INTRODUCTION
Early in neuronal development the neurotransmitter γ-aminobutyric acid (GABA) exerts an excitatory rather than inhibitory effect due to a high concentration of intracellular chloride ions (Cherubini et al., 1991). During the first two postnatal weeks in the hippocampus of rats the internal concentration of chloride ions decreases. This causes a change in the reversal potential of chloride and thus, a shift from an excitatory effect of GABAA receptor activation to an inhibitory effect (Ben-Ari et al., 1989; Leinekugel et al., 1995, 1997, 1998; Khazipov et al., 1997; Rivera et al., 1999; Ganguly et al., 2001). Evidence suggests that the upregulation of the K+Cl- co-transporter (KCC2) and the downregulation of the Na+K+2Cl- co-transporter (NKCC1) are responsible for shifting the chloride reversal potential (Plotkin et al., 1997; Lu et al., 1999; Rivera et al., 1999; Hübner et al., 2001). NKCC1 expression predominates in immature neurons and mediates chloride influx, while KCC2 expression predominates in mature neurons and mediates chloride efflux (for review see Delpire, 2000; Payne et al., 2003). Due to the high intracellular chloride concentration in immature neurons the activation of GABAA receptors depolarizes the cell, which subsequently activates voltage-dependent calcium channels, particularly L-type calcium channels (Yuste and Katz, 1991; Leinekugel et al., 1995; Khazipov et al., 1997; Ganguly et al., 2001). This GABAergic excitation is important for proper neuronal development (for review see Ben-Ari, 2002; Owens and Kriegstein, 2002; Fiumelli and Woodin, 2007; Galanopoulou, 2008; Kahle et al., 2008; Blaesse et al., 2009). As the brain matures, the number of neurons that are excitatory in response to GABA decreases and thus, the magnitude of calcium influx with GABA receptor activation decreases. Once neurons have fully developed, GABA responses are hyperpolarizing and inhibit the cell from reaching threshold. Additionally, the subunit composition of the GABAA receptor changes during development (Kanaumi et al., 2006; Liu and Wong-Riley, 2006; Rissman et al., 2006; Yu et al., 2006). This change in subunit composition should not affect the reversal potential directly since that is dependent on the internal and external chloride concentrations, but it does affect the response to various modulators such as zinc and benzodiapines.
Treatment of embryonic rat hippocampal cultures with L-type calcium channel antagonists prohibits the shift in the chloride reversal potential, suggesting that calcium influx through L-type calcium channels is involved in the changes of chloride transporter expression (Ganguly et al., 2001). However, Ganguly and co-workers did not directly look at the effect of calcium influx through L-type channels on chloride transporter expression. Previous experiments in our laboratory have demonstrated facilitation of L-type calcium current by activation of the metabotropic GABAB receptor in acutely cultured hippocampal neurons isolated from 5-7 day old rat pups (Carter and Mynlieff, 2004). Facilitation of L-type calcium current has only been observed in salamander retinal cells (Shen and Slaughter, 1999) and rat dorsal root ganglion cells (Fujikawa et al., 1997), but has not been observed by other investigators studying GABAB receptors using hippocampal tissue from either embryonic or adult rats. The main effect of GABAB receptor activation in adult hippocampus is to decrease N-type calcium current and increase potassium current (Newberry and Nicoll, 1984; Gähwiler and Brown, 1985; Dutar and Nicoll, 1988; Harrison, 1990; Lambert et al., 1991; Scholz and Miller, 1991; Thompson and Gähwiler, 1992; Davies and Collingridge, 1993; Pfrieger et al., 1994; Wu and Saggau, 1995; Takahashi et al., 1998; for review see Bettler et al., 2004). One possibility that L-type calcium current facilitation by GABAB receptor activation has not been previously observed within the rat hippocampus is that it is a phenomenon only present at a specific time point in development and may play a role in the developmental changes in gene expression such as those seen with chloride transporters.
The present study explores the potential connection between chloride transporter expression and calcium influx through L-type calcium channels in the early neonatal period. Although changes in reversal potential have been shown to be dependent on calcium influx through L-type calcium channels, this is the first study to directly investigate the effect of calcium influx on the chloride transporter protein levels in hippocampal neurons. Since calcium influx is enhanced in a subset of neonatal hippocampal neurons by activation of GABAB receptors, activation of these receptors may also alter KCC2 and NKCC1 transporter expression. Electrophysiological experiments with the GABAB agonist baclofen were performed on cultured hippocampal neurons obtained from different aged rats to identify the timecourse of L-type calcium current facilitation by GABAB receptor activation. The KCC2 and NKCC1 expression levels throughout early postnatal development were determined by Western blot analysis in the presence and absence of an L-type channel antagonist and a GABAB receptor agonist.
MATERIALS AND METHODS
Isolation of Hippocampal Neurons
All animal protocols were approved by the Marquette University Institutional Animal Care and Use Committee and followed the guidelines set forth by the U.S. Public Health Service. Neurons were isolated by a technique developed for the hippocampus of postnatal rats (Mynlieff, 1997). Briefly, Sprague-Dawley rat pups of varying ages were anesthetized by CO2 and sacrificed by decapitation. The head was immersed in 70% ethanol for 3 minutes for decontamination and rinsed in sterile rodent Ringer’s solution with glucose (146 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, 11 mM D-glucose, pH 7.4 with NaOH). The hippocampi were dissected from the brain in oxygenated, cold (~5°C), sterile rodent Ringer’s solution with glucose using sterile technique. For the electrophysiology experiments, dissections were restricted to the superior region of the hippocampus (excluding the dentate gyrus). For protein analysis of transporters, the entire hippocampus was used for cultures. The tissue was placed in PIPES-buffered saline (120 mM NaCl, 5 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 25 mM D-glucose, 20 mM piperazine-N,N’-bis[2-ethanesulfonic acid], pH 7.0 with NaOH) and sliced into ~1 mm3 sections. The tissue was transferred into a small vial and incubated at room temperature in 1 ml of 0.5% Trypsin XI and 0.01% DNase I (Sigma-Aldrich, St. Louis, MO) in PIPES buffered saline at room temperature for 20-30 minutes with 100% oxygen blown over the tissue. This incubation was followed by another for 40-60 minutes depending on the age of the pup at 35°C under continuous oxygen. The tissue was rinsed with 1 ml trypsin inhibitor solution (1 mg/ml trypsin inhibitor and 1 mg/ml bovine serum albumin) in rodent Ringer’s solution and rinsed again with 1 ml Neurobasal-A growth medium (Invitrogen, Carlsbad, CA) fortified with B27 supplement, 0.5 mM glutamine, and 0.02 mg/ml gentamicin. The tissue was triturated with a fire-polished Pasteur pipette in fresh growth medium and plated onto the center of poly-L-lysine coated dishes for both electrophysiology and protein analysis. Neurons were incubated at 37°C in a 5% CO2 water-jacketed incubator.
A total volume of 2 ml was maintained for cultures that were used for protein analysis of transporters. Twenty four hours after plating the cells, 1 ml of growth medium was removed and fresh medium was added to the dish. A volume control dish containing 2 ml of medium was placed in the incubator and the appropriate amount of fresh medium was added every other day to maintain the correct volume. For cultures treated with drugs, 2 μl of stock solutions of either 5 mM nimodipine (Sigma-Aldrich, St. Louis, MO) dissolved in 5 M HCl or 10 mM (RS)-baclofen (Tocris, Ellisville, MO) dissolved in methanol were added daily for a total of 7 days to maintain concentrations of 5 μM or 10 μM, respectively.
Electrophysiology
Whole cell patch clamp recording was used to measure calcium currents in voltage clamp mode. Data were collected using a Dagan 3900A patch clamp amplifier (Dagan Corporation, Minneapolis, MN) combined with a Digidata 1322 data acquisition system and a computer with pCLAMP 9.0 software (Molecular Devices, Sunnyvale, CA). All electrophysiological experiments were performed at room temperature 20-24 hours following dissociation. Although voltage control is best immediately following dissociation, it was necessary to wait overnight to allow the cells time to recover from enzymatic dissociation and adhere to the bottom of the dish (Mynlieff, 1997). Recording electrodes (3-7 MΩ) were made from borosilicate glass capillaries on a Flaming/Brown Micropipette Puller model P-87 (Sutter Instrument Co., Novato, CA). The internal solution used to fill the patch electrodes contained 140 mM Cs-aspartate, 5 mM MgCl2, 10 mM Cs2EGTA, 10 mM HEPES, and 2 mM ATP-Na2, 0.1 mM GTP, pH of 7.4 with CsOH and osmolarity between 310-320 mOsm. The external solution used for calcium current measurements contained 10 mM CaCl2, 145 mM TEACl, 10 mM HEPES and 1 μM tetrodotoxin (Sigma-Aldrich, St. Louis, MO) to block Na+ currents, pH of 7.4 with CsOH and osmolarity between 300-310 mOsm. The cells were held at -80 mV and depolarized with a 300 ms pulse to +10 mV in the presence or absence of the GABAB agonist, (RS)-baclofen (Tocris, Ellisville, MO), which was dissolved in the external calcium solution. Whole cell currents were electronically filtered at 1 kHz and digitized at 2 kHz. Linear components of leak current were subtracted post-hoc by the passive resistance protocol in pClamp 9.0. Drugs were applied using a double barreled U-tube delivery system, constructed with PE-10 polyethylene tubing housed in a piece of glass tubing. The U-tube application method allowed for quick application and washout of compounds, which were gravity fed onto the cell and removed by vacuum suction. Multiple control currents were measured before and after drug application. This allowed for compensation due to run-up or run-down of calcium currents. For analysis, the linear regression and 95% confidence intervals were determined for the control currents and were used to determine the effect of application of 10 μM baclofen on calcium current amplitude (Figure 1A).
Figure 1.
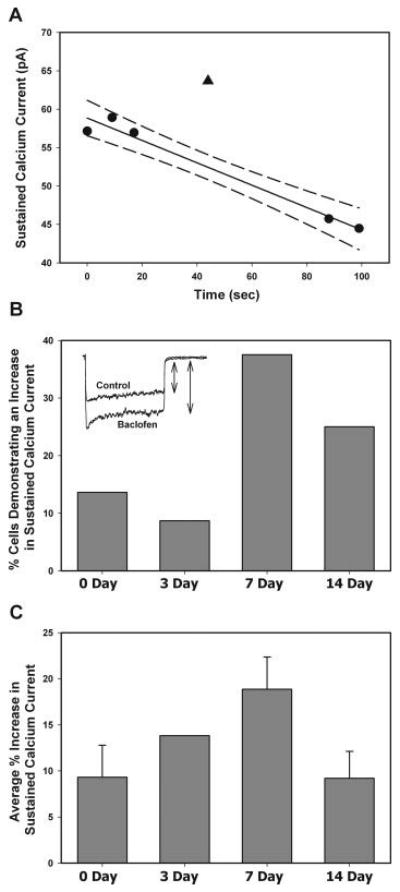
GABAB receptor facilitation of sustained calcium currents isolated from rat pups of different ages. Calcium currents were elicited by a 300 ms depolarization to +10 mV from a holding potential of -80 mV. The sustained current was measured at the end of the 300 ms pulse (see arrow on inset in B) (A) Sustained calcium currents recorded from a cultured hippocampal neuron isolated from a 14 day old rat. The linear regression (solid line) and 95% confidence interval (dashed line) were determined for the control currents (●). The percent change with 10 μM baclofen application (▲) was determined by comparison to the regression line. In all cells where the current with baclofen fell within the 95% confidence interval the effect was counted as “no change”. (B) Each bar represents the percent of cells demonstrating an increase in sustained calcium current following application of 10 μM baclofen in hippocampal cultures isolated from rats that were 0 (N=22), 3 (N=23), 7 (N=32), and 14 (N=24) days old (P = 0.054 using a Pearson Chi-Square when each age was analyzed separately; P=0.023 using a Pearson Chi-Square when day 0 and day 3 were grouped into early postnatal period and day 7 and day 14 were grouped into late postnatal period). The inset represents currents recorded in a hippocampal cell isolated from a 7 day old rat. (C) Average percent increase of sustained current in cells demonstrating facilitation in response to baclofen.
Western Blot Analysis
For Western blot analysis, whole hippocampal tissue was obtained from rats ranging from 1 to 42 days old (D1 to D42) and from hippocampal cultures that were obtained from postnatal day 0 rats and kept in culture for 1 to 15 days (C0-1 to C0-15). Proteins were extracted by homogenization in an ice-cold sucrose buffer (250 mM sucrose, 10 mM Tris, 10 mM HEPES, 1 mM EDTA, pH 7.2) with fresh protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 0.5 mg/ml pefabloc; Sigma-Aldrich, St. Louis, MO) followed by centrifugation at 3622 × g for 10 minutes at 4°C. The supernatant was centrifuged at 39,104 × g for 30 minutes at 4°C. The final pellet was resuspended in homogenizing buffer and stored at -80°C.
NuPAGE lithium dodecyl sulfate (LDS) sample buffer and sample reducing agent (Invitrogen, Carlsbad, CA) were added to the membrane extracts and heated at 37°C for 30 minutes. The membrane extracts were run on a 3-8% Tris-acetate Novex Minigel and transferred to a polyvinylidene difluoride (PVDF) membrane (pore size 0.45 μm) for KCC2 experiments and a nitrocellulose membrane (pore size 0.45 μm) for NKCC1 experiments in NuPAGE transfer buffer (Invitrogen, Carlsbad, CA). The membranes were washed with phosphate buffered saline (PBS, 134.4 mM NaCl, 4.36 mM KCl, 10.56 mM Na2HPO4, 1.66 mM NaH2PO4 and pH to 7.4 with HCl) and blocked for 1 hour in PBS containing 0.05% Tween, 5% nonfat dry milk, and 0.1% bovine serum albumin at room temperature. The membranes were decorated with primary antibodies in the PBS blocking solution against KCC2 (1:1000; Upstate Technologies, New York, NY) or NKCC1 (1:2000; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) overnight at 4°C. Following a 1 hour wash in PBS, the membranes were incubated with either a goat anti-rabbit or goat anti-mouse HRP-conjugated secondary antibody (1:1000; Pierce, Rockford, IL) in the PBS blocking solution for 2 hours at room temperature. The SuperSignal West Dura Extended Duration chemiluminescent enhancement kit (Pierce, Rockford, IL) was used to visualize the protein bands with classic blue autoradiography film (Molecular Technologies, St. Louis, MO). Protein concentration was measured with an enhanced BCA protein assay (Pierce, Rockford, IL) prior to the addition of LDS sample buffer to control for loading on the gels.
Quantification of Western Blots
To quantify protein on Western blots, the background intensity was first subtracted using ImageJ software (developed at U.S. National Institutes of Health and available at http://rsb.info.nih.gov/ij/). The integrated optical density (IOD) for each band was determined using Labworks 4.6 imaging and analysis software (UVP, Inc., Upland, CA). To control for gel loading, the IOD for each protein was divided by the IOD of a band in the same lane labeled with either anti-β-actin antibodies (1:1000; Cell Signaling Technologies, Danvers, MA) or anti-β-tubulin antibodies (1:5000; Sigma-Aldrich, St. Louis, MO). Multiple protein samples were analyzed per developmental time point studied for both whole hippocampal protein extracts and cultured hippocampal protein extracts.
RESULTS
Time Course of Calcium Current Modulation by GABAB Receptors
To determine whether the facilitation of L-type calcium current by GABAB receptor activation is developmentally regulated, calcium currents were recorded in neurons isolated from the hippocampi of 0, 3, 7, and 14 day old rat pups using whole cell voltage clamp recording in the absence and presence of a GABAB agonist baclofen (10 μM). The percentage of cells demonstrating an increase, decrease, or no change in peak calcium current depended on the age from which the neurons were isolated. At the early time points it appeared that more neurons demonstrated a decrease in current (data not shown). However, this peak current is a combination of L-type, N-type, P/Q-type, and R-type calcium current hampering interpretation of these data. Thus, the sustained current component at the end of a 300 ms depolarization pulse to +10 mV was measured minimizing the contribution of N-type, P/Q-type, and R-type calcium current while maximizing the contribution of L-type calcium current to the total measurement. Previous studies in the laboratory have already confirmed that the facilitatory effect of GABAB receptor activation on calcium currents was entirely mediated through L-type channels (Carter and Mynlieff, 2004). Hippocampal cultures isolated from 7 day old rats demonstrated the highest percentage of cells exhibiting an increase in sustained calcium current in response to 10 μM baclofen application (37.5%, N=32; Figure 1B). In contrast, hippocampal cultures isolated from 0, 3, or 14 day old rat pups demonstrated a smaller percentage of cells that exhibited an increase in sustained calcium current (0 day, 13.6%, N=22; 3 day, 8.7%, N=23; 14 day, 25.0%, N=24), but the difference was not statistically significant if each individual age was analyzed separately (P = 0.054; Figure 1B). However, since the number of cells isolated from 0 and 3 day old rats responding with facilitation were very low and similar and the numbers of cells isolated from 7 and 14 day old rats responding with facilitation were much higher, the data were re-analyzed and grouped into early postnatal (day 0-3) and late postnatal (day 7-14) time points. The number of cells responding in the early versus late postnatal period with a facilitation of the sustained current was statistically different (P = 0.023). In the cells that demonstrated an increase in calcium current from hippocampal cultures isolated from 7 day old rats, the average increase in sustained calcium current was 18.86 ± 3.48% (Figure 1C). The average percent increase was highest in cultures isolated from 7 day old rats, but the values were not significantly different from the other time points studied (P = 0.136). The cells from 3 day old animals were not included in the statistical analysis, because there were only 2 cells that demonstrated an increase in response to baclofen.
There were no significant differences in cell size (P = 0.911) as measured by cell capacitance or current density (P = 0.690) in the cells that exhibited an increase in sustained calcium current in response to 10 μM baclofen application when compared to cells that exhibited no effect or a decrease in sustained calcium current in response to 10 μM baclofen (Supplemental figure 1). These results held true when determining the current density using the sustained component (P = 0.633) and the transient component (P = 0.921) of the current (Supplemental figure 2). In addition, mean low voltage activated (LVA; P = 0.107) or high voltage activated (HVA; transient, P = 0.356; sustained, P = 0.508) current densities do not significantly change at the different ages studied (Supplemental figure 3). These experiments demonstrated that facilitation of L-type calcium current by GABAB receptor activation is most prominent in neurons isolated from 7 day old rats. However, the changes in response to baclofen can not be attributed to changes in LVA or HVA current density.
Developmental Regulation of NKCC1 Protein during the Early Postnatal Period
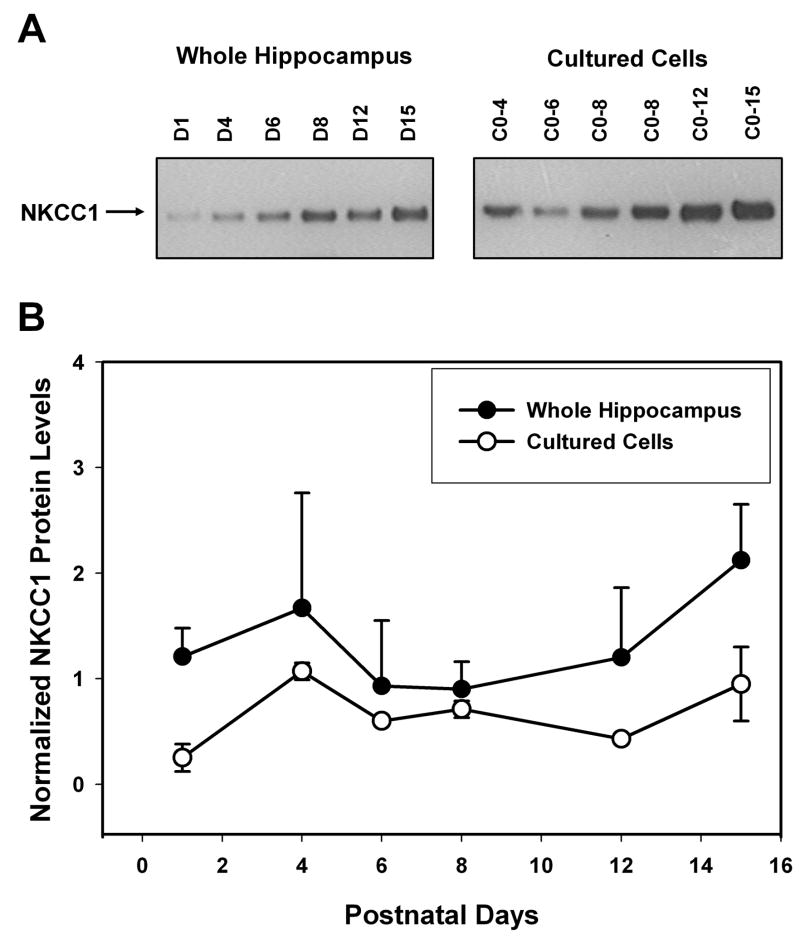
The expression level of both KCC2 and NKCC1 were determined by Western blot analysis in hippocampal neurons cultured for various time periods and were compared to that from fresh tissue of equivalent ages. Whole hippocampal tissue protein was isolated from rats ranging in ages 1 day to 42 days old and cultured hippocampal neurons were isolated from 0 day old rat pups and kept in culture for 1 to 15 days. Other investigators have demonstrated that hippocampal neurons in culture exhibit spontaneous activity and form synaptic connections within a few days following dissociation (Siebler et al., 1993; Bi and Poo, 1998; Vicario-Abejón et al., 1998). Anti-NKCC1 antibodies labeled a single band of 143 kDa on Western blots of proteins isolated from either cultured neurons or whole hippocampus. Steady state NKCC1 protein levels were determined at each time point studied by dividing the IOD for the 143 kDa band by the IOD for the band labeled with β-tubulin antibodies to control for variations in loading. The internal control was necessary since the amount of protein extracted from cultured neurons was too low for a protein assay. In contrast to KCC2 protein levels (see below), there was little change in the steady state protein levels of NKCC1 during the first two postnatal weeks of development both in fresh tissue and in cultured hippocampal neurons (P = 0.278; Figure 2). Fresh tissue that was analyzed from rats of one month or more in age demonstrated a five-fold increase in the steady state expression level of NKCC1 (data not shown).
Figure 2.
Developmentally regulated expression of NKCC1 protein in rat hippocampus and cultured hippocampal neurons. (A) Representative Western blot analysis of proteins extracted from whole hippocampus (left panel; postnatal day 1 to day 15) and cultured hippocampal neurons (right panel). Cultured neurons were obtained from day 0 rats and were kept in culture for 1 to 15 days before protein isolation (C0-1 to C0-15). (B) Summary data comparing the steady state NKCC1 protein levels isolated from whole hippocampal protein extracts (solid circles; N = 3) and cultured hippocampal neurons (open circles; N = 3 – 4, except C0-6 where N = 2). The IOD of the band labeled with NKCC1 antibodies (143 kDa) was divided by the IOD of the band labeled with β-tubulin antibodies (55 kDa) for normalization. In the first two weeks there was no difference in NKCC1 protein levels in the different ages across both tissue types (P = 0.278 using a two way ANOVA).
Developmental Regulation of KCC2 Protein during the Early Postnatal Period
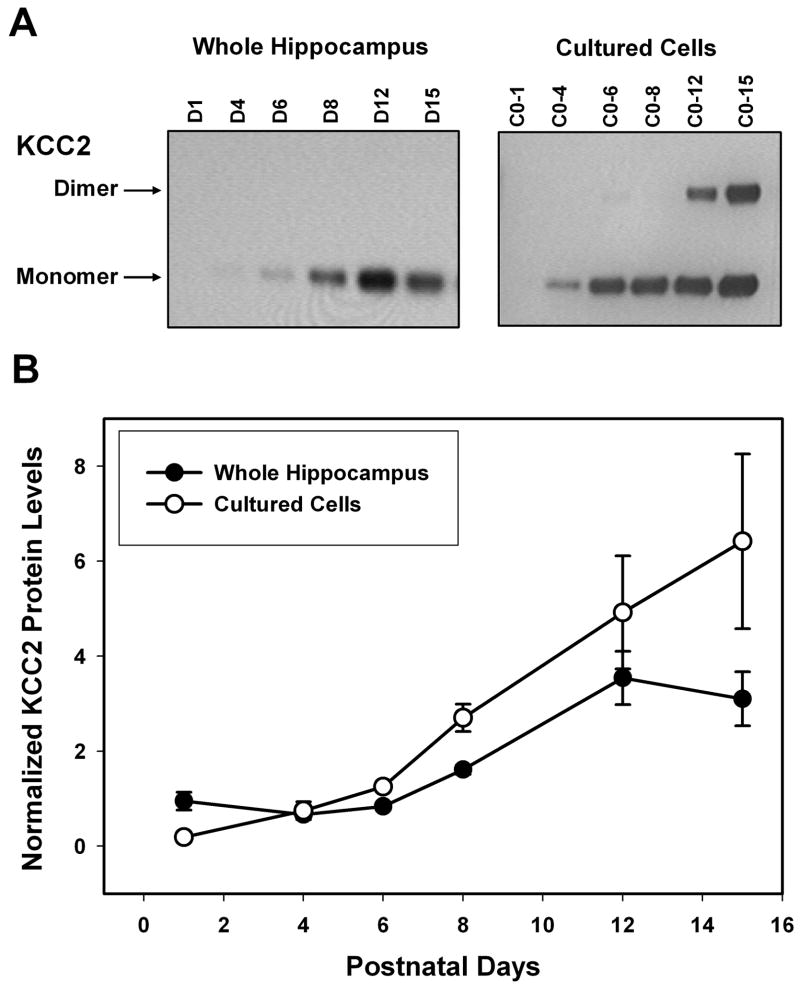
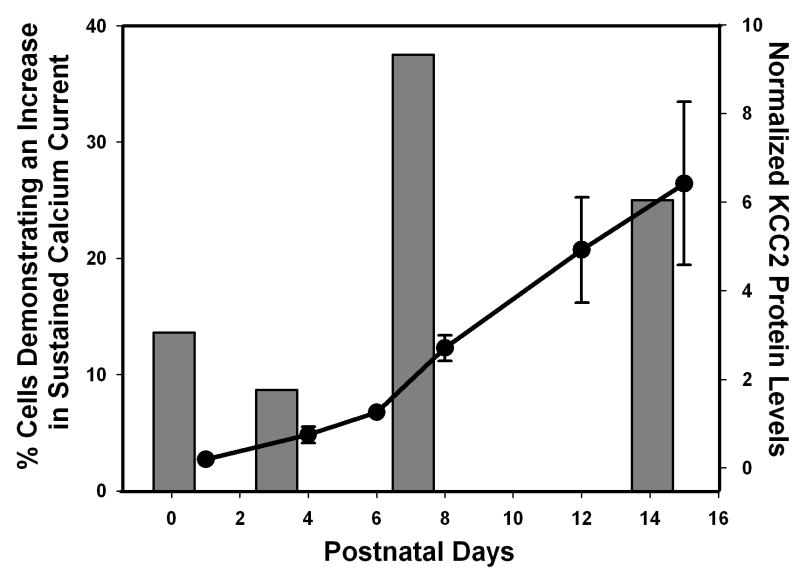
Antibodies to KCC2 labeled two bands of 248 kDa and 125 kDa representing a dimer and a monomer. As the neurons matured there were increasing amounts of the dimer in relation to the monomer. Steady state KCC2 protein levels were determined at each time point studied by adding the IOD for the band corresponding to the monomer plus the IOD for the band corresponding to the dimer and this value was further divided by the IOD for the band labeled with β-actin antibodies as a control for variations in loading. Previous studies showed that KCC2 mRNA levels within the rat hippocampus are low following birth and rapidly rise in the first few postnatal weeks (Rivera et al., 1999; Balakrishnan et al., 2003). Our protein data is consistent with the mRNA data in that both fresh tissue and cultured neurons demonstrated low KCC2 protein levels during the first postnatal week, but levels rapidly rise during the second postnatal week (P < 0.001). In fresh tissue the protein level tripled between day 15 and day 24, at which point it plateaued through adulthood (data not shown). The increase in KCC2 protein levels during the second postnatal week in development occurred both in vivo and in cultured hippocampal cells (Figure 3), supporting the use of cultured hippocampal neurons to study the developmental expression of KCC2. In addition, these results demonstrate that in hippocampal cell cultures obtained from rats of various ages L-type current facilitation peaks at approximately the same developmental time point as KCC2 expression levels begin to rise. This supports the hypothesis that facilitation of L-type calcium current by GABAB receptor activation within the hippocampus may play a role in the regulation of KCC2 during the first two postnatal weeks of development.
Figure 3.
Developmentally regulated expression of KCC2 protein in rat hippocampus and cultured hippocampal neurons. (A) Representative Western blot analysis of proteins extracted from whole hippocampus (left panel; postnatal day 1 to day 15) and cultured hippocampal neurons (right panel). Cultured neurons were obtained from day 0 rats and were kept in culture for 1 to 15 days before protein isolation (C0-1 to C0-15). (B) Summary data comparing the steady state KCC2 protein levels isolated from whole hippocampal protein extracts (solid circles; N = 3) and cultured hippocampal neurons (open circles; N = 3 – 4, except C0-6 where N = 2). The IOD of the band corresponding to the monomer (125 kDa) and the IOD of the band corresponding to the dimer (248 kDa) were added together and divided by the IOD for the band labeled with β-actin antibodies (45 kDa). There is a significant difference in KCC2 protein levels at the different ages across both tissue types (P < 0.001 using a two way ANOVA).
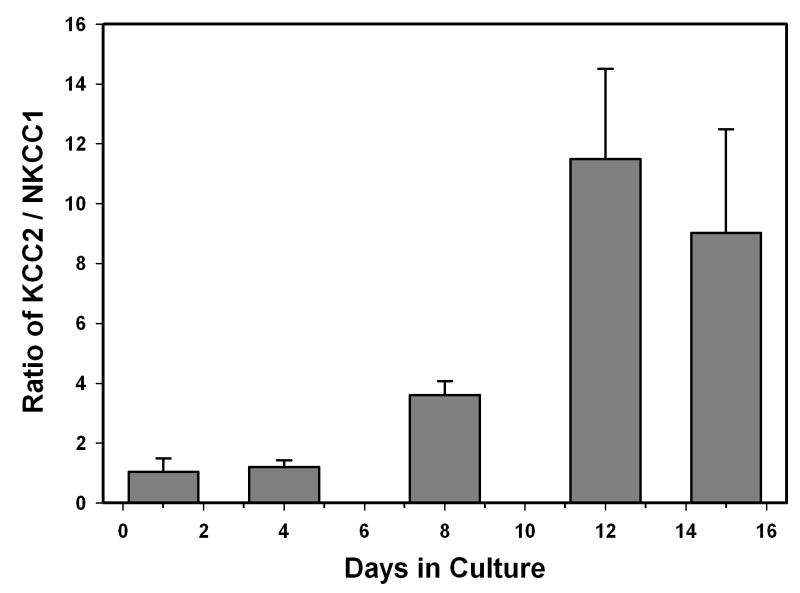
Other investigators have demonstrated that the internal chloride concentration decreases during the early postnatal period in rat hippocampal neurons. This could be due to a concurrent increase in KCC2 with a decrease in NKCC1, since these transporters move chloride in the opposite direction. However, our data suggest that only the KCC2 transporter levels are changing drastically in the first two postnatal weeks of hippocampal development and that it is the ratio of the two transporters that is important in determining the intracellular concentration of chloride and thus, the reversal potential for the GABAA response. Our data demonstrate a large increase in the KCC2 to NKCC1 ratio between postnatal day 8 and 12 (P = 0.005; Figure 4).
Figure 4.
KCC2 to NKCC1 ratio. The ratio of KCC2 to NKCC1 was determined using the normalized values for the cultured hippocampal neurons for each date that a preparation was isolated. An average of three to four preparations was determined for each time point. The ratio of KCC2 to NKCC1 is higher after 12 to 15 days in culture (P = 0.005 using an ANOVA).
Role of L-type Calcium Channels and GABAB Receptors in Modulating Chloride Transporter Expression
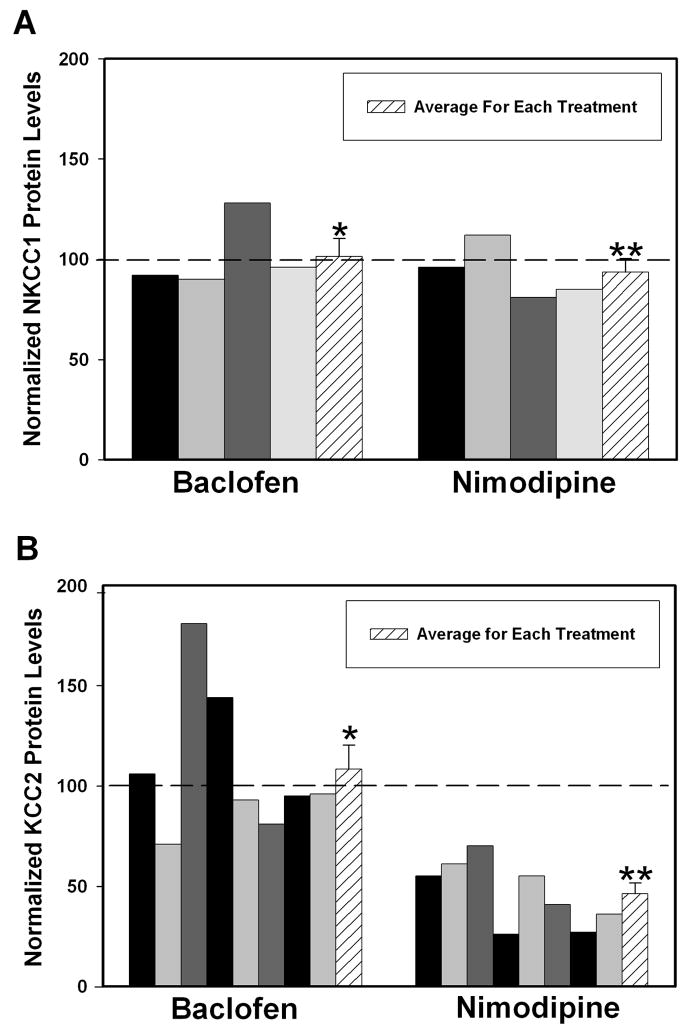
To determine whether calcium influx through L-type calcium channels and GABAB receptor activation affects the protein level of KCC2 and NKCC1 during the early neonatal period, hippocampal cultures isolated from 0 day rats were treated daily with the GABAB receptor agonist baclofen (10 μM) or the L-type calcium channel antagonist nimodipine (5 μM) for one week. Previous studies treating cultures with the vehicles for these drugs demonstrated only a small shift in pH and no effect on cell viability or protein levels (data not shown). Steady state NKCC1 protein levels were determined by Western blot analysis and the values were determined by dividing the IOD for the band corresponding to NKCC1 by the IOD for the band corresponding to β-tubulin. This value was further normalized by dividing it by the value obtained for the control culture grown in the absence of any drugs along side each set of cultures treated with drugs and exposed to the same film and multiplied by 100%. NKCC1 protein levels during the first postnatal week were not modified by treatment with the GABAB agonist baclofen (101.50 ± 8.90%; N = 4 individual cultures, P = 0.8772; Figure 5A). In addition, the L-type calcium channel antagonist nimodipine did not affect the steady state protein levels of NKCC1 during the first postnatal week of development (93.50 ± 6.90%; N = 4 individual cultures, P = 0.4177; Figure 5A). Therefore, NKCC1 protein levels in hippocampal cultures are not affected by one week treatment of the GABAB agonist, baclofen or the L-type antagonist, nimodipine.
Figure 5.
Effects of baclofen and nimodipine on chloride transporter expression using Western blot analysis. Hippocampal cultures were obtained from day 0 rats and either 10 μM baclofen, a GABAB agonist, or 5 μM nimodipine, an L-type calcium channel antagonist were added daily to the cultures for one week. Each bar represents one protein preparation that was isolated from a set of cultures treated with either drug and an average (hatched bar) was determined for both drug treatments. (A) NKCC1 steady state protein levels following treatment with a GABAB agonist or an L-type calcium channel antagonist. The IOD of the band labeled with NKCC1 antibodies was divided by the IOD of the band labeled with β-tubulin antibodies. This value was further normalized by dividing it by the value obtained for the control culture for each set of cultures and multiplied by 100%. (*P = 0.8772 and **P = 0.935 using a one sample t-test). (B) KCC2 steady state protein levels following treatment with a GABAB agonist or an L-type calcium channel antagonist. The IOD of the band corresponding to the monomer and the IOD of the band corresponding to the dimer of KCC2 were added together and divided by the IOD of the band labeled with β-actin antibodies. This value was further normalized by dividing it by the value obtained for the control culture for each set of cultures and multiplied by 100%. (*P = 0.5357 and **P = 0.0001 using a one sample t-test).
Steady state KCC2 levels were analyzed by Western blot and the values were determined by adding the IOD for the band corresponding to the monomer plus the IOD of the band corresponding to the dimer and dividing it by the IOD for the band labeled by β-actin antibodies. This value was further normalized by dividing it by the normalized IOD obtained from the control culture that was run on the same gel and exposed to the same film and multiplied by 100%. A total of 8 cultures were treated with each drug. The steady state protein levels of KCC2 after a week in culture were not significantly altered by treatment with the GABAB agonist baclofen (108.38 ± 12.03%; N = 8, P = 0.5357; Figure 5B). The steady state protein levels of KCC2 were greatly reduced by treatment with the L-type calcium channel antagonist nimodipine (46.38 ± 5.37%; N = 8, P = 0.0001; Figure 5B). Thus, the upregulation of steady state KCC2 expression that normally occurs during the first postnatal week in culture appears to be dependent, at least in part, on calcium influx through L-type channels.
DISCUSSION
GABA is the main inhibitory neurotransmitter within the adult brain. However, activation of GABAA receptors has an excitatory effect in immature neurons due to the high intracellular chloride levels (Cherubini et al., 1991). Later in development the activation of GABAA receptors has an inhibitory effect that persists into adulthood. Changes in chloride transporter expression levels during the first few postnatal weeks of development are thought to underlie this developmental switch (Plotkin et al., 1997; Lu et al., 1999; Rivera et al., 1999; Hübner et al., 2001). The mechanisms involved in signaling the shift in transporter expression are not known. It has been demonstrated that blockade of GABAA receptors or L-type calcium channels delays the change in the reversal potential of chloride in developing hippocampal neurons (Ganguly et al., 2001). Prior to the switch in reversal potential for the GABAA receptor, activation of these receptors causes calcium influx through voltage-dependent calcium channels due to the depolarizing nature of the response. These data from Ganguly and co-workers (2001) suggest that L-type calcium current may play a role in the changes in chloride transporter expression that occurs in the rat hippocampus during the first two postnatal weeks of development. Our laboratory has demonstrated that activation of the GABAB metabotropic receptor can enhance calcium influx through L-type calcium channels and attenuate calcium influx through N-type calcium channels during the early neonatal period (Carter and Mynlieff, 2004). The attenuation of N-type calcium current by GABAB receptors is known to persist into adulthood in the hippocampus and is likely to modulate neurotransmitter release from the presynaptic terminal (Dutar and Nicoll, 1988; Harrison, 1990; Lambert et al., 1991; Thompson and Gähwiler, 1992; Davies and Collingridge, 1993; Pfrieger et al., 1994; Wu and Saggau, 1995). The functional role of L-type calcium channel enhancement is less clear. In the present study we demonstrated that the facilitation of L-type calcium current by GABAB receptor activation is most prominent in the second postnatal week and that influx of calcium through L-type calcium channels is necessary for the upregulation of KCC2 protein levels in the first postnatal week within the hippocampus. Thus, these findings support the hypothesis that calcium influx through L-type channels is involved in the developmental switch of the GABAA response by affecting chloride transporter expression.
In this study we found that the facilitation of L-type calcium current by GABAB receptor activation is a developmental phenomenon and is greatest in hippocampal neurons isolated from 7 day old rats. Experiments on the HVA and LVA current density at various time points in this early postnatal period suggest that a change in the distribution of channel types in the cells cannot explain the differences in the effect of GABAB receptor activation at different time points. Even though we do not see a change in the total amount of sustained current throughout development, there may be variations in the contribution of the different forms of L-type calcium channels. It has been demonstrated that L-type Cav1.2 protein levels peak in hippocampal neurons isolated from 7 day old rats (Nuñez and McCarthy, 2007). A peak facilitation of L-type current by baclofen at approximately one week may be a reflection of the peak expression of Cav1.2 protein levels in these neurons. This idea is also supported by the fact that using immunohistochemical techniques we have shown that less than 30% of hippocampal neurons taken from 7 day old pups demonstrate significant amounts of the Cav1.2 channels (unpublished data), which may reflect the subset of cells that demonstrate the facilitation of L-type calcium current by GABAB receptors.
Our developmental timecourse of NKCC1 protein levels is consistent with previous studies looking at NKCC1 mRNA and protein within rat hippocampal neurons (Yan et al., 2001; Wang et al., 2002). These studies observed an upregulation of both NKCC1 mRNA and protein in the developing rat hippocampus with a peak level of expression within the adult. The Western blot analysis of NKCC1 by Yan et al. (2001) demonstrated a trend for an increase in the first three postnatal weeks but only in the adult was the increase statistically significant, which is very similar to the data presented here. Our preparations from fresh hippocampal protein also contain glial cells, which are one of the main cell types that express NKCC1 protein in adult hippocampus (Kanaka et al., 2001; Yan et al., 2001; Wang et al., 2002). The fact that our in vivo preparations contain glial cells could explain why NKCC1 protein expression levels are higher in the preparations isolated from adult tissue versus tissue isolated from neonates. However, some studies have seen peak expression of NKCC1 mRNA and protein within the rat hippocampus at postnatal day 7 with very little expression in the adult (Plotkin et al., 1997). Our cultures are strictly neuronal and do not support glial cells (Mynlieff, 1997). Fresh tissue consistently demonstrated higher NKCC1 levels than age matched cultures, which is likely due to the lack of glial cells in the cultures. Therefore, all NKCC1 protein levels measured in culture reflect expression within the first two postnatal weeks exclusively in neurons.
The timing of the baclofen response and Cav1.2 expression is consistent with the upregulation of KCC2 protein levels during the first two postnatal weeks both in vivo and in hippocampal cultures. In whole hippocampal protein extracts from 1, 4, and 6 day old rats and in cultures isolated on day 0 and kept in culture for 1, 4, and 6 days, KCC2 protein levels remain relatively low. However, in fresh hippocampal extracts as well as in cells kept in culture, the slope of the KCC2 protein levels drastically increases between day 6 and day 8, correlating well with the peak in the enhancement of L-type calcium current by GABAB receptor activation at day 7 (Figure 6). KCC2 protein levels continue to rise throughout the first few weeks of development and plateau by three weeks of age. This trend in KCC2 expression in vivo was similar to what was seen in our cultured hippocampal neurons as well as by others studying expression of KCC2 by Western blot analysis or mRNA levels (Rivera et al., 1999; Lu et al., 1999; Wang et al., 2002; Stein et al., 2004; Nuñez and McCarthy, 2007). The fact that we do not see dramatic changes in NKCC1 transporter protein levels during the first two postnatal weeks suggest that the ratio of KCC2 to NKCC1, versus the downregulation of NKCC1 protein levels may be responsible for the change in the reversal potential of chloride and thus, the developmental switch of the GABAA response from excitatory to inhibitory.
Figure 6.
Comparison of cell percentage demonstrating L-type current facilitation and KCC2 steady state levels in cultured hippocampal neurons. The bar graph represents the percent of cells that exhibited L-type calcium current facilitation in response to GABAB receptor activation at different developmental ages. The line graph represents the steady state KCC2 levels in cultured hippocampal neurons determined by Western blot analysis.
NKCC1 expression in hippocampal cultures was not affected by either baclofen or nimodipine treatment. While these data suggest that neither GABAB receptor activation nor L-type calcium current is directly involved in developmental changes of NKCC1 protein levels, the involvement of L-type calcium current in KCC2 expression would still cause a change in the KCC2/NKCC1 ratio. Ultimately it is the ratio of these two transporters that will determine the internal chloride concentration since they carry chloride in opposite directions. Thus, a change in just one transporter will alter the ratio and affect the chloride reversal potential. Relatively little change in the NKCC1 protein levels during the first two weeks in culture is offset by the large increase in KCC2 protein levels.
Treatment of hippocampal cultures with the L-type calcium channel blocker nimodipine significantly reduced the upregulation of KCC2 protein levels compared to the cultures not treated with the blocker. Calcium influx through L-type calcium channels appears necessary to signal a pathway involved in the upregulation of KCC2. These findings support the model that the elevation of intracellular calcium levels through voltage-dependent calcium channels promotes the developmental switch of the GABAA response from excitatory to inhibitory. This role of L-type calcium channels in regulating KCC2 protein levels also suggests that L-type calcium current facilitation by GABAB receptor activation could enhance the upregulation of KCC2 during the early neonatal period within the rat hippocampus. However, when cultures were treated with the GABAB agonist baclofen there was no significant change in KCC2 protein levels compared to the untreated cultures. There are a number of reasons that enhancement of L-type calcium current by activation of GABAB receptors may be important in this developmental switch without allowing us to demonstrate an effect in the current study. Typically, only about 25-30% of the neurons demonstrate an enhancement of the L-type calcium current with GABAB receptor activation. The effect of baclofen may have been quite significant on the KCC2 expression in these individual cells, but when the tissue was pooled for a Western blot it would not be sufficient to see a significant change in the protein levels. Ideally, one would need to perform experiments such as single cell RT-PCR to determine if the chloride transporters are affected by GABAB receptor activation differently in distinct cells. In addition, there is still a large amount of L-type calcium current in the absence of GABAB receptor activation. The activation of the GABAB receptors merely “enhances” the whole cell current that is already present. To see a difference in protein levels by Western blot analysis one needs a very robust change such as that seen with nimodipine. Perhaps the “enhancement” seen with baclofen was not sufficiently large to analyze by this method.
In conclusion, the activation of voltage-dependent calcium channels following depolarizing GABAA responses aids in signaling the developmental switch of the GABAA response from excitatory to inhibitory. Here we have demonstrated that L-type calcium current plays a role in the upregulation of KCC2 protein levels during the first few weeks of hippocampal development without causing a significant change in the NKCC1 protein levels.
Supplementary Material
Acknowledgments
The monoclonal NKCC1 antibody developed by Christian Lytle and Bliss Forbush III was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We would like to thank Matthew M. Marcetich, Eddie K. Brotkowski, and Caroline M. Freitag for their technical assistance and Pinfen Yang for critical reading of the manuscript. This work was supported by a grant from the NIH (NS 048900) and the Biological Sciences Department at Marquette University.
References
- Balakrishnan V, Becker M, Lohrke S, Nothwang HG, Guresir E, Friauf E. Expression and function of chloride transporters during development of inhibitory neurotransmission in the auditory brainstem. J Neurosci. 2003;23:4134–4145. doi: 10.1523/JNEUROSCI.23-10-04134.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: The nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant Synaptic Potentials in Immature Rat CA3 Hippocampal Neurons. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABAB receptors. Physiol Rev. 2004;84:835–837. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-Chloride Cotransporters and Neuronal Function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Carter TJ, Mynlieff M. Gamma-aminobutyric acid type B receptors facilitate L-type and attenuate N-type Ca2+ currents in isolated hippocampal neurons. J Neurosci Res. 2004;76:323–333. doi: 10.1002/jnr.20085. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Benari Y. GABA - An Excitatory Transmitter in Early Postnatal Life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Davies CH, Collingridge GL. The Physiological Regulation of Synaptic Inhibition by GABAB Autoreceptors in Rat Hippocampus. J Physiol. 1993;472:245–265. doi: 10.1113/jphysiol.1993.sp019945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpire E. Cation-chloride cotransporters in neuronal communication. News Physiol Sci. 2000;15:309–312. doi: 10.1152/physiologyonline.2000.15.6.309. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. A Physiological Role for GABAB Receptors in the Central Nervous System. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Fiumelli H, Woodin MA. Role of activity-dependent regulation of neuronal chloride homeostasis in development. Curr Opin Neurobiol. 2007;17:81–86. doi: 10.1016/j.conb.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Fujikawa S, Motomura H, Ito Y, Ogata N. GABAB-mediated upregulation of the high-voltage-activated Ca2+ channels in rat dorsal root ganglia. Pflugers Arch. 1997;434:84–90. doi: 10.1007/s004240050366. [DOI] [PubMed] [Google Scholar]
- Gähwiler BH, Brown DA. GABAB-Receptor-Activated K+ Current in Voltage-Clamped CA3 Pyramidal Cells in Hippocampal Cultures. Proc Natl Acad Sci U S A. 1985;82:1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanopoulou AS. GABAA receptors in normal development and seizures: Friends or foes? Curr Neuropharmacol. 2008;6:1–20. doi: 10.2174/157015908783769653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly K, Schinder AF, Wong ST, Poo M. GABA itself promotes the developmental switch of neuronal GABAergic responses from excitation to inhibition. Cell. 2001;105:521–532. doi: 10.1016/s0092-8674(01)00341-5. [DOI] [PubMed] [Google Scholar]
- Harrison NL. On the Presynaptic Action of Baclofen at Inhibitory Synapses Between Cultured Rat Hippocampal Neurons. J Physiol. 1990;422:433–446. doi: 10.1113/jphysiol.1990.sp017993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübner CA, Stein V, Hermans-Borgmeyer I, Meyer T, Ballanyi K, Jentsch TJ. Disruption of KCC2 reveals an essential role of K-Cl cotransport already in early synaptic inhibition. Neuron. 2001;30:515–524. doi: 10.1016/s0896-6273(01)00297-5. [DOI] [PubMed] [Google Scholar]
- Kahle KT, Staley KJ, Nahed BV, Gamba G, Hebert SC, Lifton RP, Mount DB. Roles of the cation-chloride cotransporters in neurological disease. Nat Clin Pract Neurol. 2008;4:490–503. doi: 10.1038/ncpneuro0883. [DOI] [PubMed] [Google Scholar]
- Kanaka C, Ohno K, Okabe A, Kuriyama K, Itoh T, Fukuda A, Sato K. The differential expression patterns of messenger RNAs encoding K-Cl cotransporters (KCC1,2) and Na-K-2Cl cotransporter (NKCC1) in the rat nervous system. Neuroscience. 2001;104:933–946. doi: 10.1016/s0306-4522(01)00149-x. [DOI] [PubMed] [Google Scholar]
- Kanaumi T, Takashima S, Iwasaki H, Mitsudome A, Hirose S. Developmental changes in the expression of GABAA receptor alpha 1 and gamma 2 subunits in human temporal lobe, hippocampus and basal ganglia: An implication for consideration on age-related epilepsy. Epilepsy Res. 2006;71:47–53. doi: 10.1016/j.eplepsyres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Leinekugel X, Khalilov I, Gaiarsa JL, Benari Y. Synchronization of GABAergic interneuronal network in CA3 subfield of neonatal rat hippocampal slices. J Physiol. 1997;498:763–772. doi: 10.1113/jphysiol.1997.sp021900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert NA, Harrison NL, Teyler TJ. Baclofen-Induced Disinhibition in Area CA1 of Rat Hippocampus Is Resistant to Extracellular Ba2+ Brain Res. 1991;547:349–352. doi: 10.1016/0006-8993(91)90985-5. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Tseeb V, Benari Y, Bregestovski P. Synaptic GABAA Activation Induces Ca2+ Rise in Pyramidal Cells and Interneurons from Rat Neonatal Hippocampal Slices. J Physiol. 1995;487:319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Benari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, Ben Ari Y, Khazipov R. Giant depolarizing potentials: the septal pole of the hippocampus paces the activity of the developing intact septohippocampal complex in vitro. J Neurosci. 1998;18:6349–6357. doi: 10.1523/JNEUROSCI.18-16-06349.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu QL, Wong-Riley MTT. Developmental changes in the expression of GABAA receptor subunits alpha 1, alpha 2, and alpha 3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Lu J, Karadsheh M, Delpire E. Developmental regulation of the neuronal-specific isoform of K-Cl cotransporter KCC2 in postnatal rat brains. J Neurobiol. 1999;39:558–568. [PubMed] [Google Scholar]
- Mynlieff M. Dissociation of postnatal hippocampal neurons for short term culture. J Neurosci Methods. 1997;73:35–44. doi: 10.1016/s0165-0270(96)02209-1. [DOI] [PubMed] [Google Scholar]
- Newberry NR, Nicoll RA. Direct Hyperpolarizing Action of Baclofen on Hippocampal Pyramidal Cells. Nature. 1984;308:450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Nuñez JL, McCarthy MM. Evidence for an extended duration of GABA-Mediated excitation in the developing male versus female hippocampus. Dev Neurobiol. 2007;67:1879–1890. doi: 10.1002/dneu.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- Payne JA, Rivera C, Voipio J, Kaila K. Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 2003;26:199–206. doi: 10.1016/S0166-2236(03)00068-7. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Gottmann K, Lux HD. Kinetics of GABAB Receptor-Mediated Inhibition of Calcium Currents and Excitatory Synaptic Transmission in Hippocampal Neurons In Vitro. Neuron. 1994;12:97–107. doi: 10.1016/0896-6273(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Plotkin MD, Snyder EY, Hebert SC, Delpire E. Expression of the Na-K-2Cl cotransporter is developmentally regulated in postnatal rat brains: A possible mechanism underlying GABA’s excitatory role in immature brain. J Neurobiol. 1997;33:781–795. doi: 10.1002/(sici)1097-4695(19971120)33:6<781::aid-neu6>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Nocera R, Fuller LM, Kordower JH, Armstrong DM. Age-related alterations in GABAA receptor subunits in the nonhuman primate hippocampus. Brain Res. 2006;1073:120–130. doi: 10.1016/j.brainres.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Scholz KP, Miller RJ. GABAB Receptor-Mediated Inhibition of Ca2+ Currents and Synaptic Transmission in Cultured Rat Hippocampal-Neurons. J Physiol. 1991;444:669–686. doi: 10.1113/jphysiol.1991.sp018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic GABA receptors facilitate L-type and inhibit N-type calcium channels in single salamander retinal neurons. J Physiol. 1999;516:711–718. doi: 10.1111/j.1469-7793.1999.0711u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebler M, Koller H, Stichel CC, Muller HW, Freund HJ. Spontaneous Activity and Recurrent Inhibition in Cultured Hippocampal Networks. Synapse. 1993;14:206–213. doi: 10.1002/syn.890140304. [DOI] [PubMed] [Google Scholar]
- Stein V, Hermans-Borgmeyer I, Jentsch TJ, Hubner CA. Expression of the KC1 cotransporter KCC2 parallels neuronal maturation and the emergence of low intracellular chloride. J Comp Neurol. 2004;468:57–64. doi: 10.1002/cne.10983. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kajikawa Y, Tsujimoto T. G-protein-coupled modulation of presynaptic calcium currents and transmitter release by a GABAB receptor. J Neurosci. 1998;18:3138–3146. doi: 10.1523/JNEUROSCI.18-09-03138.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SM, Gähwiler BH. Comparison of the Actions of Baclofen at Presynaptic and Postsynaptic Receptors in the Rat Hippocampus In Vitro. J Physiol. 1992;451:329–345. doi: 10.1113/jphysiol.1992.sp019167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejón C, Collin C, Mckay RDG, Segal M. Neurotrophins induce formation of functional excitatory and inhibitory synapses between cultured hippocampal neurons. J Neurosci. 1998;18:7256–7271. doi: 10.1523/JNEUROSCI.18-18-07256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Shimizu-Okabe C, Watanabe K, Okabe A, Matsuzaki H, Ogawa T, Mori N, Fukuda A, Sato K. Developmental changes in KCC1, KCC2, and NKCC1 mRNA expressions in the rat brain. Brain Res Dev Brain Res. 2002;139:59–66. doi: 10.1016/s0165-3806(02)00536-9. [DOI] [PubMed] [Google Scholar]
- Wu LG, Saggau P. GABAB Receptor-Mediated Presynaptic Inhibition in Guinea-Pig Hippocampus Is Caused by Reduction of Presynaptic Ca2+ Influx. J Physiol. 1995;485:649–657. doi: 10.1113/jphysiol.1995.sp020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YP, Dempsey RJ, Sun DD. Expression of Na+-K+-Cl- cotransporter in rat brain during development and its localization in mature astrocytes. Brain Res. 2001;911:43–55. doi: 10.1016/s0006-8993(01)02649-x. [DOI] [PubMed] [Google Scholar]
- Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- Yuste R, Katz LC. Control of Postsynaptic Ca2+ Influx in Developing Neocortex by Excitatory and Inhibitory Neurotransmitters. Neuron. 1991;6:333–344. doi: 10.1016/0896-6273(91)90243-s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.