Abstract
A growing body of clinical and epidemiological evidence suggests that low dietary intake and/or tissue levels of n-3 (omega-3) polyunsaturated fatty acids (PUFAs) are associated with postpartum depression. Low tissue levels of n-3 PUFAs, particularly docosahexaenoic acid (DHA), are reported in patients with either postpartum or nonpuerperal depression. Moreover, the physiological demands of pregnancy and lactation put childbearing women at particular risk of experiencing a loss of DHA from tissues including the brain, especially in individuals with inadequate dietary n-3 PUFA intake or suboptimal metabolic capabilities. Animal studies indicate that decreased brain DHA in postpartum females leads to several depression-associated neurobiological changes including decreased hippocampal brain-derived neurotrophic factor and augmented hypothalamic-pituitary-adrenal responses to stress. Taken together, these findings support a role for decreased brain n-3 PUFAs in the multifactorial etiology of depression, particularly postpartum depression. These findings, and their implications for research and clinical practice, are discussed.
1. Introduction
Postpartum depression is a potentially devastating disorder that occurs in 10%–20% of childbearing women [1–3]. The etiology remains to be fully elucidated; however, it is complex, most likely heterogeneous, and probably involves the interaction of environmental factors and genetic predispositions, with pregnancy or childbirth as the triggering event [4–10]. Hormonal changes during pregnancy and after childbirth appear to play a contributory role, but cannot fully explain incidence of the disorder [11–13]. Untreated, postpartum depression can lead to recurrent depressive episodes, negatively affect the development of the infant, and in severe instances, lead to maternal suicide or infanticide [14–16]. There is thus a critical need to elucidate causes and risk factors of this disorder that affects the health and well-being of both mothers and infants in order to identify means of prevention or treatment. A growing body of evidence suggests that n-3 polyunsaturated fatty acid (PUFA) status may contribute to the development of postpartum depression. That literature, and its implications for research and clinical practice, is reviewed here.
2. Methods
This paper is based on literature found in PubMed searched from 1964 through July 31, 2010. Primary outcomes of interest were the effects of pregnancy and lactation on maternal n-3 PUFA status in humans and in animals, relationships between n-3 PUFA status and postpartum depression, as well as clinical trials of n-3 PUFAs in postpartum depression. Secondary clinical outcomes of interest were relationships between n-3 PUFA status and non-puerperal depression, and clinical trials of n-3 PUFAs in non-puerperal depression. Preclinical outcomes of interest were the effects of manipulations of dietary or tissue n-3 PUFA status on neurobiological systems known to be altered in depression (i.e., hippocampal expression of brain-derived neurotrophic factor, the hypothalamic-pituitary-adrenal axis, the CNS serotonin and dopamine systems, and neuroinflammation). Effects of altered n-3 PUFA status in animal models of depression were also examined.
3. N-3 Polyunsaturated Fatty Acids
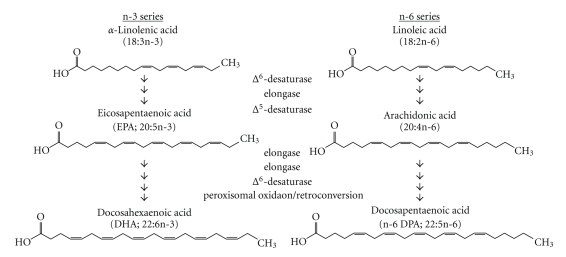
Long-chain polyunsaturated fatty acids (LC-PUFAs) are fatty acids that are 20 or more carbons in length and contain multiple double bonds. The n-3 and n-6 (or omega-3 and omega-6) families of PUFAs are synthesized from the nutritionally essential fatty acids, α-linolenic acid (18:3n-3; which indicates the number of carbons: the number of double bonds, and the fatty acid family) and linoleic acid (18:2n-6) (Figure 1). Biologically important LC-PUFAs such as docosahexaenoic acid (DHA; 22:6n-3) and arachidonic acid (20:4n-6) can either be synthesized from the essential fatty acids or consumed directly in the diet from sources such as fatty fish, which is notably rich in n-3 LC-PUFAs, and other animal products. However, humans are relatively inefficient in synthesizing LC-PUFAs (≤6% conversion) from the essential fatty acids [17, 18], which may be further exacerbated by genetic polymorphisms that render certain individuals particularly poor at synthesizing or utilizing LC-PUFAs [19, 20]. Compounding this metabolic inefficiency, diets in North America are notably low in n-3 PUFAs, particularly relative to n-6 PUFAs, which compete for metabolism into LC-PUFAs [21]. Thus, there is considerable potential for variation in n-3 PUFA status between populations and between individuals within a given population.
Figure 1.
Biosynthesis of n-3 and n-6 polyunsaturated fatty acids. The essential fatty acids α-linolenic acid and linoleic acid are metabolized by elongases and desaturases into a variety of n-3 and n-6 LC-PUFA, respectively.
LC-PUFAs are components of the phospholipids that form cell membranes. The phospholipids in brain have notably high concentrations of LC-PUFAs, with DHA being the most abundant species. Variation in fatty acid composition of the phospholipids alters the physicochemical properties of the membrane and can thus alter the function of membrane-bound proteins and lipid rafts [22, 23]. DHA and other LC-PUFAs can also be cleaved from the membrane by phospholipases to serve as precursors for inter- and intracellular signaling molecules such as prostaglandins, neuroprotectin D1, and resolvins [22, 24–26]. In addition, LC-PUFAs are agonists at nuclear receptors, such as the retinoid X receptor (RXR) and peroxisome proliferator-activated receptors (PPAR), which modulate gene expression [27, 28]. Thus, changes in the relative abundance of specific LC-PUFAs, particularly DHA, can affect neuronal function through a variety of mechanisms.
Most of the DHA in the human brain accumulates during the third trimester of gestation, and continues through the first few years of life [29–31]. DHA is supplied by the mother to the fetus in utero, and to the neonate in breast milk which contains high concentrations of DHA, though concentrations vary depending on maternal diet and other factors [32, 33]. Low availability of DHA results in increased incorporation of docosapentaenoic acid (n-6 DPA, 22:5n-6), the 22-carbon member of the n-6 PUFA family, thus altering the fatty acid composition of the phospholipids [34]. This change in the composition of brain phospholipids does not affect brain weight or overall growth [35] but is associated with suboptimal visual, attentional, and intellectual development [36–39].
4. Effects of Pregnancy and Lactation on Maternal N-3 PUFA Status
As the source of nutrition for the developing fetus and infant, there is considerable demand on pregnant and nursing women to supply DHA to their offspring [37, 38]. Without an adequate diet, mothers can become depleted of nutrients. The majority of studies reported that maternal plasma DHA levels were decreased by as much as 50% in some individuals after a single pregnancy, and were not fully replenished at 26 weeks postpartum [40–44]. Additional pregnancies resulted in further reduction of maternal DHA levels in plasma and breast milk [43, 45]. Similar decreases in the percentage of DHA in erythrocytes and liver have also been reported after pregnancy and lactation in rats [46]. Although brain fatty acid status has not been studied in humans after pregnancy, studies in rats indicated that the DHA content of brain phospholipids was reduced by roughly 25% after only a single reproductive cycle (i.e., pregnancy and lactation through weaning) if the animals were fed a diet low in n-3 PUFAs [47, 48]. The percentage of DHA in rat brain was not further decreased after multiple reproductive cycles; however, a second reproductive cycle resulted in additional incorporation of n-6 DPA [48]. While these reproduction-associated changes in brain fatty acid composition can be reversed by subsequent treatment with DHA (Figure 2), it is not yet known whether this restoration of brain fatty acid composition reverses the neurobiological changes that result from the loss of DHA (see below).
Figure 2.
Effects of dietary remediation on brain phospholipid DHA and n-6 DPA contents in postpartum dams with a reproduction- and diet-related decrease in brain DHA. Adult female rats underwent two complete reproductive cycles (pregnancy and lactation) while fed an n-3 PUFA-deficient diet as previously described [49]. At the time of weaning of the second litter, dams were placed on a remediation diet containing DHA (4% of fat by weight). Virgin controls were age-matched females fed a control diet for 12 weeks. n = 6/group. Data are presented as the mean ± SEM. *P < .05 versus virgin control by ANOVA and Tukey's test.
5. N-3 PUFAs in Depression
5.1. Postpartum Depression
Epidemiologic and clinical studies suggest that pregnancy-associated changes in n-3 LC-PUFA status contribute to the development of postpartum depression. A cross-national analysis indicated that higher fish consumption, which was reflected in higher concentrations of DHA in breast milk, correlated with a lower incidence of postpartum depression [50]. Low intake of fish and other sources of n-3 PUFAs was also associated with depression during pregnancy [51, 52]. Brain fatty acid composition in postpartum depression has not been studied. Nonetheless, in studies of plasma or serum, DHA concentrations, or the DHA:n-6 DPA ratio, was significantly lower in postpartum women experiencing depressive symptoms than those who were not [53, 54]. Similarly, women who later developed postpartum depression had lower serum DHA levels after delivery than those who did not develop depressive symptoms [55], although other studies did not find such a relationship [56–58]. Likewise, risk of postpartum depression was associated with a single-nucleotide polymorphism in the FADS1/FADS2 gene cluster [59], which encodes the rate-limiting enzymes in LC-PUFA biosynthesis, and was associated with lower proportions of DHA in breast milk even if the women were consuming fish or fish oil [60]. In addition, women with more than one child or who had short interpregnancy intervals (<24 months) were found to be at higher risk of developing postpartum depression [9, 61], consistent with the potential for greater alterations in n-3 LC-PUFA status after multiple pregnancies and/or inadequate time for replenishment between pregnancies.
5.2. Nonpuerperal Depression
Low n-3 PUFA status, particularly low DHA, has also been reported in non-puerperal depression. Analyses of dietary n-3 PUFA intake indicate an association of low n-3 PUFA consumption with depressive symptoms [62–66]. Likewise, in postmortem studies, tissue DHA content of the orbitofrontal cortex was decreased 22% in individuals with major depressive disorder compared to controls and was the only fatty acid found to be altered [67]. Similarly, the DHA content of the cingulate cortex was lower in individuals with major depression, but was one of several fatty acids found to be altered [68]. Studies of erythrocyte, serum, plasma, or adipose tissue levels of DHA and other n-3 PUFAs have found similar results [69–79]; findings that have been supported by subsequent meta-analysis [80]. Interestingly, some studies found stronger associations of low n-3 PUFA status in women than in men, or found an association only in women [67, 81, 82], in whom depression occurs with roughly twice the frequency as men [83–85]. Expression of FADS1 and several other genes involved in lipid metabolism was also decreased in individuals who completed suicide [86]; however, no alterations in any of the n-3 PUFAs were found in postmortem brains from suicide completers, even in those individuals with a diagnosis of major depression [87, 88].
6. Clinical Trials with N-3 PUFAs in Depression
Clinical trials with n-3 PUFAs in depression have yielded varying results. These differences in outcomes are likely due to the considerable variation in the n-3 PUFA preparations used, as well as numerous other differences between the studies including the dose, duration of treatment, severity of the depressive symptoms, and inclusion/exclusion criteria, percentage of male and female subjects, choice of placebo, and the inclusion of other concomitant treatments such as psychotherapy. In addition, the populations studied in these clinical trials also varied in their dietary n-3 and n-6 PUFA content, which may also have contributed to the variable outcomes. Moreover, several of the studies likely lacked adequate statistical power.
6.1. Postpartum Depression
Only a few studies have examined the effects of n-3 PUFA treatment specifically in postpartum depression. Treatment with a preparation containing DHA and eicosapentaenoic acid (EPA, 20:5n-3) (EPAX 550 [EPA : DHA, 1.5 : 1] 0.5, 1.4, or 2.8 g/day) for 8 weeks reduced depressive symptoms in postpartum depression in a dose-ranging pilot study that did not include a placebo control group [89]. In a double-blind, placebo-controlled trial, however, treatment with DHA and EPA (0.8 and 1.1 g/day, resp.) for 8 weeks was of no additional benefit in women with perinatal major depression when all subjects received concomitant psychotherapy [90]. Fish oil (2960 mg/day [EPA : DPA, 1.4 : 1] from week 34–36 of pregnancy through 12 weeks postpartum) [91] or DHA supplements (200 mg/day for 4 months after delivery [92] or 220 mg/day from week 16 of pregnancy through 3 months postpartum [93]) also failed to prevent the development of postpartum depressive symptoms [91–93].
6.2. Nonpuerperal Depression
The effects of n-3 PUFAs in non-puerperal depression have been more extensively tested (Table 1). Double-blind, placebo-controlled clinical trials in depressed patients found that various n-3 LC-PUFA preparations, such as EPA, a combination of EPA and DHA, or fish oil, were beneficial as an adjunct to the patients' current antidepressant medication [94–96] or when administered as monotherapy [97–100], for at least some doses or in certain subsets of subjects. Similarly, fish oil improved depressive symptoms in depressed Parkinson's patients [101]. In other studies that did not include a placebo control, ethyl-EPA had equal efficacy to fluoxetine, and the combination of ethyl-EPA and fluoxetine produced greater improvement than fluoxetine alone [102]. Some doses of DHA alone also improved depressive symptoms in a dose-ranging study [103]. On the other hand, other double-blind, controlled clinical trials using DHA alone, combinations of DHA and EPA, or fish oil found no antidepressant effects [104–106], and EPA produced no beneficial effects alone or as an add-on to antidepressant medication [107–109]. Despite the negative results of some trials, subsequent meta-analyses and other post hoc evaluations generally support the antidepressant efficacy of n-3 PUFAs, particularly EPA, though the efficacy of DHA remains unclear [110–114]. These analyses also highlight the need for additional controlled randomized trials with larger sample sizes and adequate doses and treatment durations.
Table 1.
Double-blind, randomized, placebo-controlled trials of n-3 PUFAs in nonpuerperal depression.
| Author | Year | Disorder | Population | Intervention | Treatment groups1 | Dose | Duration | Major finding |
|---|---|---|---|---|---|---|---|---|
| Nemets et al. [94] | 2002 | Major depressive disorder | Israel, 85% female | Add-on to current antidepressant | Ethyl-EPA, n = 10 placebo (not stated), n = 10 | 2 g/day | 4 weeks | Improvement in HDRS score over placebo (P < .05) |
| Peet and Horrobin [95] | 2002 | Major depressive disorder | UK, 84% female | Add-on to current antidepressant | ethyl-EPA 1 g/kg, n = 17 2 g/kg, n = 18 4 g/kg, n = 17 placebo (liquid paraffin), n = 14 |
1, 2, or 4 g/day | 12 weeks | Improvement in HDRS score over placebo at 1 mg/kg (P < .05). No effect at 2 or 4 mg/kg |
| Su et al. [96] | 2003 | Major depressive disorder | Taiwan, 82% female | Add-on to current antidepressat | Fish oil, n = 12, placebo (olive oil ethyl esters), n = 10 |
9.6 g/day containing EPA: 4.4 g/day DHA: 2.2 g/day | 8 weeks | Improvement in HDRS score over placebo (P < .05) |
| Marangell et al. [104] | 2003 | Major depressive disorder | USA, 80% female | Monotherapy | DHA, n = 18 placebo (not stated), n = 7 |
2 g/day | 6 weeks | No effect of treatment on HDRS, CGI or MADRS scores |
| Silvers et al. [106] | 2005 | Depression | New Zealand, 53% female | Add-on to current antidepressant | Fish oil, n = 40 placebo (olive oil), n = 37 |
8 g/day containing EPA: 0.6 g/day DHA: 2.4 g/day | 12 weeks | Improvements in HDRS and BDI scores were greater than, but not significantly different from placebo |
| Nemets et al. [97] | 2006 | Childhood depression | Israel, Gender ratio not stated | Monotherapy | EPA + DPA, n = 10 placebo (olive or safflower oil), n = 10 |
1 g/day containing EPA: 400 mg/day DHA: 200 mg/day | 16 weeks | Improvement in CDRS score over placebo (P < .05) |
| Grenyer et al. [108] | 2007 | Major depressive disorder | Australia, 74% female | Add-on to current antidepressant (74% of subjects) or Monotherapy | Fish oil, n = 32 placebo (olive oil), n = 28 |
8 g/day containing EPA: 0.6 g/day DHA: 2.2 g/day | 16 weeks | No effect of treatment on BDI score |
| Su et al. [98] | 2008 | Major depression during pregnancy | Taiwan, 100% female | Monotherapy | EPA+DHA, n = 13 placebo (olive oil ethyl esters), n = 11 | EPA: 2.2 g/day DHA: 1.2 g/day | 8 weeks | Improvement in HDRS over placebo (P < .05) and higher response rate (P < .05) |
| da Silva et al. [101] | 2008 | Depression in Parkinson's disease | Brazil, Gender ratio not stated | Monotherapy or add-on to current antidepressant | Fish oil only, n = 6, Fish oil + antidepressant, n = 8, placebo (mineral oil), n = 7, placebo + antidepressant, n = 8 | EPA: 720 mg/day DHA: 480 mg/day | 12 weeks | Improvement in MADRS score compared to placebo or placebo + antidepressant (P < .05) |
| Rogers et al. [105] | 2008 | Mild to moderate depression | UK, Gender ratio not stated | Monotherapy | EPA + DHA, n = 96, placebo (olive oil with 7.5 mg mixed tocopherols), n = 94 | EPA: 630 mg/day, DHA: 850 mg/day | 12 weeks | No effect of treatment on DASS or BDI scores |
| Lucas et al. [99] | 2009 | Middle-aged women with psycholo gical distress and depressive symptoms | Canada, 100% female | Monotherapy | Ethyl-EPA, n = 59 placebo (sunflower oil), n = 61 | 1.5 g/day containing EPA: 1.05 g/day DHA: 0.15 g/day | 8 weeks | Improvement in HDRS score was greater than placebo only for subjects not meeting criteria for major depression (P < .05) |
| Mischoulon et al. [107] | 2009 | Major depressive disorder | USA, 65% female | Monotherapy | Ethyl-EPA, n = 11 placebo (paraffin oil with 0.2% α-tocopherol), n = 3 | 1 g/day | 8 weeks | Improvement in HDRS score was greater than, but not significantly different from placebo |
| Rondanelli et al. [100] | 2010 | Elderly women with depression | Italy, 100% female | Monotherapy | EPA + DHA, n = 22, placebo (paraffin oil), n = 24 | EPA: 1.67 g/day, DHA: 0.83 g/day | 8 weeks | Improvement in GDS score over placebo (P < .05). |
| Bot et al. [109] | 2010 | Major depression in diabetes mellitus | The Netherlands, 52% female | Add-on to current antidepressant | Ethyl-EPA, n = 12 placebo (rapeseed oil with medium chain triglycerides), n = 12 | 1 g/day | 12 weeks | No effect of treatment on MADRS score |
1Sample size at end of study.
HDRS:Hamilton Depression Rating Scale, CGI:Clinical Global Impression, MADRS:Montgomery-Åsberg Depression Rating Scale, BDI:Beck Depression Inventory, CDRS:Childhood Depression Rating Scale, DASS:Depression, Anxiety, and Stress Scales, GDS:Geriatric Depression Scale.
7. Biological Mechanisms for N-3 PUFAs in Postpartum Depression
Experimental studies in animals and correlational studies in humans indicate several biological mechanisms by which variation in n-3 PUFA consumption and/or tissue n-3 PUFA status may contribute to the pathogenesis of depression. The vast majority of animal studies have used diets to modulate the availability of specific LC-PUFAs, and thus, tissue fatty acid compositions. On the other hand, altered brain LC-PUFAs in humans may result from genetic variation in PUFA metabolism or utilization, perhaps also in combination with inadequate diet. Nevertheless, the effects of altered brain LC-PUFA composition should likely be similar regardless of the underlying cause. However, the neurobiological consequences of variation in n-3 PUFA status do vary depending on the magnitude of the change, the point in the lifespan at which the manipulation occurred, and in some instances, the physiological state (e.g., postpartum). Accordingly, the effects of variation in brain n-3 PUFA status on neurobiological parameters known to be of importance in depression are reviewed here with a focus on the effects in postpartum females.
7.1. Effects on Hippocampal Expression of Brain-Derived Neurotrophic Factor (BDNF)
Decreased expression of BDNF in the hippocampus, a component of the limbic system involved in memory, affect, and regulation of the hypothalamic-pituitary-adrenal axis [115], is strongly implicated in the pathophysiology of depression. Of note, hippocampal BDNF levels were decreased in suicide completers [116, 117]. This decrease in BDNF expression results in decreased hippocampal neurogenesis [118], and may consequently contribute to the hippocampal atrophy observed in depression [119]. Furthermore, BDNF levels were higher in postmortem hippocampus of antidepressant-treated patients than in untreated patients, suggesting a role for BDNF in the mechanism of antidepressants [120]. Similar effects have been observed in animal studies with various models of depression, stress paradigms, and antidepressant treatments [121–127].
When examined at the time of weaning a litter, which is at the end of the period of greatest offspring demand for DHA in rodents [128], and thus roughly comparable to the postpartum period in humans, female rats that experienced a decrease in brain DHA as a result of pregnancy and lactation while being fed an n-3 PUFA-deficient diet exhibited decreased hippocampal BDNF mRNA and peptide levels [49]. These animals had a decrease in brain DHA content of about 25% which is similar to the decrease observed in postmortem brain samples from individuals with major depressive disorder [67]. Furthermore, the magnitude of the decrease in BDNF mRNA (−32%) was similar to that observed in suicide victims [116, 117]. A decrease in hippocampal BDNF mRNA levels was also observed in virgin female rats that were fed an n-3 PUFA-deficient diet for a sufficient period of time (6 months) to decrease brain DHA content by about 25% [49]. These effects on BDNF could not be attributed to differences in general health, weight gain, maternal offspring burden, or serum estradiol levels [49, 129]. This suggests that the decrease in hippocampal BDNF expression is related to brain DHA status specifically, not an interaction of brain DHA level and reproductive status. Even so, this effect was somewhat greater in parous females [49], suggesting that there may be some augmentation in the postpartum state.
Findings in other animal models and in humans also indicate a role for n-3 PUFAs in the regulation of hippocampal BDNF expression and function. Notably, a DHA-enriched diet in rats increased hippocampal expression of BDNF and also increased concentrations of molecules involved in BDNF signaling such as calmodulin kinase II and activated Akt [130]. Similarly, an increase in hippocampal BDNF was observed in adult mice treated with α-linoleic acid injections [131]. Consistent with this observation, mice or rats fed diets enriched in n-3 PUFAs had increased expression of hippocampal BDNF and either increased hippocampal neurogenesis or hippocampal volume [132, 133]. Likewise, in humans, higher consumption of n-3 LC-PUFAs was associated with increased gray matter volume in hippocampus and other corticolimbic structures, indicating maintenance of cells in those brain regions [134]. Thus, these data suggest that n-3 PUFAs support expression of hippocampal BDNF, which in turn fosters optimal hippocampal function.
7.2. Effects on the Hypothalamic-Pituitary-Adrenal Axis
Dysregulation of the hypothalamic-pituitary-adrenal axis is another major clinical finding in depression [135]. These findings include elevated basal levels of serum cortisol, increased corticotrophin-releasing factor in cerebral spinal fluid [136–138], and disruption of negative feedback mechanisms [139].
In postpartum rats with decreased brain DHA levels, stress-induced corticosterone secretion was higher than in postpartum rats with normal brain DHA levels [49]. In addition, both postpartum and virgin female rats with decreased brain DHA exhibited greater relative increases in corticosterone secretion over baseline when subjected to an intense stressor [49], although stressed corticosterone levels were not different between virgin females with decreased brain DHA and virgin females with normal brain DHA. This suggests that a loss of DHA from the adult brain contributes to dysregulation of the hypothalamic-pituitary-adrenal axis and that this effect may be more pronounced in the postpartum state.
Consistent with these findings, other animal and clinical studies support a role for dietary and tissue n-3 PUFA status in the modulation of the hypothalamic-pituitary-adrenal axis. In rats, an n-3 PUFA-enriched diet resulted in lower levels of anxiety- and stress-like behavioral effects in the elevated plus maze and the open field test after treatment with interleukin-1, an inflammatory cytokine that increases corticosterone levels [140]. Similarly, in human studies, fish oil supplements decreased stress responses such as increased plasma epinephrine, norepinephrine, and cortisol, in normal subjects [141, 142]. Furthermore, in a study of perpetrators of domestic violence, DHA levels were inversely related to concentrations of corticotropin-releasing factor in cerebrospinal fluid [143]. Thus, dietary and tissue n-3 PUFA levels appear to modulate the function of the hypothalamic-pituitary-adrenal axis in both the non-puerperal and postpartum states, and the effects of lower n-3 PUFAs are similar to the alterations observed in depressed patients.
7.3. Effects on the CNS Serotonin Systems
Decreased serotonergic function plays a central role in the theories of the pathogenesis of depression. This is supported by observations such as decreased concentrations of serotonin in the brainstem and increased densities of serotonin receptors, such as 5-HT1A and 5-HT2A, in the prefrontal cortex of postmortem depressives and suicide victims [144–150]. These receptors downregulate after treatment with antidepressant drugs which increase synaptic availability of serotonin [151].
Postpartum rats with decreased brain DHA levels had increased expression of 5-HT1A receptors in the hippocampus [49]. No alterations in hippocampal 5-HT1A binding were observed in virgin females with decreased brain DHA indicating that this represents an interaction of decreased brain DHA content with the postpartum state. However, this observation differs from findings in depressed humans in whom densities of hippocampal 5-HT1A receptors were either not altered or were decreased [152–155]. Furthermore, the densities of 5-HT1A and 5-HT2A receptors were not altered in the frontal cortex of either postpartum or virgin female rats with decreased brain DHA [49]. Thus, these findings are inconsistent with findings in humans with non-puerperal depression. Nevertheless, the increase in hippocampal 5-HT1A receptors may represent a unique effect of a loss of brain DHA in postpartum dams that may contribute specifically to the yet-to-be determined etiology of postpartum depression.
Other studies in animals and humans indicate that various aspects of the serotonin system are affected by n-3 PUFA status. In animal studies, adult female rats with a diet-induced decrease in brain DHA content of about 25% initiated after adulthood had decreased concentrations of serotonin in the frontal cortex [49]. Similarly, rats fed an n-3 PUFA-deficient diet from birth, which produced brain DHA levels 61% lower than controls as a result of inadequate accumulation during postnatal development, exhibited decreased midbrain expression of tryptophan hydroxylase, the enzyme that synthesizes serotonin, and increased serotonin turnover in the prefrontal cortex [156]. Consistent with these findings, piglets fed formula lacking both α-linolenic and linoleic acids exhibited lower cortical serotonin concentrations than those fed a formula containing the essential fatty acids [157], further suggesting a role for brain LC-PUFA composition in modulating serotonin levels though the specific role of n-3 PUFAs was not addressed. In another model, rats raised for two generations on an n-3 PUFA-deficient diet, which resulted in a 75% decrease in brain DHA content, had increased density of 5-HT2A receptors in the frontal cortex [158, 159]. Conversely, an n-3 PUFA-supplemented diet reversed decreases in brain serotonin levels in mice subjected to unpredictable chronic mild stress [160]. In humans, low plasma DHA levels in normal subjects and alcoholics were correlated with lower concentrations of the serotonin metabolite 5-hydroxyindoleacetic acid in cerebrospinal fluid, a marker of altered serotonergic neurotransmission associated with depression and suicide [161, 162]. Similarly, the density of platelet serotonin transporter binding, another marker of depression and suicide, was also correlated with plasma DHA levels [163]. Thus, many of the serotonergic alterations associated with low dietary or tissue n-3 PUFAs are consistent with those observed in depression.
7.4. Effects on the CNS Dopamine Systems
Although the monoamine theory of depression focuses on serotonin and norepinephrine [164], the CNS dopamine systems also appear to play a role in the disease. Decreased dopaminergic function, particularly of the mesolimbic system, appears to underlie anhedonic behavior in several animal models [165–168]. Notably, concentrations of homovanillic acid, a dopamine metabolite, in cerebrospinal fluid were decreased in depressed patients and in suicide victims, and were inversely related to depression scores [169–172]. Depression is also common in Parkinson's disease, a neurodegenerative disease involving the loss of nigrostriatal dopamine neurons [173, 174]. Accordingly, decreased dopaminergic function has been hypothesized to contribute to the anhedonia and motivational deficits associated with depression [175].
Postpartum rats with decreased brain DHA levels exhibited decreased density of D2-like dopamine receptors in the ventral striatum (nucleus accumbens and olfactory tubercle) [129]. A trend towards a decrease in D2-like receptor binding was also observed in virgin females with decreased brain DHA, suggesting that the decrease in D2-like receptor binding in this brain region resulted from the change in brain DHA status, but may be augmented in the postpartum female. While this observation is consistent with the proposed hypoactivity of the mesolimbic dopamine system in depression, a postmortem study of drug-naïve patients with major depressive disorder found no differences in the density of D2 receptors in either the ventral striatum or the caudate nucleus [176]. Nevertheless, decreased densities of D2-like receptors or D2 receptor mRNA in the nucleus accumbens have been reported in several putative rat models of depression including chronic mild stress-induced anhedonia, the socially isolated Flinders sensitive line rat, and the learned helplessness model [177–179]. Decreased density of D2-like receptors was also observed in the nucleus accumbens core of the Wistar-Kyoto rat, another depression model, though D2-like receptor binding was increased in the nucleus accumbens shell [180].
Variation in diet and tissue n-3 PUFA content in other animals models also results in alterations in the CNS dopamine systems, but these effects vary considerably depending on the magnitude of the change and the point in development when the manipulation was made. For example, in contrast to the effects of a loss of brain DHA in adult animals, either an increase or no change in the density of D2 receptors in the nucleus accumbens was observed in rats with inadequate accumulation of brain DHA during development, depending on the magnitude of the change in DHA [181–183]. Thus, the effects of modulation of brain DHA on the CNS dopamine systems appear to be more dependent on the specific manipulation than the other systems discussed here.
7.5. Effects on Neuroinflammation
Neuroinflammation is becoming increasingly recognized as another likely contributor to the underlying pathology of depression. Of note, higher circulating levels of several NFκB-regulated inflammatory mediators including interleukin-1β, interleukin-6, tumor necrosis factor-α, and interferon-γ have been noted in depressed patients [184–186]. Depressed patients also exhibited augmented NFκB and interleukin-6 responses to psychological stressors [187]. Postmortem studies of brain from patients with major depression, or who completed suicide, also indicated increased levels of transmembrane tumor necrosis factor-α in some cortical regions, as well as increased expression of genes involved in inflammatory responses [188–191]. Furthermore, studies in postpartum women indicated increased levels of inflammatory mediators in those with depressive symptoms or who had previously suffered from major depression [192–194].
N-3 PUFAs have a variety of anti-inflammatory activities [195]. DHA is the precursor of neuroprotectin D1, a mediator formed in brain that inhibits the production of tumor necrosis factor-α and interferon-γ by activated T cells [25, 196]. DHA and EPA are also precursors of a variety of resolvins which control the magnitude and duration of the inflammatory response [197]. In addition, DHA and EPA inhibit the NFκB-mediated inflammation cascade through actions at the toll-like 4 receptor and PPARs [198, 199]. Consistent with these activities, treatment with either DHA or EPA reduced expression of a number of inflammatory mediators including tumor necrosis factor-α, interleukin-6, nitric oxide synthase, and cyclooxygenase 2, and induced expression of heme oxygenase-1 in cultured BV-2 microglia [200], indicating potential anti-inflammatory mechanisms through which n-3 PUFAs could exert antidepressant effects. However, the interaction of low tissue and/or dietary n-3 PUFAs with the postpartum state has not been investigated.
7.6. Effects on Depression-Related Behavior
Although there is debate regarding the extent to which subhuman species can experience depression [201], several rodent models have been proven highly reliable as drug screens for the prediction of antidepressant efficacy. Among these tests, the forced swim test is perhaps the most validated [202] and is sometimes also used as a putative rodent model of depression. In the test, rats placed in an inescapable cylindrical tank of cool water are evaluated for time spent climbing, swimming, floating immobile, and, in some studies, latency to immobility. Drugs that decrease the time spent floating immobile, or increase the latency to immobility, are likely to have antidepressant effects in humans [202, 203].
In the forced swim test, postpartum rats with decreased brain DHA content exhibited shorter latencies to immobility than postpartum rats with normal brain DHA levels [49]. This effect was not observed in virgin females with decreased brain DHA indicating that it represents an interaction of the decrease in brain DHA content with the postpartum state. Shorter latency to immobility is also consistent with an interpretation of a more “depressed” phenotype in the postpartum rats with decreased brain DHA to the extent possible within the limitations of the test.
Concordant with these findings, manipulation of n-3 PUFAs in other rodent models also point to a role for lower dietary and tissue n-3 PUFA status contributing to “depressed” behavior in antidepressant drug screens. For example, adult rats fed an n-3 PUFA-deficient diet beginning at weaning, which resulted in brain DHA levels 36% lower than controls, exhibited more time immobile in the forced swim test [204]. Conversely, rats or mice that were fed n-3 PUFA-supplemented diets exhibited less immobility [132, 205–207]. Likewise, adult male mice that were treated with injections of α-linoleic acid also exhibited less immobility [131]. Similar effects have also been reported in the tail suspension test, another rodent antidepressant drug screen [131, 132].
8. Conclusion
Although a confluence of genetic and environmental factors may be required to cause depression, an individual factor (e.g., reduced brain DHA content), may create a state of vulnerability that contributes to the development of the disease when the other appropriate factors are present. The preponderance of the literature indicates that changes in brain LC-PUFA status, particularly decreased DHA, are associated with both non-puerperal and postpartum depression. Furthermore, experimentally induced reductions in brain DHA content result in neurobiological alterations in rats similar to those observed in depressed humans. These effects of decreased brain DHA interact with the postpartum state such that the number of neurobiological alterations in postpartum rats with decreased brain DHA is greater than in virgin females with decreased brain DHA, and the magnitude of some of the alterations appears to be greater in the postpartum state. With the low n-3 PUFA content of the North American diet, there is considerable potential for individuals to have suboptimal availability of these fatty acids. Genetic polymorphisms that confer suboptimal metabolism or utilization of LC-PUFA, or the physiological demands of pregnancy and lactation, may place certain individuals at even greater risk. Accordingly, decreased brain DHA, and perhaps other n-3 PUFAs, represents an important potential risk factor for depression generally, and postpartum depression in particular.
Despite this growing body of evidence, the role(s) of LC-PUFA in the pathogenesis of postpartum depression and other depressive illnesses remains to be fully elucidated. In addition to determining specifically how changes in brain LC-PUFA composition contribute to the etiology of depression (e.g., altered membrane properties, actions of LC-PUFA-derived mediators, etc.), it must be determined whether n-3 PUFA status contributes to the etiology of depression in all, or only a subset of, patients (e.g., postpartum females). Importantly, the reversibility of the neurobiological consequences of a pregnancy-associated loss of brain DHA must be determined. Should these changes prove to be reversible, this will support the use of n-3 PUFA supplements in the treatment of postpartum depression. On the other hand, should the neurobiological consequences of a pregnancy-associated loss of brain DHA be irreversible, this will indicate the imperative of preventing the loss of DHA during pregnancy and lactation through appropriate nutrition and/or supplementation. Finally, should such findings support the viability of preventing postpartum depression and/or treating existing depressive illness with n-3 LC-PUFAs, the appropriate formulation, optimal dose, and treatment duration also remain to be determined in well-designed, adequately powered clinical trials.
Acknowledgments
The author thanks Heather Spalding for assistance in the preparation of this paper. The supported by NIH MH071599, P30 HD02528, and P20 RR016475 from the INBRE Program of the National Center for Research Resources.
References
- 1.Brockington I. Postpartum psychiatric disorders. Lancet. 2004;363(9405):303–310. doi: 10.1016/S0140-6736(03)15390-1. [DOI] [PubMed] [Google Scholar]
- 2.Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics and Gynecology. 2005;106(5, part 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 3.Leung BMY, Kaplan BJ. Perinatal depression: prevalence, risks, and the nutrition link—a review of the literature. Journal of the American Dietetic Association. 2009;109(9):1566–1575. doi: 10.1016/j.jada.2009.06.368. [DOI] [PubMed] [Google Scholar]
- 4.Gale S, Harlow BL. Postpartum mood disorders: a review of clinical and epidemiological factors. Journal of Psychosomatic Obstetrics and Gynecology. 2003;24(4):257–266. doi: 10.3109/01674820309074690. [DOI] [PubMed] [Google Scholar]
- 5.Pfuhlmann B, Stoeber G, Beckmann H. Postpartum psychoses: prognosis, risk factors, and treatment. Current Psychiatry Reports. 2002;4(3):185–190. doi: 10.1007/s11920-002-0025-6. [DOI] [PubMed] [Google Scholar]
- 6.Stowe ZN, Nemeroff CB. Women at risk for postpartum-onset major depression. American Journal of Obstetrics and Gynecology. 1995;173(2):639–645. doi: 10.1016/0002-9378(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 7.McCoy SJ, Beal M, Miller Shipman SB, Payton ME, Watson GH. Risk factors for postpartum depression: a retrospective investigation at 4-weeks postnatal and a review of the literature. Journal of the American Osteopathic Association. 2006;106(4):193–198. [PubMed] [Google Scholar]
- 8.Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. General Hospital Psychiatry. 2004;26(4):289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Berle JØ, Aarre TF, Mykletun A, Dahl AA, Holsten F. Screening for postnatal depression: validation of the Norwegian version of the Edinburgh Postnatal Depression Scale, and assessment of risk factors for postnatal depression. Journal of Affective Disorders. 2003;76(1–3):151–156. doi: 10.1016/s0165-0327(02)00082-4. [DOI] [PubMed] [Google Scholar]
- 10.Gürel SA, Gürel H. The evaluation of determinants of early postpartum low mood: the importance of parity and inter-pregnancy interval. European Journal of Obstetrics Gynecology and Reproductive Biology. 2000;91(1):21–24. doi: 10.1016/s0301-2115(99)00224-9. [DOI] [PubMed] [Google Scholar]
- 11.Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Comprehensive Psychiatry. 2003;44(3):234–246. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- 12.McCoy SJ, Beal JM, Watson GH. Endocrine factors and postpartum depression: a selected review. Journal of Reproductive Medicine for the Obstetrician and Gynecologist. 2003;48(6):402–408. [PubMed] [Google Scholar]
- 13.Zonana J, Gorman JM. The neurobiology of postpartum depression. CNS Spectrums. 2005;10(10):792–805. doi: 10.1017/s1092852900010312. [DOI] [PubMed] [Google Scholar]
- 14.Logsdon MC, Wisner KL, Pinto-Foltz MD. The impact of postpartum depression on mothering. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2006;35(5):652–658. doi: 10.1111/j.1552-6909.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- 15.Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Archives of Women’s Mental Health. 2003;6(4):263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- 16.Miller LJ. Postpartum depression. Journal of the American Medical Association. 2002;287(6):762–765. doi: 10.1001/jama.287.6.762. [DOI] [PubMed] [Google Scholar]
- 17.Brenna JT. Efficiency of conversion of alpha-linolenic acid to long chain n-3 fatty acids in man. Current Opinion in Clinical Nutrition and Metabolic Care. 2002;5(2):127–132. doi: 10.1097/00075197-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Gerster H. Can adults adequately convert α-linolenic acid (18:3n-3) to eicosapentaenoic acid (20:5n-3) and docosahexaenoic acid (22:6n-3)? International Journal for Vitamin and Nutrition Research. 1998;68(3):159–173. [PubMed] [Google Scholar]
- 19.Ross BM. ω-3 fatty acid deficiency in major depressive disorder is caused by the interaction between diet and a genetically determined abnormality in phospholipid metabolism. Medical Hypotheses. 2007;68(3):515–524. doi: 10.1016/j.mehy.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 20.Simopoulos AP. Genetic variants in the metabolism of omega-6 and omega-3 fatty acids: their role in the determination of nutritional requirements and chronic disease risk. Experimental Biology and Medicine. 2010;235(7):785–795. doi: 10.1258/ebm.2010.009298. [DOI] [PubMed] [Google Scholar]
- 21.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomedicine and Pharmacotherapy. 2002;56(8):365–379. doi: 10.1016/s0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 22.Salem N, Jr., Litman B, Kim H-Y, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36(9):945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- 23.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassal SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reproduction Nutrition Development. 2005;45(5):559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 24.Horrocks LA, Farooqui AA. Docosahexaenoic acid in the diet: its importance in maintenance and restoration of neural membrane function. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;70(4):361–372. doi: 10.1016/j.plefa.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Bazan NG. Neuroprotectin D1 (NPD1): a DHA-derived mediator that protects brain and retina against cell injury-induced oxidative stress. Brain Pathology. 2005;15(2):159–166. doi: 10.1111/j.1750-3639.2005.tb00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bannenberg G, Arita M, Serhan CN. Endogenous receptor agonists: resolving inflammation. TheScientificWorldJournal. 2007;7:1440–1462. doi: 10.1100/tsw.2007.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Urquiza AM, Liu S, Sjoberg M, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290(5499):2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 28.Clarke SD, Thuillier P, Baillie RA, Sha X. Peroxisome proliferator-activated receptors: a family of lipid-activated transcription factors. American Journal of Clinical Nutrition. 1999;70(4):566–571. doi: 10.1093/ajcn/70.4.566. [DOI] [PubMed] [Google Scholar]
- 29.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Extrauterine fatty acid accretion in infant brain: implications for fatty acid requirements. Early Human Development. 1980;4(2):131–138. doi: 10.1016/0378-3782(80)90016-x. [DOI] [PubMed] [Google Scholar]
- 30.Clandinin MT, Chappell JE, Leong S, Heim T, Swyer PR, Chance GW. Intrauterine fatty acid accretion rates in human brain: implications for fatty acid requirements. Early Human Development. 1980;4(2):121–129. doi: 10.1016/0378-3782(80)90015-8. [DOI] [PubMed] [Google Scholar]
- 31.Martinez M. Developmental profiles of polyunsaturated fatty acids in the brain of normal infants and patients with peroxisomal diseases: severe deficiency of docosahexaenoic acid in Zellweger’s and pseudo-Zellweger’s syndromes. World Review of Nutrition and Dietetics. 1991;66:87–102. doi: 10.1159/000419282. [DOI] [PubMed] [Google Scholar]
- 32.Innis SM. Human milk and formula fatty acids. Journal of Pediatrics. 1992;120(4, part 2):56–61. doi: 10.1016/s0022-3476(05)81237-5. [DOI] [PubMed] [Google Scholar]
- 33.Innis SM. Polyunsaturated fatty acids in human milk: an essential role in infant development. Advances in Experimental Medicine and Biology. 2004;554:27–43. doi: 10.1007/978-1-4757-4242-8_5. [DOI] [PubMed] [Google Scholar]
- 34.Galli C, Trzeciak HI, Paoletti R. Effects of dietary fatty acids on the fatty acid composition of brain ethanolamine phosphoglyceride: reciprocal replacement of n-6 and n-3 polyunsaturated fatty acids. Biochimica et Biophysica Acta. 1971;248(3):449–454. [Google Scholar]
- 35.Gordon N. Nutrition and cognitive function. Brain and Development. 1997;19(3):165–170. doi: 10.1016/s0387-7604(96)00560-8. [DOI] [PubMed] [Google Scholar]
- 36.McNamara RK, Carlson SE. Role of omega-3 fatty acids in brain development and function: potential implications for the pathogenesis and prevention of psychopathology. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75(4-5):329–349. doi: 10.1016/j.plefa.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Developmental Medicine and Child Neurology. 2000;42(3):174–181. doi: 10.1017/s0012162200000311. [DOI] [PubMed] [Google Scholar]
- 38.Willatts P, Forsyth JS, DiModugno MK, Varma S, Colvin M. Influence of long-chain polyunsaturated fatty acids on infant cognitive function. Lipids. 1998;33(10):973–980. doi: 10.1007/s11745-998-0294-7. [DOI] [PubMed] [Google Scholar]
- 39.Makrides M, Smithers LG, Gibson RA. Role of long-chain polyunsaturated fatty acids in neurodevelopment and growth. Nestle Nutrition Workshop Series. 2010;65:123–136. doi: 10.1159/000281154. [DOI] [PubMed] [Google Scholar]
- 40.Otto SJ, Van Houwelingen AC, Antal M, et al. Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. European Journal of Clinical Nutrition. 1997;51(4):232–242. doi: 10.1038/sj.ejcn.1600390. [DOI] [PubMed] [Google Scholar]
- 41.Holman RT, Johnson SB, Ogburn PL. Deficiency of essential fatty acids and membrane fluidity during pregnancy and lactation. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(11):4835–4839. doi: 10.1073/pnas.88.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al MDM, Van Houwelingen AC, Kester ADM, Hasaart THM, De Jong AEP, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. British Journal of Nutrition. 1995;74(1):55–68. doi: 10.1079/bjn19950106. [DOI] [PubMed] [Google Scholar]
- 43.Al MDM, Van Houwelingen AC, Hornstra G. Relation between birth order and the maternal and neonatal docosahexaenoic acid status. European Journal of Clinical Nutrition. 1997;51(8):548–553. doi: 10.1038/sj.ejcn.1600444. [DOI] [PubMed] [Google Scholar]
- 44.Van den Ham EC, Van Houwelingen AC, Hornstra G. Evaluation of the relation between n-3 and n-6 fatty acid status and parity in nonpregnant women from the Netherlands. American Journal of Clinical Nutrition. 2001;73(3):622–627. doi: 10.1093/ajcn/73.3.622. [DOI] [PubMed] [Google Scholar]
- 45.Hornstra G, Al MDM, Van Houwelingen AC, Foreman-van Drongelen MMHP. Essential fatty acids in pregnancy and early human development. European Journal of Obstetrics Gynecology and Reproductive Biology. 1995;61(1):57–62. doi: 10.1016/0028-2243(95)02153-j. [DOI] [PubMed] [Google Scholar]
- 46.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity affect liver and erythrocyte phospholipid fatty acid composition in female rats. Journal of Nutrition. 2007;137(11):2425–2430. doi: 10.1093/jn/137.11.2425. [DOI] [PubMed] [Google Scholar]
- 47.Levant B, Ozias MK, Carlson SE. Diet (n-3) polyunsaturated fatty acid content and parity interact to alter maternal rat brain phospholipid fatty acid composition. Journal of Nutrition. 2006;136(8):2236–2242. doi: 10.1093/jn/136.8.2236. [DOI] [PubMed] [Google Scholar]
- 48.Levant B, Radel JD, Carlson SE. Reduced brain DHA content after a single reproductive cycle in female rats fed a diet deficient in N-3 polyunsaturated fatty acids. Biological Psychiatry. 2006;60(9):987–990. doi: 10.1016/j.biopsych.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Levant B, Ozias MK, Davis PF, et al. Decreased brain docosahexaenoic acid content produces neurobiological effects associated with depression: interactions with reproductive status in female rats. Psychoneuroendocrinology. 2008;33(9):1279–1292. doi: 10.1016/j.psyneuen.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hibbeln JR. Seafood consumption, the DHA content of mothers’ milk and prevalence rates of postpartum depression: a cross-national, ecological analysis. Journal of Affective Disorders. 2002;69(1–3):15–29. doi: 10.1016/s0165-0327(01)00374-3. [DOI] [PubMed] [Google Scholar]
- 51.Golding J, Steer C, Emmett P, Davis JM, Hibbeln JR. High levels of depressive symptoms in pregnancy with low omega-3 fatty acid intake from fish. Epidemiology. 2009;20(4):598–603. doi: 10.1097/EDE.0b013e31819d6a57. [DOI] [PubMed] [Google Scholar]
- 52.Sontrop J, Avison WR, Evers SE, Speechley KN, Campbell MK. Depressive symptoms during pregnancy in relation to fish consumption and intake of n-3 polyunsaturated fatty acids. Paediatric and Perinatal Epidemiology. 2008;22(4):389–399. doi: 10.1111/j.1365-3016.2008.00941.x. [DOI] [PubMed] [Google Scholar]
- 53.Otto SJ, De Groot RHM, Hornstra G. Increased risk of postpartum depressive symptoms is associated with slower normalization after pregnancy of the functional docosahexaenoic acid status. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2003;69(4):237–243. doi: 10.1016/s0952-3278(03)00090-5. [DOI] [PubMed] [Google Scholar]
- 54.Rees A-M, Austin M-P, Owen C, Parker G. Omega-3 deficiency associated with perinatal depression: case control study. Psychiatry Research. 2009;166(2-3):254–259. doi: 10.1016/j.psychres.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 55.De Vriese SR, Christophe AB, Maes M. Lowered serum n-3 polyunsaturated fatty acid (PUFA) levels predict the occurrence of postpartum depression: further evidence that lowered n-PUFAs are related to major depression. Life Sciences. 2003;73(25):3181–3187. doi: 10.1016/j.lfs.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 56.Miyake Y, Sasaki S, Yokoyama T, et al. Risk of postpartum depression in relation to dietary fish and fat intake in Japan: the Osaka Maternal and Child Health Study. Psychological Medicine. 2006;36(12):1727–1735. doi: 10.1017/S0033291706008701. [DOI] [PubMed] [Google Scholar]
- 57.Browne JC, Scott KM, Silvers KM. Fish consumption in pregnancy and omega-3 status after birth are not associated with postnatal depression. Journal of Affective Disorders. 2006;90(2-3):131–139. doi: 10.1016/j.jad.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 58.Strøm M, Mortensen EL, Halldorsson TI, Thorsdottir I, Olsen SF. Fish and long-chain n-3 polyunsaturated fatty acid intakes during pregnancy and risk of postpartum depression: a prospective study based on a large national birth cohort. American Journal of Clinical Nutrition. 2009;90(1):149–155. doi: 10.3945/ajcn.2009.27552. [DOI] [PubMed] [Google Scholar]
- 59.Xie L, Innis SM. Association of fatty acid desaturase gene polymorphisms with blood lipid essential fatty acids and perinatal depression among canadian women: a pilot study. Journal of Nutrigenetics and Nutrigenomics. 2009;2(4-5):243–250. doi: 10.1159/000255636. [DOI] [PubMed] [Google Scholar]
- 60.Moltó-Puigmartí C, Plat J, Mensink RP, et al. FADS1 FADS2 gene variants modify the association between fish intake and the docosahexaenoic acid proportions in human milk. American Journal of Clinical Nutrition. 2010;91(5):1368–1376. doi: 10.3945/ajcn.2009.28789. [DOI] [PubMed] [Google Scholar]
- 61.Flores DL, Hendrick VC. Etiology and treatment of postpartum depression. Current Psychiatry Reports. 2002;4(6):461–466. doi: 10.1007/s11920-002-0074-x. [DOI] [PubMed] [Google Scholar]
- 62.Hibbeln JR. Fish consumption and major depression. Lancet. 1998;351(9110):p. 1213. doi: 10.1016/S0140-6736(05)79168-6. [DOI] [PubMed] [Google Scholar]
- 63.Tanskanen A, Hibbeln JR, Tuomilehto J, et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatric Services. 2001;52(4):529–531. doi: 10.1176/appi.ps.52.4.529. [DOI] [PubMed] [Google Scholar]
- 64.Appleton KM, Woodside JV, Yarnell JWG, et al. Depressed mood and dietary fish intake: direct relationship or indirect relationship as a result of diet and lifestyle? Journal of Affective Disorders. 2007;104(1–3):217–223. doi: 10.1016/j.jad.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Van De Rest O, De Goede J, Sytsma F, et al. Association of n-3 long-chain PUFA and fish intake with depressive symptoms and low dispositional optimism in older subjects with a history of myocardial infarction. British Journal of Nutrition. 2010;103(9):1381–1387. doi: 10.1017/S0007114509993308. [DOI] [PubMed] [Google Scholar]
- 66.Suominen-Taipale AL, Partonen T, Turunen AW, Männistö S, Jula A, Verkasalo PK. Fish consumption and omega-3 polyunsaturated fatty acids in relation to depressive episodes: a cross-sectional analysis. PloS One. 2010;5(5, article e10530) doi: 10.1371/journal.pone.0010530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McNamara RK, Hahn C-G, Jandacek R, et al. Selective deficits in the omega-3 fatty acid docosahexaenoic acid in the postmortem orbitofrontal cortex of patients with major depressive disorder. Biological Psychiatry. 2007;62(1):17–24. doi: 10.1016/j.biopsych.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 68.Conklin SM, Runyan CA, Leonard S, Reddy RD, Muldoon MF, Yao JK. Age-related changes of n-3 and n-6 polyunsaturated fatty acids in the anterior cingulate cortex of individuals with major depressive disorder. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2010;82(2-3):111–119. doi: 10.1016/j.plefa.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edwards R, Peet M, Shay J, Horrobin D. Omega-3 polyunsaturated fatty acid levels in the diet and in red blood cell membranes of depressed patients. Journal of Affective Disorders. 1998;48(2-3):149–155. doi: 10.1016/s0165-0327(97)00166-3. [DOI] [PubMed] [Google Scholar]
- 70.Maes M, Christophe A, Delanghe J, Altamura C, Neels H, Meltzer HY. Lowered ω3 polyunsaturated fatty acids in serum phospholipids and cholesteryl esters of depressed patients. Psychiatry Research. 1999;85(3):275–291. doi: 10.1016/s0165-1781(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 71.Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biological Psychiatry. 1998;43(5):315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- 72.Schiepers OJG, de Groot RHM, Jolles J, van Boxtel MPJ. Plasma phospholipid fatty acid status and depressive symptoms: association only present in the clinical range. Journal of Affective Disorders. 2009;118(1–3):209–214. doi: 10.1016/j.jad.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 73.Mamalakis G, Kalogeropoulos N, Andrikopoulos N, et al. Depression and long chain n-3 fatty acids in adipose tissue in adults from Crete. European Journal of Clinical Nutrition. 2006;60(7):882–888. doi: 10.1038/sj.ejcn.1602394. [DOI] [PubMed] [Google Scholar]
- 74.Sarri KO, Linardakis M, Tzanakis N, Kafatos AG. Adipose DHA inversely associated with depression as measured by the Beck Depression Inventory. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;78(2):117–122. doi: 10.1016/j.plefa.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Astorg P, Bertrais S, Laporte F, et al. Plasma n-6 and n-3 polyunsaturated fatty acids as biomarkers of their dietary intakes: a cross-sectional study within a cohort of middle-aged French men and women. European Journal of Clinical Nutrition. 2008;62(10):1155–1161. doi: 10.1038/sj.ejcn.1602836. [DOI] [PubMed] [Google Scholar]
- 76.Fitten LJ, Ortiz F, Fairbanks L, et al. Depression, diabetes and metabolic-nutritional factors in elderly hispanics. Journal of Nutrition, Health and Aging. 2008;12(9):634–640. doi: 10.1007/BF03008274. [DOI] [PubMed] [Google Scholar]
- 77.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. Journal of Affective Disorders. 2010;126(1-2):303–311. doi: 10.1016/j.jad.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Assies J, Pouwer F, Lok A, et al. Plasma and erythrocyte fatty acid patterns in patients with recurrent depression: a matched case-control study. PloS One. 2010;5(5, article e10635) doi: 10.1371/journal.pone.0010635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Riemer S, Maes M, Christophe A, Rief W. Lowered ω-3 PUFAs are related to major depression, but not to somatization syndrome. Journal of Affective Disorders. 2009;123(1–3):173–180. doi: 10.1016/j.jad.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 80.Lin P-Y, Huang S-Y, Su K-P. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biological Psychiatry. 2010;68(2):140–147. doi: 10.1016/j.biopsych.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 81.Timonen M, Horrobin D, Jokelainen J, Laitinen J, Herva A, Räsänen P. Fish consumption and depression: the Northern Finland 1966 birth cohort study. Journal of Affective Disorders. 2004;82(3):447–452. doi: 10.1016/j.jad.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Colangelo LA, He K, Whooley MA, Daviglus ML, Liu K. Higher dietary intake of long-chain ω-3 polyunsaturated fatty acids is inversely associated with depressive symptoms in women. Nutrition. 2009;25(10):1011–1019. doi: 10.1016/j.nut.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51(1):8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 84.Marcus SM, Young EA, Kerber KB, et al. Gender differences in depression: findings from the STAR*D study. Journal of Affective Disorders. 2005;87(2-3):141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 85.Grigoriadis S, Robinson GE. Gender issues in depression. Annals of Clinical Psychiatry. 2007;19(4):247–255. doi: 10.1080/10401230701653294. [DOI] [PubMed] [Google Scholar]
- 86.Lalovic A, Klempan T, Sequeira A, Luheshi G, Turecki G. Altered expression of lipid metabolism and immune response genes in the frontal cortex of suicide completers. Journal of Affective Disorders. 2010;120(1–3):24–31. doi: 10.1016/j.jad.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 87.Lalovic A, Levy É, Canetti L, Sequeira A, Montoudis A, Turecki G. Fatty acid composition in postmortem brains of people who completed suicide. Journal of Psychiatry and Neuroscience. 2007;32(5):363–370. [PMC free article] [PubMed] [Google Scholar]
- 88.McNamara RK, Jandacek R, Rider T, et al. Fatty acid composition of the postmortem prefrontal cortex of adolescent male and female suicide victims. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2009;80(1):19–26. doi: 10.1016/j.plefa.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 89.Freeman MP, Hibbeln JR, Wisner KL, Brumbach BH, Watchman M, Gelenberg AJ. Randomized dose-ranging pilot trial of omega-3 fatty acids for postpartum depression. Acta Psychiatrica Scandinavica. 2006;113(1):31–35. doi: 10.1111/j.1600-0447.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 90.Freeman MP, Davis M, Sinha P, Wisner KL, Hibbeln JR, Gelenberg AJ. Omega-3 fatty acids and supportive psychotherapy for perinatal depression: a randomized placebo-controlled study. Journal of Affective Disorders. 2008;110(1-2):142–148. doi: 10.1016/j.jad.2007.12.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marangell LB, Martinez JM, Zboyan HA, Chong H, Puryear LJ. Omega-3 fatty acids for the prevention of postpartum depression: negative data from a preliminary, open-label pilot study. Depression and Anxiety. 2004;19(1):20–23. doi: 10.1002/da.10148. [DOI] [PubMed] [Google Scholar]
- 92.Llorente AM, Jensen CL, Voigt RG, Fraley JK, Berretta MC, Heird WC. Effect of maternal docosahexaenoic acid supplementation on postpartum depression and information processing. American Journal of Obstetrics and Gynecology. 2003;188(5):1348–1353. doi: 10.1067/mob.2003.275. [DOI] [PubMed] [Google Scholar]
- 93.Doornbos B, van Goor SA, Dijck-Brouwer DAJ, Schaafsma A, Korf J, Muskiet FAJ. Supplementation of a low dose of DHA or DHA + AA does not prevent peripartum depressive symptoms in a small population based sample. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33(1):49–52. doi: 10.1016/j.pnpbp.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 94.Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. American Journal of Psychiatry. 2002;159(3):477–479. doi: 10.1176/appi.ajp.159.3.477. [DOI] [PubMed] [Google Scholar]
- 95.Peet M, Horrobin DF. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Archives of General Psychiatry. 2002;59(10):913–919. doi: 10.1001/archpsyc.59.10.913. [DOI] [PubMed] [Google Scholar]
- 96.Su K-P, Huang S-Y, Chiu C-C, Shen WW. Omega-3 fatty acids in major depressive disorder: a preliminary double-blind, placebo-controlled trial. European Neuropsychopharmacology. 2003;13(4):267–271. doi: 10.1016/s0924-977x(03)00032-4. [DOI] [PubMed] [Google Scholar]
- 97.Nemets H, Nemets B, Apter A, Bracha Z, Belmaker RH. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. American Journal of Psychiatry. 2006;163(6):1098–1100. doi: 10.1176/ajp.2006.163.6.1098. [DOI] [PubMed] [Google Scholar]
- 98.Su K-P, Huang S-Y, Chiu T-H, et al. Omega-3 fatty acids for major depressive disorder during pregnancy: results from a randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2008;69(4):644–651. doi: 10.4088/jcp.v69n0418. [DOI] [PubMed] [Google Scholar]
- 99.Lucas M, Asselin G, Mérette C, Poulin M-J, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. American Journal of Clinical Nutrition. 2009;89(2):641–651. doi: 10.3945/ajcn.2008.26749. [DOI] [PubMed] [Google Scholar]
- 100.Rondanelli M, Giacosa A, Opizzi A, et al. Effect of omega-3 fatty acids supplementation on depressive symptoms and on health-related quality of life in the treatment of elderly women with depression: a double-blind, placebo-controlled, randomized clinical trial. Journal of the American College of Nutrition. 2010;29(1):55–64. doi: 10.1080/07315724.2010.10719817. [DOI] [PubMed] [Google Scholar]
- 101.da Silva TM, Munhoz RP, Alvarez C, et al. Depression in Parkinson’s disease: a double-blind, randomized, placebo-controlled pilot study of omega-3 fatty-acid supplementation. Journal of Affective Disorders. 2008;111(2-3):351–359. doi: 10.1016/j.jad.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 102.Jazayeri S, Tehrani-Doost M, Keshavarz SA, et al. Comparison of therapeutic effects of omega-3 fatty acid eicosapentaenoic acid and fluoxetine, separately and in combination, in major depressive disorder. Australian and New Zealand Journal of Psychiatry. 2008;42(3):192–198. doi: 10.1080/00048670701827275. [DOI] [PubMed] [Google Scholar]
- 103.Mischoulon D, Best-Popescu C, Laposata M, et al. A double-blind dose-finding pilot study of docosahexaenoic acid (DHA) for major depressive disorder. European Neuropsychopharmacology. 2008;18(9):639–645. doi: 10.1016/j.euroneuro.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 104.Marangell LB, Martinez JM, Zboyan HA, Kertz B, Kim HFS, Puryear LJ. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. American Journal of Psychiatry. 2003;160(5):996–998. doi: 10.1176/appi.ajp.160.5.996. [DOI] [PubMed] [Google Scholar]
- 105.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. British Journal of Nutrition. 2008;99(2):421–431. doi: 10.1017/S0007114507801097. [DOI] [PubMed] [Google Scholar]
- 106.Silvers KM, Woolley CC, Hamilton FC, Watts PM, Watson RA. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;72(3):211–218. doi: 10.1016/j.plefa.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 107.Mischoulon D, Papakostas GI, Dording CM, et al. A double-blind, randomized controlled trial of ethyl-eicosapentaenoate for major depressive disorder. Journal of Clinical Psychiatry. 2009;70(12):1636–1644. doi: 10.4088/JCP.08m04603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grenyer BFS, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2007;31(7):1393–1396. doi: 10.1016/j.pnpbp.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Bot M, Pouwer F, Assies J, et al. Eicosapentaenoic acid as an add-on to antidepressant medication for co-morbid major depression in patients with diabetes mellitus: a randomized, double-blind placebo-controlled study. Journal of Affective Disorders. 2010;126(1-2):282–286. doi: 10.1016/j.jad.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 110.Lin P-Y, Su K-P. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. Journal of Clinical Psychiatry. 2007;68(7):1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- 111.Ross BM, Seguin J, Sieswerda LE. Omega-3 fatty acids as treatments for mental illness: which disorder and which fatty acid? Lipids in Health and Disease. 2007;6, article 21 doi: 10.1186/1476-511X-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martins JG. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: evidence from a meta-analysis of randomized controlled trials. Journal of the American College of Nutrition. 2009;28(5):525–542. doi: 10.1080/07315724.2009.10719785. [DOI] [PubMed] [Google Scholar]
- 113.Rocha Araujo DM, Vilarim MM, Nardi AE. What is the effectiveness of the use of polyunsaturated fatty acid omega-3 in the treatment of depression? Expert Review of Neurotherapeutics. 2010;10(7):1117–1129. doi: 10.1586/ern.10.77. [DOI] [PubMed] [Google Scholar]
- 114.Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. American Journal of Clinical Nutrition. 2010;91(3):757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- 115.Sapolsky RM. The possibility of neurotoxicity in the hippocampus in major depression: a primer on neuron death. Biological Psychiatry. 2000;48(8):755–765. doi: 10.1016/s0006-3223(00)00971-9. [DOI] [PubMed] [Google Scholar]
- 116.Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Molecular Brain Research. 2005;136(1-2):29–37. doi: 10.1016/j.molbrainres.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 117.Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Archives of General Psychiatry. 2003;60(8):804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- 118.Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59(12):1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 119.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(9):3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen B, Dowlatshahi D, MacQueen GM, Wang J-F, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biological Psychiatry. 2001;50(4):260–265. doi: 10.1016/s0006-3223(01)01083-6. [DOI] [PubMed] [Google Scholar]
- 121.Smith MA, Makino S, Kim S-Y, Kvetnansky R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology. 1995;136(9):3743–3750. doi: 10.1210/endo.136.9.7649080. [DOI] [PubMed] [Google Scholar]
- 122.Grønli J, Bramham C, Murison R, et al. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacology Biochemistry and Behavior. 2006;85(4):842–849. doi: 10.1016/j.pbb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 123.Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. Journal of Neuroscience. 1995;15(11):7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Johnson RA, Rhodes JS, Jeffrey SL, Garland T, Jr., Mitchell GS. Hippocampal brain-derived neurotrophic factor but not neurotrophin-3 increases more in mice selected for increased voluntary wheel running. Neuroscience. 2003;121(1):1–7. doi: 10.1016/s0306-4522(03)00422-6. [DOI] [PubMed] [Google Scholar]
- 125.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacology Biochemistry and Behavior. 1997;56(1):131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 126.Shirayama Y, Chen AC-H, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. Journal of Neuroscience. 2002;22(8):3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Adachi M, Barrot M, Autry AE, Theobald D, Monteggia LM. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biological Psychiatry. 2008;63(7):642–649. doi: 10.1016/j.biopsych.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Green P, Yavin E. Fatty acid composition of late embryonic and early postnatal rat brain. Lipids. 1996;31(8):859–865. doi: 10.1007/BF02522981. [DOI] [PubMed] [Google Scholar]
- 129.Davis PF, Ozias MK, Carlson SE, et al. Dopamine receptor alterations in female rats with diet-induced decreased brain docosahexaenoic acid (DHA): interactions with reproductive status. Nutritional Neuroscience. 2010;13(4):161–169. doi: 10.1179/147683010X12611460764282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu A, Ying Z, Gomez-Pinilla F. Omega-3 fatty acids supplementation restores mechanisms that maintain brain homeostasis in traumatic brain injury. Journal of Neurotrauma. 2007;24(10):1587–1595. doi: 10.1089/neu.2007.0313. [DOI] [PubMed] [Google Scholar]
- 131.Blondeau N, Nguemeni C, Debruyne DN, et al. Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke. Neuropsychopharmacology. 2009;34(12):2548–2559. doi: 10.1038/npp.2009.84. [DOI] [PubMed] [Google Scholar]
- 132.Venna VR, Deplanque D, Allet C, Belarbi K, Hamdane M, Bordet R. PUFA induce antidepressant-like effects in parallel to structural and molecular changes in the hippocampus. Psychoneuroendocrinology. 2009;34(2):199–211. doi: 10.1016/j.psyneuen.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 133.Cysneiros RM, Ferrari D, Arida RM, et al. Qualitative analysis of hippocampal plastic changes in rats with epilepsy supplemented with oral omega-3 fatty acids. Epilepsy and Behavior. 2010;17(1):33–38. doi: 10.1016/j.yebeh.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 134.Conklin SM, Gianaros PJ, Brown SM, et al. Long-chain omega-3 fatty acid intake is associated positively with corticolimbic gray matter volume in healthy adults. Neuroscience Letters. 2007;421(3):209–212. doi: 10.1016/j.neulet.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 135.Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression: hypothalamic-pituitary-adrenal axis. Psychiatric Clinics of North America. 1998;21(2):293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- 136.Carpenter WT, Jr., Bunney WE., Jr. Adrenal cortical activity in depressive illness. American Journal of Psychiatry. 1971;128(1):31–40. doi: 10.1176/ajp.128.1.31. [DOI] [PubMed] [Google Scholar]
- 137.Carroll BJ, Curtis GC, Davies BM, Mendels J, Sugerman AA. Urinary free cortisol excretion in depression. Psychological Medicine. 1976;6(1):43–50. doi: 10.1017/s0033291700007480. [DOI] [PubMed] [Google Scholar]
- 138.Nemeroff CB, Widerlov E, Bissette G, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226(4680):1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 139.Carroll BJ, Martin FI, Davies B. Pituitary-adrenal function in depression. Lancet. 1968;1(7556):1373–1374. doi: 10.1016/s0140-6736(68)92072-2. [DOI] [PubMed] [Google Scholar]
- 140.Song C, Leonard BE, Horrobin DF. Dietary ethyl-eicosapentaenoic acid but not soybean oil reverses central interleukin-1-induced changes in behavior, corticosterone and immune response in rats. Stress. 2004;7(1):43–54. doi: 10.1080/10253890410001667188. [DOI] [PubMed] [Google Scholar]
- 141.Hamazaki T, Itomura M, Sawazaki S, Nagao Y. Anti-stress effects of DHA. BioFactors. 2000;13(1–4):41–45. doi: 10.1002/biof.5520130108. [DOI] [PubMed] [Google Scholar]
- 142.Delarue J, Matzinger O, Binnert C, Schneiter P, Chioléro R, Tappy L. Fish oil prevents the adrenal activation elicited by mental stress in healthy men. Diabetes and Metabolism. 2003;29(3):289–295. doi: 10.1016/s1262-3636(07)70039-3. [DOI] [PubMed] [Google Scholar]
- 143.Hibbeln JR, Bissette G, Umhau JC, George DT. Omega-3 status and cerebrospinal fluid corticotrophin releasing hormone in perpetrators of domestic violence. Biological Psychiatry. 2004;56(11):895–897. doi: 10.1016/j.biopsych.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 144.Beskow J, Gottfries CG, Roos BE, Winblad B. Determination of monoamine and monoamine metabolites in the human brain: post mortem studies in a group of suicides and in a control group. Acta Psychiatrica Scandinavica. 1976;53(1):7–20. doi: 10.1111/j.1600-0447.1976.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 145.Lloyd KG, Farley IJ, Deck JH, Hornykiewicz O. Serotonin and 5-hydroxyindoleacetic acid in discrete areas of the brainstem of suicide victims and control patients. Advances in Biochemical Psychopharmacology. 1974;11:387–397. [PubMed] [Google Scholar]
- 146.Shaw DM, Camps FE, Eccleston EG. 5-hydroxytryptamine in the hind-brain of depressive suicides. British Journal of Psychiatry. 1967;113(505):1407–1411. doi: 10.1192/bjp.113.505.1407. [DOI] [PubMed] [Google Scholar]
- 147.Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and β-adrenergic receptor binding sites in the brain of suicide victims. Archives of General Psychiatry. 1990;47(11):1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- 148.Mann JJ, Arango V. Abnormalities of brain structure and function in mood disorders. In: Charney DS, Neslter EJ, Bunney BS, editors. Neurobiology of Mental Illness. New York, NY, USA: Oxford University Press; 1999. pp. 385–393. [Google Scholar]
- 149.Yates M, Ferrier IN. 5-HT1(A) receptors in major depression. Journal of Psychopharmacology. 1990;4(2):69–74. doi: 10.1177/026988119000400204. [DOI] [PubMed] [Google Scholar]
- 150.Yates M, Leake A, Candy JM, Fairbairn AF, McKeith IG, Ferrier IN. 5HT2 receptor changes in major depression. Biological Psychiatry. 1990;27(5):489–496. doi: 10.1016/0006-3223(90)90440-d. [DOI] [PubMed] [Google Scholar]
- 151.Baldessarini RJ. Drugs and the treatment of psychiatric disorders: depression and anxiety disorders. In: Hardman JG, Limbird LE, Gilman AG, editors. The Pharmacological Basis of Therapeutics. New York, NY, USA: McGraw-Hill; 2001. pp. 447–484. [Google Scholar]
- 152.Stockmeier CA. Involvement of serotonin in depression: evidence from postmortem and imaging studies of serotonin receptors and the serotonin transporter. Journal of Psychiatric Research. 2003;37(5):357–373. doi: 10.1016/s0022-3956(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 153.Sargent PA, Kjaer KH, Bench CJ, et al. Brain serotonin(1A) receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Archives of General Psychiatry. 2000;57(2):174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 154.Drevets WC, Frank E, Price JC, Kupfer DJ, Greer PJ, Mathis C. Serotonin type-1A receptor imaging in depression. Nuclear Medicine and Biology. 2000;27(5):499–507. doi: 10.1016/s0969-8051(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 155.Savitz J, Lucki I, Drevets WC. 5-HT1A receptor function in major depressive disorder. Progress in Neurobiology. 2009;88(1):17–31. doi: 10.1016/j.pneurobio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McNamara RK, Able J, Liu Y, et al. Omega-3 fatty acid deficiency during perinatal development increases serotonin turnover in the prefrontal cortex and decreases midbrain tryptophan hydroxylase-2 expression in adult female rats: dissociation from estrogenic effects. Journal of Psychiatric Research. 2009;43(6):656–663. doi: 10.1016/j.jpsychires.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De La Presa Owens S, Innis SM. Docosahexaenoic and arachidonic acid prevent a decrease in dopaminergic and serotoninergic neurotransmitters in frontal cortex caused by a linoleic and α-linolenic acid deficient diet in formula-fed piglets. Journal of Nutrition. 1999;129(11):2088–2093. doi: 10.1093/jn/129.11.2088. [DOI] [PubMed] [Google Scholar]
- 158.Delion S, Chalon S, Herault J, Guilloteau D, Bresnard JC, Durand G. Chronic dietary alpha-linolenic acid deficiency alters dopaminergic and serotonergic neurotransmission in rats. Journal of Nutrition. 1994;124(12):2466–2475. doi: 10.1093/jn/124.12.466. [DOI] [PubMed] [Google Scholar]
- 159.Delion S, Chalon S, Guilloteau D, Besnard J-C, Durand G. α-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. Journal of Neurochemistry. 1996;66(4):1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- 160.Vancassel S, Leman S, Hanonick L, et al. n-3 polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. Journal of Lipid Research. 2008;49(2):340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- 161.Hibbeln JR, Linnoila M, Umhau JC, Rawlings R, George DT, Salem N., Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late- onset alcoholics. Biological Psychiatry. 1998;44(4):235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 162.Hibbeln JR, Umhau JC, Linnoila M, et al. A replication study of violent and nonviolent subjects: cerebrospinal fluid metabolites of serotonin and dopamine are predicted by plasma essential fatty acids. Biological Psychiatry. 1998;44(4):243–249. doi: 10.1016/s0006-3223(98)00143-7. [DOI] [PubMed] [Google Scholar]
- 163.De Vriese SR, Christophe AB, Maes M. In humans, the seasonal variation in poly-unsaturated fatty acids is related to the seasonal variation in violent suicide and serotonergic markers of violent suicide. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2004;71(1):13–18. doi: 10.1016/j.plefa.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 164.Duman RS. The neurochemistry of mood disorders. In: Charney DS, Neslter EJ, editors. The Neurobiology of Mental Illness. New York, NY, USA: Oxford University Press; 2004. pp. 221–239. [Google Scholar]
- 165.Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Archives of General Psychiatry. 2007;64(3):327–337. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- 166.Gershon AA, Vishne T, Grunhaus L. Dopamine D2-like receptors and the antidepressant response. Biological Psychiatry. 2007;61(2):145–153. doi: 10.1016/j.biopsych.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 167.aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. Canadian Medical Association Journal. 2009;180(3):305–313. doi: 10.1503/cmaj.080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biological Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 169.Post RM, Kotin J, Goodwin FK, Gordon EK. Psychomotor activity and cerebrospinal fluid amine metabolites in affective illness. American Journal of Psychiatry. 1973;130(1):67–72. doi: 10.1176/ajp.130.1.67. [DOI] [PubMed] [Google Scholar]
- 170.Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine transmission among patients with depression who attempted suicide. Archives of General Psychiatry. 1992;49(6):447–450. doi: 10.1001/archpsyc.1992.01820060027004. [DOI] [PubMed] [Google Scholar]
- 171.Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Sridhara Rama Rao BS. CSF amine metabolites in depression. Biological Psychiatry. 1992;31(2):112–118. doi: 10.1016/0006-3223(92)90198-9. [DOI] [PubMed] [Google Scholar]
- 172.Brown AS, Gershon S. Dopamine and depression. Journal of Neural Transmission. 1993;91(2-3):75–109. doi: 10.1007/BF01245227. [DOI] [PubMed] [Google Scholar]
- 173.Van Praag HM, Korf J, Lakke JPWF, Schut T. Dopamine metabolism in depression, psychoses, and Parkinson's disease: the problem of specificity of biological variables in behavior disorders. Psychological Medicine. 1975;4(2):21–29. doi: 10.1017/s0033291700056385. [DOI] [PubMed] [Google Scholar]
- 174.Guze BH, Barrio JC. The etiology of depression in Parkinson’s disease patients. Psychosomatics. 1991;32(4):390–395. doi: 10.1016/S0033-3182(91)72039-2. [DOI] [PubMed] [Google Scholar]
- 175.Harvey P-O, Pruessner J, Czechowska Y, Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Molecular Psychiatry. 2007;12(8):767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- 176.Hirvonen J, Karlsson H, Kajander J, et al. Striatal dopamine D2 receptors in medication-naive patients with major depressive disorder as assessed with [11C]raclopride PET. Psychopharmacology. 2008;197(4):581–590. doi: 10.1007/s00213-008-1088-9. [DOI] [PubMed] [Google Scholar]
- 177.Papp M, Klimek V, Willner P. Parallel changes in dopamine D2 receptor binding in limbic forebrain associated with chronic mild stress-induced anhedonia and its reversal by imipramine. Psychopharmacology. 1994;115(4):441–446. doi: 10.1007/BF02245566. [DOI] [PubMed] [Google Scholar]
- 178.Bjørnebekk A, Mathé AA, Brené S. Isolated flinders sensitive Line rats have decreased dopamine D2 receptor mRNA. NeuroReport. 2007;18(10):1039–1043. doi: 10.1097/WNR.0b013e3281668bf7. [DOI] [PubMed] [Google Scholar]
- 179.Kram ML, Kramer GL, Ronan PJ, Steciuk M, Petty F. Dopamine receptors and learned helplessness in the rat: an autoradiographic study. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26(4):639–645. doi: 10.1016/s0278-5846(01)00222-6. [DOI] [PubMed] [Google Scholar]
- 180.Yaroslavsky I, Colletti M, Jiao X, Tejani-Butt S. Strain differences in the distribution of dopamine (DA-2 and DA-3) receptor sites in rat brain. Life Sciences. 2006;79(8):772–776. doi: 10.1016/j.lfs.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 181.Zimmer L, Delion-Vancassel S, Durand G, et al. Modification of dopamine neurotransmission in the nucleus accumbens of rats deficient in n-3 polyunsaturated fatty acids. Journal of Lipid Research. 2000;41(1):32–40. [PubMed] [Google Scholar]
- 182.Kuperstein F, Eilam R, Yavin E. Altered expression of key dopaminergic regulatory proteins in the postnatal brain following perinatal n-3 fatty acid dietary deficiency. Journal of Neurochemistry. 2008;106(2):662–671. doi: 10.1111/j.1471-4159.2008.05418.x. [DOI] [PubMed] [Google Scholar]
- 183.Levant B, Zarcone TJ, Fowler SC. Developmental effects of dietary n-3 fatty acids on activity and response to novelty. Physiology and Behavior. 2010;101(1):176–183. doi: 10.1016/j.physbeh.2010.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Anisman H. Cascading effects of stressors and inflammatory immune system activation: implications for major depressive disorder. Journal of Psychiatry and Neuroscience. 2009;34(1):4–20. [PMC free article] [PubMed] [Google Scholar]
- 185.Maes M, Yirmyia R, Noraberg J, et al. The inflammatory & neurodegenerative (I&ND) hypothesis of depression: leads for future research and new drug developments in depression. Metabolic Brain Disease. 2009;24(1):27–53. doi: 10.1007/s11011-008-9118-1. [DOI] [PubMed] [Google Scholar]
- 186.Dinan T, Siggins L, Scully P, O’Brien S, Ross P, Stanton C. Investigating the inflammatory phenotype of major depression: focus on cytokines and polyunsaturated fatty acids. Journal of Psychiatric Research. 2009;43(4):471–476. doi: 10.1016/j.jpsychires.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 187.Pace TWW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry. 2006;163(9):1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 188.Tonelli LH, Stiller J, Rujescu D, et al. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatrica Scandinavica. 2008;117(3):198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dean B, Tawadros N, Scarr E, Gibbons AS. Regionally-specific changes in levels of tumour necrosis factor in the dorsolateral prefrontal cortex obtained postmortem from subjects with major depressive disorder. Journal of Affective Disorders. 2010;120(1–3):245–248. doi: 10.1016/j.jad.2009.04.027. [DOI] [PubMed] [Google Scholar]
- 190.Gaiteri C, Guilloux J-P, Lewis DA, Sibille E. Altered gene synchrony suggests a combined hormone-mediated dysregulated state in major depression. PLoS ONE. 2010;5(4, article e9970):1–9. doi: 10.1371/journal.pone.0009970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, et al. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. doi: 10.1038/mp.2010.52. Molecular Psychiatry. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Maes M, Lin A-H, Ombelet W, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25(2):121–137. doi: 10.1016/s0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 193.Maes M, Ombelet W, De Jongh, R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. Journal of Affective Disorders. 2001;63(1–3):85–92. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- 194.Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biological Research for Nursing. 2008;10(2):128–133. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- 195.Farooqui AA, Horrocks LA, Farooqui T. Modulation of inflammation in brain: a matter of fat. Journal of Neurochemistry. 2007;101(3):577–599. doi: 10.1111/j.1471-4159.2006.04371.x. [DOI] [PubMed] [Google Scholar]
- 196.Ariel A, Li P-L, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing promotes human T cell via lipid raft clustering. Journal of Biological Chemistry. 2005;280(52):43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 197.Bannenberg GL. Resolvins: current understanding and future potential in the control of inflammation. Current Opinion in Drug Discovery and Development. 2009;12(5):644–658. [PubMed] [Google Scholar]
- 198.Lee JY, Plakidas A, Lee WH, et al. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. Journal of Lipid Research. 2003;44(3):479–486. doi: 10.1194/jlr.M200361-JLR200. [DOI] [PubMed] [Google Scholar]
- 199.Pascual G, Fong AL, Ogawa S, et al. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ . Nature. 2005;437(7059):759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lu D-Y, Tsao Y-Y, Leung Y-M, Su K-P. Docosahexaenoic acid suppresses neuroinflammatory responses and induces heme oxygenase-1 expression in BV-2 microglia: implications of antidepressant effects for omega-3 fatty acids. Neuropsychopharmacology. 2010;35(11):22438–2248. doi: 10.1038/npp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Frazer A, Morilak DA. What should animal models of depression model? Neuroscience and Biobehavioral Reviews. 2005;29(4-5):515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 202.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neuroscience and Biobehavioral Reviews. 2005;29(4-5):547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 203.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr. Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. Journal of Neuroscience. 2001;21(18):7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.DeMar JC, Jr., Ma K, Bell JM, Igarashi M, Greenstein D, Rapoport SI. One generation of n-3 polyunsaturated fatty acid deprivation increases depression and aggression test scores in rats. Journal of Lipid Research. 2006;47(1):172–180. doi: 10.1194/jlr.M500362-JLR200. [DOI] [PubMed] [Google Scholar]
- 205.Lakhwani L, Tongia SK, Pal VS, Agrawal RP, Nyati P, Phadnis P. Omega-3 fatty acids have antidepressant activity in forced swimming test in Wistar rats. Acta Poloniae Pharmaceutica. 2007;64(3):271–276. [PubMed] [Google Scholar]
- 206.Huang S-Y, Yang H-T, Chiu C-C, Pariante CM, Su K-P. Omega-3 fatty acids on the forced-swimming test. Journal of Psychiatric Research. 2008;42(1):58–63. doi: 10.1016/j.jpsychires.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 207.Ferraz AC, Kiss A, Araújo RLF, et al. The antidepressant role of dietary long-chain polyunsaturated n-3 fatty acids in two phases in the developing brain. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2008;78(3):183–188. doi: 10.1016/j.plefa.2008.02.001. [DOI] [PubMed] [Google Scholar]