Synopsis
Age-related changes in immune function leave older adults at risk for a host of inflammatory diseases. Immune-mediated inflammatory processes are regulated by neuroendocrine hormones, including glucocorticoids, dehydroepiandrosterone (DHEA), and the catecholamines, epinephrine and norepinephrine. This regulation, however, becomes impaired in older adults in light of age-related changes in endocrine function. Chronic stress shows similarly harmful effects on neuroendocrine and immune function and may, therefore, combine with age to further increase disease risk in older adults. This article highlights evidence for the impact of age and stress on neuroendocrine regulation of inflammatory processes that may substantially increase risk for inflammatory disease at older ages.
Keywords: stress, HPA axis, inflammation, inflammatory disease, aging, older adults
Introduction
Aging is accompanied by immunosenescence, a gradual and natural change in immune system structure and function. Many of these changes lead to substantial immune dysregulation, leaving older adults at increased risk for infection, compromised wound healing, and poor oral health. Immunosenescence is also implicated in chronic, low level inflammation that is linked to a host of chronic, age-related diseases, including rheumatoid arthritis, atherosclerosis, osteoporosis, and type-2 diabetes [1-3]. The relative balance of the production of inflammatory mediators by subsets of T-helper (Th) cells, namely Th1 and Th2 cells, also shifts with aging and has consequences for immunity and health in the elderly [4]. Psychological stress has similar costs: chronic stress, such as ongoing interpersonal strain or caregiving for a spouse with dementia, has many of the same dysregulating effects on inflammatory processes as seen with aging [5]. Thus, there may be potentially additive or synergistic effects of immunosenescence and stress on immune and inflammatory dysregulation in older adults, further increasing disease risk.
The physiological stress response is a function of the dynamic interplay among the nervous, endocrine and immune systems, and is intended to promote adaptation and homeostasis in the face of environmental challenges (a violent aggressor) or physical stressors (tissue injury or infection). Psychological stressors, like worry, anxiety and perceived lack of control, also activate stress systems to support adaptation by the organism. When chronically or repeatedly activated, however, the products of these systems can damage tissues and contribute to disease development [6].
For older adults, physiological activation in response to stressors is transposed upon age-related dysregulation of stress response systems. Neuroendocrine activation is the primary stimulus for fight-flight responses, originating in the hypothalamus and resulting in secretion of the so-called stress hormones by the adrenal glands. Adrenal hormones released in response to stress, including glucocorticoids, dehydroepiandrosterone (DHEA), and the catecholamines, epinephrine and norepinephrine, can each independently modulate immune function. Importantly, neuroendocrine function, like immune function, is altered with both aging and chronic stress [7,8]. Accordingly, aging and stress-related neuroendocrine dysregulation may combine to further disrupt immune function, increasing risk for or exacerbating inflammatory disease in older adults. The current review highlights evidence for the impact of age and stress on adrenal stress hormone regulation of inflammatory processes that may substantially increase risk for inflammatory disease at older ages.
Aging, Stress, and Inflammatory Processes
Immunosenescence is characterized by two, inter-related changes in inflammatory activity that can place older adults at risk for chronic, inflammatory diseases. The first relates to chronic activation of innate, inflammatory responses, marked by increasing levels and impaired synthesis of pro-inflammatory cytokines, especially interleukin-6 (IL-6) [9]. Indeed, IL-6 is a potent predictor of mortality in older adults [10,11], and IL-6 and other pro-inflammatory mediators have been implicated in the development of a host of inflammatory diseases, including cardiovascular disease, type 2 diabetes, osteoporosis, and arthritis [12-14].
Age-related changes in adaptive or acquired cell-mediated immunity also occur and are accompanied by changes in Th1 and Th2 effector cell activity. Th1 cells primarily secrete interleukin-2 (IL-2), interferon gamma (IFN-γ), and tumor necrosis factor beta (TNF-β), required for cell-mediated inflammatory reactions that can efficiently eliminate intracellular pathogens. Th2 cells primarily secrete IL-4, IL-5, IL-10, and IL-13, required for humoral immunity. IL-4 and IL-10 stimulate B cells to produce antigen and immunoglobulin switching to IgE, as well as mast cell and eosinophils growth and activation [15,16].
Th1 and Th2 cytokines are mutually inhibitory, and, ideally, maintain a homeostatic equilibrium between cell-mediated and humoral responses [17,18]. Aging, however, is associated with a decline in this equilibrium. Th1 cytokine secretion declines with advancing age, especially impaired expression of IL-2 [19], and there is a shift toward a Th2 anti-inflammatory response, marked by increases in IL-10 secretion and expression [20,21]. Such shifts have proposed links to increased risk for or exacerbation of atopic allergy, allergic rhinitis, asthma, autoimmunity and chronic infection in susceptible individuals [17,22-24].
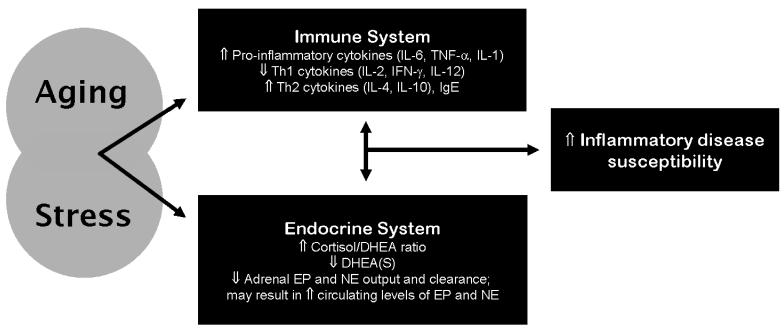
Notably, chronic stress, like age, is associated with increases in circulating levels of pro-inflammatory cytokines [4,12,25,26], as well as a shift toward Th2 responses [17,18,21]. Age and chronic stress are also each associated with changes in adrenal stress hormone function which, in light of substantial hormonal modulation of inflammatory activity, may play a prominent role in exacerbating effects of stress on inflammation, Th1 to Th2 shift, and relevant disease risk in older adults. Changes in these relevant aspects of immunological and endocrinological function are depicted in Figure 1.
Figure 1.
Aging and stress each impact endocrine and immune function, affecting regulation of inflammatory mediators and adrenal stress hormones. Stress-related dysregulation of these systems may combine with age-related dysregulation to render older adults particularly vulnerable to inflammatory disease. Abbreviations: IL, interleukin; TNF, tumor necrosis factor; Th, T helper; Ig, immunoglobulin; DHEA(S), dehydroepiandrosterone (sulfate); EP, epinephrine; NE, norepinephrine.
The Neuroendocrine Stress Response and Modulation of Immunity: Age Effects
The aging and remodeling of the endocrine system – endocrinosenescence -- is closely related to immunosenescence, due to the immunomodulating properties of endocrine hormones [27]. Endocrine dysregulation affects the course and consequences of activation of the hypothalamic-pituitary-adrenal (HPA) axis and the sympathetic-adrenal-medullary (SAM) axis, which together coordinate physiological responses to stress.
Comprehensive reviews of the physiological stress response are available [28,29]. Briefly, in response to both physical and psychological stressors, and integrating input from processing centers including the amygdala, prefrontal cortex, and hippocampus, the hypothalamus releases corticotropin-releasing hormone (CRH) from the paraventricular nucleus. CRH stimulates the pituitary gland to release adrenocorticotropic hormone (ACTH). From the brain, ACTH enters circulation and ultimately stimulates the cortex of the adrenal glands to produce glucocorticoids, of which cortisol is the primary stress hormone secreted in humans. Other androgens can be released as well, including dehydroepiandrosterone (DHEA), an endogenous hormone that regulates activities of cortisol, although its physiological significance and role in disease are not well understood [30,31]. In parallel, due to hypothalamic sympathetic innervation of the adrenal gland's medulla, sympathetic activation results in release of catecholamines into circulation, including epinephrine and norepinephrine. Together, cortisol and epinephrine upregulate glucose metabolism and cardiovascular activity to support fight or flight. Cortisol also suppresses aspects of immune activity during stress, whereas DHEA may serve to moderate these immunosuppressive affects, as DHEA is shown to antagonize effects of glucocorticoids.
Thus, the HPA axis is a dynamic, regulatory system that supports life-sustaining adjustments aimed at maintaining homeostasis, and is, thus, one of the most important allostatic or adaptive systems [32]. Aging, however, is associated with changes in HPA axis morphology and function that affect cortisol, DHEA, and catecholamine regulation and responses to stress, and, consequently, regulation of inflammatory processes.
Cortisol
Age-related changes
Secretion of glucocorticoids by the HPA axis occurs through spontaneous, pulsatile, circadian fluctuations, as well as in response to stress, and has widespread affects on immune function under both basal and stressful conditions. Cortisol secretion is maintained with advancing age. Evidence from animal and human studies suggests that glucocorticoid levels remain constant throughout adulthood [32,33] and may even increase [7,34,35], although observed diurnal increases may be more evident among distressed older adults [36] or individuals with impairments in physical functioning [37]. Other studies indicate higher nocturnal levels of cortisol among older compared to younger adults [32,38], resulting in greater overall cortisol circulation across the diurnal cycle. Further, cortisol remains elevated relative to age-related declines in other adrenal hormones [39].
Aging also appears to affect glucocorticoid output in response to HPA activation, although evidence is mixed and may depend on the source of activation as well as subject characteristics. In pharmacological challenge studies, CRH administration increases circulating and salivary cortisol levels to a greater degree in older compared to younger adults [40-42]. Age is also associated with a reduced suppression of cortisol secretion by dexamethasone, though age-related effects may be stronger in women [40]. Psychological challenge studies show greater inconsistency, with some studies supporting age-related increases in cortisol response [43,44], while others show no age differences [41,45] or reduced responsiveness in older adults [46] There are apparent gender differences, as well as other moderators of aging effects on cortisol response, such as fitness level [47]. In a recent meta-analysis [42], age had stronger effects on cortisol responses to pharmacological and psychosocial challenges in women compared to men. However, older men overall show larger cortisol responses to psychological stress compared to older women [44,48], a gender effect consistently observed across the age range [49]. Mixed findings from stress reactivity studies comparing men versus women, and older and younger adults, may be due to a host of factors. For example, there is evidence that sex steroids, which of course vary by age and gender, modulate HPA reactivity [49]. Further, different kinds of stressors (e.g., interpersonal versus cognitive) and other psychosocial factors (e.g., social environment) modulate cortisol reactivity differently in men versus women [50], and older versus younger adults [51]. Thus, the nature of the stressor used in research must be considered when interpreting age and gender differences.
Major contributors to age-related changes in cortisol regulation and responses to stress include age-related impairments in negative feedback sensitivity of the HPA axis to cortisol [40,52], increased adrenal sensitivity to ACTH [53], and the decline of adrenal hormones, such as DHEA, that regulate cortisol production [8]. Glucocortiocoids are key regulators of inflammatory responses during and following stress , and contribute to stress-related shifts toward Th2 responses [16]. Thus, age-related dysregulation of cortisol secretion may substantially impact regulation of inflammatory responses during a stress response, thereby increasing older adults' risk for inflammatory disease.
Regulation of pro-inflammatory cytokines
Glucocorticoids have strong anti-inflammatory effects, primarily mediated through their interaction with glucocorticoid receptors (GR) maintained in the cytoplasm of immune cells. Glucocorticoid receptor activation inhibits inflammatory cytokine production by blocking the activation of transcription factors, such as NF-κB, that are responsible for cytokine gene expression.
The functional effects of glucocorticoids, however, depend on the sensitivity of target tissues to the hormones. For example, as noted above, aging reduces negative feedback sensitivity of the HPA axis, characterized by reduced glucocorticoid inhibition of CRH and ACTH secretion [41,52]. Stress and aging modulate glucocorticoid sensitivity in the immune system as well. Evidence suggests that glucocorticoid sensitivity of cytokine-producing immune cells is rapidly increased by physical stressors [54], as well as acute psychological stressors [45], resulting in substantial anti-inflammatory effects of glucocorticoids. Glucocorticoid sensitivity appears to be retained [55] or even increased at older ages [45]. On the contrary, more chronically stressful and distressing circumstances, such as low SES in early life [56], caring for a child with cancer [57], or caregiving for a spouse with dementia [36], are associated with resistance to anti-inflammatory effects of glucocorticoids. Further, glucocorticoid inhibition of IL-6 production was lower in older compared to younger men following psychological stress-induced HPA activation [58], and treatment with testosterone diminished the age-related reduction in stress-induced glucocorticoid resistance. Finally, glucocorticoid resistance in certain inflammatory diseases, such as steroid-resistant asthma, rheumatoid arthritis, and inflammatory bowel disease, is well-documented [59,60].
Glucocorticoid resistance can develop through various pathways, including genetic susceptibility, down regulation of glucocorticoid receptors and alterations in the expression of transcription factors necessary for glucocorticoid signaling [61,62]. These pathways are regulated by both glucocorticoids and pro-inflammatory cytokines [62]. For example, glucocorticoids can down regulate glucocorticoid receptor expression [63], and pro-inflammatory cytokines can activate transcription pathways that inhibit glucocorticoid receptor signaling [64]. Thus, combined influences of age and stress on increases in pro-inflammatory cytokine production and cortisol may further exacerbate alterations in glucocorticoid sensitivity and consequent inflammation in older adults.
Regulation of Th1 and Th2 responses
Glucocortiocids have strong effects on Th1 and Th2 responses. Cortisol can inhibit production of IL-12 [17], a major inducer of Th1 responses. Glucocorticoids also suppress Th1 cytokines directly, including production of IFN-γ and IL-2, leaving intact or augmenting production of Th2 cytokines, including IL-4 and IL-10 [65,66]. As a result, glucocorticoids can enhance Th2 functions, such as production of immunoglobulin E (IgE). In prior clinical studies, stress was associated with elevated IgE [67,68], and we recently demonstrated the ability of stress and anxiety to enhance allergen-specific IgE responses [69]. Further, altered HPA responsiveness is observed in inflammatory diseases [23,70]. Thus, altered stress responsiveness of the HPA axis with advancing age may contribute to dysregulation of Th1 and Th2 cytokine production and increase older adults' risk for Th1 and Th2-mediated inflammatory disease.
In addition, activation and termination of an immune response depends on complex and interactions between the innate and adaptive arms of immunity, specifically, interactions of antigen presenting cells (APCs) and T cells, respectively. As already noted, IL-12 is the primary stimulator of TH1 responses. IL-12 is produced by APCs, including monocytes/macrophages and dendritic cells, and these cells also secrete the anti-inflammatory cytokine IL-10. Studies show increases in both IL-12 and IL-10 with aging, suggesting a possible compensatory process [20]. There is also evidence for age-related impairment of communication between APCs and T cells [20]. The role of stress hormones in changes in APC cell-to-T cell communication as a function of age or stress remains to be characterized.
DHEA
Age related changes
Dehydroepiandrosterone (DHEA) and its inactive precursor, DHEA sulfate (DHEAS) are the most abundant adrenal steroid hormones in circulation in humans [71], and their steady decline with advancing age is a well-recognized pattern [30]. DHEA/DHEAS peak during the third decade of life and by the end of the eighth decade are at 10-30% of peak levels [32,72,73]. The age-related decline is believed to be due primarily to the morphological changes of the adrenal cortex, particularly the reduction in size of the zona reticularis [74,75], the exclusive source of DHEA. The extent of age-related decline in DHEA(S), however, shows marked interindividual variability [76] may also be gender differences. In two studies women showed less decline relative to men [77,78], though Mazat et al. [79] found greater decline in women.
Like cortisol, DHEA is secreted by the adrenal cortex in response to CRH and ACTH stimulation [33], and there is evidence that both pharmacological challenges and psychological stressors provoke increases in circulating and salivary levels of DHEA(S) in humans [80,81] and non-human primates [82]. Less is known about how stress-responsivity of DHEA(S) is affected by age, but older individuals show reduced DHEA secretion in response to ACTH stimulation compared to [83]. Importantly, DHEA and cortisol have opposing effects on immune function [84], and cortisol/DHEA(S) ratio increases with age. Thus, cortisol/DHEAS ratio may provide more information about neuroendocrine-immune interactions that compromise health at older ages [7,85].
Regulation of inflammatory processes
DHEAs have direct effects on cytokine-producing monocytes and lymphocytes, and evidence suggests its potential role in reducing the inflammatory affects of immunosenescence. DHEA diminished IL-6 secretion by lymphoid cells of aged mice [86] and murine macrophages stimulated with LPS [87]. DHEA was shown to increase Th1 cytokines [86,88,89] and inhibit in vitro IL-6 secretion in a study of postmenopausal women [90].
DHEA may also affect inflammatory cytokine production indirectly through its suppressive effects on cortisol production [8]. Animal models of trauma show that supplementation with DHEA can attenuate the trauma-related rise in corticosterone and enhance T-cell secretion of IL-2, IL-3 and IFN-γ, thereby restoring splenocyte proliferation that is typically depressed following traumatic injury [88]. As noted, the ratio of cortisol to DHEA increases with age; thus, the decline in DHEA and resulting glucocorticoid excess together may impact on inflammatory function under basal conditions and in response to stress.
In addition, downstream products of DHEA synthesis may be primarily responsible for attenuation of age-related inflammatory dysregulation, as others have shown sex steroids and other androgens for which DHEA is a precursor to modulate inflammatory cytokine production [91]. For example, age-related immunosuppression was associated with a Th1 (IFN-γ) to Th2 (IL-4) shift in burn-injured mice [92], and a significantly greater increase in IL-6 [93]. In both cases, the effect of age on cytokine production was reduced with estrogen treatment. Indeed, evidence has accumulated that sex steroids are key inflammatory regulators [94].
Evidence regarding the suppressive versus enhancing effects of DHEA on pro-inflammatory cytokine production is equivocal, however. IL-6 production by human monocytes stimulated by LPS was enhanced by DHEA [95]. T-cells from DHEA-treated older men, when stimulated with non-specific mitogen, also showed enhanced IL-6 production, but IL-2 production was also improved [96], suggesting an enhanced Th1 response. In contrast, DHEA supplementation had little influence on mitogen-stimulated IL-6 production in a study of post-menopausal women [97], although other immune effects of DHEA were found, including enhancement of natural killer cell cytotoxicity. It has been suggested that the mixed findings in human studies may be due to inconsistent DHEA exposure (for example, longer-term in vivo administration versus short-term administration in vitro [98]) and supplementation dosage [8]. In spite of mixed findings, DHEA supplementation continues to be regarded as a promising approach to reduce age associated risks for inflammatory diseases [8,98,99].
Sympathoadrenal Hormones
Age-related changes
Compared to the other adrenal stress hormones, much less is known about aging effects on the SAM axis regulation of inflammatory processes. The sympathetic nervous system (SNS) shows changes with age, with increased overall tonic activity at rest, primarily indexed by norepinephrine (NE) output at neuroeffector junctions (that is, NE in its neurotransmitter form) using tracer technologies [100,101]. Enhanced SNS activity, however, is seen in some tissue regions but not others [102-104]. Higher basal circulating levels of NE have also been observed in older compared to younger men [100,105]. In contrast, epinephrine (EP) output by the adrenal medulla under resting conditions was shown to decline with age [106], whereas others had reported a slight decline or no change in circulating levels of EP with aging [107,108]. However, age-related reductions in EP clearance from circulation can obscure interpretations about EP output by the adrenal medulla when measuring circulating catecholamine levels [103].
The same interpretation constraints may also contribute to mixed findings regarding age effects on SAM responses to stress. Adrenal catecholamine output in response to stress also appears to decline with age [103,106]. Again, however, age-related declines in catecholamine clearance may have led to the larger increases in circulating levels of NE observed in older relative to younger adults following physical and psychological stressors [109,110]. Others have not found age to affect circulating NE in response to stress [100,111]. In addition, NE spillover from neuroeffector junctions into circulation increases with age [103]; thus it is unclear whether circulating levels of NE are more a function of spillover or age-related changes in NE output by the adrenal medulla.
In sum, relatively little is known about the effects of age on sympathoadrenal activity during stress, but evidence suggests there may be age-related differences in adrenal output and clearance of catecholamines. The health implications of these age-related changes remain to be determined.
Regulation of inflammatory processes
The SNS has immunomodulating properties [112-114], but less is known about regulation of inflammatory cytokines by in vivo actions of catecholamines secreted by the adrenal medulla during stress. However, epinephrine and cortisol appear to combine to regulate inflammatory cytokine production. For example, under basal conditions, epinephrine was recently shown to enhance LPS-induced production of the anti-inflammatory cytokine IL-10 by monocytes, while inhibiting pro-inflammatory TNF-α and IL-12 [22]; basal cortisol levels did not regulate production of these cytokines ex vivo. In contrast, in vitro studies indicate that both physiologic stress levels of glucocorticoids and epinephrine inhibit production of IL-12, the potent stimulator of Th1 responses [66]. Further, epinephrine and corticosteroids in vitro decrease Th1 cytokine production and increase Th2 cytokine production to a significantly greater degree compared to either adrenal hormone alone [115]. It is unclear how age-related changes in HPA function affect these regulatory pathways.
Taken together, adrenally-secreted catecholamines, specifically epinephrine, play a role in both innate pro-inflammatory cytokine regulation, as well as adaptive Th responses, and may act in concert with cortisol during stress to modulate cytokine activity. Indeed, hyporesponsiveness of the HPA axis and hyperresponsiveness of the SAM axis to psychological stress have been observed in patients with atopic dermatitis, a chronic inflammatory disease primarily mediated by Th2 inflammatory responses [70], further underscoring co-regulatory contributions of these stress hormones. The age-related decline in epinephrine output might be expected to affect inflammatory cytokine regulation and subsequent disease susceptibility; alternatively, age-related reductions in epinephrine clearance may serve a compensatory function.
Summary and Conclusions
Advances in our understanding of neuroendocrine-immune interactions suggest that endocrinosenescence and immunosenescence are tightly linked through hormones and inflammatory cytokines [116]. Evidence further suggests that some of the biological sequelae of stress mimic those observed in human aging, and include increased production of pro-inflammatory cytokines and an impairment of the Th1/Th2 balance. Age-related changes in the HPA axis and in regulatory control of inflammatory processes by stress hormones render older adults vulnerable to inflammatory disease. Transposing stress onto age-related changes in endocrine and inflammatory function likely exacerbates these disease risks. Indeed, greater immunosuppressive effects of stress, such as antibody response to vaccination, have been observed in older compared to younger adults [117].
There remain important questions about the potential additive or synergistic affects of stress and biological aging that may increase older adults' risk for inflammatory disease. First, although this review highlighted the regulatory control of stress hormones on inflammatory processes, inflammatory cytokines regulate the HPA axis as well [118], and so elucidation of age effects on this regulatory pathway is needed. Further, a systems-level approach is necessary to fully understand the pathophysiology of age-related changes in immunity [13,116], and its contribution to disease development. A full model of the inflammatory disease implications of stress and aging must account for the mutual regulation of the endocrine and immune systems. Finally, it has become increasingly clear that immunosenescence is a function of lifetime exposure to pathogens [119]. Models of stress and health suggest that lifetime exposure to stress also contribute to biological aging [120,121]. Thus, developmental changes in the interplay of stress hormones and inflammatory processes must be considered to fully understand the implications of neuroendocrine stress responses for inflammatory disease risk in the elderly.
Acknowledgments
This work was supported by National Institute on Aging grant R24 AG031089-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author has nothing to disclose.
References
- 1.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 2.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6; Proceedings of the National Academy of Sciences of the United States of America; 2003. pp. 9090–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papanicolaou DA, Wilder RL, Manolagas SC, et al. The pathophysiologic roles of interleukin-6 in human disease. Ann Intern Med. 1998;128:127–137. doi: 10.7326/0003-4819-128-2-199801150-00009. [DOI] [PubMed] [Google Scholar]
- 4.Elenkov IJ, Iezzoni DG, Daly A, et al. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 5.Hawkley LC, Cacioppo JT. Stress and the aging immune system. Brain, Behavior, and Immunity. 2004;18:114–9. doi: 10.1016/j.bbi.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. European Journal of Pharmacology. 2008;583:174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bauer ME. Stress, glucocorticoids and ageing of the immune system. Stress. 2005;8:69–83. doi: 10.1080/10253890500100240. [DOI] [PubMed] [Google Scholar]
- 8.Butcher SK, Lord JM, Butcher SK, et al. Stress responses and innate immunity: aging as a contributory factor. Aging Cell. 2004;3:151–60. doi: 10.1111/j.1474-9728.2004.00103.x. [DOI] [PubMed] [Google Scholar]
- 9.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annual Review of Medicine. 2000;51:245–70. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 10.Gruenewald TL, Seeman TE, Ryff CD, et al. Combinations of biomarkers predictive of later life mortality; Proceedings of the National Academy of Sciences of the United States of America; 2006. pp. 14158–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. American Journal of Medicine. 1999;106:506–12. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 12.Black PH. The inflammatory consequences of psychologic stress: relationship to insulin resistance, obesity, atherosclerosis and diabetes mellitus, type II. Medical Hypotheses. 2006;67:879–91. doi: 10.1016/j.mehy.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Giunta S. Exploring the complex relations between inflammation and aging (inflamm-aging): anti-inflamm-aging remodelling of inflamm- aging, from robustness to frailty. Inflammation Research. 2008;57:558–63. doi: 10.1007/s00011-008-7243-2. [DOI] [PubMed] [Google Scholar]
- 14.van Leeuwen MA, Westra J, Limburg PC, et al. Clinical significance of interleukin-6 measurement in early rheumatoid arthritis: relation with laboratory and clinical variables and radiological progression in a three year prospective study. Annals of the Rheumatic Diseases. 1995;54:674–7. doi: 10.1136/ard.54.8.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diehl S, Rincon M. The two faces of IL-6 on Th1/Th2 differentiation. Molecular Immunology. 2002;39:531–6. doi: 10.1016/s0161-5890(02)00210-9. [DOI] [PubMed] [Google Scholar]
- 16.Elenkov IJ. Neurohormonal-cytokine interactions: implications for inflammation, common human diseases and well-being. Neurochemistry International. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov IJ, Chrousos GP. Stress Hormones, Th1/Th2 patterns, Pro/Anti-inflammatory Cytokines and Susceptibility to Disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 18.Marshall GD, Jr., Agarwal SK, Lloyd C, et al. Cytokine dysregulation associated with exam stress in healthy medical students. Brain, Behavior, and Immunity. 1998;12:297–307. doi: 10.1006/brbi.1998.0537. [DOI] [PubMed] [Google Scholar]
- 19.Rink L, Cakman I, Kirchner H. Altered cytokine production in the elderly. Mechanisms of Ageing and Development. 1998;102:199–209. doi: 10.1016/s0047-6374(97)00153-x. [DOI] [PubMed] [Google Scholar]
- 20.Castle SC. Clinical relevance of age-related immune dysfunction. Clinical Infectious Diseases. 2000;31:578–85. doi: 10.1086/313947. [DOI] [PubMed] [Google Scholar]
- 21.Glaser R, MacCallum RC, Laskowski BF, et al. Evidence for a shift in the Th-1 to Th-2 cytokine response associated with chronic stress and aging. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2001;56:M477–82. doi: 10.1093/gerona/56.8.m477. [DOI] [PubMed] [Google Scholar]
- 22.Elenkov IJ, Kvetnansky R, Hashiramoto A, et al. Low- versus high-baseline epinephrine output shapes opposite innate cytokine profiles: presence of Lewis- and Fischer-like neurohormonal immune phenotypes in humans? Journal of Immunology. 2008;181:1737–45. doi: 10.4049/jimmunol.181.3.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eskandari F, Webster JI, Sternberg EM. Neural immune pathways and their connection to inflammatory diseases. Arthritis Res Ther. 2003;5:251–65. doi: 10.1186/ar1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall GD. Neuroendocrine mechanisms of immune dysregulation: applications to allergy and asthma. Annals of Allergy, Asthma & Immunology : Official Publication of the American College of Allergy, Asthma, & Immunology. 2004;93:S11–7. doi: 10.1016/s1081-1206(10)61482-2. [DOI] [PubMed] [Google Scholar]
- 25.Brydon L, Walker C, Wawrzyniak A, et al. Synergistic effects of psychological and immune stressors on inflammatory cytokine and sickness responses in humans. Brain, Behavior, and Immunity. 2009;23:217–24. doi: 10.1016/j.bbi.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou D, Kusnecov AW, Shurin MR, et al. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–30. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 27.Straub RH, Gluck T, Cutolo M, et al. The adrenal steroid status in relation to inflammatory cytokines (interleukin-6 and tumour necrosis factor) in polymyalgia rheumatica. Rheumatology. 2000;39:624–31. doi: 10.1093/rheumatology/39.6.624. [DOI] [PubMed] [Google Scholar]
- 28.Chrousos GP. Stress and disorders of the stress system. Nat Rev Endocrinol. 2009;5:374–81. doi: 10.1038/nrendo.2009.106. [DOI] [PubMed] [Google Scholar]
- 29.Lovallo WR. Stress & health: Biological and psychological interactions. ed 2nd Sage; Thousand Oaks, CA: 2005. [Google Scholar]
- 30.Chahal HS, Drake WM. The endocrine system and ageing. Journal of Pathology. 2007;211:173–80. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- 31.Maninger N, Wolkowitz OM, Reus VI, et al. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) Frontiers in Neuroendocrinology. 2009;30:65–91. doi: 10.1016/j.yfrne.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrari E, Cravello L, Muzzoni B, et al. Age-related changes of the hypothalamic-pituitary-adrenal axis: pathophysiological correlates. European Journal of Endocrinology / European Federation of Endocrine Societies. 2001;144:319–29. doi: 10.1530/eje.0.1440319. [DOI] [PubMed] [Google Scholar]
- 33.Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mechanisms of Ageing and Development. 2002;123:1191–201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 34.Born J, Ditschuneit I, Schreiber M, et al. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. European Journal of Endocrinology / European Federation of Endocrine Societies. 1995;132:705–11. doi: 10.1530/eje.0.1320705. [DOI] [PubMed] [Google Scholar]
- 35.Seeman TE, Singer B, Wilkinson CW, et al. Gender differences in age-related changes in HPA axis reactivity. Psychoneuroendocrinology. 2001;26:225–40. doi: 10.1016/s0306-4530(00)00043-3. [DOI] [PubMed] [Google Scholar]
- 36.Bauer ME, Vedhara K, Perks P, et al. Chronic stress in caregivers of dementia patients is associated with reduced lymphocyte sensitivity to glucocorticoids. Journal of Neuroimmunology. 2000;103:84–92. doi: 10.1016/s0165-5728(99)00228-3. [DOI] [PubMed] [Google Scholar]
- 37.Kumari M, Badrick E, Sacker A, et al. Identifying patterns in cortisol secretion in an older population. Findings from the Whitehall II study. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.01.010. in press. [DOI] [PubMed] [Google Scholar]
- 38.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. Journal of Clinical Endocrinology and Metabolism. 1996;81:2468–73. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 39.Straub RH, Miller LE, Scholmerich J, et al. Cytokines and hormones as possible links between endocrinosenescence and immunosenescence. Journal of Neuroimmunology. 2000;109:10–5. doi: 10.1016/s0165-5728(00)00296-4. [DOI] [PubMed] [Google Scholar]
- 40.Heuser IJ, Gotthardt U, Schweiger U, et al. Age-associated changes of pituitary-adrenocortical hormone regulation in humans: importance of gender. Neurobiology of Aging. 1994;15:227–31. doi: 10.1016/0197-4580(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 41.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, et al. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70:422–30. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- 42.Otte C, Hart S, Neylan TC, et al. A meta-analysis of cortisol response to challenge in human aging: Importance of gender. Psychoneuroendocrinology. 2005;30:80–91. doi: 10.1016/j.psyneuen.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 43.Gotthardt U, Schweiger U, Fahrenberg J, et al. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. American Journal of Physiology. 1995;268:R865–73. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- 44.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, et al. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 45.Rohleder N, Kudielka BM, Hellhammer DH, et al. Age and sex steroid-related changes in glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Journal of Neuroimmunology. 2002;126:69–77. doi: 10.1016/s0165-5728(02)00062-0. [DOI] [PubMed] [Google Scholar]
- 46.Nicolson N, Storms C, Ponds R, et al. Salivary cortisol levels and stress reactivity in human aging. Journals of Gerontology: Series A: Biological Sciences & Medical Sciences. 1997;52:M68–M75. doi: 10.1093/gerona/52a.2.m68. [DOI] [PubMed] [Google Scholar]
- 47.Traustadottir T, Bosch PR, Matt KS. The HPA axis response to stress in women: effects of aging and fitness. Psychoneuroendocrinology. 2005;30:392–402. doi: 10.1016/j.psyneuen.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Traustadottir T, Bosch PR, Matt KS. Gender Differences in Cardiovascular and Hypothalamic- Pituitary-Adrenal Axis Responses to Psychological Stress in Healthy Older Adult Men and Women. Stress: The International Journal on the Biology of Stress. 2003;6:133–140. doi: 10.1080/1025389031000111302. [DOI] [PubMed] [Google Scholar]
- 49.Kudielka BM, Hellhammer DH, Wust S. Why do we respond so differently? Reviewing determinants of human salivary cortisol responses to challenge. Psychoneuroendocrinology. 2009;34:2–18. doi: 10.1016/j.psyneuen.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52:318–27. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- 51.Heffner KL, Kiecolt-Glaser JK, Loving TJ, et al. Spousal support satisfaction as a modifier of physiological responses to marital conflict in younger and older couples. Journal of Behavioral Medicine. 2004;27:233–254. doi: 10.1023/b:jobm.0000028497.79129.ad. [DOI] [PubMed] [Google Scholar]
- 52.Wilkinson CW, Petrie EC, Murray SR, et al. Human glucocorticoid feedback inhibition is reduced in older individuals: evening study. Journal of Clinical Endocrinology and Metabolism. 2001;86:545–50. doi: 10.1210/jcem.86.2.7232. [DOI] [PubMed] [Google Scholar]
- 53.Bornstein SR, Engeland WC, Ehrhart-Bornstein M, et al. Dissociation of ACTH and glucocorticoids. Trends Endocrinol Metab. 2008;19:175–80. doi: 10.1016/j.tem.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 54.DeRijk RH, Petrides J, Deuster P, et al. Changes in corticosteroid sensitivity of peripheral blood lymphocytes after strenuous exercise in humans. Journal of Clinical Endocrinology and Metabolism. 1996;81:228–35. doi: 10.1210/jcem.81.1.8550757. [DOI] [PubMed] [Google Scholar]
- 55.Daun JM, Ball RW, Cannon JG. Glucocorticoid sensitivity of interleukin-1 agonist and antagonist secretion: the effects of age and gender. Am J Physiol Regul Integr Comp Physiol. 2000;278:R855–62. doi: 10.1152/ajpregu.2000.278.4.R855. [DOI] [PubMed] [Google Scholar]
- 56.Miller GE, Chen E, Fok AK, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling; Proceedings of the National Academy of Sciences of the United States of America; 2009. pp. 14716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: A glucocorticoid-resistance model. Health Psychology. 2002;21:531–541. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- 58.Rohleder N, Wolf JM, Kirschbaum C. Glucocorticoid sensitivity in humans-interindividual differences and acute stress effects. Stress. 2003;6:207–22. doi: 10.1080/1025389031000153658. [DOI] [PubMed] [Google Scholar]
- 59.Chrousos GP, Detera-Wadleigh SD, Karl M. Syndromes of glucocorticoid resistance. Annals of Internal Medicine. 1993;119:1113–24. doi: 10.7326/0003-4819-119-11-199312010-00009. [DOI] [PubMed] [Google Scholar]
- 60.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. New England Journal of Medicine. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 61.Heijnen CJ. Receptor regulation in neuroendocrine-immune communication: current knowledge and future perspectives. Brain, Behavior, and Immunity. 2007;21:1–8. doi: 10.1016/j.bbi.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 62.Barnes PJ. Mechanisms and resistance in glucocorticoid control of inflammation. Journal of Steroid Biochemistry and Molecular Biology. 2010 doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Rosewicz S, McDonald AR, Maddux BA, et al. Mechanism of glucocorticoid receptor down-regulation by glucocorticoids. Journal of Biological Chemistry. 1988;263:2581–4. [PubMed] [Google Scholar]
- 64.Webster JC, Oakley RH, Jewell CM, et al. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance; Proceedings of the National Academy of Sciences of the United States of America; 2001. pp. 6865–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agarwal SK, Marshall GD., Jr. Dexamethasone promotes type 2 cytokine production primarily through inhibition of type 1 cytokines. Journal of Interferon and Cytokine Research. 2001;21:147–55. doi: 10.1089/107999001750133159. [DOI] [PubMed] [Google Scholar]
- 66.Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Annals of the New York Academy of Sciences. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 67.Buske-Kirschbaum A, Fischbach S, Rauh W, et al. Increased responsiveness of the hypothalamus-pituitary-adrenal (HPA) axis to stress in newborns with atopic disposition. Psychoneuroendocrinology. 2004;29:705–11. doi: 10.1016/S0306-4530(03)00100-8. [DOI] [PubMed] [Google Scholar]
- 68.Wright RJ, Finn P, Contreras JP, et al. Chronic caregiver stress and IgE expression, allergen-induced proliferation, and cytokine profiles in a birth cohort predisposed to atopy. Journal of Allergy and Clinical Immunology. 2004;113:1051–7. doi: 10.1016/j.jaci.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 69.Kiecolt-Glaser JK, Heffner KL, Glaser R, et al. How stress and anxiety can alter immediate and late phase skin test responses in allergic rhinitis. Psychoneuroendocrinology. 2009;34:670–80. doi: 10.1016/j.psyneuen.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Buske-Kirschbaum A, Geiben A, Hollig H, et al. Altered responsiveness of the hypothalamus-pituitary-adrenal axis and the sympathetic adrenomedullary system to stress in patients with atopic dermatitis. Journal of Clinical Endocrinology and Metabolism. 2002;87:4245–51. doi: 10.1210/jc.2001-010872. [DOI] [PubMed] [Google Scholar]
- 71.Orentreich N, Brind JL, Rizer RL, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. Journal of Clinical Endocrinology and Metabolism. 1984;59:551–5. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 72.Orentreich N, Brind JL, Vogelman JH, et al. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. Journal of Clinical Endocrinology and Metabolism. 1992;75:1002–4. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 73.Vermeulen A. Dehydroepiandrosterone sulfate and aging. Annals of the New York Academy of Sciences. 1995;774:121–7. doi: 10.1111/j.1749-6632.1995.tb17376.x. [DOI] [PubMed] [Google Scholar]
- 74.Hornsby PJ. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Annals of the New York Academy of Sciences. 1995;774:29–46. doi: 10.1111/j.1749-6632.1995.tb17370.x. [DOI] [PubMed] [Google Scholar]
- 75.Parker CR, Jr., Mixon RL, Brissie RM, et al. Aging alters zonation in the adrenal cortex of men. Journal of Clinical Endocrinology and Metabolism. 1997;82:3898–901. doi: 10.1210/jcem.82.11.4507. [DOI] [PubMed] [Google Scholar]
- 76.Arlt W, Hewison M. Hormones and immune function: implications of aging. Aging Cell. 2004;3:209–16. doi: 10.1111/j.1474-9728.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- 77.Kahonen MH, Tilvis RS, Jolkkonen J, et al. Predictors and clinical significance of declining plasma dehydroepiandrosterone sulfate in old age. Aging. 2000;12:308–14. doi: 10.1007/BF03339852. [DOI] [PubMed] [Google Scholar]
- 78.Tannenbaum C, Barrett-Connor E, Laughlin GA, et al. A longitudinal study of dehydroepiandrosterone sulphate (DHEAS) change in older men and women: the Rancho Bernardo Study. European Journal of Endocrinology / European Federation of Endocrine Societies. 2004;151:717–25. doi: 10.1530/eje.0.1510717. [DOI] [PubMed] [Google Scholar]
- 79.Mazat L, Lafont S, Berr C, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality; Proceedings of the National Academy of Sciences of the United States of America; 2001. pp. 8145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Izawa S, Sugaya N, Shirotsuki K, et al. Salivary dehydroepiandrosterone secretion in response to acute psychosocial stress and its correlations with biological and psychological changes. Biological Psychology. 2008;79:294–8. doi: 10.1016/j.biopsycho.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 81.Morgan CA, 3rd, Southwick S, Hazlett G, et al. Relationships among plasma dehydroepiandrosterone sulfate and cortisol levels, symptoms of dissociation, and objective performance in humans exposed to acute stress. Archives of General Psychiatry. 2004;61:819–25. doi: 10.1001/archpsyc.61.8.819. [DOI] [PubMed] [Google Scholar]
- 82.Maninger N, Capitanio JP, Mason WA, et al. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology. doi: 10.1016/j.psyneuen.2010.01.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parker CR, Jr., Slayden SM, Azziz R, et al. Effects of aging on adrenal function in the human: responsiveness and sensitivity of adrenal androgens and cortisol to adrenocorticotropin in premenopausal and postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 2000;85:48–54. doi: 10.1210/jcem.85.1.6265. [DOI] [PubMed] [Google Scholar]
- 84.Kalimi M, Shafagoj Y, Loria R, et al. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA) Molecular and Cellular Biochemistry. 1994;131:99–104. doi: 10.1007/BF00925945. [DOI] [PubMed] [Google Scholar]
- 85.Hechter O, Grossman A, Chatterton RT., Jr. Relationship of dehydroepiandrosterone and cortisol in disease. Medical Hypotheses. 1997;49:85–91. doi: 10.1016/s0306-9877(97)90258-9. [DOI] [PubMed] [Google Scholar]
- 86.Daynes RA, Araneo BA, Ershler WB, et al. Journal of immunology. Vol. 150. Baltimore Md: 1993. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative; pp. 5219–30. 1950. [PubMed] [Google Scholar]
- 87.Padgett DA, Loria RM. Endocrine regulation of murine macrophage function: effects of dehydroepiandrosterone, androstenediol, and androstenetriol. Journal of Neuroimmunology. 1998;84:61–8. doi: 10.1016/s0165-5728(97)00244-0. [DOI] [PubMed] [Google Scholar]
- 88.Catania RA, Angele MK, Ayala A, et al. Dehydroepiandrosterone restores immune function following trauma-haemorrhage by a direct effect on T lymphocytes. Cytokine. 1999;11:443–50. doi: 10.1006/cyto.1998.0458. [DOI] [PubMed] [Google Scholar]
- 89.Suzuki T, Suzuki N, Daynes RA, et al. Dehydroepiandrosterone enhances IL2 production and cytotoxic effector function of human T cells. Clinical Immunology and Immunopathology. 1991;61:202–11. doi: 10.1016/s0090-1229(05)80024-8. [DOI] [PubMed] [Google Scholar]
- 90.Gordon CM, LeBoff MS, Glowacki J. Adrenal and gonadal steroids inhibit IL-6 secretion by human marrow cells. Cytokine. 2001;16:178–86. doi: 10.1006/cyto.2001.0962. [DOI] [PubMed] [Google Scholar]
- 91.Loria RM. Beta-androstenes and resistance to viral and bacterial infections. Neuroimmunomodulation. 2009;16:88–95. doi: 10.1159/000180263. [DOI] [PubMed] [Google Scholar]
- 92.Kovacs EJ, Duffner LA, Plackett TP. Immunosuppression after injury in aged mice is associated with a TH1-TH2 shift, which can be restored by estrogen treatment. Mechanisms of Ageing and Development. 2004;125:121–3. doi: 10.1016/j.mad.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 93.Kovacs EJ, Plackett TP, Witte PL. Estrogen replacement, aging, and cell-mediated immunity after injury. Journal of Leukocyte Biology. 2004;76:36–41. doi: 10.1189/jlb.1103538. [DOI] [PubMed] [Google Scholar]
- 94.Gilliver S. Sex steroids as inflammatory regulators. Journal of Steroid Biochemistry and Molecular Biology. 2010 doi: 10.1016/j.jsbmb.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 95.Delpedro AD, Barjavel MJ, Mamdouh Z, et al. Activation of human monocytes by LPS and DHEA. Journal of Interferon and Cytokine Research. 1998;18:125–35. doi: 10.1089/jir.1998.18.125. [DOI] [PubMed] [Google Scholar]
- 96.Khorram O, Vu L, Yen SS. Activation of immune function by dehydroepiandrosterone (DHEA) in age-advanced men. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 1997;52:M1–7. doi: 10.1093/gerona/52a.1.m1. [DOI] [PubMed] [Google Scholar]
- 97.Casson PR, Andersen RN, Herrod HG, et al. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. American Journal of Obstetrics and Gynecology. 1993;169:1536–9. doi: 10.1016/0002-9378(93)90431-h. [DOI] [PubMed] [Google Scholar]
- 98.Dillon JS. Dehydroepiandrosterone, dehydroepiandrosterone sulfate and related steroids: their role in inflammatory, allergic and immunological disorders. Curr Drug Targets Inflamm Allergy. 2005;4:377–85. doi: 10.2174/1568010054022079. [DOI] [PubMed] [Google Scholar]
- 99.Bauer ME, Jeckel CM, Luz C. The role of stress factors during aging of the immune system. Annals of the New York Academy of Sciences. 2009;1153:139–52. doi: 10.1111/j.1749-6632.2008.03966.x. [DOI] [PubMed] [Google Scholar]
- 100.Esler M, Kaye D, Thompson J, et al. Effects of aging on epinephrine secretion and regional release of epinephrine from the human heart. Journal of Clinical Endocrinology and Metabolism. 1995;80:435–42. doi: 10.1210/jcem.80.2.7852502. [DOI] [PubMed] [Google Scholar]
- 101.Iwase S, Mano T, Watanabe T, et al. Age-related changes of sympathetic outflow to muscles in humans. Journal of Gerontology. 1991;46:M1–5. doi: 10.1093/geronj/46.1.m1. [DOI] [PubMed] [Google Scholar]
- 102.Christensen NJ, Jensen EW. Effect of psychosocial stress and age on plasma norepinephrine levels: a review. Psychosomatic Medicine. 1994;56:77–83. doi: 10.1097/00006842-199401000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–17. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ng AV, Callister R, Johnson DG, et al. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 105.Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, et al. Psychosocial stress and HPA functioning: no evidence for a reduced resilience in healthy elderly men. Stress. 2000;3:229–40. doi: 10.3109/10253890009001127. [DOI] [PubMed] [Google Scholar]
- 106.Esler M, Lambert G, Kaye D, et al. Influence of ageing on the sympathetic nervous system and adrenal medulla at rest and during stress. Biogerontology. 2002;3:45–9. doi: 10.1023/a:1015203328878. [DOI] [PubMed] [Google Scholar]
- 107.Franco-Morselli R, Elghozi JL, Joly E, et al. Increased plasma adrenaline concentrations in benign essential hypertension. British Medical Journal. 1977;2:1251–4. doi: 10.1136/bmj.2.6097.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weidmann P, Beretta-Piccoli C, Ziegler WH, et al. Age versus urinary sodium for judging renin, aldosterone, and catecholamine levels: studies in normal subjects and patients with essential hypertension. Kidney International. 1978;14:619–28. doi: 10.1038/ki.1978.171. [DOI] [PubMed] [Google Scholar]
- 109.Palmer GJ, Ziegler MG, Lake CR. Response of norepinephrine and blood pressure to stress increases with age. Journal of Gerontology. 1978;33:482–7. doi: 10.1093/geronj/33.4.482. [DOI] [PubMed] [Google Scholar]
- 110.Aslan S, Nelson L, Carruthers M, et al. Stress and age effects on catecholamines in normal subjects. Journal of Psychosomatic Research. 1981;25:33–41. doi: 10.1016/0022-3999(81)90081-7. [DOI] [PubMed] [Google Scholar]
- 111.Mazzeo RS, Rajkumar C, Jennings G, et al. Norepinephrine spillover at rest and during submaximal exercise in young and old subjects. Journal of Applied Physiology. 1997;82:1869–74. doi: 10.1152/jappl.1997.82.6.1869. [DOI] [PubMed] [Google Scholar]
- 112.Friedman EM, Irwin MR. Modulation of immune cell function by the autonomic nervous system. Pharmacology & Therapeutics. 1997;74:27–38. doi: 10.1016/s0163-7258(96)00200-8. [DOI] [PubMed] [Google Scholar]
- 113.Nance DM, Sanders VM. Autonomic innervation and regulation of the immune system (1987-2007) Brain, Behavior, and Immunity. 2007;21:736–45. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Callahan TA, Moynihan JA. Contrasting pattern of cytokines in antigen- versus mitogen-stimulated splenocyte cultures from chemically denervated mice. Brain, Behavior, and Immunity. 2002;16:764–73. doi: 10.1016/s0889-1591(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 115.Salicru AN, Sams CF, Marshall GD. Cooperative effects of corticosteroids and catecholamines upon immune deviation of the type-1/type-2 cytokine balance in favor of type-2 expression in human peripheral blood mononuclear cells. Brain, Behavior, and Immunity. 2007;21:913–20. doi: 10.1016/j.bbi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 116.Straub RH, Cutolo M, Zietz B, et al. The process of aging changes the interplay of the immune, endocrine and nervous systems. Mechanisms of Ageing and Development. 2001;122:1591–611. doi: 10.1016/s0047-6374(01)00289-5. [DOI] [PubMed] [Google Scholar]
- 117.Kiecolt-Glaser JK, Glaser R, Gravenstein S, et al. Chronic stress alters the immune response to influenza virus vaccine in older adults; Proceedings of the National Academy of Sciences of the United States of America; 1996. pp. 3043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Besedovsky H, Sorkin E, Felix D, et al. Hypothalamic changes during the immune response. European Journal of Immunology. 1977;7:323–5. doi: 10.1002/eji.1830070516. [DOI] [PubMed] [Google Scholar]
- 119.Pawelec G, Larbi A, Pawelec G, et al. Immunity and ageing in man: Annual Review 2006/2007. Experimental Gerontology. 2008;43:34–8. doi: 10.1016/j.exger.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 120.McEwen BS. Sex, stress and the hippocampus: Allostasis, allostatic load and the aging process. Neurobiology of Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 121.Seeman TE, McEwen BS, Rowe JW, et al. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging; Proceedings of the National Academy of Sciences of the United States of America; 2001. pp. 4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]