Natural killer cell recognition of “missing self” contributes meaningfully to control of mouse cytomegalovirus infection in vivo.
Abstract
Cytomegaloviruses (CMVs) are renowned for interfering with the immune system of their hosts. To sidestep antigen presentation and destruction by CD8+ T cells, these viruses reduce expression of major histocompatibility complex class I (MHC I) molecules. However, this process sensitizes the virus-infected cells to natural killer (NK) cell–mediated killing via the “missing self” axis. Mouse cytomegalovirus (MCMV) uses m152 and m06 encoded proteins to inhibit surface expression of MHC I molecules. In addition, it encodes another protein, m04, which forms complexes with MHC I and escorts them to the cell surface. This mechanism is believed to prevent NK cell activation and killing by restoring the “self” signature and allowing the engagement of inhibitory Ly49 receptors on NK cells. Here we show that MCMV lacking m04 was attenuated in an NK cell– and MHC I–dependent manner. NK cell–mediated control of the infection was dependent on the presence of NK cell subsets expressing different inhibitory Ly49 receptors. In addition to providing evidence for immunoevasion strategies used by CMVs to avoid NK cell control via the missing-self pathway, our study is the first to demonstrate that missing self–dependent NK cell activation is biologically relevant in the protection against viral infection in vivo.
A balance of activating and inhibitory signals, mediated through distinct classes of receptors found on their surface, regulates NK cell activity (Ortaldo and Young, 2005; Lanier, 2008). Activating signals are interrupted when inhibitory receptors on NK cells engage MHC class I (MHC I) molecules on target cells. This dual receptor system, also known as the “missing self” activation mechanism (Kärre et al., 1986), enables NK cells to detect virally infected cells that exhibit a reduced expression of MHC I molecules. To compromise antigen presentation in the context of MHC I molecules, and thus evade the attack by CD8+ T cells, cytomegaloviruses (CMVs) encode immunoevasion proteins that can downmodulate the expression of MHC I on the cell surface (Hengel et al., 1999), most likely by a recently described mechanism demonstrating that the down-regulation of MHC I from the surface of infected cell is a result of a MHC I turnover and inhibited supply of newly synthesized molecules, rather than the active downmodulation of the surface-resident portion (Lemmermann et al., 2010). However, this lowers inhibitory interactions and triggering through inhibitory Ly49 receptors, and might activate NK cells through a missing self–dependent mechanism. In addition, CMVs actively compromise NK cell activation using other strategies, such as the downmodulation of cellular ligands for activating NK cell receptors (e.g., NKG2D) or miRNAs (Jonjić et al., 2008). Because of an efficient and redundant viral immunoevasion from NK cells, most laboratory mouse strains, as well as feral mice, fail to mount a significant NK cell response toward mouse CMV (MCMV; Scalzo et al., 2005), with the exception of a few mouse strains that possess an activating Ly49 receptor, which specifically recognizes infected cells and therefore circumvents viral immunoevasion (Arase et al., 2002; Smith et al., 2002; Adam et al., 2006; Kielczewska et al., 2009).
The aim of this study was to explain the mechanisms by which MCMV avoids NK cell control, in spite of the down-regulation of MHC I molecules on the surface of infected cells. As shown previously, MCMV encodes three proteins involved in the regulation of MHC I expression (Wagner et al., 2002; Holtappels et al., 2006; Pinto et al., 2006). The m152-encoded gp40 glycoprotein arrests MHC I at the level of the ERGIC/cis-Golgi compartment (Ziegler et al., 1997), whereas m06-encoded gp48 redirects MHC I complexes to lysosomes for degradation (Reusch et al., 1999). The third MCMV regulator of MHC I expression is gp34, encoded by the m04 gene (Kleijnen et al., 1997). Unlike the other two, m04/gp34 does not prevent the surface expression of MHC I, but instead binds to these proteins in the ER, forming complexes that can reach the cell surface (Kleijnen et al., 1997). Although it has been proposed that m04/gp34 cooperates with two other immunoevasins to compromise antigen presentation to CD8+ T cells (Kavanagh et al., 2001a), recently published data indicate that m04 is not a CD8+ T cell immunoevasin on its own, but rather an antagonist of m152 function (Holtappels et al., 2006; Pinto et al., 2006). It has been suggested that m04/gp34 might inhibit NK cell activation through its ability to escort MHC I to the cell surface to engage inhibitory NK cell receptors (Kleijnen et al., 1997). We have recently shown that m04/gp34 is essential for the recognition of infected cells by the activating Ly49P receptor, which recognizes H-2Dk together with m04 product and another, thus far unidentified viral component (Kielczewska et al., 2009).
Here, we provide evidence that MCMV lacking the m04 gene (Δm04) is attenuated in an NK cell– and MHC I–dependent manner in several mouse strains. By using reporter cells expressing the inhibitory receptor Ly49A, we have shown that the expression of m04 in infected cells allows the engagement of this receptor by enabling MHC I molecules to reach the cell surface, despite the two viral MHC I inhibitors. Our studies provide the first evidence that NK cell recognition of the missing self is relevant in the recognition and control of a viral pathogen in vivo.
RESULTS
Δm04 MCMV is attenuated in vivo in an NK cell– and MHC I–dependent manner
Based on the ability of m04 to escort MHC I molecules to the surface of infected cells, we have hypothesized that its primary function is blocking NK cell activation via the missing self mechanism. If this is correct, the deletion of m04 should attenuate MCMV in an NK cell–dependent manner by decreasing the expression of ligands for inhibitory NK cell receptors. To test this, we infected BALB/c mice with either WT MCMV or virus lacking m04 (Δm04). A portion of mice in each group was depleted of NK cells, and the virus titer in the spleen was determined 3 d post infection (dpi). We have observed a significantly lower viral replication of the Δm04 mutant, which was reverted to WT MCMV titer after depletion of NK cells (Fig. 1 A). These results clearly support the initial hypothesis that m04/gp34 causes NK cell inhibition. We have also tested whether this phenotype is dependent on the expression of MHC I molecules. The aforementioned difference in viral titers between Δm04 and WT MCMV was absent in TAP1-deficient mice (Tap1−/−) and mice deficient for β2 microglobulin (β2m−/−), both on BALB/c background (Fig. 1 B).
Figure 1.
Δm04 is attenuated in vivo in an NK cell– and MHC I–dependent manner. (A–D) Indicated strains of mice were injected i.v. with 2 × 105 PFU (except C57BL/6, which received a dose of 3 × 105 PFU) of WT or Δm04 MCMV and 3 dpi viral titers in spleens were assessed. Where indicated, mice were depleted of NK cells by injection of 20 µl of anti-AGM1. P values, unpaired two-tailed Mann-Whitney test. A circle depicts the titer for each individual mouse; a small horizontal line indicates the mean. Data are representative of at least two independent experiments with four to five mice per group.
Moreover, we have tested the impact of m04 in several other mouse strains (Fig. 1 C). Even more pronounced than in BALB/c mice, the absence of m04 resulted in an NK cell–dependent viral attenuation in C3H/J (H-2k), CBA/J (H-2k), BALB.K (H-2k), and B10.M x BALB/c (BALB.F; H-2f) mouse strains. However, in C57BL/6 (H-2b) mice no difference in viral titers was observed between Δm04 and WT MCMV (Fig. 1 D). To avoid a strong activation of C57BL/6 NK cells through the Ly49H receptor, which can abolish the impact of m04, we have used BALB.B mice, of the identical H-2 haplotype but different NKC background. As expected, without Ly49H, BALB.B mice showed a high virus titer; however, no attenuation with Δm04 observed (Fig. 1 D). Therefore, we concluded that the attenuation of Δm04 mutant is NK cell– and MHC I–dependent. This phenomenon is clearly seen in mice of the H-2k and, to a lesser extent, the H-2d backgrounds, but is most likely completely absent in H-2b animals.
MCMV m04 protects infected cells from NK cell–mediated killing
To further elaborate the role of m04 in protecting infected cell from NK cell–mediated recognition, we have tested ex vivo the ability of splenic cells from naive BALB/c and C3H/J mice to lyse B12 (SV40-transformed BALB/c fibroblasts; H-2d) and SVEC4-10 (C3H/J-derived SV40 transformed endothelial cells; H-2k) cells, infected with WT or Δm04 MCMV or left uninfected. WT infected B12 and SVEC4-10 cells were both lysed less efficiently as compared with noninfected targets. However, Δm04-infected targets were more susceptible to NK cell lysis than WT-infected cells (Fig. 2 A).
Figure 2.
m04 protects cells from NK cell–mediated killing. (A) Target B12 and SVEC4-10 cells were CFSE-labeled, infected with WT or Δm04 MCMV for 10 h, or were left uninfected, and co-cultured with splenocytes from naive BALB/c or C3H/J mice, respectively. Specific lysis of target cells was assessed by staining with 7-AAD and analyzed by flow cytometry. Data points on the graph represent the mean of a triplicate for each sample. Error bars represent SD. Data are representative of five (SVEC4-10) or three (B12) independent experiments. Statistical significance applies for the effector-to-target ratio of 16:1 and is indicated by asterisks. *, P < 0.05; **, P < 0.001; ***, P < 0.0001. (B) B12 and SVEC4-10 cells were infected with WT or Δm04 MCMV or left uninfected. Cells were harvested 10 hpi and stained for surface MHC I and NKG2D ligand expression. Data are representative of two independent experiments.
Although it is difficult to determine if viral titers in the spleen are always correlated with the ex vivo function of NK cells, it has to be pointed out that the contribution of m04 to the concealment of infected cells in vitro is much less pronounced. Even though in vivo MCMV can infect many different cell types, the antiviral activity of NK cells differs depending on the affected organ or tissue. Therefore, it is not completely unexpected that the extent of cytolytic susceptibility of certain cell lines does not directly correlate with NK cell efficacy in vivo.
Because both B12 and SVEC4-10 target cells constitutively express NKG2D ligands (Fig. 2 B), uninfected cells are likely lysed by NK cells via an NKG2D-dependent mechanism, but the role of other activating receptors cannot be excluded. It is notable that MCMV down-regulates ligands for NKG2D receptor on these cells, with the exception of surface-resident RAE-1δ in SVEC4-10 cells, which is constitutively resistant to MCMV (Arapovic et al., 2009). Because m04 does not interfere with the expression of these ligands (Fig. 2 B), the decrease in NK cell lysis of WT MCMV–infected cells as compared with uninfected targets might be caused by a decreased signal from an activating receptor (e.g., NKG2D) and/or NK cell inhibition provided by m04–MHC I complexes. Regardless, in the absence of m04, MCMV infected cells regain susceptibility to NK killing. Together with in vivo results, this finding confirms the inhibitory role of m04 in NK cell–mediated virus control.
We have also performed the same assay on the macrophage cell line RAW264.7 derived from BALB/c mice. Contrary to B12 and SVEC4-10 cells, WT MCMV–infected RAW264.7 cells were lysed to a greater extent than noninfected targets, whereas Δm04-infected targets were again more susceptible to lysis than WT-infected cells (Fig. S1).
m04 rescues the engagement of inhibitory Ly49 receptors
Because m04-modified H-2Dk has been shown to interact with the activating Ly49P receptor (Desrosiers et al., 2005; Kielczewska et al., 2009), on the basis of our findings, we have hypothesized that m04-modified MHC I can associate with the inhibitory Ly49 receptors as well. Ly49A is a strong inhibitory receptor that recognizes H-2Dd, H-2Dk, and H-2Dp (Chung et al., 2000). It can be found in most NK haplotypes (including BALB/c, CBA/J, and C57BL/6) and is expressed on ∼5–20% of NK cells (Ortaldo et al., 1999; Chung et al., 2000). The ligand-binding domain of Ly49A is closely related to that of Ly49P (94% amino acid sequence homology; Makrigiannis et al., 2005); therefore, Ly49A seemed to be an obvious candidate to test our hypothesis.
We constructed a reporter cell line expressing the ectodomain of Ly49A isolated from the BALB/c strain fused to the CD3ζ intracellular domain (Ly49ACD3ζ). Co-culture of Ly49ACD3ζ reporter cells with uninfected primary mouse embryonic fibroblasts (MEFs) derived from H-2k background (CBA/J mice) resulted in the activation of reporter cells (Fig. 3 A, middle). Next, we infected MEFs with WT MCMV and co-cultured them with Ly49ACD3ζ reporter cells. Surprisingly, despite a dramatic down-regulation of MHC I molecules from the surface of infected cells as early as 8 h post infection (hpi; Fig. 4 B and unpublished data), Ly49ACD3ζ carrying reporter cells were activated to a similar extent as when co-cultured with uninfected cells. Moreover, we observed an increase in GFP fluorescence intensity of the reporter cells, indicating that the same percentage of reporter cells was activated, but with even greater signal strength (Fig. 3 A). Furthermore, when reporter cells were co-cultured with Δm04-infected cells, the activation of Ly49A reporter cells was dramatically reduced or even abolished (Fig. 3 A). This activation was H-2 dependent because Ly49ACD3ζ reporters co-cultured with H-2d targets showed a decreased stimulatory potential and therefore a less dramatic impact of m04 absence, which is in agreement with the results obtained in vivo (Fig. 3 A, middle). As a control, Ly49P-expressing reporter cells were not activated after co-culture with uninfected or Δm04-infected cells (Fig. 3 A, left), which further supports our previous results (Kielczewska et al., 2009).
Figure 3.
m04 allows engagement of inhibitory Ly49 receptor. (A) Ly49ACD3ζ or Ly49PCD3ζ NFAT-GFP reporter cells were co-cultured with CBA/J, BALB/c, or C57BL/6 MEFs and either infected with indicated viruses or left uninfected. GFP expression was assessed (in the case of infected cells, overlay was made with uninfected cells presented by open histograms). Data are representative of two to four independent experiments. (B) Ly49AHCTS reporter cells were co-cultured with the indicated MEF cells, left uninfected, or infected with WT or Δm04 MCMV. GFP expression was assessed. Data are representative of two to four independent experiments. (C) Ly49C NFAT-GFP reporter cells were co-cultured with BALB/c MEFs and BALB.F MEFs that were infected with indicated viruses or left uninfected and GFP expression was assessed. P values, unpaired two-tailed Student’s t test. Data are means of four independent experiments. Error bars represent SD.
Figure 4.
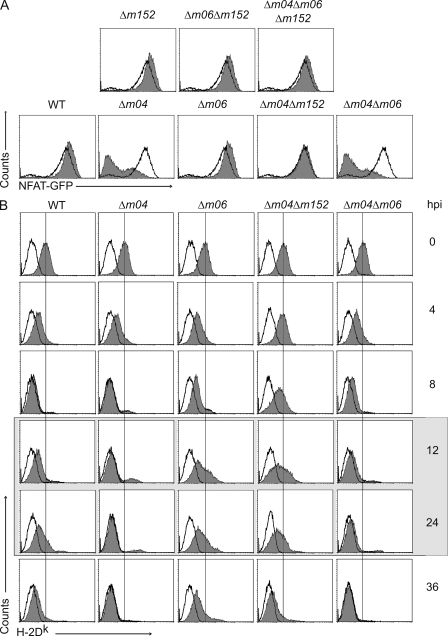
m04 antagonizes the function of m152 in the downmodulation of H-2Dk. (A) Ly49A NFAT-GFP reporter cells were co-cultured with CBA/J MEFs that were infected with indicated viruses or left uninfected, and GFP expression was assessed (shaded histograms, infected; open histograms, uninfected). Data are representative of three independent experiments. (B) CBA/J MEFs were infected with indicated viruses or uninfected. Cells were harvested at the indicated time points (hpi, hours post infection) and stained for H-2Dk expression (shaded histograms). Irrelevant isotype matched antibody was used as a control (open histogram). Shaded box represents the time window of cocultivation of target cells with reporter cells (experiment shown in A). Data are representative of three independent experiments.
Because Δm04 was not attenuated in C57BL/6 or in BALB.B mice, we tested the ability of H-2b cells, either infected or uninfected, to engage the Ly49A receptor. As shown in Fig. 3 A (right), in contrast to Ly49ACD3ζ reporter cells co-cultured with CBA/J or BALB/c MEFs, MEFs derived from C57BL/6 mice failed to activate reporter cells, confirming that Ly49A does not bind to MHC I molecules of the H-2b haplotype (Karlhofer et al., 1992), and again supporting our in vivo finding. Consequently, the infection of C57BL/6 cells with either WT or Δm04 MCMV did not result in the activation of Ly49ACD3ζ reporter cells. Altogether, these findings demonstrate that m04 plays an essential role in preventing NK cell activation via the missing self mechanism by providing ligands for inhibitory NK cell receptors, at least in the context of H-2d and H-2k haplotype.
A consistent increase in GFP expression by Ly49A reporter cells co-cultured with WT MCMV-infected cells made us conclude that association of m04/gp34 with MHC I molecules increases their binding to Ly49A. To test this assumption, we constructed a reporter cell line expressing the Ly49A ectodomain fused to the stalk, transmembrane, and cytoplasmic domains of Ly49H, which associates with DAP12 adaptor protein containing one ITAM motif (Ly49AHCTS) contrary to Ly49A-CD3ζ chimera (Ly49ACD3ζ), which by itself encodes three ITAM motifs. This Ly49AHCTS chimeric receptor shows less binding to MHC I molecules and generates a weaker signal (Ito et al., 2009). Therefore, Ly49AHCTS reporter cells were poorly activated by uninfected or Δm04 MCMV-infected cells (of H-2k or H-2d haplotype), but were still strongly activated when co-cultured with WT MCMV-infected cells (Fig. 3 B). Similar results were obtained with reporter cells expressing the Ly49A allele from C57BL/6 mice (Fig. 3 B), which also indicated that the lack of interaction between Ly49A and MHC I in C57BL/6 mice is determined by MHC I and not by the Ly49A allele. Therefore, we concluded that m04–MHC I complex might have a higher affinity for Ly49A as compared with native MHC I.
To assess whether the effect of m04 is restricted to Ly49A, we generated 2B4 NFAT-GFP reporter cells expressing Ly49C. Ly49C is another inhibitory receptor with high affinity for H-2Kb, H-2Db, H-2Kd, H-2Dd, and H-2Dk; Ly49C is found in most NK haplotypes (including BALB/c, CBA/J, and C57BL/6; Brennan et al., 1996a). However, whereas Ly49A is present on ∼5–10% of NK cells, Ly49C is expressed on ∼50% of NK cells in BALB/c mice. Co-culturing these reporter cells with target cells infected with WT or uninfected cells of H-2b, H-2d, or H-2k origin, we observed high activation of Ly49C reporter cells (Fig. 3 C, left, and not depicted). An only slightly lower activation of Ly49C reporter cells was observed upon co-culture with Δm04-infected BALB/c MEFs when compared with WT-infected targets (Fig. 3 C, left). However, co-culture of Ly49C reporter cells with MEFs of H-2f origin showed significantly lower activation of the Ly49C reporter cells co-cultured with Δm04-infected targets (Fig. 3 C, right). Thus, although the effect of m04 is most evident with Ly49A reporter cells, it is also visible with Ly49C reporter cells.
m04 antagonizes the function of m152 in the downmodulation of H-2Dk
To study the efficiency of the m04 gene product in the context of MCMV infection, we investigated the role of m04 alone, or in relation to two other MCMV MHC I regulators and used viral mutants individually lacking m04, m06, or m152, and different combinations of these three genes, or a triple mutant (Wagner et al., 2002). Contrary to the deletion of m04, which strongly reduced the activation of Ly49A reporter cells in the case of WT infection, the deletion of either m06 or m152 immunoevasins did not affect the capacity of infected cells to activate the reporter cell line (Fig. 4 A, bottom). As expected, the deletion of m06 and m152 together (Δm06Δm152), or the deletion of all three viral regulators of MHC I (Δm04Δm06Δm152) resulted in Ly49A reporter cell activation (Fig. 4 A, top). When the deletion of m04 was combined with a deletion of m06 (Δm04Δm06), the reporter cell activation was also lost, indicating that m152 alone is sufficient to down-regulate MHC I expression and prevent Ly49A engagement, when unopposed by m04. However, when both m04 and m152 were missing (Δm04Δm152), Ly49A reporter cells were activated to the same extent as the reporter cells co-cultured with noninfected cells. To elucidate this somewhat unexpected finding we followed the kinetics of surface H-2Dk down-regulation in cells infected with WT MCMV or mutants lacking m06, m04 alone, or in combination with the deletion of m06 or m152 genes (Fig. 4 B). We have observed differences in the kinetics of H-2Dk surface downmodulation, showing a more rapid and efficient activity of m152 (infection with Δm04Δm06) in comparison to m06 (infection with Δm04Δm152). In addition, these results confirm the previous findings of Holtappels et al. (2006), which showed that m04 antagonizes the MHC I retention function of m152 (as seen upon infection with Δm06). Only at 36 hpi does m06 induce levels of H-2Dk down-regulation comparable to those of m152. Therefore, given the results shown in Fig. 4 A, the absence of m04 in the case of Δm04Δm152 MCMV infection did not prevent Ly49A binding because of the differential kinetics of MHC I down-regulation by m06 and m152.
Differential contribution of NK cell subsets expressing various combinations of inhibitory Ly49 receptors in control of Δm04 MCMV in vivo
We hypothesized that a lack of NK cell–dependent control of WT MCMV in some mouse strains could be, for the most part, the consequence of a strong inhibition imposed by m04 via inhibitory Ly49 receptors. To assess the contribution of individual NK cell subsets expressing inhibitory Ly49 receptors we depleted NK cells expressing Ly49A, Ly49C, or Ly49G2 in BALB/c mice and infected them with either WT or Δm04 MCMV. The results show that each of the subsets contributes in part to a control of Δm04 MCMV, although to a different extent (Fig. 5 A, right). Depletion of Ly49C+ and Ly49A+ NK cells results in a moderate control of Δm04 MCMV, whereas the Ly49G2+ NK cell depletion results in a loss of control of the mutant virus. This suggests that Ly49G2+ NK cells play a dominant role in the control of Δm04 MCMV replication.
Figure 5.
NK cells expressing inhibitory Ly49 receptors control Δm04 MCMV in vivo. (A) BALB/c mice were i.v. injected with 2 × 105 PFU of WT or Δm04 MCMV and 3 dpi viral loads in spleen were assessed. Where indicated, mice were depleted of the indicated NK cell subsets by injecting 150 µg of the corresponding antibody. Mice in the control group received equivalent amount of PBS. P values, unpaired two-tailed Mann-Whitney test. A circle depicts the titer for each individual mouse; the horizontal line indicates the mean. Data are representative of two independent experiments with five mice per group. (B) BrdU incorporation by total NK cells in BALB/c and BALB.K mice that were left uninfected or infected with WT or Δm04 MCMV was assessed 2.5 d after infection. P values, unpaired two-tailed Student’s t test. Error bars represent SD. Data are means of three independent experiments with three mice per infection. (C) Proportions of CD3−NKp46+ cells expressing Ly49A, Ly49C, or Ly49G2 in BALB/c mice that were left uninfected or infected with WT or Δm04 MCMV were determined 2.5 dpi by flow cytometry. Data are means of two independent experiments. Error bars represent SD. (D) BrdU incorporation by CD3−NKp46+ cells expressing indicated Ly49 proteins in BALB/c and BALB.K mice infected with either WT or Δm04 MCMV was assessed 2.5 dpi. P values, unpaired two-tailed Student’s t test. Error bars represent SD. Data are representative of one (BALB.K) or two (BALB/c) experiments with three mice per infection.
The presence of m04 impairs the proliferation of NK cells
To test whether the enhanced control of Δm04 MCMV infection as opposed to WT MCMV infection is accompanied by increased proliferation and/or alterations in the size of NK cell subpopulations, we have infected BALB/c and BALB.K mice with WT and Δm04 MCMV and monitored the proliferation of total NK cells by BrdU incorporation at 3 dpi. There was an enhanced incorporation of BrdU by NK cells upon infection with both viruses as opposed to uninfected controls. However, NK cell proliferation was impaired in both mouse strains after WT infection as compared with mice infected with Δm04 MCMV (Fig. 5 B). Therefore, we have concluded that the presence of m04 impairs the proliferation of NK cells, and this impairment is correlated with poor control of WT MCMV compared with the virus lacking the m04 gene (Δm04; Fig. 1). Again, the impact of m04 is more pronounced in BALB.K than in BALB/c mice, which corresponds to the more pronounced differences in viral titers between WT and Δm04 observed in these mouse strains. No major differences in the proportion of various NK cell subpopulations expressing different inhibitory Ly49 receptors was observed between WT and Δm04 MCMV infection, suggesting that all three NK cell subsets contribute to overall increased NK cell proliferation after infection with Δm04 virus (Fig. 5 C). Indeed, this was visible after assessing BrdU incorporation by NK cell subpopulations expressing individual Ly49 inhibitory receptors (Fig. 5 D), both in BALB/c and BALB.K mice, although it was more pronounced in BALB.K mice.
The control of the virus lacking m04 is partially mediated via NKG2D
NK cells require an activating signal to respond to an insult, despite the absence of MHC I–mediated inhibitory signals. BALB/c mice do not possess an MCMV-specific, highly expressed, and strong activating receptor, such as Ly49H, which is present in C57BL/6 mice. Therefore, we tested whether NKG2D might play a role in the missing self-mediated recognition in mice infected with either WT MCMV or the Δm04 mutant (Fig. 6). In contrast to WT MCMV, blocking of NKG2D in H-2d or H-2k mice infected with Δm04 MCMV resulted in a significant increase in virus titer. This suggests that lowered inhibition leads to NK cell activation via stimulatory receptors capable of recognizing ligands expressed on infected cells.
Figure 6.
Control of Δm04 MCMV is partially mediated via NKG2D. The indicated strains of mice were i.v. injected with 2 × 105 PFU of WT or Δm04 MCMV and 3 dpi viral loads in spleen were assessed. Mice were either untreated or NKG2D blockade was performed 6 h before infection by applying 300 µg of anti-NKG2D mAb. P values, unpaired two-tailed Mann-Whitney test. A circle depicts the titer for each individual mouse; the horizontal line indicates the mean. Data are representative of two independent experiments with five mice per group.
We have not observed an effect of NKG2D blocking after infection with WT MCMV. This might be explained not only by NKG2D subversion mechanisms developed by MCMV, but also by a strong integration of inhibitory signals provided by m04–MHC I–mediated ligation of Ly49 receptors. Because m04 is not involved in the down-regulation of NKG2D ligands, one might wonder how NKG2D-blocking results in increased virus titers in Δm04 but not in WT MCMV–infected mice. We speculate that down-regulation of NKG2D ligands within different cell types in vivo is not as absolute as in permissively infected cells in vitro. To assess a possible contribution of another activating receptor, we performed a similar experiment in Ncr1−/− BALB/c mice (Gazit et al., 2006), but the results were comparable to the ones described in normal BALB/c mice. This indicated that NKp46 activating receptor does not participate in the missing self–mediated recognition of MCMV-infected cells (unpublished data).
DISCUSSION
To avoid destruction by MHC I–restricted CD8+ T cells, many viruses have evolved mechanisms to modulate MHC I expression. However, infected cells down-regulating MHC I should be immediately recognized by NK cells because of the absence of inhibitory receptor engagement (e.g., Ly49s, KIRs, or CD94/NKG2A), thus shifting a balance of signals toward NK cell activation. MCMV encodes three regulators of MHC I expression. Although the function of m152 and m06 as negative regulators of MHC I expression is clear, the role of m04 remains enigmatic. Kleijnen et al. (1997) showed that m04 biochemically antagonizes the MHC I retention function of m152, a finding confirmed functionally in subsequent studies (Wagner et al., 2002; Holtappels et al., 2006), thus showing m04 as a positive regulator of MHC I cell surface expression and antigenic peptide presentation. However, it has also been proposed that the binding of m04 to MHC I proteins might interfere with the peptide loading onto MHC I or with the recognition by the TCR (Kavanagh et al., 2001b).
We observed that m04 is one of the most abundantly produced proteins of MCMV (unpublished data). Furthermore, a great number of polymorphic variants of m04 have been identified from MCMV strains isolated from wild mice, suggesting strong selective pressure (Smith et al., 2006). m04 in complex with H-2Dk molecules is essential for the recognition of infected cells by the activating Ly49P receptor (Kielczewska et al., 2009). m157, an MCMV glycoprotein recognized by the activating Ly49H and inhibitory Ly49I receptors, is also under intense selective pressure and easily mutated (Voigt et al., 2003; French et al., 2004). We propose that m04 originally evolved to function as an agonist of certain inhibitory Ly49 receptors and that the genesis of the activating Ly49P receptor recognizing m04 was a consequence of counteracting selective pressure in the host.
In this study, we have described a mechanism by which MCMV evades NK cell–mediated control in spite of a marked down-regulation of MHC I proteins. By enabling MHC I molecules to escape complete down-regulation from the surface of infected cells, the m04 protein allows engagement of inhibitory Ly49 receptors, thereby preventing NK cell activation via the missing self process. Therefore, m04 might be a key player in determining the threshold level of NK cell inhibition. Our present findings are in accordance with recent studies demonstrating that the expression of inhibitory Ly49 receptors recognizing self-MHC I ligands restrict the proliferation and effector functions of Ly49H+ NK cells in response to MCMV infection in C57BL/6 mice (Orr et al., 2010).
The results obtained after NK cell subset depletion indicate the importance of a missing self recognition of virally infected cells by each of the NK cell subsets examined, although the stochastic expression of inhibitory Ly49 receptors precludes the exclusive depletion of one specific subset. We observed a loss of control of Δm04 MCMV replication in the absence of Ly49G2+ NK cells in BALB/c mice. The involvement of Ly49G2+ NK cells has been previously described to be important in the control of WT MCMV in C57L.M-H2k mice (Xie et al., 2009). The fact that Ly49G2+ NK cell–depleted mice infected with WT or Δm04 MCMV demonstrated increased viral titers could indicate that Ly49G2+ NK cells might be more easily triggered during infection and contribute to the control of viral spread. Indeed, previous work has demonstrated that Ly49G2+ NK cells preferentially express high-levels of IL12Rβ2 subunit, which may render them more responsive to IL-12 during infection (Chakir et al., 2000). However, we have not observed significant differences in the percentage of IFN-γ+ NK cells expressing different inhibitory Ly49 receptors during WT MCMV infection (unpublished data). Furthermore, it has been shown that Ly49G2+ NK cells expand not only during MCMV infection but also during LCMV, mouse hepatitis virus, and vaccinia virus infection of C57BL/6 mice (Tay et al., 1999; Daniels et al., 2001). All these viruses modulate MHC I expression (Brutkiewicz et al., 1992; Hewitt et al., 2002; Sevilla et al., 2004; Hahm et al., 2005; Dasgupta et al., 2007). Moreover, Ly49G2 lacks a ligand in mice bearing H-2b where we detect no attenuation in Δm04 viral load. More recently, it was shown that the interaction between Ly49G2 and H-2Dk mediates resistance to MCMV in H-2Dk transgenic mice otherwise sensitive to MCMV infection (Xie et al., 2010). Our results show that in mice expressing H-2k where Ly49G2 recognizes only H-2Dk (Silver et al., 2002), absence of m04 results in low MHC I expression, reduced inhibitory signals, and a significant decrease in viral load. On the other hand, in mice expressing H-2d, Ly49G2 is able to interact with both H-2Ld and H-2Dd (Silver et al., 2002); thus, even though MHC I expression is low during Δm04 infection, the inhibitory signal is transmitted, resulting in attenuation in viral load in vivo.
Ly49A+ NK cells represent a minor population of NK cells (∼5% in BALB/c mice; Ortaldo et al., 1999), and although they are able to detect the missing self, they only contribute moderately to the control of the virus. In mice expressing H-2b, Ly49A lacks a ligand. In mice of the H-2k or H-2d background, Ly49A recognizes H-2Dk or H-2Dd, respectively (Karlhofer et al., 1992). Our present studies using Ly49A reporter cells suggest more efficient recognition of H-2k rather than H-2d, a difference that is augmented by the presence of m04. As such, the absence of m04 should have greatest impact in mice expressing H-2k, intermediate impact in mice expressing H-2d, and no effect in mice bearing H-2b, as supported by both our in vivo and in vitro results. Furthermore, it has been shown that Ly49A+ NK cells are unable to reject H-2b bone marrow grafts, but they act synergistically with Ly49G2+ cells (Raziuddin et al., 2000).
Ly49C+ cells, on the other hand, represent almost 50% of the NK cell population in BALB/c mice, but contribute only moderately to the control of the virus. Ly49C is able to interact with H-2Kb and H-2Db in the H-2b background and, with H-2Kd and H-2Dd in the H-2d background, but only with H-2Dk in the H-2k background (Brennan et al., 1996b). Therefore, in the absence of m04 and reduced MHC I expression, NK cells of H-2k mice should show the greatest reduction in the inhibitory threshold. It is well established that MCMV encodes many MHC I–like molecules that are expressed on the surface of infected cells, and one can speculate that ligation of Ly49C by these molecules possibly results in the inhibition of Ly49C+ NK cell subset.
Ly49G2+ NK cells might be more efficient in detecting missing self, but inhibitory signals mediated by these cells might be weaker, as the strength of their interaction with MHC I is inferior to Ly49A, Ly49C, or Ly49I (Hanke et al., 1999). The robust response of Ly49G2+ NK cells raises the possibility of the existence of an activating receptor that is preferentially coexpressed on these cells and drives their proliferation. Because a preferential coexpression of Ly49D and Ly49H activation receptors has been shown (Smith et al., 2000), we cannot exclude the possible coexpression of an MCMV-specific activating receptor on these NK cells. We showed that NKG2D receptor blocking resulted in a significant increase in the Δm04 virus titer, although it did not completely eliminate NK cell–mediated resistance by itself, suggesting the involvement of other activating NK cell receptors.
It has been shown that Ly49A+ NK cell subsets are only weakly licensed in C57BL/6 mice (Brodin et al., 2009; Jonsson et al., 2010), which in the absence of an H-2b ligand is also expected for Ly49G2+ NK cells. Therefore, these cells are probably not operational in the missing self–mediated recognition of virally infected cells and this might be the reason why we have not observed the phenotype of Δm04 mutant in C57BL/6 mice. The quantitative differences of the m04 effect between H-2k and H-2d mice are not easy to explain in the absence of detailed knowledge of the binding of MHC I molecules for their cognate receptors. However, several nonexclusive mechanisms could be at play, including quantitative differences in the strength of licensing. A recent study by Jonsson et al. (2010) showing better licensing of Ly49A+ NK cells in mice of the H-2k compared with the H-2d haplotype supports this hypothesis. And yet, in our experiments shown in Fig. 3 (A and B), we observed that the impact of the absence of m04 was less dramatic in cells of H-2d background, both for Ly49A and Ly49C reporter cells, which could indicate the presence of residual Ly49 inhibition in H2d mice. Also, the fact that Ly49G2 possesses two H-2d ligands, H-2D and H-2L, in BALB/c mice but only one, H-2D, in BALB.K or C3H/J, would further contribute to an increased threshold of activation in H-2d mice infected with Δm04 MCMV.
Together, our findings highlight the importance of viral modulation of MHC I to prevent missing self signals from being detected by NK cells. This study presents the first evidence that NK cell recognition of the missing self is operational in the recognition and control of a viral pathogen in vivo.
MATERIALS AND METHODS
Mice and infection conditions.
Mice used in experiments were housed and bred under SPF conditions. All the protocols used for the breeding of mice and different kinds of treatment were approved by the Ethical Committee of the University of Rijeka and were performed in accordance with Croatian Law for the Protection of Laboratory Animals that has been harmonized with the existing EU legislation (EC Directive 86/609 EEC). Infections were performed by intravenous injection of the virus and, where indicated, NK cells were depleted by intraperitoneal injection of 20 µl of rabbit anti-asialo GM1 (Wako Chemicals) 24 h before infection. NKG2D blockade was performed by injection of 300 µg of anti-NKG2D antibody (C7 clone; Ho et al., 2002). Ly49 subsets were depleted by injections of 150 µg of YE1/48 (anti-Ly49A), 5E6 (anti-Ly49C and Ly49I), or 4D11 (anti-Ly49G2) antibodies (5E6 and 4D11 mAbs were a gift from W. Held, Ludwig Institute for Cancer Research Ltd., Lausanne Branch, Epalinges, Switzerland; Sentman et al., 1989; Takei, 1983; Mason et al., 1995). The viral titers in organs were quantified by the standard plaque assay.
Construction of chimeric Ly49 receptors.
The full-length cDNA of Ly49aBALB was cloned into the pGEM-Teasy vector (Promega). Ly49cC57BL/6 was a gift from F. Takei (University of British Columbia, Vancouver, Canada) and Ly49aC57BL/6 was a gift from J.R. Carlyle (University of Toronto, Ontario, Canada). CD3ζ-NKR-P1A construct was a gift from W.M. Yokoyama (Washington University School of Medicine, St. Louis, MO). The construction of Ly49A-CD3ζ chimera was performed by PCR amplification of the CD3ζ intracellular domain and Ly49A CTLD-Stalk domain with Esp3I designed restriction sites at 5′ and 3′, respectively, subsequent Esp3I digestion and ligation (MBI Fermentas) such that the ligation site resided in the transmembrane region. The generation of the chimeric proteins of inhibitory Ly49 CTLD with an activating Ly49H backbone was performed as described in the paper by Kielczewska et al. (2007). In brief, the C-terminal CTLD domain of the inhibitory Ly49 receptors was amplified by PCR and fused via an Esp3I (MBI Fermentas) restriction site to the stalk, transmembrane region, and intracellular domain of the activating Ly49H. The chimeric receptor obtained with this approach acquires the ability to transmit activating signals upon ligation via the adapter protein DAP12. All cDNAs encoding the chimeric Ly49 receptors were subcloned into BamHI and NotI sites in the retroviral expression pMx-puro vector.
Reporter cell assay.
2B4 NFAT-GFP reporter cells were generated by retroviral transduction, as previously described (Arase et al., 2002). Primary CBA/J, BALB/c, BALB.K, BALB.F, and C57BL/6 MEFs were prepared by using a standard procedure (Brune et al., 1999). For the reporter cell assay, stimulator cells were infected (1.5 PFU/cell) with MCMV or MCMV deletion mutants (Wagner et al., 2002) for 12 h before the addition of reporter cells. NFAT-GFP reporter cells were co-cultured for 24 h with stimulators in 24-well plates at a ratio of 1:3 and analyzed by flow cytometry.
MHC I and NKG2D expression analysis.
Target B12 (H-2Dd) and SVEC4-10 (H-2Dk) cells were infected with 3 and 1.5 PFU/cell, respectively, of WT or Δm04 MCMV or were uninfected. Cells were harvested 10 hpi and stained for MHC I expression using anti–H-2Dd (clone 34–5-8S), anti–H-2Kd (clone SF1-1.1), anti–H-2Ld (clone 28–14-8), or anti–H-2Dk (clone 15–5-5S), followed by FITC-conjugated goat anti–mouse IgG (BD). Isotype-matched irrelevant antibodies were used as control. NKG2D ligand expression was analyzed using PE-labeled NKG2D tetramers (Krmpotić et al., 2002). PE-conjugated streptavidin (eBioscience) was used as a control staining antibody.
MHC I kinetics.
MCMV-infected (1.5 PFU/cell) CBA/J MEFs were harvested at the indicated time points and stained with anti–H-2Dk (clone 15–5-5S), followed by FITC-conjugated goat anti–mouse IgG (BD).
Cytotoxicity assay.
Target B12 (H-2d) and SVEC4-10 (H-2k) cells were infected with 3 and 1.5 PFU/cell, respectively, of WT or Δm04 MCMV, followed by centrifugal enhancement of infection. Uninfected cells were used as control. After 10-h infection, cells were labeled with CFSE and co-cultured with BALB/c or C3H splenocytes, respectively, at an effector-to-target ratio adjusted to the number of NK cells, where ratios 16:1, 8:1, and 4:1 roughly correspond to ratios 500:1, 250:1, and 125:1 of bulk splenocytes. After a 4-h co-culture in a 5% CO2 atmosphere at 37°C, specific lysis was determined by flow cytometric analysis as measured by 7-aminoactinomycin D (7-AAD) incorporation (BD) in individual samples, as well as samples containing target cells only, to measure spontaneous death of cells. Cytotoxic activity was expressed as % specific lysis calculated by the following formula: (% CFSE+ 7-AAD+ cell specific lysis - % CFSE+ 7-AAD+ cell spontaneous lysis) / (100 - % CFSE+ 7-AAD+ cell spontaneous lysis) × 100. The experiment was done in triplicate for each effector-to-target ratio, and data points on the graphs represent the means.
Cell proliferation assay and immunofluorescence.
BALB/c and BALB.K mice were injected with 2 × 105 PFU of WT or Δm04 MCMV and two and a half dpi received 2 mg of bromodeoxyuridine (BrdU). After 2 h BrdU stimulation isolated mouse splenocytes were either surface stained or BrdU incorporation was assessed by FACS analysis (FACSCalibur; BD). The following antibodies were used: anti–CD3-PerCP Cy5.5, anti–NKp46-PE (both eBioscience), and anti–BrdU-APC (BD). Anti-Ly49A (JR9.318 clone), anti-Ly49C/I (5E6 clone), anti-Ly49G2 (4D11 clone), all kind gifts of W. Held, were purified from the hybridoma supernatant and FITC labeled following the manufacturer’s instructions (Alexa Fluor 488 Monoclonal Antibody Labeling kit; Invitrogen).
Statistical analysis.
Statistical significance was determined by GraphPad Prism5 software. Differences in viral titers between experimental groups were determined by the unpaired two-tailed Mann-Whitney test. Statistical significance of differences in the percentage of specific lysis and in BrdU proliferation assay was determined by the unpaired two-tailed Student’s t test.
Online supplemental material.
Fig. S1 shows that the presence of m04 during WT MCMV infection protects RAW264.7 macrophage cell line from specific lysis. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100921/DC1.
Acknowledgments
We thank Antonija Zurunić and Dr. Tihana Lenac Roviš for purifying the antibodies and our laboratory engineers for technical help. We thank Dr. Ofer Mandelboim, Dr. Lars Doelken, and Dr. Matthias J. Reddehase for critically reading the manuscript.
This study was supported by EU FP7, REGPOT-2008-1-01 (S. Jonjić) and Croatian Ministry of Science grants 0621261-1263 (S. Jonjić) and 0621261-1268 (A. Krmpotić). A. Krmpotić is supported by the Howard Hughes Medical Institute International Research Scholars grant. M. Pyzik is supported by Frederick Banting and Charles Best Canada Graduate Scholarships. S.M. Vidal is a Canada Research Chair and is funded by the Canadian Institutes of Health Research MOP-778. L.L. Lanier is an American Cancer Society Professor and is supported by National Institutes of Health grant AI068129.
The authors have no competing financial interests.
Footnotes
Abbreviations used:
- 7-AAD
- 7-aminoactinomycin D
- β2m
- β2-microglobulin
- CMV
- cytomegalovirus
- MCMV
- mouse CMV
- pi
- post infection
References
- Adam S.G., Caraux A., Fodil-Cornu N., Loredo-Osti J.C., Lesjean-Pottier S., Jaubert J., Bubic I., Jonjic S., Guénet J.L., Vidal S.M., Colucci F. 2006. Cmv4, a new locus linked to the NK cell gene complex, controls innate resistance to cytomegalovirus in wild-derived mice. J. Immunol. 176:5478–5485 [DOI] [PubMed] [Google Scholar]
- Arapovic J., Lenac T., Antulov R., Polic B., Ruzsics Z., Carayannopoulos L.N., Koszinowski U.H., Krmpotic A., Jonjic S. 2009. Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus. J. Virol. 83:8198–8207 10.1128/JVI.02549-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase H., Mocarski E.S., Campbell A.E., Hill A.B., Lanier L.L. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 296:1323–1326 10.1126/science.1070884 [DOI] [PubMed] [Google Scholar]
- Brennan J., Lemieux S., Freeman J.D., Mager D.L., Takei F. 1996a. Heterogeneity among Ly-49C natural killer (NK) cells: characterization of highly related receptors with differing functions and expression patterns. J. Exp. Med. 184:2085–2090 10.1084/jem.184.6.2085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan J., Mahon G., Mager D.L., Jefferies W.A., Takei F. 1996b. Recognition of class I major histocompatibility complex molecules by Ly-49: specificities and domain interactions. J. Exp. Med. 183:1553–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P., Lakshmikanth T., Johansson S., Kärre K., Höglund P. 2009. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 113:2434–2441 [DOI] [PubMed] [Google Scholar]
- Brune W., Hengel H., Koszinowski U.H. 1999. A mouse model for cytomegalovirus infection. Current protocols in immunology. John Wiley & Sons, New York: 19.17.11-19.17.13 [DOI] [PubMed] [Google Scholar]
- Brutkiewicz R.R., Klaus S.J., Welsh R.M. 1992. Window of vulnerability of vaccinia virus-infected cells to natural killer (NK) cell-mediated cytolysis correlates with enhanced NK cell triggering and is concomitant with a decrease in H-2 class I antigen expression. Nat. Immun. 11:203–214 [PubMed] [Google Scholar]
- Chakir H., Camilucci A.A., Filion L.G., Webb J.R. 2000. Differentiation of murine NK cells into distinct subsets based on variable expression of the IL-12R beta 2 subunit. J. Immunol. 165:4985–4993 [DOI] [PubMed] [Google Scholar]
- Chung D.H., Natarajan K., Boyd L.F., Tormo J., Mariuzza R.A., Yokoyama W.M., Margulies D.H. 2000. Mapping the ligand of the NK inhibitory receptor Ly49A on living cells. J. Immunol. 165:6922–6932 [DOI] [PubMed] [Google Scholar]
- Daniels K.A., Devora G., Lai W.C., O’Donnell C.L., Bennett M., Welsh R.M. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta A., Hammarlund E., Slifka M.K., Früh K. 2007. Cowpox virus evades CTL recognition and inhibits the intracellular transport of MHC class I molecules. J. Immunol. 178:1654–1661 [DOI] [PubMed] [Google Scholar]
- Desrosiers M.P., Kielczewska A., Loredo-Osti J.C., Adam S.G., Makrigiannis A.P., Lemieux S., Pham T., Lodoen M.B., Morgan K., Lanier L.L., Vidal S.M. 2005. Epistasis between mouse Klra and major histocompatibility complex class I loci is associated with a new mechanism of natural killer cell-mediated innate resistance to cytomegalovirus infection. Nat. Genet. 37:593–599 10.1038/ng1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A.R., Pingel J.T., Wagner M., Bubic I., Yang L., Kim S., Koszinowski U., Jonjic S., Yokoyama W.M. 2004. Escape of mutant double-stranded DNA virus from innate immune control. Immunity. 20:747–756 10.1016/j.immuni.2004.05.006 [DOI] [PubMed] [Google Scholar]
- Gazit R., Gruda R., Elboim M., Arnon T.I., Katz G., Achdout H., Hanna J., Qimron U., Landau G., Greenbaum E., et al. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7:517–523 10.1038/ni1322 [DOI] [PubMed] [Google Scholar]
- Hahm B., Trifilo M.J., Zuniga E.I., Oldstone M.B. 2005. Viruses evade the immune system through type I interferon-mediated STAT2-dependent, but STAT1-independent, signaling. Immunity. 22:247–257 [DOI] [PubMed] [Google Scholar]
- Hanke T., Takizawa H., McMahon C.W., Busch D.H., Pamer E.G., Miller J.D., Altman J.D., Liu Y., Cado D., Lemonnier F.A., et al. 1999. Direct assessment of MHC class I binding by seven Ly49 inhibitory NK cell receptors. Immunity. 11:67–77 [DOI] [PubMed] [Google Scholar]
- Hengel H., Reusch U., Gutermann A., Ziegler H., Jonjic S., Lucin P., Koszinowski U.H. 1999. Cytomegaloviral control of MHC class I function in the mouse. Immunol. Rev. 168:167–176 10.1111/j.1600-065X.1999.tb01291.x [DOI] [PubMed] [Google Scholar]
- Hewitt E.W., Duncan L., Mufti D., Baker J., Stevenson P.G., Lehner P.J. 2002. Ubiquitylation of MHC class I by the K3 viral protein signals internalization and TSG101-dependent degradation. EMBO J. 21:2418–2429 10.1093/emboj/21.10.2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho E.L., Carayannopoulos L.N., Poursine-Laurent J., Kinder J., Plougastel B., Smith H.R., Yokoyama W.M. 2002. Costimulation of multiple NK cell activation receptors by NKG2D. J. Immunol. 169:3667–3675 [DOI] [PubMed] [Google Scholar]
- Holtappels R., Gillert-Marien D., Thomas D., Podlech J., Deegen P., Herter S., Oehrlein-Karpi S.A., Strand D., Wagner M., Reddehase M.J. 2006. Cytomegalovirus encodes a positive regulator of antigen presentation. J. Virol. 80:7613–7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D., Iizuka Y.M., Katepalli M.P., Iizuka K. 2009. Essential role of the Ly49A stalk region for immunological synapse formation and signaling. Proc. Natl. Acad. Sci. USA. 106:11264–11269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonjić S., Babić M., Polić B., Krmpotić A. 2008. Immune evasion of natural killer cells by viruses. Curr. Opin. Immunol. 20:30–38 10.1016/j.coi.2007.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson A.H., Yang L., Kim S., Taffner S.M., Yokoyama W.M. 2010. Effects of MHC class I alleles on licensing of Ly49A+ NK cells. J. Immunol. 184:3424–3432 10.4049/jimmunol.0904057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlhofer F.M., Ribaudo R.K., Yokoyama W.M. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature. 358:66–70 10.1038/358066a0 [DOI] [PubMed] [Google Scholar]
- Kärre K., Ljunggren H.G., Piontek G., Kiessling R. 1986. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 319:675–678 [DOI] [PubMed] [Google Scholar]
- Kavanagh D.G., Koszinowski U.H., Hill A.B. 2001a. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J. Immunol. 167:3894–3902 [DOI] [PubMed] [Google Scholar]
- Kavanagh D.G., Gold M.C., Wagner M., Koszinowski U.H., Hill A.B. 2001b. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J. Exp. Med. 194:967–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielczewska A., Kim H.S., Lanier L.L., Dimasi N., Vidal S.M. 2007. Critical residues at the Ly49 natural killer receptor’s homodimer interface determine functional recognition of m157, a mouse cytomegalovirus MHC class I-like protein. J. Immunol. 178:369–377 [DOI] [PubMed] [Google Scholar]
- Kielczewska A., Pyzik M., Sun T., Krmpotic A., Lodoen M.B., Munks M.W., Babic M., Hill A.B., Koszinowski U.H., Jonjic S., et al. 2009. Ly49P recognition of cytomegalovirus-infected cells expressing H2-Dk and CMV-encoded m04 correlates with the NK cell antiviral response. J. Exp. Med. 206:515–523 10.1084/jem.20080954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen M.F., Huppa J.B., Lucin P., Mukherjee S., Farrell H., Campbell A.E., Koszinowski U.H., Hill A.B., Ploegh H.L. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 16:685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krmpotić A., Busch D.H., Bubić I., Gebhardt F., Hengel H., Hasan M., Scalzo A.A., Koszinowski U.H., Jonjić S. 2002. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat. Immunol. 3:529–535 [DOI] [PubMed] [Google Scholar]
- Lanier L.L. 2008. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 9:495–502 10.1038/ni1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmermann N.A., Gergely K., Böhm V., Deegen P., Däubner T., Reddehase M.J. 2010. Immune evasion proteins of murine cytomegalovirus preferentially affect cell surface display of recently generated peptide presentation complexes. J. Virol. 84:1221–1236 10.1128/JVI.02087-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrigiannis A.P., Patel D., Goulet M.L., Dewar K., Anderson S.K. 2005. Direct sequence comparison of two divergent class I MHC natural killer cell receptor haplotypes. Genes Immun. 6:71–83 10.1038/sj.gene.6364154 [DOI] [PubMed] [Google Scholar]
- Mason L.H., Ortaldo J.R., Young H.A., Kumar V., Bennett M., Anderson S.K. 1995. Cloning and functional characteristics of murine large granular lymphocyte-1: a member of the Ly-49 gene family (Ly-49G2). J. Exp. Med. 182:293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr M.T., Murphy W.J., Lanier L.L. 2010. ‘Unlicensed’ natural killer cells dominate the response to cytomegalovirus infection. Nat. Immunol. 11:321–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortaldo J.R., Young H.A. 2005. Mouse Ly49 NK receptors: balancing activation and inhibition. Mol. Immunol. 42:445–450 10.1016/j.molimm.2004.07.024 [DOI] [PubMed] [Google Scholar]
- Ortaldo J.R., Mason A.T., Winkler-Pickett R., Raziuddin A., Murphy W.J., Mason L.H. 1999. Ly-49 receptor expression and functional analysis in multiple mouse strains. J. Leukoc. Biol. 66:512–520 [DOI] [PubMed] [Google Scholar]
- Pinto A.K., Munks M.W., Koszinowski U.H., Hill A.B. 2006. Coordinated function of murine cytomegalovirus genes completely inhibits CTL lysis. J. Immunol. 177:3225–3234 [DOI] [PubMed] [Google Scholar]
- Raziuddin A., Bennett M., Winkler-Pickett R., Ortaldo J.R., Longo D.L., Murphy W.J. 2000. Synergistic effects of in vivo depletion of Ly-49A and Ly-49G2 natural killer cell subsets in the rejection of H2(b) bone marrow cell allografts. Blood. 95:3840–3844 [PubMed] [Google Scholar]
- Reusch U., Muranyi W., Lucin P., Burgert H.G., Hengel H., Koszinowski U.H. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo A.A., Manzur M., Forbes C.A., Brown M.G., Shellam G.R. 2005. NK gene complex haplotype variability and host resistance alleles to murine cytomegalovirus in wild mouse populations. Immunol. Cell Biol. 83:144–149 10.1111/j.1440-1711.2005.01311.x [DOI] [PubMed] [Google Scholar]
- Sentman C.L., Hackett J., Jr Kumar V., Bennett M. 1989. Identification of a subset of murine natural killer cells that mediates rejection of Hh-1d but not Hh-1b bone marrow grafts. J. Exp. Med. 170:191–202 10.1084/jem.170.1.191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla N., McGavern D.B., Teng C., Kunz S., Oldstone M.B. 2004. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J. Clin. Invest. 113:737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver E.T., Lavender K.J., Gong D.E., Hazes B., Kane K.P. 2002. Allelic variation in the ectodomain of the inhibitory Ly-49G2 receptor alters its specificity for allogeneic and xenogeneic ligands. J. Immunol. 169:4752–4760 [DOI] [PubMed] [Google Scholar]
- Smith H.R., Chuang H.H., Wang L.L., Salcedo M., Heusel J.W., Yokoyama W.M. 2000. Nonstochastic coexpression of activation receptors on murine natural killer cells. J. Exp. Med. 191:1341–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H.R., Heusel J.W., Mehta I.K., Kim S., Dorner B.G., Naidenko O.V., Iizuka K., Furukawa H., Beckman D.L., Pingel J.T., et al. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA. 99:8826–8831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L.M., Shellam G.R., Redwood A.J. 2006. Genes of murine cytomegalovirus exist as a number of distinct genotypes. Virology. 352:450–465 10.1016/j.virol.2006.04.031 [DOI] [PubMed] [Google Scholar]
- Takei F. 1983. Two surface antigens expressed on proliferating mouse T lymphocytes defined by rat monoclonal antibodies. J. Immunol. 130:2794–2797 [PubMed] [Google Scholar]
- Tay C.H., Yu L.Y., Kumar V., Mason L., Ortaldo J.R., Welsh R.M. 1999. The role of LY49 NK cell subsets in the regulation of murine cytomegalovirus infections. J. Immunol. 162:718–726 [PubMed] [Google Scholar]
- Voigt V., Forbes C.A., Tonkin J.N., Degli-Esposti M.A., Smith H.R., Yokoyama W.M., Scalzo A.A. 2003. Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc. Natl. Acad. Sci. USA. 100:13483–13488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner M., Gutermann A., Podlech J., Reddehase M.J., Koszinowski U.H. 2002. Major histocompatibility complex class I allele-specific cooperative and competitive interactions between immune evasion proteins of cytomegalovirus. J. Exp. Med. 196:805–816 10.1084/jem.20020811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Stadnisky M.D., Brown M.G. 2009. MHC class I Dk locus and Ly49G2+ NK cells confer H-2k resistance to murine cytomegalovirus. J. Immunol. 182:7163–7171 10.4049/jimmunol.0803933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Stadnisky M.D., Coats E.R., Ahmed Rahim M.M., Lundgren A., Xu W., Makrigiannis A.P., Brown M.G. 2010. MHC class I D(k) expression in hematopoietic and nonhematopoietic cells confers natural killer cell resistance to murine cytomegalovirus. Proc. Natl. Acad. Sci. USA. 107:8754–8759 10.1073/pnas.0913126107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler H., Thäle R., Lucin P., Muranyi W., Flohr T., Hengel H., Farrell H., Rawlinson W., Koszinowski U.H. 1997. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity. 6:57–66 10.1016/S1074-7613(00)80242-3 [DOI] [PubMed] [Google Scholar]