing a CPC disk of 2 mm in thickness and 12 mm in diameter. The culture was incubated for 1 d, 4 d, or 8 d, following a previous study [48].
2.5. hUCMSC live/dead staining
After 1, 4 or 8 days, the media was removed and the cells were washed two times in 2 mL of Tyrode’s Hepes buffer (140 mmol/L NaCl, 0.34 mmol/L Na2HPO4, 2.9 mmol/L KCl, 10 mmol/L Hepes, 12 mmol/L NaHCO3, 5 mmol/L glucose, pH 7.4). Cells were then stained and viewed by epifluorescence microscopy (Eclipse TE300, Nikon, Melville, NY). Staining was done for 1 h with 2 mL of Tyrode’s Hepes buffer containing 2 μmol/L calcein-AM and 2 μmol/L ethidium homodimer-1 (Molecular Probes, Eugene, OR). Calcein-AM is a nonfluorescent, cell-permeant fluorescein derivative, which is converted by cellular enzymes into cell-impermeant and highly fluorescent calcein. Calcein accumulates inside live cells having intact membranes causing them to fluoresce green. Ethidium homodimer-1 enters dead cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to their DNA causing the nuclei of dead cells to fluoresce red [43].
Two parameters were measured. First, the percentage of live cells was measured. Three randomly-chosen fields of view were photographed from each disk (A total of five disks yielded 15 photos per material). The cells were counted. NLive is the number of live cells, and NDead is the number of dead cells. The percentage of live cells, PLive = NLive/(NLive + NDead) [49].
The second parameter was cell attachment, CAttach [49]. It is the number of live cells attached on the specimen divided by the area, A: CAttach = NLive/A. Both PLive and CAttach were measured, because a high value of PLive only means that there are few dead cells; it does not necessarily mean a large number of live cells that are attached to the specimens. CAttach quantifies the absolute number of live cells anchored on the CPC specimen per surface area.
2.6. Wst-1 viability assay of hUCMSCs
hUCMSC viability was assessed using the Wst-1 colorimetric assay, which measures the cellular mitochondrial dehydrogenase activity (Dojindo, Gaithersburg, MD) [43]. At 8 days, CPC control and CPC–chitosan–fiber specimens with cells were transferred to wells in a 24-well plate and rinsed with 1 mL of Tyrode’s Hepes buffer. One mL of Tyrode’s Hepes buffer and 0.1 mL of Wst-1 solution (5 mmol/L Wst-1 and 0.2 mmol/L 1-methoxy-5-methylphenazinium methylsulfate in water) were added to each well and incubated at 37 °C for 2 h. Then, 200 μL of each reaction mixture was transferred to a 96-well plate. The absorbance at 450 nm was measured with a microplate reader (Wallac 1420 Victor2, PerkinElmer, Gaithersburg, MD) [43].
A scanning electron microscope (SEM, JEOL 5300, Peabody, MA) was used to examine the hUCMSCs attached on CPC specimens. Cells cultured for 4 days on specimens were rinsed with saline, fixed with 1% glutaraldehyde, subjected to graded alcohol dehydrations, rinsed with hexamethyldisilazane, sputter coated with gold, and examined in SEM [43].
One-way and two-way ANOVA were performed to detect significant effects of the variables. Tukey’s multiple comparison tests were used to compare the data at p of 0.05.
3. Results
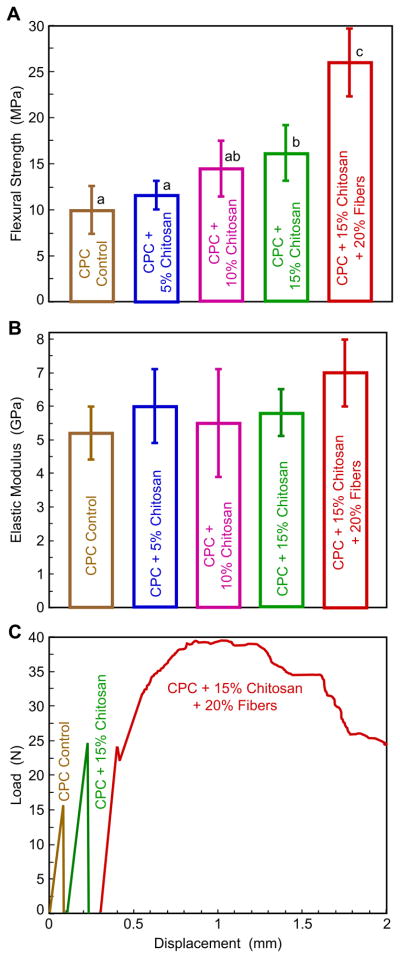
Fig. 1 plots the results from the single-load, three-point flexure test (mean ± sd; n = 6). In (A), CPC with 15% chitosan reached a strength of (16.2 ± 3.0) MPa, higher than (10.2 ± 2.1) MPa for CPC control (p < 0.05). CPC with 15% chitosan and 20% fibers had a strength of (26.0 ± 3.7) MPa, higher than all other materials (p < 0.05). In (B), elastic modulus ranged from about 5.5–7 GPa, similar for all materials (p > 0.1). In (C), the load-displacement curves of CPC control and CPC with chitosan showed a brittle, catastrophic failure mode. In contrast, the CPC with 15% chitosan and 20% fibers showed a tough, non-catastrophic failure mode.
Fig. 1.
Results of single-load fast fracture. (A) Flexural strength, (B) elastic modulus, and (C) load-displacement curves. Each strength or modulus value is the mean of six measurements, with the error bar showing one standard deviation (mean ± sd; n = 6). In (A), values indicated by dissimilar letters are significantly different (p < 0.05). In (B) all the values are statistically similar (p > 0.1). In (C), CPC with 15% chitosan and 20% fibers showed a tough, non-catastrophic failure mode.
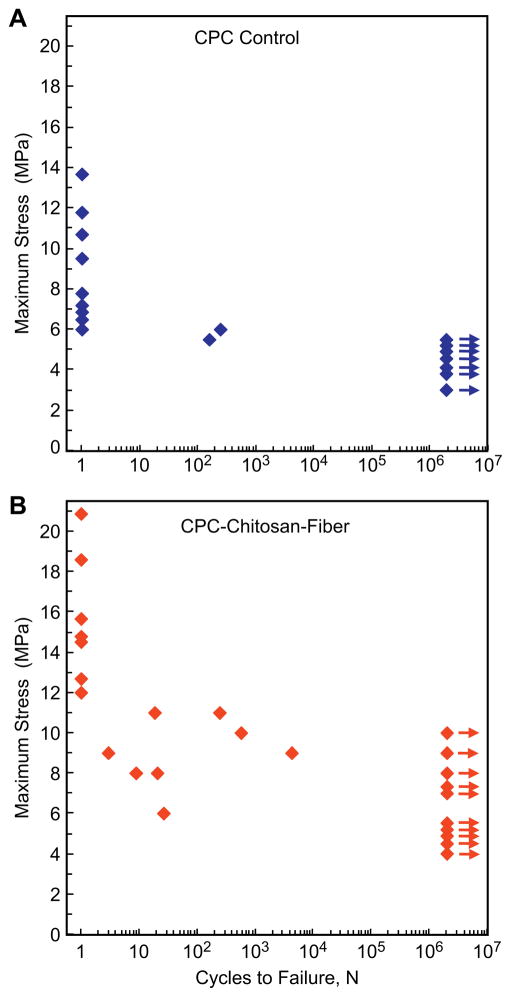
The fatigue results are plotted in Fig. 2 for (A) CPC control, and (B) CPC with 15% chitosan and 20% fibers (designated as CPC–chitosan–fiber). CPC control failed in a single cycle at stresses ≥ 6 MPa. On the other hand, at a slightly lower stress of ≤ 5 MPa, none of the specimens failed after 2 ×106 cycles. CPC–chitosan–fiber failed at higher stresses. CPC–chitosan–fiber specimens that survived 2 ×106 cycles reached the highest stress of 10 MPa. This was 2-fold the highest stress of 5 MPa for CPC control.
Fig. 2.
Fatigue of (A) CPC control, and (B) CPC with 15% chitosan and 20% fibers (designated as CPC–chitosan–fiber). Data points near the left axis are for specimens that failed at 1 cycle. Arrows near the right axis indicate specimens that survived 2 ×106 cycles without fracture. CPC–chitosan–fiber specimens failed at higher stresses than CPC control.
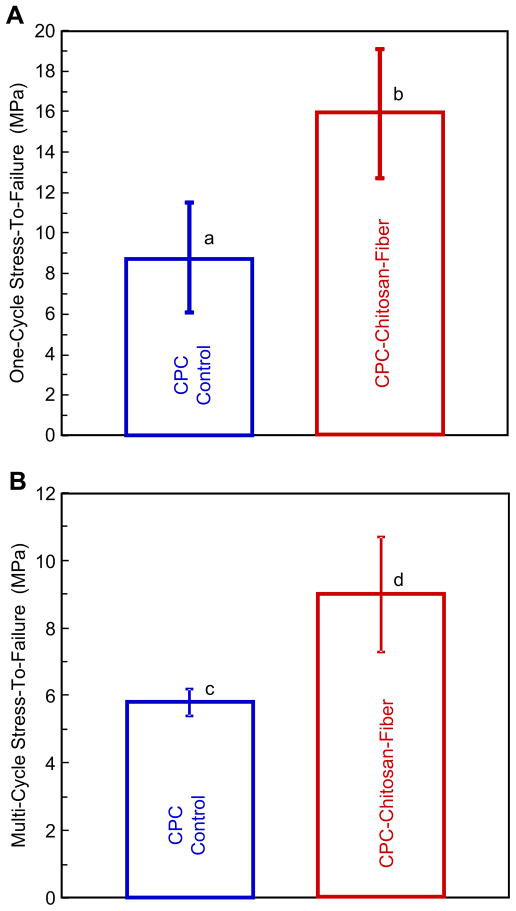
To better compare these materials, Fig. 3 plots the mean and standard deviation for specimens failed after one cycle (A), or after multiple cycles (B). In each case, CPC–chitosan–fiber had significantly higher stress-to-failure values than CPC control (p < 0.05).
Fig. 3.
Stress-to-failure values for CPC control and CPC–chitosan–fiber specimens failed after (A) one cycle, and (B) multiple cycles. In each plot, values with dissimilar letters are significant different (p < 0.05).
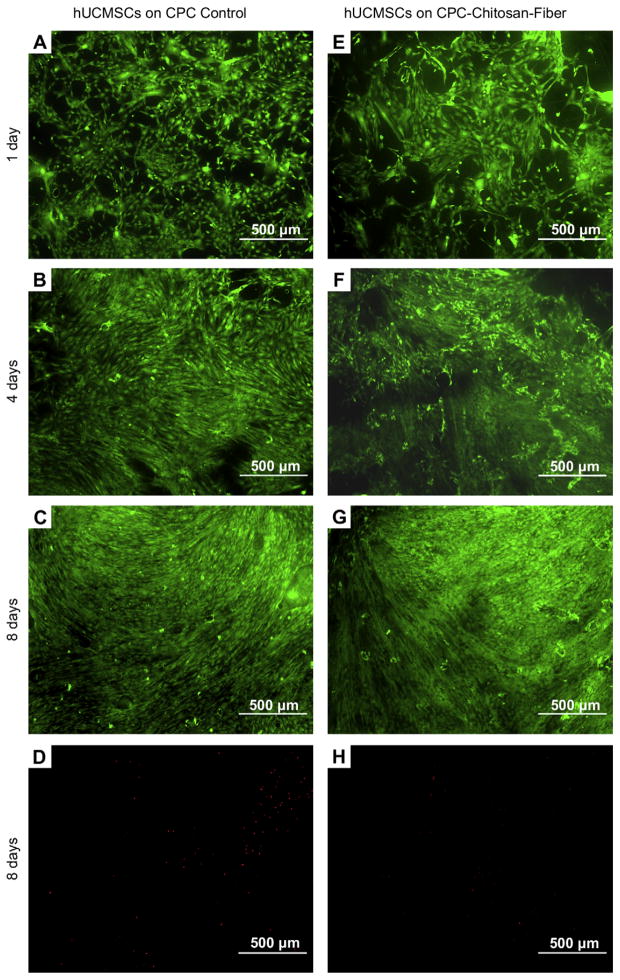
hUCMSCs seeded on CPC control and CPC–chitosan–fiber are shown in Fig. 4. Live cells (stained green) appeared to have adhered and attained a normal, polygonal morphology on both materials. Visual examination revealed that the density of live cells adherent to each material was similar at the same time point. Over time, live cells increased in numbers due to cell proliferation. Dead cells (stained red) were very few on both materials.
Fig. 4.
Live/dead staining of hUCMSCs cultured on CPC control and CPC–chitosan–fiber for 1 day, 4 days, and 8 days. Live cells, stained green, were numerous on both materials. Dead cells, stained red, were few on both materials. Three randomly-chosen fields of view were photographed from each disk. A total of five disks yielded 15 photos per material at each time period. Representative photos are shown here.
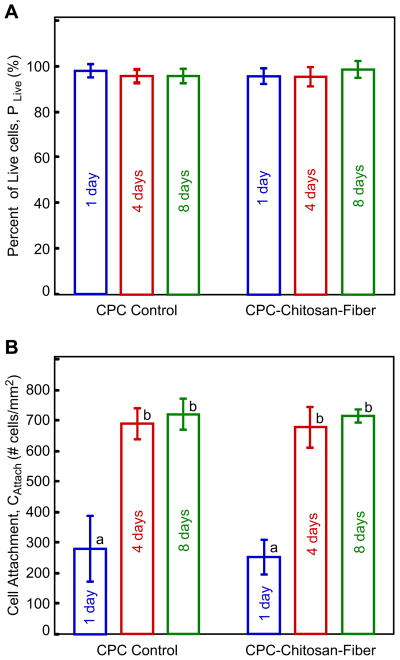
Fig. 5 plots (A) percent of live cells, and (B) live cell attachment. PLive reached 96–99%, not significantly different from each other (p > 0.1), consistent with the observation that there were few dead cells. CAttach was less than 300 cells/mm2 at day 1; it more than doubled to nearly 700 cells/mm2 at day 4, due to hUCMSC proliferation. Further culture to day 8 only slightly increased CAttach (p > 0.1), likely because the cells were nearly confluent on the specimens at day 4, and hence further proliferation was slowed down due to cell contact inhibition.
Fig. 5.
hUCMSCs were cultured on CPC control and CPC–chitosan–fiber for 1, 4, and 8 days: (A) Percent of live cells, and (B) live cell attachment (mean ± sd; n = 5). PLive reached 96–99%, not different from each other (p > 0.1). CAttach was less than 300 cells/mm2 at day 1; it more than doubled to 700 cells/mm2 at day 4, due to hUCMSC proliferation. In (B), dissimilar letters indicate values that are significantly different (p < 0.05).
The Wst-1 assay quantified the metabolic activity of the hUCMSCs cultured for 8 days on CPC control and CPC–chitosan–fiber scaffold. The cell viability, measured using the absorbance at 450 nm, was proportional to the amount of dehydrogenase activity in the cells. This absorbance was measured (mean ± sd; n = 5) to be (1.3 ± 0.2) for CPC control, and (1.2 ± 0.1) for CPC–chitosan–fiber scaffold (p > 0.1). Hence the stronger and tougher CPC–chitosan–fiber scaffold yielded hUCMSC viability that matched that of the CPC control.
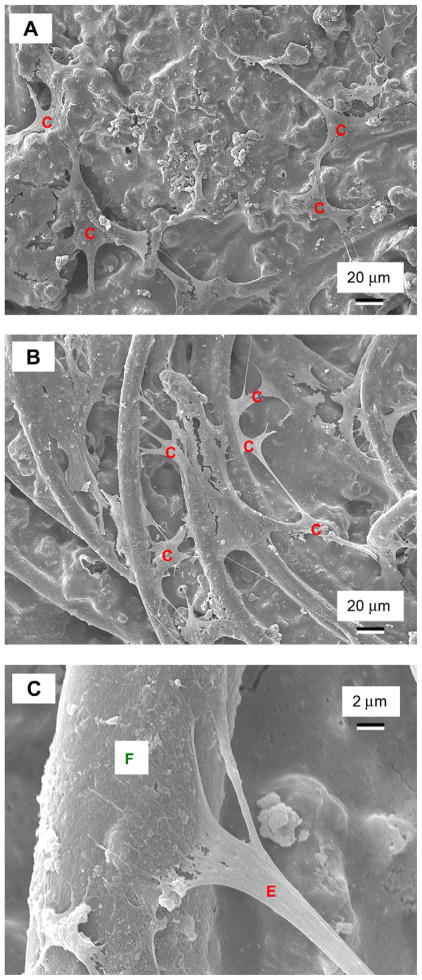
Fig. 6 shows SEM micrographs of hUCMSCs seeded on: (A) CPC control, and (B) CPC–chitosan–fiber. In (A), cells (designated as “C”) had healthy polygonal shapes and were anchored on CPC. In (B), cells attached to the fibers of CPC–chitosan–fiber scaffold. The cells had developed long cytoplasmic extensions “E”, which are visible in (A) and (B), and are shown at a higher magnification in (C) attaching to the fiber “F”. These extensions are regions of the cell plasma membrane that contain a meshwork or bundles of actin-containing microfilaments, which permit the movement of the migrating cells along a substratum [50]. While the hUCMSC body had a spread size of approximately 20 μm, the cytoplasmic extensions had additional lengths of about 20–50 μm.
Fig. 6.
SEM of hUCMSC attachment on: (A) CPC control, and (B) CPC–chitosan–fiber scaffold. Cells are designated as “C”, which anchored to CPC in (A), and to the fibers in the scaffold in (B). Cells developed long, cytoplasmic extensions “E”, shown in (C) at a higher magnification, attaching firmly to the fiber in the CPC–chitosan–fiber scaffold.
4. Discussion
Calcium phosphate cements are advantageous because they can be injected or molded to the desired shape, set to form a scaffold in situ, and then be gradually resorbed and replaced by new bone [30,37]. Hence, extensive studies have been performed on their compositions and mechanical properties [26–29], injectable cements [30,31], growth factors delivery via these cements [32], calcium phosphate-polymer composites [33], and reinforced calcium phosphate scaffolds [38–40]. However, mechanical properties have usually been measured using single-load, fast fracture methods, while implants in vivo undergo repeated loadings. A literature search revealed that the present study represented the first report on the fatigue behavior of CPC. When a tough material is loaded cyclically, micro-damage such as microcracks would be created in the material. As the number of cycles continues to increase, microcracks would accumulate and coalesce, eventually leading to specimen failure. The present study showed that for CPC control, at stresses ≤ 5 MPa, no specimens failed after 2 million cycles; however, with a slight increase in stress (to ≥ 6 MPa), all the specimens failed at a single cycle. There was little room in the stress level for gradual damage accumulation without specimen failure, indicating that the CPC control has a very low damage tolerance. This is likely because CPC is extremely brittle, and as soon as a microcrack is formed, it propagates catastrophically through the entire specimen.
Incorporation of chitosan and polyglactin fibers progressively improved the CPC strength (Fig. 1). The elastic modulus was not improved, because chitosan and the polyglactin fibers did not have a high stiffness. The load-displacement curves indicate that CPC and CPC–chitosan failed catastrophically in a single crack. In contrast, for CPC–chitosan–fiber, after the first cracking (the first load drop on the load-displacement curve), its load-bearing ability continued to increase, due to the fibers bridging and supporting the applied load [38–40]. In fatigue (Fig. 2), the highest stress reached 10 MPa for CPC–chitosan–fiber to survive 2 million cycles, compared to the highest stress of 5 MPa for CPC control. After one cycle, the stress-to-failure for CPC–chitosan–fiber was nearly 2-fold that of CPC control. After multiple cycles, the stress-to-failure for CPC–chitosan–fiber was 1.5 times that for CPC control.
The stress-to-failure at one cycle (Fig. 3A) was slightly lower than the flexural strength in Fig. 1A, likely due to two factors. First, the fatigue test was performed using four-point flexure, while the flexural strength in Fig. 1 was determined using three-point flexure. The former test sampled a larger volume of the specimen with an increased probability of containing a large flaw, yielding a slightly lower strength. Second, the single-load fracture for Fig. 1 was done in air, to serve as a screening test for the different materials. The fatigue test, on the other hand, was performed with the specimens always immersed in the physiological solution, to simulate in vivo conditions. The immersion may have slightly weakened the specimens, particularly the absorbable fibers which undergo hydrolytic dissolution. Therefore, the single-load test and the fatigue test were two different tests, yet they both confirmed that the CPC–chitosan–fiber scaffold was much more resistant to failure, both in fast fracture and in fatigue.
A load-bearing scaffold can help deliver stem cells to a wide range of load-bearing locations to enhance bone regeneration. Stem cell-based tissue engineering is promising as the weapon of mass salvation, and bone marrow MSCs are commonly studied [6–10]. However, bone marrow MSCs for autogenous use can cause donor site morbidity, are limited in number, and have lower self-renewal capacity and differentiation potential with aging. Therefore, there is a strong need for alternative MSCs. Recent studies have shown that hUCMSCs could be guided to differentiate down the osteogenic lineage with a high potential for bone regeneration [11–16]. However, to date, there has little study on hUCMSC interactions with bioactive scaffolds for bone tissue engineering.
The present study showed that hUCMSCs attached well on the CPC control, as well as on the stronger and tougher CPC–chitosan–fiber scaffold. The CPC–chitosan–fiber bone graft was non-cytotoxic in the cell culture studies. After 1-day incubation, the hUCMSCs were able to adhere, spread and remain viable on CPC–chitosan–fiber and CPC control. After 8 days, fluorescence microscopy and Wst-1 assay showed that cell adhesion, proliferation and viability were equivalent on both materials. Therefore, these in vitro cell culture results suggest that the new CPC–chitosan–fiber composite is non-cytotoxic and compatible with hUCMSCs. Cell proliferation and viability were equivalent on both materials, suggesting that the CPC strength and resistance to fatigue can be greatly improved, without compromising its hUCMSC compatibility. This study showed that chitosan and fibers substantially increased the fatigue and fracture resistance of CPC, without compromising the hUCMSC attachment, proliferation and viability. Hence, hUCMSCs delivered via bioactive scaffolds may be a superior, inexhaustible and low-cost alternative to the gold-standard bone marrow MSCs, and may broadly impact the field of stem cell-based regenerative medicine. Further studies should investigate the osteogenic differentiation and mineralization of hUCMSCs delivered via CPC-based scaffolds, and bone regeneration in animal models.
5. Conclusions
This study showed that chitosan and polyglactin fibers substantially increased the fatigue resistance of CPC, and that the CPC-based scaffolds supported hUCMSC attachment, proliferation and viability. hUCMSCs are highly promising for bone repair; however, little has been reported on hUCMSC seeding on bioactive scaffolds. In this study, CPC and CPC–chitosan–fiber scaffolds showed excellent hUCMSC compatibility, manifested by nearly 99% live cell density, and rapid cell proliferation from day 1 to day 4. The addition of chitosan and polyglactin fibers into CPC did not adversely affect its hUCMSC viability, compared to the CPC control. Cells showed healthy spreading and anchored on the fibers in CPC via cytoplasmic extensions. These results suggest that the strong CPC–chitosan–fiber scaffold supports hUCMSC attachment and viability, and may be suitable for stem cell delivery and bone tissue engineering.
Acknowledgments
We are indebted to Dr. M. D. Weir at the University of Maryland Dental School for discussions and help. We also thank Drs. S. Takagi and L. C. Chow at the Paffenbarger Research Center and Carl G. Simon at the National Institute of Standards and Technology for discussions, and Anthony Giuseppetti for help with the SEM. This study was supported by NIH R01 grants DE14190 (HX), DE17974 (HX), DE16904 (DA), Maryland Stem Cell Research Fund (HX), and the University of Maryland Dental School.
Appendix
All Figures of this article may be difficult to interpret in black and white. The full colour images can be found in the online version, at doi:10.1016/j.biomaterials.2009.09.106.
References
- 1.Praemer A, Furner S, Rice DP. Musculoskeletal conditions in the United States. Chapter 1 Rosemont, Illinois: Amer Acad Orthop Surg; 1999. [Google Scholar]
- 2.Ambrosio AMA, Sahota JS, Khan Y, Laurencin CT. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J Biomed Mater Res. 2001;58B:295–301. doi: 10.1002/1097-4636(2001)58:3<295::aid-jbm1020>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Laurencin CT, Ambrosio AMA, Borden MD, Cooper JA. Tissue engineering: orthopedic applications. Annu Rev Biomed Eng. 1999;1:19–46. doi: 10.1146/annurev.bioeng.1.1.19. [DOI] [PubMed] [Google Scholar]
- 4.Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res. 2001;56:228–33. doi: 10.1002/1097-4636(200108)56:2<228::aid-jbm1089>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Yao J, Radin S, Reilly G, Leboy PS, Ducheyne P. Solution-mediated effect of bioactive glass in poly (lactic-co-glycolic acid)-bioactive glass composites on osteogenesis of marrow stromal cells. J Biomed Mater Res. 2005;75A:794–801. doi: 10.1002/jbm.a.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Datta N, Holtorf HL, Sikavitsas VI, Jansen JA, Mikos AG. Effect of bone extra-cellular matrix synthesized in vitro on the osteoblastic differentiation of marrow stromal cells. Biomaterials. 2005;26:971–7. doi: 10.1016/j.biomaterials.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006;85:966–79. doi: 10.1177/154405910608501101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Kim HJ, Vunjak-Novakovic G, Kaplan DL. Stem cell based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–82. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogeneic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Mao JJ, Vunjak-Novakovic G, Mikos AG, Atala A. Regenerative medicine: translational approaches and tissue engineering. Boston and London: Artech House; 2007. [Google Scholar]
- 11.Wang HS, Hung SC, Peng ST. Mesenchymal stem cells in the Wharton’s jelly of the human umbilical cord. Stem Cells. 2004;22:1330–7. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- 12.Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886–95. doi: 10.1634/stemcells.2007-0417. [DOI] [PubMed] [Google Scholar]
- 13.Baksh D, Yao R, Tuan RS. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–92. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- 14.Bailey MM, Wang L, Bode CJ, Mitchell KE, Detamore MS. A comparison of human umbilical cord matrix stem cells and temporomandibular joint condylar chondrocytes for tissue engineering temporomandibular joint condylar cartilage. Tissue Eng. 2007;13:2003–10. doi: 10.1089/ten.2006.0150. [DOI] [PubMed] [Google Scholar]
- 15.Karahuseyinoglu S, Kocaefe C, Balci D, Erdemli E, Can A. Functional structure of adipocytes differentiated from human umbilical cord stroma-derived stem cells. Stem Cells. 2008;26:682–91. doi: 10.1634/stemcells.2007-0738. [DOI] [PubMed] [Google Scholar]
- 16.Wang L, Singh M, Bonewald LF, Detamore MS. Signalling strategies for osteogenic differentiation of human umbilical cord mesenchymal stromal cells for 3D bone tissue engineering. J Tissue Eng Regen Med. 2009;3:398–404. doi: 10.1002/term.176. [DOI] [PubMed] [Google Scholar]
- 17.Ducheyne P, Qiu Q. Bioactive ceramics: the effect of surface reactivity on bone formation and bone cell function. Biomaterials. 1999;20:2287–303. doi: 10.1016/s0142-9612(99)00181-7. [DOI] [PubMed] [Google Scholar]
- 18.Murphy WL, Hsiong S, Richardson TP, Simmons GA, Mooney DJ. Effects of a bone-like mineral film on phenotype of adult human mesenchymal stem cells in vitro. Biomaterials. 2005;26:303–10. doi: 10.1016/j.biomaterials.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 19.LeGeros RZ. Biodegradation and bioresorption of calcium phosphate ceramics. Clin Mater. 1993;14:65–88. doi: 10.1016/0267-6605(93)90049-d. [DOI] [PubMed] [Google Scholar]
- 20.Hing KA, Best SM, Bonfield W. Characterization of porous hydroxyapatite. J Mater Sci Mater in Med. 1999;10:135–45. doi: 10.1023/a:1008929305897. [DOI] [PubMed] [Google Scholar]
- 21.Pilliar RM, Filiaggi MJ, Wells JD, Grynpas MD, Kandel RA. Porous calcium polyphosphate scaffolds for bone substitute applications – in vitro characterization. Biomaterials. 2001;22:963–72. doi: 10.1016/s0142-9612(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 22.Chu TMG, Orton DG, Hollister SJ, Feinberg SE, Halloran JW. Mechanical and in vivo performance of hydroxyapatite implants with controlled architectures. Biomaterials. 2002;23:1283–93. doi: 10.1016/s0142-9612(01)00243-5. [DOI] [PubMed] [Google Scholar]
- 23.Radin S, Reilly G, Bhargave G, Leboy PS, Ducheyne P. Osteogenic effects of bioactive glass on bone marrow stromal cells. J Biomed Mater Res. 2005;73A:21–9. doi: 10.1002/jbm.a.30241. [DOI] [PubMed] [Google Scholar]
- 24.Russias J, Saiz E, Deville S, Gryn K, Liu G, Nalla RK, et al. Fabrication and in vitro characterization of three-dimensional organic/inorganic scaffolds by robo-casting. J Biomed Mater Res. 2007;83A:434–45. doi: 10.1002/jbm.a.31237. [DOI] [PubMed] [Google Scholar]
- 25.Miranda P, Pajares A, Saiz E, Tomsia AP, Guiberteau F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J Biomed Mater Res. 2008;85A:218–27. doi: 10.1002/jbm.a.31587. [DOI] [PubMed] [Google Scholar]
- 26.Durucan C, Brown PW. Low temperature formation of calcium-deficient hydroxyapatite-PLA/PLGA composites. J Biomed Mater Res. 2000;51:717–25. doi: 10.1002/1097-4636(20000915)51:4<717::aid-jbm21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 27.Ginebra MP, Rilliard A, Fernández E, Elvira C, Román JS, Planell JA. Mechanical and rheological improvement of a calcium phosphate cement by the addition of a polymeric drug. J Biomed Mater Res. 2001;57:113–8. doi: 10.1002/1097-4636(200110)57:1<113::aid-jbm1149>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 28.Barralet JE, Gaunt T, Wright AJ, Gibson IR, Knowles JC. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J Biomed Mater Res. 2002;63B:1–9. doi: 10.1002/jbm.1074. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama A, Yamamoto S, Kawasaki T, Kohgo T, Nakasu M. Development of calcium phosphate cement using chitosan and citric acid for bone substitute materials. Biomaterials. 2002;23:1091–101. doi: 10.1016/s0142-9612(01)00221-6. [DOI] [PubMed] [Google Scholar]
- 30.Bohner M, Gbureck U, Barralet JE. Technological issues for the development of more efficient calcium phosphate bone cements: a critical assessment. Biomaterials. 2005;26:6423–9. doi: 10.1016/j.biomaterials.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 31.Bohner M, Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–63. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Link DP, van den Dolder J, van den Beucken JJ, Wolke JG, Mikos AG, Jansen JA. Bone response and mechanical strength of rabbit femoral defects filled with injectable CaP cements containing TGF-β1 loaded gelatin microspheres. Biomaterials. 2008;29:675–82. doi: 10.1016/j.biomaterials.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 33.Link DP, van den Dolder J, van den Beucken JJ, Cuijpers VM, Wolke JG, Mikos AG, et al. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res. 2008;87:A760–9. doi: 10.1002/jbm.a.31831. [DOI] [PubMed] [Google Scholar]
- 34.Brown WE, Chow LC. A new calcium phosphate water setting cement. In: Brown PW, editor. Cements research progress. Westerville, OH: Am Ceram Soc; 1986. pp. 352–79. [Google Scholar]
- 35.Shindo ML, Costantino PD, Friedman CD, Chow LC. Facial skeletal augmentation using hydroxyapatite cement. Arch Otolaryngol Head Neck Surg. 1993;119:185–90. doi: 10.1001/archotol.1993.01880140069012. [DOI] [PubMed] [Google Scholar]
- 36.Friedman CD, Costantino PD, Takagi S, Chow LC. Bone source hydroxyapatite cement: a novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J Biomed Mater Res (Appl Biomater) 1998;43:428–32. doi: 10.1002/(sici)1097-4636(199824)43:4<428::aid-jbm10>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Chow LC. Calcium phosphate cements: chemistry, properties, and applications. Mat Res Symp Proc. 2000;599:27–37. [Google Scholar]
- 38.Xu HHK, Quinn JB. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials. 2002;23:193–202. doi: 10.1016/s0142-9612(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 39.Xu HHK, Takagi S, Quinn JB, Chow LC. Fast-setting and anti-washout calcium phosphate scaffolds with high strength and controlled macropore formation rates. J Biomed Mater Res. 2004;68A:725–34. doi: 10.1002/jbm.a.20093. [DOI] [PubMed] [Google Scholar]
- 40.Burguera EF, Xu HHK, Takagi S, Chow LC. High early-strength calcium phosphate bone cement: effects of dicalcium phosphate dehydrate and absorbable fibers. J Biomed Mater Res. 2005;75A:966–75. doi: 10.1002/jbm.a.30497. [DOI] [PubMed] [Google Scholar]
- 41.Muzzarelli RAA, Biagini G, Bellardini M, Simonelli L, Castaldini C, Fraatto G. Osteoconduction exerted by methylpyrolidinone chitosan in dental surgery. Biomaterials. 1993;14:39–43. doi: 10.1016/0142-9612(93)90073-b. [DOI] [PubMed] [Google Scholar]
- 42.Xu HHK, Quinn JB, Takagi S, Chow LC. Processing and properties of strong and non-rigid calcium phosphate cement. J Dent Res. 2002;81:219–24. [PubMed] [Google Scholar]
- 43.Xu HHK, Simon CG., Jr Fast setting calcium phosphate-chitosan scaffold: mechanical properties and biocompatibility. Biomaterials. 2005;26:1337–48. doi: 10.1016/j.biomaterials.2004.04.043. [DOI] [PubMed] [Google Scholar]
- 44.American Society for Testing and Materials. ASTM D 790-03: standard test methods for flexural properties of unreinforced and reinforced plastic and electrical insulating materials. West Conshohocken, PA: ASTM International; 2004. [Google Scholar]
- 45.Arola D, Reprogel R. Tubule orientation and the fatigue strength of human dentin. Biomaterials. 2006;27:2131–40. doi: 10.1016/j.biomaterials.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Arola D, Reprogel R. Effects of aging on the mechanical behavior of human dentin. Biomaterials. 2005;26:4051–61. doi: 10.1016/j.biomaterials.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 48.Kim K, Dean D, Mikos AG, Fisher JP. Effect of initial cell seeding density on early osteogenic signal expression of rat bone marrow stromal cells cultured on cross-linked poly(propylene fumarate) disks. Biomacromolecules. 2009;10:1810–7. doi: 10.1021/bm900240k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moreau JL, Xu HHK. Mesenchymal stem cell proliferation and differentiation on an injectable calcium phosphate-chitosan composite scaffold. Biomaterials. 2009;30:2675–82. doi: 10.1016/j.biomaterials.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodish H, Berk A, Zipursky SL, Matsudaira P, Baltimore D, Darnell J. Molecular cell biology. 4. Chapters 18–19 New York: Freeman; 2000. [Google Scholar]