IL-12 is enriched at the immunological synapse in TLR-activated dendritic cells interacting with antigen-specific CD8+ T cells; synaptic delivery of IL-12 induces IFN-γ production in the T cell.
Abstract
The immune synapse (IS) forms as dendritic cells (DCs) and T cells interact in lymph nodes during initiation of adaptive immunity. Factors that contribute to the formation and maintenance of IS stability and function have been mostly studied in T cells, whereas little is known about events occurring during synapse formation in DCs. Here, we show that DCs activated by Toll-like receptor (TLR) agonists reorient the microtubule-organizing center (MTOC) toward the interacting T cell during antigen-specific synapse formation through a mechanism that depends on the Rho GTPase Cdc42. IL-12, a pivotal cytokine produced by DCs, is found enriched around the MTOC at early time points after TLR ligation and is dragged to the DC–T cell interface in antigen-specific synapses. Synaptic delivery of IL-12 induces activation of pSTAT4 and IFN-γ neosynthesis in CD8+ naive T cells engaged in antigen-specific conjugates and promotes the survival of antigen-primed T cells. We propose that DC polarization increases the local concentration of proinflammatory mediators at the IS and that this represents a new mechanism by which T cell priming is controlled.
During initiation of adaptive immunity, signals arising from MHC–peptide complexes and co-stimulatory molecules expressed on DCs are transmitted to naive T cells at the immune synapse (IS). Besides presenting antigen and expressing ligands for co-stimulation, DCs modulate the extent and the nature of the T cell response by secreting large amounts of soluble cytokines in response to ligation of TLRs (Medzhitov, 1997, 2001). Formation of the IS is accompanied by extensive reorganization of molecules and organelles that is well characterized in T cells. TCR ligation induces redistribution of membrane receptors at the contact site and polarization of microtubules and polarity proteins underneath the contact region (Krummel and Macara, 2006). In contrast, little is known about the mechanisms that coordinate transfer of membrane bound and secreted signals from DCs to T cells. Few studies suggest that, in DCs, membrane receptors and intracellular components distribute asymmetrically during interaction with naive T cells. For instance, actin is enriched at the contact site and MHC class II molecules become clustered in the synaptic area, thereby increasing the density of TCR ligands (Boes et al., 2002; Al-Alwan et al., 2003; de la Fuente et al., 2005). Spinophilin, a PDZ domain protein that serves as scaffold in the neuronal synapse, was shown to be recruited in DCs at the IS, where it modulates antigen presentation to T cells (Bloom et al., 2008). Furthermore, the recruitment of pro-survival factors at the contact site after synapse formation was recently shown to be critical to protect DCs from apoptosis (Riol-Blanco et al., 2009).
Cell polarity is a highly conserved mechanism common to various cellular processes like asymmetric cell division and directional migration that serves to generate specialized shapes and functions. A common molecular regulator of cell polarity is Cdc42, a small GTPase of the Rho family that plays a central role in establishing cell polarity in all eukaryotic cells (Etienne-Manneville, 2004). A major feature of cell polarity is the organized distribution of the microtubule cytoskeleton that allows directional flow of proteins and organelles to specific location. Polarization of the microtubule organizing center (MTOC) during synapse formation is well defined in T cells, where it regulates delivery of cytokines and lytic granules toward target cells and it was recently shown to be important to sustain TCR signaling (Kupfer et al., 1985; Stinchcombe and Griffiths, 2003; Chen et al., 2006; Huse et al., 2006; Banerjee et al., 2007; Martín-Cófreces et al., 2008). Cells of the myeloid lineage, like macrophages, move the MTOC toward the site of particle internalization during phagocytosis, and this event is important to position the antigen-processing machinery close to the ingested particle (Eng et al., 2007).
To gain further insight into DC properties as antigen-presenting cells, we asked whether the microtubule system of DCs become polarized during the interaction with naive CD8+ T cells. We show that DCs stimulated by TLR agonists acquire the ability to polarize the MTOC and the associated Golgi toward the DC–T cell contact site in antigen-specific synapses. This mechanism depends on the small Rho GTPase Cdc42. IL-12, a key T cell priming cytokine produced by DCs upon TLR ligation, is recruited at the IS and induce early events of IL-12–dependent signaling in T cells. Blocking MTOC polarization does not interfere with synapse formation, but selectively affects the transmission of IL-12–dependent signals, resulting in reduced IFN-γ secretion by activated T cells.
RESULTS
MTOC polarization in DCs during synapse formation
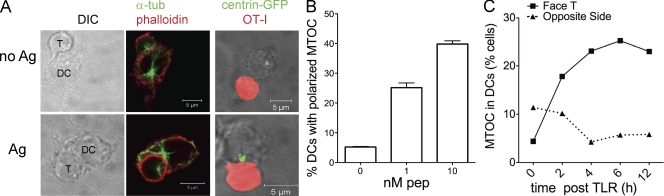
We examined the reorganization of the microtubule cytoskeleton in DCs during interaction with naive CD8+ T cells. Bone marrow–derived DCs were activated by TLR agonists to induce maturation and were loaded or not loaded with OVA class I peptide. DCs were plated on a fibronectin matrix and CD8+, OVA-specific naive T cells (OT-I cells) were layered over DCs for 30 min. After washing off nonadherent cells, slides were fixed and analyzed by confocal microscopy. To visualize the microtubule cytoskeleton, we performed immunolabeling with antitubulin antibodies. DCs differentiated from the bone marrow of centrin GFP knock-in mice were used to allow a sharper visualization of the MTOC. Under these experimental conditions, most of the conjugates corresponded to 1:1 DC/T cell doublets. In several DC–T cell conjugates, we observed the DC’s MTOC (DC-MTOC hereafter) in close proximity to the synaptic membrane. The percentage of cells showing a polarized MTOC was quantified according to the criteria described in the Material and methods. As shown in Fig. 1 (A and B), DC-MTOC polarization depended on antigen dose. Only a few DCs (5.3 ± 0.3%) were polarized in the absence of peptide, a figure that increased to 20 ± 2.02% and 42 ± 1.8% at 1 and 10 nM peptide, respectively. Thus, DCs engaged in antigen-specific synapses undergo remodeling of the microtubule cytoskeleton by redirecting the MTOC toward the interacting T cell in an antigen dose-dependent manner.
Figure 1.
DCs polarize the MTOC in antigen-specific synapses. (A) DCs were stimulated with a combination of TLR agonists (CpG and LPS) and loaded (Ag) or not (no Ag) with OVA class I peptide before mixing to OVA-specific CD8+ T cells (OT-I). Confocal sections showing the MTOC position detected by staining with anti–α-tubulin antibodies (left) or using DCs differentiated from centrin-GFP knock-in mice (right) mixed with carboxylic acid, succinimidyl ester (SNARF)–labeled T cells (red). (B) DC–T cell conjugates were formed using TLR-stimulated DCs loaded with the indicated doses of peptide. The percentage of DCs with the MTOC polarized toward the DC–T cell interface was quantified as described in the experimental procedure. Values are plotted as means ± SEM of >100 conjugates/condition. (C) DCs were treated with TLR agonist for the indicated periods and loaded with 1 nM peptide before synapse formation. The percentage of polarized DCs was scored in at least 50 conjugates for each condition in 3 independent experiments.
It is established that TLR-stimulated DCs are more efficient in inducing T cell activation than immature DCs. We have previously shown that this correlates to the formation of stronger and longer lasting DC–T cell interactions that in turn depend on an intact actin cytoskeleton (Benvenuti et al., 2004a,b). To understand whether microtubules were preferentially polarized in mature DC–T cell contacts, we formed synapses using antigen-loaded DCs (1 nM peptide) that were incubated or not with TLR agonist before mixing with antigen-specific T cells. DC–T cell conjugates formed by DCs that had not been stimulated by TLRs agonist showed a low degree of MTOC polarization. As soon as 2 h after activation, the number of conjugates with the MTOC facing the T cell increased, reaching maximal levels at 6 h after stimulation (Fig. 1 C). At this time point (6 h after TLR engagement), DCs have reached the highest capacity to cluster and activate T cells as shown by FACS analysis of conjugate formation and by the levels of IL-2 produced by antigen-specific T cells upon co-culture (Fig. S1).
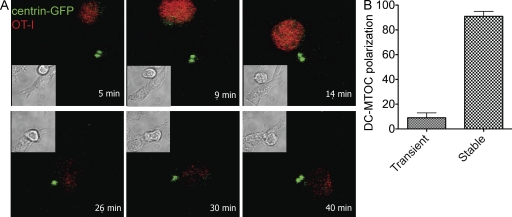
We next asked whether the DC-MTOC translocates toward the synaptic region upon contact formation. To address this issue, we made time-lapse recordings of centrin-GFP DCs mixed with labeled OT-I cells during the first 40 min of interaction (Fig. 2 A and Video 1). The MTOC spot localized mostly to a central position in isolated DCs. Upon contact with a T cell, we observed the MTOC traveling toward the membrane contacting the T cell in about half of the conjugates that formed during the recording period. Full MTOC polarization required 7.5 ± 1.2 min after the initial contact. In the majority of cases (82 ± 5.6%), once the MTOC became polarized it remained close to the T cell membrane for the rest of the movie (up to 40 min), with little oscillation forward and back. This was true even when the DC–T cell doublet moved rapidly along the x–y plane (Video 2). In only a few cases (9 ± 4%), the MTOC moved to a distal position after repeated contact with the membrane facing the T cell (Fig. 2 B).
Figure 2.
Dynamics of MTOC polarization in DCs. (A) Time-lapse video microscopy sequence showing DC-MTOC movements during the interaction of DCs derived from centrin-GFP knock-in mice (the green spots correspond to DCs MTOCs) with OT-I (labeled with SNARF; red). TLR-stimulated DCs were loaded with OVA class I peptide (10 nM) and mixed with OT-I cells. Movies were recorded during the first 50 min of interaction. (B) Stability of MTOC polarization. Bars show the percentage of DC–T cell conjugates (measured on contacts lasting >20 min) in which the DC-MTOC remained oriented during the entire movie length (stable) or moved back to a distal position (transient; 60 cells in four independent experiments were scored).
Together, these data show that DC maturation induced by TLR ligation confers the ability to polarize the MTOC at the IS rapidly after formation of antigen-specific conjugates with T cells.
DC-MTOC polarization drags cytokines and secretory organelles to the DC–T cell interface
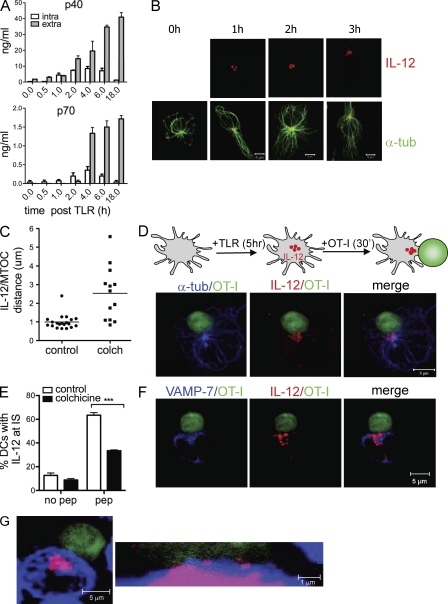
MTOC polarization has been functionally associated to directed secretion of cytokines and lytic granules in T cells and NK cells (Kupfer et al., 1991; Stinchcombe and Griffiths, 2003). We thus asked whether DC-MTOC polarization is functionally linked to polarized secretion at the synapse. To test this hypothesis we focused on IL-12, a cytokine that is produced in high amounts by DCs upon TLR stimulation. IL-12 has key roles in Th1/2 fate determination of CD4+ T cells and it is involved in clonal expansion and survival of CD8+ T cells (Valenzuela et al., 2002; Trinchieri et al., 2003; Pearce and Shen, 2007). We first analyzed the kinetics of production and the intracellular distribution of IL-12 in DCs after TLR engagement. The bioactive form of IL-12 is the heterodimeric IL-12p70 composed of the p40 and the p35 chains. Cells were pulsed with TLR agonist and harvested at different time points to analyze IL12p40 and IL12p70 content in supernatants and cell lysates. As shown in Fig. 3 A, both IL-12 p40 and IL12p70 are present in the intracellular fraction as early as 1 h after stimulation and reach the highest intracellular concentration at 4–6 h after TLR ligation. At 18 h, the intracellular content has been almost completely emptied. In agreement, intracellular staining by FACS showed a peak in the number of IL-12–positive cells at early time points (4 h after TLR = 45 ± 3%) that declined with time (35.4 ± 4% at 6 h and 7.57 ± 5% at 18 h; not depicted). Confocal analysis of the intracellular distribution of IL-12 (revealed using an anti p40/p70 antibody hereafter referred to as anti–IL-12) showed that most of the IL-12 signal is found in a ring that surrounds the MTOC at 1, 2, and 4 h after TLR induction with little punctuate staining in other areas of the cell (Fig. 3 B). Staining with anti-giantin antibodies indicates that IL-12 signal localizes with the Golgi complex around the MTOC (not depicted). Association of IL-12–containing vesicles to the MTOC depends on microtubule integrity as treatment of DCs with colchicine, a drug that inhibits microtubule polimerization, disrupted IL-12/MTOC association (Fig. 3 C). Collectively, these data show that a few hours after TLR ligation intracellular IL-12 is mostly distributed around the MTOC in DCs.
Figure 3.
Polarization of IL-12 at the IS. (A) Kinetic of IL-12 production and secretion in DCs. DCs were stimulated with a combination of TLR agonist for the indicated times (time post TLR). The relative content of IL-12 p40 and p70 in cell culture supernatants (gray bars) and cell lysates (white bars) was determined by ELISA. Bars show means ± SEM of five independent experiments. (B) Intracellular localization of IL-12 in DCs. DCs stimulated with TLR agonist for the indicated times were immunolabeled with anti–α-tubulin (green) and anti–IL-12 p40/70 (red) antibodies. Images are z projections of confocal sections. (C) Association between IL-12–containing vesicles and MTOC. TLR-stimulated DCs (4-6 h) were immunolabeled as in B. The distance (μm) of IL-12 containing vesicles from the MTOC was measured in each cell in normal (control) or colchicine (colch)-treated DCs. Each dot represents the mean distance of vesicles/MTOC in a single cell. Mean values ± SEM of 25 cells/condition. (D) Confocal images showing recruitment of IL-12 at the DC–T cell interface. Experimental outline: DCs were stimulated with TLR agonist (5 h), loaded with peptide, and mixed with OT-I cells to induce synapse formation. (top) Confocal planes showing IL-12 (red) and α-tubulin (blue) distribution in DC–T cell doublets. T cells are in green (CFSE labeling). (E) DCs were treated with colchicine before synapse formation. The percentage of conjugates with IL-12 enriched at the contact in control and treated cells was measured in at least 50 conjugates/condition in three independent experiments (P < 0.001, Student’s t test). (F) Labeling with anti–VAMP-7 (Ti-VAMP) and anti–IL-12 antibodies on fixed DC–T cell synapses shows alignment of secretory organelles close to the DC–T cell interface. (G) DCs prelabeled with WGA (blue) to demark the membrane were mixed with CFSE-labeled T cells. The image shows one representative high-magnification projection of z stacks along the contact zone which depicts vesicles of IL-12 (red) crossing the DC plasma membrane.
We next asked whether the intracellular pool of IL-12 is translocated to the synaptic region in concert with MTOC polarization when DCs encounter T cells. DCs were stimulated with TLR agonists for 5 h to reach the highest intracellular levels of IL-12. DCs were loaded with OVA peptide and mixed with OVA-specific CD8+ T cells (prelabeled with CFSE to facilitate detection of DC–T cell couples) for 30 min to allow synapse formation. In DC–T cell antigen-specific conjugates, intracellular IL-12 remained tightly associated to the MTOC (Fig. S2) and it was transported to the synaptic region close to the T cell membrane (Fig. 3 D). In the presence of peptide, up to 67% of conjugates showed enrichment of IL-12 at the DC–T cell interface. We also found a proportion of cells (21 ± 3%; unpublished data) with polarized IL-12 and a not fully oriented MTOC, suggesting that IL-12 vesicles may also travel in front of the MTOC. Pretreatment of DCs with colchicine significantly inhibited recruitment of IL-12 at the IS (Fig. 3 E), indicating that MTOC and IL-12 polarization are linked. In NK and T cells, it has been shown that different trafficking routes are associated with polarized or multidirectional delivery of soluble mediators toward a target. However, whereas cytotoxic granules are released in a strictly polarized fashion in NK and cytotoxic T cells, the release of cytokines seem to be less spatially confined. For instance, in NK cells interacting with a target, IFN-γ is released toward the synapse, but it can also be released elsewhere. Two evidences indicate that in DCs recruitment of the intracellular pool of IL-12 toward the IS leads to preferential secretion in the synaptic area (not necessarily exclusive). First, we observed that VAMP-7–positive vesicles (VAMP-7 is a Ti-VAMP that marks late endocytic vesicles and mediates fusion of intracellular vesicles with the plasma membrane; Braun et al., 2004), were enriched at the DC–T cell interfaces of antigen-specific conjugates in close proximity to IL-12–positive vesicles, suggesting that secretory organelles align at the contact site (Fig. 3 F). Second, high magnification sections taken through the interaction site revealed vesicles of IL-12 crossing the DC membrane toward the T cell (Fig. 3 G). Thus, DC polarization reorients the secretory apparatus and increases the concentration of IL-12 vesicles directed toward the IS.
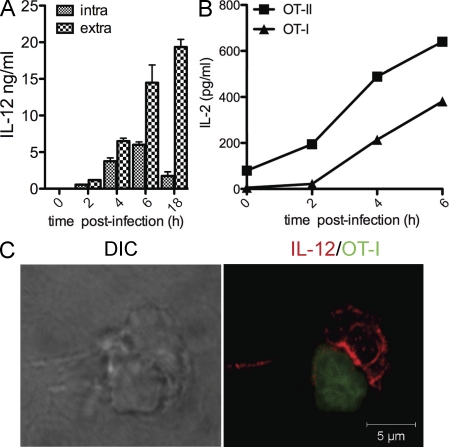
To confirm the data in a relevant model of infection, we studied cytokine polarization in DCs exposed to Escherichia coli expressing the OVA antigen. Intracellular and extracellular IL-12 levels induced by pathogen-associated molecular patterns were measured at different time points after infection. In parallel, DCs were fixed and incubated with OVA-specific class I and II T cells to assess antigen presentation as the ability to activate T cells. As shown in Fig. 4, exposure to bacteria induced a peak of intracellular IL-12 between 4 and 6 h after infection, which coincides with the peak of antigen presentation of MHC–OVA complexes derived from ingested bacteria. To follow polarization of intracellular IL-12 in infected DCs, DC and OVA T cells were mixed 5 h after infection and fixed for confocal analysis. As shown in Fig. 4 C, IL-12 staining was highly enriched at the contact site with antigen-specific T cells.
Figure 4.
IL-12 polarization upon bacterial infection. DCs were infected with E. coli expressing OVA for 2, 4, or 6 h (DC/bacteria ratio of 1:20). (A) Intracellular accumulation (intra) and secretion (extra) of IL-12 were measured by ELISA at every time point after infection. (B) Kinetic of antigen presentation after infection. DCs were infected with E. coli -OVA and fixed after 2, 4, and 6 h. OT-I and OT-II cells were added to DCs, and the levels of IL-2 in the cell culture supernatant was tested by ELISA after 12 h of incubation. Results are presented as mean values of triplicate culture wells. One representative of three experiments with similar results is shown. (C) Staining profile of IL-12 (red) in the DC–T cell synapse formed using E. coli–infected DCs and OT-I cells (CFSE labeled).
Therefore, we conclude that at early time points after infection, intracellular IL-12 is dragged at the DC–T cell contact site during antigen recognition in a microtubule-dependent fashion.
IL-12 signaling at the DC–T cell synapse
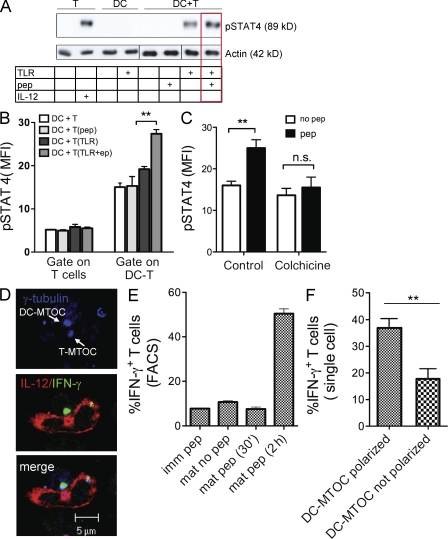
Binding of IL-12 to its receptor initiates a signaling cascade that, via the Janus-associated kinases, leads to phosphorylation of the STAT4 transcription factor and transactivation of IL-12 regulated genes, such as IFN-γ (Bacon et al., 1995). Moreover, downstream target of IL-12 include activation of Bcl-2 and Bcl-3, which promote the survival of antigen-activated CD8+ T cells, inducing their clonal expansion (Li et al., 2006). To investigate the functional impact of IL-12 recruitment at the IS, we studied IL-12–dependent events in T cells. To this aim, we set up an assay to measure phosphorylated STAT4 (pSTAT4) in T cells during synapse formation. DCs were left untreated (immature) or stimulated with TLR agonist for 5 h (mature) and loaded or not with peptide. DCs were then mixed with antigen-specific OT-I cells and lysed after 30 min of interaction. Analysis of cell lysates by immunoblot showed a clearly detectable pSTAT4 signal upon incubation of T cells with mature DCs, but not with immature DCs that do not contain IL-12. Most importantly, T cells mixed with mature DCs pulsed with antigen showed higher pSTAT4 levels than T cells incubated in the absence of antigen (Fig. 5 A), indicating that synapse formation promotes STAT4 activation. To quantify the extent of pSTAT4 signaling, we used intracellular staining and FACS analysis. DCs were pulsed or not pulsed with TLR agonist and peptide and mixed with T cells for 30 min. The percentage of pSTAT4-positive T cells was determined by gating on the region of DC–T cell doublets and, as a control, on T cells not engaged in synapse, as described in the experimental procedure. T cells alone showed a low background level similar in all cases. In the gate of DC–T cell doublets, we observed an overall higher background and a specific pSTAT4 signal that varied depending on the DC state. Immature DCs induced an equivalent signal regardless of the presence of peptide. In contrast, incubation with mature DCs induced a clear increase in the number of pSTAT4+ T cells that was significantly higher in conjugates formed with antigen-loaded DCs as compared with not loaded DCs (P = 0.0029; (Fig. 5 B). Treatment with colchicine to disrupt IL-12/MTOC association and translocation to the IS caused a significant reduction in the number of pSTAT4+ T cells in antigen-specific conjugates (Fig. 5 C).
Figure 5.
Antigen-specific synapse formation induces STAT4 signaling and IFN-γ neosynthesis in T cells. (A) DCs were pretreated with TLR agonist, loaded with 10 nM of OVA peptide, and mixed with OT-I cells. After 30 min of incubation, cells were lysed and analyzed by Western blotting using an antibody against pSTAT4. Control lanes (1–4) are T cells alone (1) or incubated with soluble IL-12 (2) and DCs alone not stimulated (3) or stimulated (4) with TLR agonist. Lanes 5–8 are DCs coincubated with T cells in different conditions as indicated in the table. (B) Detection of pSTAT4 by intracellular FACS analysis. DCs were either left untreated or stimulated with TLR agonist (TLR), loaded (pep) or not with OVA peptide, and mixed with OT-I for 30 min. Cells were fixed and labeled intracellularly with anti-pSTAT4 Alexa Fluor 488 antibody. The MFI was determined by gating on isolated T cells (gate on T cells) or on DC–T cell doublets (gate on DC–T cell) as described in experimental procedure. Data represent the mean values ± SEM (subtracted for the isotype control values) obtained in three independent experiments. (C) Control DCs or DCs treated with colchicine were used in the assay described in B. Data show the MFI ± SEM of pSTAT4 signal determined on T cells engaged in doublets in four independent experiments (P < 0.01; Student’s t test). (D) DC–T cell antigen-specific interactions trigger IFN-γ neosynthesis in T cells. DCs activated by TLR agonist and loaded with peptide were allowed to interact with OT-I for 2 h, fixed, and labeled. Confocal images show polarized IL-12 (red) and DC-MTOC (blue) facing IFN-γ staining around the T cell MTOC (green). (E) Quantification of IFN-γ neosynthesis in T cells engaged in antigen-specific synapses was measured by intracellular FACS analysis under different conditions (as specified). Values plotted indicate the percentage of IFN-γ+/CD8+ T cells gated in the region of DC–T cell doublets (isolated T cells show no IFN-γ signal), subtracted for the isotype control (one of three experiments with identical results is presented). (F) Early expression of IFN-γ in T cells correlates to DC-MTOC polarization at the IS. Cells were labeled as in (D) and quantified. Bars show the distribution of IFN-γ–positive T cells in synapse with DCs that present (polarized) or not (not polarized) the MTOC facing the T cell membrane. The data comes from quantification of >100 cells in three independent experiments (**, P = 0.052).
To causally link polarization of the intracellular pool of IL-12 to activation of IL-12–dependent events, we performed analysis at the single cell level. As the signal of pSTAT4 was too weak to allow quantification by immunofluorescence, we tracked neosynthesis of IFN-γ, the first gene induced by IL-12 in a STAT4-dependent manner (Lund et al., 2004). DCs were stimulated to induce accumulation of intracellular IL-12, washed extensively, and mixed with OT-I cells for either 30 min or 2 h. At 2 h, a clear IFN-γ+ ring focused around the T-MTOC was visible in many T cells engaged in synapses (Fig. 5 D). IFN-γ in DC–T cell conjugates was specifically induced by 2 h of interaction with IL-12 containing DCs, as no signal was detected when T cells were incubated with immature DCs, when the interaction was stopped after 30 min, or when T cells and DCs were mixed in the absence of antigen (Fig. 5 E). Importantly, single-cell quantification by confocal microscopy showed that IFN-γ was preferentially induced in T cells in synapse with polarized DCs (Fig. 5 F). Therefore, synapse formation and polarization of the intracellular pool of IL-12 controls activation of STAT4 and IFN-γ neosynthesis in T cells.
MTOC polarization in DCs is regulated by Cdc42
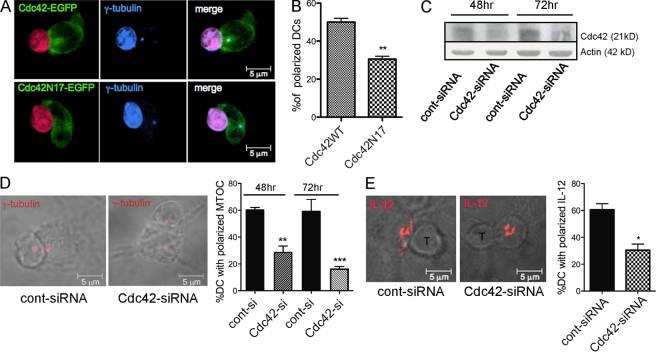
To elucidate the molecular mechanism of MTOC polarization in DCs, we studied the Rho GTPase Cdc42, a master regulator of cell polarity in several cellular models. Previous studies showed that Cdc42 regulates MTOC polarization in immune cells (T cells, NK cells, and macrophages) through multiple effectors that control actin dynamics and anchoring of MT to the plasma membrane, providing the forces that pull the MTOC (Stinchcombe et al., 2006; Banerjee et al., 2007; Eng et al., 2007). To assess the role of Cdc42 in DCs polarization, we overexpressed a plasmid encoding Cdc42 fused to GFP (Cdc42WTGFP) or its corresponding dominant inactive mutant (Cdc42N17GFP). In DCs expressing Cdc42WTGFP, most of the GFP signal was focused at the DC–T cell interface in a pocket-like shape and the MTOC localized together with the peak of GFP intensity. The GFP signal in cells expressing Cdc42N17GFP was more diffused with a central area of high GFP intensity surrounded by bands extending toward the cell periphery (Fig. 6 A and Fig. S3). The MTOC remained associated to the area of strongest Cdc42 signal, but its translocation was inhibited in respect to cells expressing the WT counterpart (30% of reduction, n = 70 cells analyzed; Fig. 6 B). Thus, activated Cdc42 is necessary to induce MTOC polarization in DCs. To firmly establish the role of Cdc42, we silenced its expression by delivery of a previously described Cdc42-specific siRNA (Gasman et al., 2004). This oligo efficiently reduced the levels of endogenous Cdc42 in DCs at 48 and 72 h after transfection (Fig. 6 C). To assess the functional consequences of Cdc42 depletion on cell polarization, DCs were plated on fibronectin and mixed with OT-I cells to induce synapse formation. DCs with reduced levels of Cdc42 showed some morphological differences with a flat and spread shape and, on average, occupied a wider area on the slide. This is in line with a recent report showing that Cdc42-deficient DCs cannot coordinate leading and trailing edge and develop multiple competing leading edges (Lämmermann et al., 2009). DC-MTOC polarization was significantly reduced in cells expressing the Cdc42-specific siRNA (51 and 64% of reduction in respect to control cells when cells were harvested for synapse 48 and 72 h after transfection, respectively). DC-MTOC polarization at the IS was partially rescued by coexpressing a Cdc42 mutant insensitive to siRNA silencing, excluding off-target effects of Cdc42 oligo (Fig. S4). Depletion of Cdc42 did not interfere with the association of intracellular IL-12 with the MTOC; however, as expected, translocation of MTOC-associated IL-12 vesicles was reduced in Cdc42-depleted cells (Fig. 6 E). Based on these data, we conclude that expression of Cdc42 is required to trigger MTOC and intracellular IL-12 polarization at the DC–T cell interface in antigen-specific conjugates.
Figure 6.
Cdc42 controls MTOC/IL-12 polarization at the IS. Cdc42 is recruited at the IS and is required for DC-MTCO polarization. (A) DCs were transfected with WT (Cdc42WTGFP) or dominant-negative mutant (Cdc42N17GFP) Cdc42 fused to GFP. GFP+ cells were enriched by cell sorting and mixed with SNARF-labeled OT-I cells (emits in the blue and red in this acquisition setting). Confocal images show a representative example of Cdc42WTGFP recruited at the IS and colocalized with polarized γ-tubulin (blue), and one example of diffused Cdc42N17GFP staining in a nonpolarized cell (Fig. S3). (B) The percentage of DCs with the MTOC polarized toward the contact region in the two groups was scored in n > 50 cells/condition in three independent experiments (**, P = 0.0124). (C) DCs were transfected with a siRNA targeted against Cdc42 (Cdc42-siRNA) or an unrelated siRNA (cont-siRNA). Protein depletion was assessed on total cell extracts by Western blot analysis at 48 and 72 h after transfection. (D) A representative confocal z slice showing the DC-MTOC position at the IS in control and Cdc42-depleted DCs (anti-γ-tubulin, red). The percentage of DCs showing a polarized MTOC was quantified on at least 90 conjugates in 3 independent experiments. Values are plotted as mean ± SEM (**, P = 0.0032 and **, P = 0.0045 at 48 and 72 h, respectively; Student’s t test). (E) Representative images and quantification of IL-12 recruitment at the IS in control and Cdc42-silenced cells. Values are plotted as mean ± SEM of two independent experiments (40 cells/condition; *, P = 0.0365, Student’s t test).
Cdc42 silencing in DCs results in reduced T cell proliferation and IFN-γ production
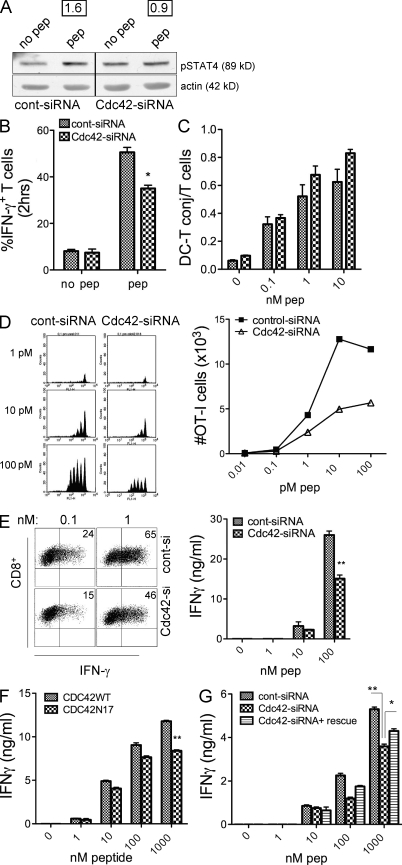
Cdc42-silenced DCs were finally used to investigate the impact of reduced DC-MTOC polarization on T cell activation. For this, we used the assays presented in Fig. 5 to measure early IL-12–dependent signals induced in T cells upon synapse formation. Control and depleted cells were pulsed with TLR agonist, incubated with CD8+ T cells for 30 min, lysed, and probed for the levels of phosphorylated STAT4. Antigen-specific induction of pSTAT4 in T cells was observed in control cells but not in T cells incubated with Cdc42-depleted DCs (Fig. 7 A). Importantly, the activation of IFN-γ neosynthesis after 2 h of interaction was significantly reduced in T cells stimulated by Cdc42-silenced cells as compared with control cells treated with control siRNA (Fig. 7 B). To rule out that depletion of Cdc42-disturbed DC–T cell interaction, we measured conjugate formation by FACS. As shown in Fig. 7 C, DCs and T cells aggregate in a peptide dose-dependent manner. Conjugate formation was not hampered by Cdc42 depletion at the different peptide doses tested; instead, we observed a slight increase in the percentage of T cells engaged in synapses with silenced cells. Consistent with this, the up-regulation of CD69 on T cells at 18 h was not affected by Cdc42 depletion, indicating that transmission of type 1 signals through the TCR was intact (Fig. S5). Moreover, IL-12 levels were equal in control and silenced cells, except that reduced IFN-γ responses were caused by a nonspecific reduction in cytokine content (Fig. S6). Hence, we conclude that blocking DC-MTOC polarization selectively interferes with the delivery of IL-12–containing vesicles at the IS and impairs the activation of the IFN-γ response in CD8+ T cells. As IL-12 produced by DCs regulates survival of primed T cells, we finally assessed the fate of T cells at later time points. DCs transfected with plasmids coding for unrelated-siRNA (control DCs) or for Cdc42 siRNA were stimulated by TLR agonist, loaded with graded peptide doses, and mixed with OT-I cells that had been prelabeled with the vital dye CFSE to follow cell division. At day 3, cells were analyzed for cell division profile, total number of cells, and secretion of IFN-γ. OT-I cells entered division starting at 1 pM and underwent a maximum of 5 cycles. The number of OT-I cells in culture with control DCs increased steadily reaching a maximum at 100 pM. In sharp contrast, DCs treated with Cdc42-siRNA induced OT-I cells to enter division (we observed one cycle of delay only at the 10 pM dose), but divided cells did not accumulate as shown by the marked reduction in the total number of cells (Fig. 7 D). This indicates that Cdc42-silenced cells are not competent to promote survival of primed T cells. T cells activated by Cdc42-depleted DCs show an intrinsic per-cell reduction in the percentage of IFN-γ–positive cells at day 3 after priming, which is reflected in the lower levels of IFN-γ in day 3 cell culture supernatant (Fig. 7 E). A similar reduction in IFN-γ levels was obtained using DCs expressing the dominant-negative mutant of Cdc42 (Cdc42N17) proving that inhibition of Cdc42-mediated MTOC polarization by two distinct approaches has the same impact on T cell functions (Fig. 7 F). Moreover, by coexpressing a rescue plasmid insensitive to Cdc42 silencing, we partially rescued the levels of IFN-γ secreted by T cells stimulated by silenced cells, proving the specificity of the Cdc42 effect (Fig. 7 G).
Figure 7.
Cdc42 depletion in DCs selectively impairs IL-12–dependent events in T cells. (A) Control and Cdc42-silenced cells activated by TLRs agonist and loaded (peptide) or not loaded (no peptide) with 10 nM of class I peptide were mixed with OT-I cells for 30 min and lysed to analyze the levels of activated STAT4 by W.B. Numbers indicate the intensity values (ratio pSTAT4 peptide/pSTAT4 no peptide normalized for actin levels) obtained from density scans. (B) Neosynthesis of IFN-γ in T cells engaged in synapse with control or Cdc42-silenced cells. DCs treated as in A were incubated for 2 h, fixed, and analyzed for intracellular IFN-γ in T cells engaged in synapse (gated on DC–T cell doublets) by FACS. Values represent average of three independent experiments (*, P = 0.0133). (C) Conjugate formation is not affected by Cdc4 silencing. 105 control or Cdc42-silenced DCs were loaded with graded doses of peptide as indicated, labeled with SNARF and mixed with 105 CFSE labeled OT-I for 20 min at 37°C. The number of DC–T cell doublets was measured by FACS gating on the double-positive events and normalized to the number of T cells. Values shown are mean ± SD of three experiments. (D) T cells proliferation and survival are affected by Cdc42 silencing in DCs. 104 control (control-siRNA) or Cdc42-depleted (Cdc42-siRNA) DCs were TLR stimulated, loaded with graded doses of peptide, and mixed with OVA-specific OT-I cells that had been prelabeled with CFSE. (left) Day 3 CFSE dilution profiles at the indicated doses of peptide. (right) The plot shows the total number of cells recovered at day 3. One of three independent experiments with similar results is shown. (E) Dot plots show the percentage of IFN-γ–producing cells at day 3 after priming, when T cells were primed using control or Cdc42-depleted DCs. Data show one representative of two experiments. Cell culture supernatants were harvested at day 3, and the levels of IFN-γ were measured by ELISA (values are mean ± SD of three independent experiments (**, P = 0.001). IFN-γ levels secreted by T cells are inhibited by DCs expressing dominant-negative Cdc42. Cdc42WT or the dominant-negative Cdc42N17 were stimulated as in D and mixed with OT-I. Bars show the levels of IFN-γ in day 3 cell culture supernatants. Data are the means ± SD of three independent experiments (**, P = 0.0017). (G) Expression of a rescue construct partially restores IFN-γ levels. DCs were cotransfected with control or Cdc42-specific siRNA, plus a plasmid encoding a Cdc42 resistant to silencing (rescue). Cells were treated as in F. Bars shows values of IFN-γ in day 3 cell culture supernatants from 2 independent experiments (means ± SD; *, P = 0.0385, **, P = 0.0068).
Collectively, we conclude that synapse formation and Cdc42-mediated polarization of intracellular stores f IL-12 is required for the acquisition of effector functions by CD8+ naive T cells.
DISCUSSION
Productive T cell activation by antigen-presenting DCs depends on coordinated delivery of signals from antigen (signal 1), adhesion and co-stimulatory molecules (signal 2), and soluble mediators (signal 3). In this work, we propose that by recruiting the MTOC and intracellular cytokine vesicles toward the interacting T cell DCs can coordinate the transmission of signal 1, 2 and 3 at the IS.
Our results show that synapse formation with DCs containing intracellular stores of IL-12 induces the activation of STAT4 and the early production of IFN-γ in T cells. This proves that the DC–T cell synapse is the platform in which antigen-specific T cells receive soluble mediators that determine their differentiation. At the single cell level, early activation of IFN-γ is preferentially induced in T cells engaged in synapse with polarized DCs. We propose that establishment of polarity in DCs and the consequent directional delivery of newly formed intracellular cytokines to synapses optimize the delivery of T cell priming cytokines to cells that recognize antigens presented by DCs.
Cytotoxic granules in NK cells and CTLs exit the cell via a truly polarized secretion mode at the IS to avoid release of dangerous mediators in the extracellular space. The release of cytokines seems to be less spatially confined and depends on the cargo and cell type analyzed. In T cells, it has been demonstrated that cytokines that work in an antigen-specific way (IFN-γ and IL-2), accumulate in compartments tightly associated with the MTOC, like IL-12 in DCs, and are release at the IS. In contrast, proinflammatory cytokines (TNF) and chemokines that act at a distance to recruit other cell types are found dispersed in the cytosol and are secreted in a multidirectional fashion with no synaptic bias (Huse et al., 2006). In NK cells, it has recently been shown that exocytosis of cytokines (IFN-γ and TNF), although not strictly confined at the IS like cytotoxic granules, also take place at the IS (Reefman et al., 2010).
Our data in DCs do not provide a formal demonstration that IL-12 is secreted at the synapse because the receptor/receptors that trigger DCs polarization have not been identified, thus hampering the reconstitution of artificial surfaces on which the cytokine can be captured. Still, the high concentration of cytokine at the IS and the localization with markers of the exocytic pathway indicate that, if not exclusive, a preferential secretion must operate in the synaptic area. Consistent with this, blocking MTOC polarization by silencing Cdc42 prevents IL-12 recruitment at the IS and cause a selective reduction in early IL-12–dependent events in T cells (STAT4 phosphorylation and IFN-γ neosynthesis). At later time points, interfering with MTOC polarization resulted in a strong impairment in the number of T cells that survive after priming. In the context of naive CD8+ T cell priming, IL-12 has been shown to provide a third signal that promotes full activation and survival of activated T cells (Valenzuela et al., 2002). IL-12 also promotes enhanced DC–T cell interaction times (Henry et al., 2008) a factor that in turn favors full acquisition of effector functions (Hugues et al., 2004; Scholer et al., 2008). The requirement for IL-12 as a third signal for full T cell proliferation is especially relevant when the antigen dose is low, whereas at high antigen doses IL-12 is important to develop cytolytic effector functions (Curtsinger et al., 2003). Interestingly, our data show that blocking DC-MTOC polarization results in similar T cell proliferation but highly reduced survival of primed T cells, especially at low antigen doses. At higher antigen doses, when defective proliferation is rescued, the levels of IFN-γ produced by T cells primed by Cdc42-silenced DCs are lower than in control cells.
Previous studies reported that IL-12 and IL-18 in DCs polarize at the interface between the two cells during interaction with NK cells. Moreover, CD8+ T lymphocytes induce the exocytosis of IL-1β secretory lysosomes from DCs, reinforcing the notion that DCs undergo functional polarization in response to interaction with other cells (NK or T cells; Gardella et al., 2001; Borg et al., 2003; Semino et al., 2005). Our study extends these observations to the context of CD8+ naive T cell priming and provides a molecular mechanism to explain cytokine recruitment at the IS via polarization of the microtubule cytoskeleton. MTOC polarization in DCs is strongly dependent on antigen recognition, i.e., nonspecific DC–T cell doublets rarely display a polarized MTOC. The percentage of DCs with polarized MTOC increases as soon as antigen is added and occurs preferentially in those synapses that display an organized IS (with enriched TCR). Time-lapse videos showed that polarization of the DC centrosome is rather stable and the MTOCs remained confined to the area underneath the T cell membrane with little movements on the spot. Interestingly, we captured conjugates with the DC-MTOC very near to the T-MTOC/TCR region, suggesting that the secretory domains of the two cells may become contiguous at some point during the interaction.
The question of which signals trigger MTOC movements in DCs remains unanswered. We failed to reconstitute specific MTOC polarization using beads coated with anti–MHC class I or class II antibodies (unpublished data), indicating that the signal to induce polarization in DCs is not triggered directly by engagement of MHC molecules. One likely hypothesis is that TCR triggering by DCs presenting specific antigens changes the affinity of adhesion molecules in T cells that, in turn, induce antigen-independent signals mediated by integrins in DCs. In line with this, in migrating astrocytes MTOC/Golgi polarization is triggered by integrins through recruitment of Cdc42 and activation of the mPar6–PKCζ complex (Etienne-Manneville and Hall, 2001). Also in T cells, microtubule dynamics are regulated by the LFA-1–ICAM-1 cross talk (Rodríguez-Fernández et al., 1999). In DCs, triggering of LFA-1 on DCs by ICAM-3–coated beads induce clustering of MHC class II at the IS, further highlighting the role of integrins on inducing molecular reorganization in DCs (de la Fuente et al., 2005).
The other requirement for MTOC repositioning in DCs is a maturation signal through ligation of TLRs by bacterial compounds. The ability to reorient the MTOC in antigen-specific conjugates therefore adds to the list of properties acquired by DCs upon maturation. This may depend on an indirect effect caused by the fact that mature DCs form more stable synapses and induce stronger signaling in T cells than immature DCs (Benvenuti et al., 2004b; Hugues et al., 2004). Alternatively, intrinsic remodeling of the actin cytoskeleton induced by TLR ligation (West et al., 2004) may in turn affect microtubule dynamics, enabling MTOC movements.
The exact molecular pathways that control MTOC polarization in different cell types have not been fully unraveled and depend on the context. Still, in several different cellular models, the Rho GTPase Cdc42 has emerged as a common central regulator of MTOC polarization. In cells of the immune system, Cdc42 is important for polarization of the MTOC during phagocytosis in macrophages (Eng et al., 2007). In T cells, MTOC polarization during synapse formation depends on several adaptor molecules associated to TCR-specific signaling (Dustin et al., 1998; Blanchard et al., 2002) and is mediated by Cdc42 effectors, such as IQGAP, which mediate linkage of microtubules to specific cortical regions (Stinchcombe et al., 2006). In this study, we show that Cdc42 also regulates the ability to reorient in response to synapse formation in DCs. We also found that DC-MTOC polarization is strongly reduced in cells deficient for the Wiskott-Aldrich syndrome protein (WASp; unpublished data), in line with studies that identified WASp as a major molecular link between actin cytoskeleton and microtubule network during MTOC polarization in NK cells through the Cdc42 interactor protein CIP4 (Orange et al., 2002; Banerjee et al., 2007). Because Cdc42 determines polarity by regulating the activity of different effectors depending on the cell system analyzed, further studies are required to delineate the precise mechanism by which Cdc42 controls MTOC polarization in DCs.
The model of DC polarity that we propose is valid for 1:1 interactions. Studies by several groups that imaged DC–T cell interactions in vivo demonstrated that stable DC–T cell contacts are causally linked to the acquisition of effector functions (Celli et al., 2007; Henrickson et al., 2008). Inspection of the videos reveals that even under superphysiological concentrations of antigen-bearing DCs and antigen-specific T cells, these interactions are mostly monogamous. Therefore, it is highly likely that 1:1 interactions occur early in response, given the low frequencies of antigen-specific T cells (20–200 CD4+ T cells and 80–1,200 for CD8+ T cells; Moon et al., 2007; Obar et al., 2008) and the low level of occupancy of MHC peptide complexes by foreign antigens in vivo.
Our data also predict that synaptic transmission would be limited to a short period of time in the DC maturation program, i.e., early after TLR ligation (4-6 h), when DCs contain abundant intracellular IL-12. Importantly, at this time point, DCs are fully competent to interact with T cells because they have processed and presented pathogen-derived antigens and they have acquired full capacity to interact with T cells. At later time points, fully mature DCs are devoid of intracellular IL-12, suggesting that interaction with T cells at this stage would not result in a strong burst in IL-12 signaling transmitted through the synapse. A further restriction in the number of T cells that would receive synaptic IL-12 comes from the relatively low percentage of DCs that reorient the MTOC in synapses, especially at low, physiologically relevant antigen doses. Therefore, these findings may provide an additional basis to understand the generation of diversity in the fate of T cells.
In summary, this study provides evidences of a previously unappreciated function of DCs at the IS, i.e., the targeted delivery of soluble mediators at the DC–T cell interface to enrich the local concentration of cytokines received by antigen-specific T cells.
MATERIALS AND METHODS
Mice.
6–8-wk-old C57BL/6 females were purchased from Harlan. GFP-centrin mice were generated from a construct provided by M. Bornens (Institut Curie, Paris, France) and were a gift from C. Desdouets (Institut Cochin, Paris, France).
OVA-specific, MHC class I restricted and MHC class II, TCR transgenic OT I and OT II mice were purchased from the Jackson ImmunoResearch Laboratories. CD45.1 congenic C57BL/6 (a gift from P. Guermonprez, Institut Curie, Paris, France) were bred to OT-I mice to obtain OT-I/CD45.1.
Mice were bred and maintained in sterile isolators. Animal care and treatment were conducted in conformity with institutional guidelines in compliance with national and international laws and policies (European Economic Community [EEC] Council Directive 86/609; OJL 358; December 12, 1987). Protocols were approved by the Italian Ministry of Health.
Cells.
Bone marrow–derived DCs were differentiated in vitro from the bone marrow of C57BL/6 or centrin-GFP knockin mice using culture medium containing Fms-like tyrosine kinase 3 ligand. DCs were used for experiments between days 7 and 8, when expression of Cd11c was higher than 80%. For experiments with endogenous DCs, spleens from mice were extracted, homogenized, and digested with Collagenase D (1.6 mg/ml; Roche) and DNase I (0.1 mg/ml; Roche). Enrichment of DCs was performed by density gradient in 1,068 g/cm3 of OptiPrep solution (Sigma-Aldrich).The very low density fraction mainly composed by DCs was recovered and subjected to purification using CD11c microbeads (Miltenyi Biotec). OT-I and OT-II cells were isolated from total lymph nodes suspension by negative selection using MACS isolation kit.
Synapse formation and immunolabeling.
To induce maturation, DCs were stimulated with a mixture of the TLR agonists CpG and LPS (10 µg/ml). Cells were used for synapses between 4 and 5 h after TLR triggering. DCs were pulsed with graded dose of the MHC class I restricted peptide of OVA 257–264(SIINFEKL) and transferred to slides coated with fibronectin (Sigma-Aldrich; 10 µg/ml). OT-I cells were added to DCs in a 1:1 ratio and incubated at 37°C for 30 min. In some experiments, OT-I were labeled with 2 µm CFSE to facilitate detection of DC–T cell doublets. Nonadherent T cells where removed by washing the slides with PBS several times. Cells were fixed (4% paraformaldehyde), permeabilized (PBS/BSA 0.1%/saponin 0.05%), and immunolabeled. The following antibodies were used: rat α-tubulin (AbD; Serotec), rat anti–IL-12 p40/p70 (BD), hamster anti-CD3 (BD), mouse anti–VAMP-7 (provided by T. Gally, Institut Jaques Monod, Paris, France), and rabbit anti–γ-tubulin (provided by M. Bornen, UMR144, Institute Curie, Paris). All secondary antibodies were obtained from Invitrogen. Phalloidin-Texas red (Sigma-Aldrich) was used to detect polymerized F-actin. Confocal images were acquired in a LSM510 META Axiovert 200M reverse microscope with a 63× objective (Carl Zeiss, Inc.). Z projection of slices, three-dimensional reconstruction, and image analysis were performed using an LSM image examiner (Carl Zeiss, Inc.) and ImageJ software (National Institutes of Health).
Time-lapse video microscopy.
For time lapse analysis, 2 × 105 centrin-GFP DCs pulsed for 5 h with CpG (1 µg/ml), LPS (1 ng/ml), and SIINFEKL peptide (10 nm) were plated on a fibronectin-coated coverslip, placed into a chamber with IMDM medium on a LSM510 META Axiovert 200M reverse microscope at 37°C in a 5% CO2 atmosphere. OT-I cells labeled with the vital dye SNARF (Invitrogen) were added a few minutes before starting the record. Transmitted light and fluorescence images were taken with a 63× objective and a three-charge-coupled device camera every 30 s for at least 40 min. The dynamics of centrin-GFP spots corresponding to the MTOC were tracked frame by frame in every cell, choosing the plane with the brightest GFP spot. Number of cells that reoriented the MTOC, elapsed time between the establishment of the contact and polarization, and duration of the polarized condition were analyzed using the ImageJ software.
Analysis of polarization.
The analysis of polarization was performed on DCs conjugated to a single T cell. This is the most represented condition when a 1:1 DC/T cell ratio is used. To score conjugates with polarized MTOCs we calculated the ratio between the DC’s diameter and the distance of the MTOC to the synapse region. Conjugates in which such value was <0.3 were considered “polarized.” In Fig. 3 B, we defined IL12-containing vesicles using a standardized threshold calculated with ImageJ on Z projections of confocal sections. The distance between the MTOC and all vesicles was measured on individual cells and plotted as the mean distance in at least 30 cells/condition. In Fig. 3 E, we measured the distance between the synapse region and each cytokine vesicle. The ratio between the mean distances of cytokine vesicles and synapse region/diameter of the DC was calculated. The cytokine was considered polarized when this ratio was <0.3. To evaluate whether DC polarization is linked to activation of IFN-γ expression in T cells, we first collected pictures of DC–T cell single conjugates with transmitted light/γ-tubulin staining channel (red or blue depending on the experiment), and then we measured the position of the MTOC spot to score it as polarized or not. We next switched on the green channel to visualize the signal of IFN-γ in the T cell.
ELISA.
5 × 105 DCs were stimulated with CpG/LPS for different periods. At the end of the incubation period, cell culture supernatant was harvested and the cells pellets were washed 2 times in PBS and lysed in 120 µl of TNN + 1 µl of protease inhibitor cocktail. The levels of IL-12p40 and IL-12p70 in supernatants and cell lysates were determined by commercial ELISA kits (BD and eBioscience) according to the manufacturer’s instructions.
Cytoskeletal disruption.
To inhibit microtubule polymerization, cells were treated with 1 µg/ml colchicine (Sigma-Aldrich) for the last 5 min of the pulsing period with TLR agonist. The cells were extensively washed before mixing to T cell to avoid carry over of the drug.
Silencing of Cdc42 and expression of Cdc42 mutants.
The control siRNA (ATTCTATCACTAGCGTGAC) and specific Cdc42 siRNA (GGGCAAGAGGATTATGACATT) were as previously reported (Malacombe et al., 2006; Momboisse et al., 2009). 6 × 106 DCs were transfected with 1 µM of siRNA using the Amaxa Nucleofector according to the manufacturer’s instructions. Cells were collected 48 or 72 h after transfection. For the expression of GFP-tagged construct of Cdc42 WT or N17 (Gasman et al., 2004). 10 × 106 DCs were transfected with 2–3 µg of endotoxin-free DNA. Cells were collected after 48 h and live GFP+ cells were enriched by cell sorting. For rescue experiments, DCs were cotransfected with siRNA directed against Cdc42 and a plasmid coding for the HA-tagged mutated rescue Cdc42, resistant to siRNA degradation, which has been generated by mutagenesis (QuickChange mutagenesis kit; Stratagene) of the codon GAT encoding Asp-63 to GAC. To assess depletion of Cdc42 1 × 105 cells were lysed and analyzed by SDS page using anti-Cdc42 antibody (BD). To assess maturation and cytokine production, 105 cells were plated for 4 h in TLR agonist–containing medium. The levels of IL-12 were measured in the cell culture supernatants by ELISA. The up-regulation of surface maturation markers (MHC class II and B72) was evaluated by FACS analysis at 4 and 12 h after transfection.
Bacterial infection.
DCs were infected with 3.6 × 106 CFU of Escherichia coli-OVA (gift from A. Savina, Institut Curie, Paris, France; DC/bacteria ratio was 1:20) for 1 h. After infection, the medium was removed and the cells were washed with cold PBS and medium with gentamicin was added for 1, 3, or 5 h (total time of infection: 2, 4, and 6 h). At the end of each chasing period, the cell culture supernatant was harvested and the cell pellets were lysed. The content of IL-12 in the extracellular and intracellular fraction was quantified as described above. For T cell activation, after the chasing period the cells were fixed in 0.008% gluteraldehyde for 3 min on ice, quenched with 0.2 M glycine, and washed extensively. OT-I and OT-II cells were added to the wells and incubated for 12 h. Cell culture supernatant was harvested and the content of IL-2 was measured according to standard procedures.
STAT4 phosphorylation.
For analysis of STAT4 phosphorylation by Western blot, 2 × 105 control of Cdc42-silenced DCs were mixed with 4 × 106 OT-I/CD45.1 in 96-well plates by spinning at 800 rpm for 1 min. After 30 min of incubation at 37°C, the cells were lysed and cell lysates were resolved by 10% SDS-PAGE. Membranes were blocked in TBS-5% BSA and developed using anti pSTAT4-ser721 (sc-22160; Santa Cruz Biotechnology, Inc.), followed by anti–rabbit horse radish peroxidase (Sigma-Aldrich). For FACS analysis, DC–T cell synapses were formed as for STAT6 analysis. At the end of incubation at 37°C, cells were fixed with 1% PFA (10 min) and permeabilized with methanol 80% (20 min). T cells were incubated with supernatant of TLR-stimulated DCs as a control (referred to as soluble IL-12 in Fig. 5 B). After washing, cells were stained with Alexa Fluor 488 mouse anti-STAT4 (pY693; BD) or mouse IgG-FITC isotype control (BD), CD45.1-rhodamine (1:400), and CD8-Cy5 (1:400). For FACS analysis, we gated on CD8-CD45.1 double-positive events. Inside this population, T cells alone or conjugated with DCs were distinguished by the FSC/SSC profile.
FACS analysis of IFN-γ in synapses.
Control of Cdc42-silenced DCs were stimulated with 10 µg/ml CpG+LPS and loaded with 10 nM OVA class I peptide. As controls, we used DCs without TLR agonist or without peptide. After 3 h of stimulation, DCs were washed extensively to eliminate extracellular IL-12 and mixed with OVA-specific OT-I cells. After 30 min or 2 h of interaction, cells were fixed, and the cell surface was labeled with anti CD8 antibodies. Cells were permeabilized and labeled with Alexa Fluor 488 anti–mouse IFN-γ (XMG1.2; eBioscience) or the corresponding isotype control. For FACS analysis, we gated on CD8+ events, and inside this population, T cells alone or conjugated with DCs were distinguished by the FSC/SSC profile. Values are expressed as the percentage of cells positive for IFN-γ in respect to the corresponding isotype control.
T cell proliferation and INF-γ production.
To analyze T cell proliferation and IFN-γ production, 1.2 × 104 control or Cdc42-depleted DCs, DCs expressing WT or dominant-negative N17 mutant or DCs cotransfected with silencing and rescue plasmid were plated on 96 wells and stimulated for 4 h with 1 µg/ml CpG/LPS. Cells were loaded with serial dilution of SIINFEKL peptide. After 4 h, cells were washed and 105 OT-I/CD45 that had been prelabeled with CFSE were added to the culture wells. At day 3, the supernatant was collected and the cells were labeled with anti-CD8 and anti-CD45.1 antibodies to gate on T cells. All cells were acquired to determine the CFSE dilution profile, and the total number of cells in each well. To determine the intracellular levels of IFN-γ at day 3, cultures were restimulated with 10 µg/ml BFA and 1µM OVA class I peptide for 4 h. Cells were fixed and the surface was labeled with anti-CD45.1/anti-CD8 antibodies, permeabilized, and labeled with Alexa Fluor 488 anti–mouse IFN-γ (XMG1.2; eBioscience). The content in IFN-γ in cell culture supernatants was analyzed by ELISA using standard procedures.
Statistical analysis.
All data were reported as the mean ± SD as calculated using GraphPad Prism 5 software. The unpaired Student’s t test was used as indicated in the text to assess significance.
Online supplemental material.
Fig. S1 shows DC–T cell conjugate formation and T cell activation at different times after TLR stimulation. Fig. S2 illustrates the relative distribution of IL-12 and tubulin in a representative DC–T cell synapse. Fig. S3 shows the distribution of a GFP-tagged dominant-negative mutant Cdc42 in DCs. Fig. S4 shows that inhibition of MTOC polarization by silencing Cdc42 is specific and does not depend on off-targets effects. Fig. S5 indicates that Cdc42 silencing does not affect up-regulation of CD69 on T cells. Fig. S6 shows that Cdc42 silencing has no impact on the levels of IL-12 produced by DCs. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100007/DC1.
Acknowledgments
We thank V. Calco (Centre National de la Recherche Scientifique UPR3212, INCI, Strasbourg) for technical assistance, Paolo Maiuri for help with time-lapse video microscopy, and Mauro Sturnega for help with animal handling. We also thank Thierry Gally for providing anti–VAMP-7 antibodies and Claire Hivroz for scientific advice and helpful discussions.
This work is supported by Italian Telethon Grant GGP06267. Julian Pulecio and Jelena Petrovic have been supported by an ICGEB pre-doctoral fellowship. Francesca Prete and Giulia Chiaruttini were supported by Associazione Italiana Ricerca Cancro.
The authors have no competing financial interest.
Footnotes
Abbreviations used:
- Ag
- antigen
- IS
- immune synapse
- MTOC
- microtubule organizing center
- TLR
- Toll-like receptors
References
- Al-Alwan M.M., Liwski R.S., Haeryfar S.M., Baldridge W.H., Hoskin D.W., Rowden G., West K.A. 2003. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J. Immunol. 171:4479–4483 [DOI] [PubMed] [Google Scholar]
- Bacon C.M., McVicar D.W., Ortaldo J.R., Rees R.C., O’Shea J.J., Johnston J.A. 1995. Interleukin 12 (IL-12) induces tyrosine phosphorylation of JAK2 and TYK2: differential use of Janus family tyrosine kinases by IL-2 and IL-12. J. Exp. Med. 181:399–404 10.1084/jem.181.1.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee P.P., Pandey R., Zheng R., Suhoski M.M., Monaco-Shawver L., Orange J.S. 2007. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J. Exp. Med. 204:2305–2320 10.1084/jem.20061893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benvenuti F., Hugues S., Walmsley M., Ruf S., Fetler L., Popoff M., Tybulewicz V.L., Amigorena S. 2004a. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 305:1150–1153 10.1126/science.1099159 [DOI] [PubMed] [Google Scholar]
- Benvenuti F., Lagaudrière-Gesbert C., Grandjean I., Jancic C., Hivroz C., Trautmann A., Lantz O., Amigorena S. 2004b. Dendritic cell maturation controls adhesion, synapse formation, and the duration of the interactions with naive T lymphocytes. J. Immunol. 172:292–301 [DOI] [PubMed] [Google Scholar]
- Blanchard N., Di Bartolo V., Hivroz C. 2002. In the immune synapse, ZAP-70 controls T cell polarization and recruitment of signaling proteins but not formation of the synaptic pattern. Immunity. 17:389–399 10.1016/S1074-7613(02)00421-1 [DOI] [PubMed] [Google Scholar]
- Bloom O., Unternaehrer J.J., Jiang A., Shin J.S., Delamarre L., Allen P., Mellman I. 2008. Spinophilin participates in information transfer at immunological synapses. J. Cell Biol. 181:203–211 10.1083/jcb.200711149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boes M., Cerny J., Massol R., Op den Brouw M., Kirchhausen T., Chen J., Ploegh H.L. 2002. T-cell engagement of dendritic cells rapidly rearranges MHC class II transport. Nature. 418:983–988 10.1038/nature01004 [DOI] [PubMed] [Google Scholar]
- Borg C., Taieb J., Terme M., Maruyama K., Flament C., Angevin E., Zitvogel L. 2003. NK cell-based immunotherapy: new prospects and involvement of dendritic cells. Bull. Cancer. 90:699–705 [PubMed] [Google Scholar]
- Braun V., Fraisier V., Raposo G., Hurbain I., Sibarita J.B., Chavrier P., Galli T., Niedergang F. 2004. TI-VAMP/VAMP7 is required for optimal phagocytosis of opsonised particles in macrophages. EMBO J. 23:4166–4176 10.1038/sj.emboj.7600427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli S., Lemaître F., Bousso P. 2007. Real-time manipulation of T cell-dendritic cell interactions in vivo reveals the importance of prolonged contacts for CD4+ T cell activation. Immunity. 27:625–634 10.1016/j.immuni.2007.08.018 [DOI] [PubMed] [Google Scholar]
- Chen X., Allan D.S., Krzewski K., Ge B., Kopcow H., Strominger J.L. 2006. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc. Natl. Acad. Sci. USA. 103:10346–10351 10.1073/pnas.0604236103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtsinger J.M., Lins D.C., Mescher M.F. 2003. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197:1141–1151 10.1084/jem.20021910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente H., Mittelbrunn M., Sánchez-Martín L., Vicente-Manzanares M., Lamana A., Pardi R., Cabañas C., Sánchez-Madrid F. 2005. Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol. Biol. Cell. 16:3314–3322 10.1091/mbc.E05-01-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M.L., Olszowy M.W., Holdorf A.D., Li J., Bromley S., Desai N., Widder P., Rosenberger F., van der Merwe P.A., Allen P.M., Shaw A.S. 1998. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 94:667–677 10.1016/S0092-8674(00)81608-6 [DOI] [PubMed] [Google Scholar]
- Eng E.W., Bettio A., Ibrahim J., Harrison R.E. 2007. MTOC reorientation occurs during FcgammaR-mediated phagocytosis in macrophages. Mol. Biol. Cell. 18:2389–2399 10.1091/mbc.E06-12-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. 2004. Cdc42—the centre of polarity. J. Cell Sci. 117:1291–1300 10.1242/jcs.01115 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S., Hall A. 2001. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 106:489–498 10.1016/S0092-8674(01)00471-8 [DOI] [PubMed] [Google Scholar]
- Gardella S., Andrei C., Lotti L.V., Poggi A., Torrisi M.R., Zocchi M.R., Rubartelli A. 2001. CD8(+) T lymphocytes induce polarized exocytosis of secretory lysosomes by dendritic cells with release of interleukin-1beta and cathepsin D. Blood. 98:2152–2159 10.1182/blood.V98.7.2152 [DOI] [PubMed] [Google Scholar]
- Gasman S., Chasserot-Golaz S., Malacombe M., Way M., Bader M.F. 2004. Regulated exocytosis in neuroendocrine cells: a role for subplasmalemmal Cdc42/N-WASP-induced actin filaments. Mol. Biol. Cell. 15:520–531 10.1091/mbc.E03-06-0402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson S.E., Mempel T.R., Mazo I.B., Liu B., Artyomov M.N., Zheng H., Peixoto A., Flynn M.P., Senman B., Junt T., et al. 2008. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat. Immunol. 9:282–291 10.1038/ni1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry C.J., Ornelles D.A., Mitchell L.M., Brzoza-Lewis K.L., Hiltbold E.M. 2008. IL-12 produced by dendritic cells augments CD8+ T cell activation through the production of the chemokines CCL1 and CCL17. J. Immunol. 181:8576–8584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S., Fetler L., Bonifaz L., Helft J., Amblard F., Amigorena S. 2004. Distinct T cell dynamics in lymph nodes during the induction of tolerance and immunity. Nat. Immunol. 5:1235–1242 10.1038/ni1134 [DOI] [PubMed] [Google Scholar]
- Huse M., Lillemeier B.F., Kuhns M.S., Chen D.S., Davis M.M. 2006. T cells use two directionally distinct pathways for cytokine secretion. Nat. Immunol. 7:247–255 10.1038/ni1304 [DOI] [PubMed] [Google Scholar]
- Krummel M.F., Macara I. 2006. Maintenance and modulation of T cell polarity. Nat. Immunol. 7:1143–1149 10.1038/ni1404 [DOI] [PubMed] [Google Scholar]
- Kupfer A., Dennert G., Singer S.J. 1985. The reorientation of the Golgi apparatus and the microtubule-organizing center in the cytotoxic effector cell is a prerequisite in the lysis of bound target cells. J. Mol. Cell. Immunol. 2:37–49 [PubMed] [Google Scholar]
- Kupfer A., Mosmann T.R., Kupfer H. 1991. Polarized expression of cytokines in cell conjugates of helper T cells and splenic B cells. Proc. Natl. Acad. Sci. USA. 88:775–779 10.1073/pnas.88.3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lämmermann T., Renkawitz J., Wu X., Hirsch K., Brakebusch C., Sixt M. 2009. Cdc42-dependent leading edge coordination is essential for interstitial dendritic cell migration. Blood. 113:5703–5710 10.1182/blood-2008-11-191882 [DOI] [PubMed] [Google Scholar]
- Li Q., Eppolito C., Odunsi K., Shrikant P.A. 2006. IL-12-programmed long-term CD8+ T cell responses require STAT4. J. Immunol. 177:7618–7625 [DOI] [PubMed] [Google Scholar]
- Lund R.J., Chen Z., Scheinin J., Lahesmaa R. 2004. Early target genes of IL-12 and STAT4 signaling in th cells. J. Immunol. 172:6775–6782 [DOI] [PubMed] [Google Scholar]
- Malacombe M., Ceridono M., Calco V., Chasserot-Golaz S., McPherson P.S., Bader M.F., Gasman S. 2006. Intersectin-1L nucleotide exchange factor regulates secretory granule exocytosis by activating Cdc42. EMBO J. 25:3494–3503 10.1038/sj.emboj.7601247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Cófreces N.B., Robles-Valero J., Cabrero J.R., Mittelbrunn M., Gordón-Alonso M., Sung C.H., Alarcón B., Vázquez J., Sánchez-Madrid F. 2008. MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell Biol. 182:951–962 10.1083/jcb.200801014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. 2001. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 1:135–145 10.1038/35100529 [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Preston-Hurlburt P., Janeway C.A., Jr 1997. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 388:394–397 10.1038/41131 [DOI] [PubMed] [Google Scholar]
- Momboisse F., Ory S., Calco V., Malacombe M., Bader M.F., Gasman S. 2009. Calcium-regulated exocytosis in neuroendocrine cells: intersectin-1L stimulates actin polymerization and exocytosis by activating Cdc42. Ann. N. Y. Acad. Sci. 1152:209–214 10.1111/j.1749-6632.2008.03998.x [DOI] [PubMed] [Google Scholar]
- Moon J.J., Chu H.H., Pepper M., McSorley S.J., Jameson S.C., Kedl R.M., Jenkins M.K. 2007. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 27:203–213 10.1016/j.immuni.2007.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar J.J., Khanna K.M., Lefrançois L. 2008. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 28:859–869 10.1016/j.immuni.2008.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange J.S., Ramesh N., Remold-O’Donnell E., Sasahara Y., Koopman L., Byrne M., Bonilla F.A., Rosen F.S., Geha R.S., Strominger J.L. 2002. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc. Natl. Acad. Sci. USA. 99:11351–11356 10.1073/pnas.162376099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Shen H. 2007. Generation of CD8 T cell memory is regulated by IL-12. J. Immunol. 179:2074–2081 [DOI] [PubMed] [Google Scholar]
- Reefman E., Kay J.G., Wood S.M., Offenhäuser C., Brown D.L., Roy S., Stanley A.C., Low P.C., Manderson A.P., Stow J.L. 2010. Cytokine secretion is distinct from secretion of cytotoxic granules in NK cells. J. Immunol. 184:4852–4862 10.4049/jimmunol.0803954 [DOI] [PubMed] [Google Scholar]
- Riol-Blanco L., Delgado-Martín C., Sánchez-Sánchez N., Alonso-C L.M., Gutiérrez-López M.D., Del Hoyo G.M., Navarro J., Sánchez-Madrid F., Cabañas C., Sánchez-Mateos P., Rodríguez-Fernández J.L. 2009. Immunological synapse formation inhibits, via NF-kappaB and FOXO1, the apoptosis of dendritic cells. Nat. Immunol. 10:753–760 10.1038/ni.1750 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Fernández J.L., Gómez M., Luque A., Hogg N., Sánchez-Madrid F., Cabañas C. 1999. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol. Biol. Cell. 10:1891–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholer A., Hugues S., Boissonnas A., Fetler L., Amigorena S. 2008. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 28:258–270 10.1016/j.immuni.2007.12.016 [DOI] [PubMed] [Google Scholar]
- Semino C., Angelini G., Poggi A., Rubartelli A. 2005. NK/iDC interaction results in IL-18 secretion by DCs at the synaptic cleft followed by NK cell activation and release of the DC maturation factor HMGB1. Blood. 106:609–616 10.1182/blood-2004-10-3906 [DOI] [PubMed] [Google Scholar]
- Stinchcombe J.C., Griffiths G.M. 2003. The role of the secretory immunological synapse in killing by CD8+ CTL. Semin. Immunol. 15:301–305 10.1016/j.smim.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Stinchcombe J.C., Majorovits E., Bossi G., Fuller S., Griffiths G.M. 2006. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 443:462–465 10.1038/nature05071 [DOI] [PubMed] [Google Scholar]
- Trinchieri G., Pflanz S., Kastelein R.A. 2003. The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity. 19:641–644 10.1016/S1074-7613(03)00296-6 [DOI] [PubMed] [Google Scholar]
- Valenzuela J., Schmidt C., Mescher M. 2002. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 169:6842–6849 [DOI] [PubMed] [Google Scholar]
- West M.A., Wallin R.P., Matthews S.P., Svensson H.G., Zaru R., Ljunggren H.G., Prescott A.R., Watts C. 2004. Enhanced dendritic cell antigen capture via toll-like receptor-induced actin remodeling. Science. 305:1153–1157 10.1126/science.1099153 [DOI] [PubMed] [Google Scholar]