Abstract
OBJECTIVE: Human embryonic stem (hES) cells can be differentiated into pancreatic endoderm structures in vitro. The study was performed to determine whether induced pluripotent stem (iPS) cells can be differentiated into similar structures with comparable efficiency. METHODS: We compared the ability of hES cells and iPS cells derived from human epidermal keratinocytes to progressively differentiate into pancreatic endoderm. Human foreskin keratinocytes were reprogrammed to pluripotency by transduction with retroviruses encoding Oct4, Sox2, and Klf4. The resulting keratinocyte-derived iPS (KiPS) cell lines and a hES cell line were subjected to a modified pancreatic endoderm differentiation protocol. Cells and embryoid-body structures derived from both hES and KiPS cells were compared at different stages of development for expression of stem cell and differentiation markers, including Sox2, Oct4, Mixl1, Brachyury, Gsc, FoxA2, Sox17, Hnf4α, Hnf1β, Nkx2.2, Nkx6.1, Hex, Isl1, Pdx1, and Slc2A, via Taqman real-time PCR, flow-cytometry, and/or immunocytochemistry. RESULTS: hES cells and KiPS cells expressed similar levels of the stem cell factors Sox2 and Oct4. Upon differentiation, both cell types underwent remarkably similar changes in gene expression. They acquired the definitive endoderm markers Sox17 and FoxA2. Most Sox17+ and FoxA2+ cells co-expressed Hnf4α and Hnf1β, found in the primitive gut tube, a pancreas precursor. Most FoxA2+ cells were also Pdx1+, and many expressed Nkx2.2, Nkx6.1, and Isl1. CONCLUSIONS: Keratinocyte-derived iPS cells can be differentiated into pancreatic endoderm, and the efficiency of this process is comparable to that seen for hES cells. Thus keratinocytes have the potential to serve as a source of patient-specific pancreatic endoderm for transplantation.
Keywords: type 1 diabetes, embryonic stem cells, induced pluripotent stem cells, pancreatic endoderm, beta-cell, keratinocytes, differentiation
Abbreviations: B27 - serum-free cell culture supplement; bFGF - basic fibroblast growth factor; c-Myc - cellular version of the myelocytomatosis oncogene (transcription factor regulating expression of many genes); DAPI - 4'-6-diamidino-2-phenylindole (fluorescence stain); DMEM - Dulbecco's modified Eagle medium; EB - embyoid body; ES cell - embryonic stem cell; FBS - fetal bovine serum; FGF7 - fibroblast growth factor 7; FoxA2 - forkhead-box protein A2 (also termed hepatocyte-nuclear factor 3β, Hnf3β); GCG - glucagon; Gsc - goosecoid (homeodomain-containing protein); hES cell - human embryonic stem cell; Hex - hematopoietically expressed homeobox protein (transcription factor; regulator of cell fate); Hnf1β - hepatocyte nuclear factor 1β (nuclear receptor protein/transcription factor; essential for liver development); Hnf4α - hepatocyte nuclear factor 4α (nuclear receptor protein/transcription factor; essential for liver development); HSA - human serum albumin; HUES - human embryonic stem cell; IgG - immunoglobulin G; iPS cell - induced pluripotent stem cell; Isl1 - insulin gene enhancer protein 1 (important in embryogenesis of pancreatic islets); KAAD-cyclopamine - 3-Keto-N-(aminoethyl-aminocaproyl-dihydrocinnamoyl)cyclopamine; KiPS cell - keratinocyte-derived iPS cell; KiPS3F1 - KiPS cell line transduced with Oct4, Sox2, and Klf4; KiPS4F1 - KiPS cell line transduced with Oct4, Sox2, Klf4, and c-Myc; Klf4 - Krüppel-like transcription factor 4; KO - knockout; MEF - mouse embryonic fibroblast; Mixl1 - Mix-like 1 homeodomain protein (transcription factor; required for mesendoderm morphogenesis); Nkx2.2 - homeobox protein involved in morphogenesis of the central nervous system; Nkx6.1 - homeobox protein required for β-cell development; Oct4 - octamer binding transcription factor 4 (transcription factor, expressed by embryonic stem cells); Pdx1 - pancreatic and duodenal homeobox 1 (transcription factor necessary for pancreas development); PFA - paraformaldehyde (used to fix cells); RA - retinoic acid; RNA - ribonucleic acid; RPMI-1640 - Roswell Park Memorial Institute 1640 (cell culture medium); RT - room temperature; PCR - polymerase chain reaction; Slc2A - glucose transporter (also GLUT; membrane protein required for glucose transport into cells); Sox2 - sex determining region Y box 2 (transcription factor, essential to maintain self-renewal of embryonic stem cells); Sox17 - sex determining region Y box 17 (endodermal transcription factor); T - symbol and gene name of brachyury (transcription factor expressed in
Introduction
At present, the transplantation of pancreatic islets, or whole pancreas from a cadaveric donor, are the only alternatives to insulin therapy, to restore normoglycemia in type 1 diabetic patients. Islet transplantation can afford insulin-independence to some patients, and can improve the quality of life of others, even when insulin independence is not achieved [1]. However, islet transplantation is not currently the standard treatment for type 1 diabetes. Possibly one day, the development of immunosuppressive strategies capable of selectively blunting autoreactivity, without impairing systemic immunity, coupled with improved islet isolation and preservation techniques, may justify a more general application of this approach. However, the realization of this dream will only be possible if the problem of limited organ availability is eventually solved.
Differentiation of human embryonic stem (hES) cells into pancreatic β-cells has been long considered as a possible solution to this problem. ES cells, derived from the inner cell mass of pre-implantation embryos, can both be propagated indefinitely without loss of the undifferentiated state, and prompted to differentiate into cells belonging to endoderm, mesoderm, or ectoderm lineages. Early studies showed that mouse ES cells could be differentiated in vitro into cells displaying phenotypic and morphological properties of islet cells [2-6]. More recently, it has been shown that hES cells can be differentiated into pancreatic endoderm and, much less efficiently, into clusters containing insulin-producing cells [7-13].
The need to use human embryos to generate hES cells as a source of transplantation material poses significant ethical concerns. The discovery that mouse fibroblasts can be reprogrammed into induced pluripotent stem (iPS) cells by transduction of four key transcription factors (Oct4, Sox2, Klf4, and c-Myc) provided a potential solution to this ethical issue, and brought forth the notion that derivation of tissues from patient-specific stem cells is possible [14]. These studies were replicated by others [15], and extended to the generation of human iPS cells from fibroblasts [16-18]. However, it has not been addressed whether iPS cells prompted to differentiate into pancreatic endoderm progressively upregulate markers of differentiation with similar kinetics/rate as ES cells.
The purpose of this study was to determine the feasibility of differentiating pancreatic endoderm from human iPS cells. More specifically, we examined iPS cells generated from human skin keratinocytes, as these are significantly more amenable to iPS generation than fibroblasts. Also, we monitored the kinetics of marker expressions at different stages of differentiation.
Materials and methods
Cell lines and cell culture
The hES cell line ES4 was maintained, as described [19]. The generation and characterization of the KiPS cell lines KiPS4F1 (transduced with Oct4, Sox2, Klf4, and c-Myc) and KiPS3F1 (with Oct4, Sox2, and Klf4) has been reported [20]. hES and KiPS cells were maintained in Matrigel-coated plates with hES cell medium, conditioned by irradiated mouse embryonic fibroblasts (MEFs). hES cell medium consisted on KO-DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% KO serum replacement (Invitrogen), 0.5% human albumin (Grifols, Barcelona, Spain), 2 mM Glutamax, 50 μM 2-mercaptoethanol, non-essential amino acids, and 10 ng/ml bFGF (Peprotech, London, UK).
Antibodies
Primary antibodies were:
- mouse anti-hOct4 (1:60), rabbit anti-hSox2 (1:400), mouse anti-hNkx6.1 and anti-hNkx2.2 ascites from Developmental Studies Hybridoma Bank (University of Iowa) (1:2).
- goat anti-hHnf3β/FoxA2 (R&D Biosystems, Minneapolis, MN; 1:100)
- goat anti-hSox17 (1:100) (Neuromics, Bloomington, MN)
- rabbit anti-hGlucagon (1:100) (Dako, Barcelona, Spain)
- rabbit anti-hHnf4α (1:100) and rabbit anti-hHnf1β (1:100) (Santa Cruz Biotechnology, Santa Cruz, CA)
- anti-hProinsulin C-peptide (1:200) and rabbit anti-hPdx1 (1:500) (Millipore, Madrid, Spain) and
- rabbit anti-hIsl1 (J. Ferrer - University of Barcelona, Spain).
Secondary antibodies were:
- donkey anti-mouse IgG-Cy3 or -Cy2
- anti-rabbit IgG-Cy2 and
- donkey anti-goat IgG-Cy3 or -Cy5
all from Jackson Immunoresearch (Suffolk, UK) (1:200).
Pancreatic endoderm differentiation
hES and iPS cells were differentiated into pancreatic endoderm, as described by Kroon et al. [13], with modifications [9]. Briefly, cells were progressively adapted to growth on RPMI-1640 containing 2% B27, 0.25% HSA-20%, and MEF-conditioned HUES (from 50% to 10%), grown to ~90% confluency on matrigel-coated dishes, and then cultured in RPMI containing 1% B27, Wnt3a (25 ng/ml), activin A (100 ng/ml) and 0.5 mM sodium butyrate (day 1). During the next 2 days, cells were cultured in RPMI with activin A (100 ng/ml) and 0.2% FBS. On days 4-6, cells were cultured in RPMI with 2% FBS and fibroblast growth factor 7 (FGF7; 50 ng/ml). The medium was replaced with DMEM containing 1% B27, KAAD-cyclopamine (0.25 µM), retinoic acid (2 µM) and noggin (50 ng/ml) for 3-4 days. On day 10, cells were sliced into aggregates (embryoid body-like structures, EBs), and cultured in ultra-low attachment plates in DMEM containing 1% B27 until day 14. Activin A, FGF7, noggin, and Wnt3a were from R&D Biosystems, KAAD-cyclopamine from Calbiochem (Nottingham, UK), and retinoic acid from Sigma-Aldrich (Madrid, Spain).
Immunoflourescence and flow cytometry
EBs and monolayers were fixed in 4% PFA for 2 h at 4ºC, washed 3 times with PBS, and treated with Target Retrieval Solution, pH 9 (Dako) for 1h at 60ºC and 30 min at RT. Samples were washed 3 times with Tris-buffered saline (TBS), and incubated with block buffer (TBS, 0.5% Triton X-100, 6% donkey serum) for 2 h at RT, followed by overnight storage at 4º C, and 2 h at RT. Subsequently, samples were washed with TBS++ (TBS, 0.1% Triton X-100, 6% donkey serum), and incubated with primary Abs diluted in TBS++ for 2 h at RT, and again stored overnight at 4ºC, and 2 h at RT. After 4 TBS++ washes, EBs were incubated with secondary Abs in TBS++ for 2h at RT, followed by overnight storage at 4ºC, and 2 h at RT. Lastly, samples were incubated with DAPI, then mounted and analyzed on a confocal microscope. For flow cytometry, cells were incubated with goat anti-human FoxA2 or Sox17 antibodies and AF488-conjugated anti-goat Abs, and analyzed with a MoFlo High-Speed Sorter.
Taqman real-time polymerase chain reaction
RNA was extracted with TRIZOL. TaqMan real-time polymerase chain reaction (PCR) was performed on an ABI-7300 machine (Applied Biosystems, Madrid, Spain). Assay IDs were: Pou5f1, Hs00742896; Nanog, Hs02387400; Mixl1, Hs00430824; T-brachyury, Hs00610080; Gsc, Hs00418279; Hex, Hs00242160; Aft, Hs00173490; Hnf4α, Hs00766846; FoxA2, Hs00232764; Pdx1, Hs00236830; Isl1, Hs00158126; Slc2a2, Hs00165775; Gcg, Hs00174967; Gapdh, Hs99999905.
Results and discussion
The pancreas arises from the definitive endoderm, a specialized epithelium that forms the primitive gut tube, and specifies the structures that differentiate into the organs arising from this germ layer. Dorsal and ventral buds originating from the gut tube fuse to form the posterior foregut. Factors produced by the notochord, such as activin A and FGF7, coupled with retinoic acid (RA) signaling and inhibition of sonic hedgehog signaling, specify expression of the key transcription factor Pdx1 by the dorsal pancreatic endoderm [21, 22]. The latter gives rise to all the endocrine, exocrine, and ductal cells of the pancreas. Specific sets of transcription factors are expressed at each of these stages. The definitive endoderm, for example, expresses Sox17 and FoxA2. As it progressively differentiates into the primitive gut tube, posterior foregut, and pancreatic endoderm, it acquires expression of Hnf1β and Hnf4α, followed by Pdx1, HlxB9, and Hnf6, and ultimately, Ngn3, Pax4, Nkx6.1, Nkx2.1, and Isl1, among others [22].
We first attempted to differentiate the ES4 cell line using Kroon's protocol [13], but with modifications [9]. Undifferentiated ES4 cells expressed the stem cell factors Sox2 and Oct4 (Figure 1), and lacked expression of ectoderm, mesoderm, and endoderm markers (not shown). Also, they were invariably negative for endocrine markers, or markers of definitive or pancreatic endoderm, including Hnf4α, Hnf1β, Sox17, Pdx1, C-peptide, and glucagon (Figure 1). On day 10 of differentiation, the monolayers were sliced into fragments, detached, and cultured for 3-4 days in ultra-low attachment plates to promote formation of EB-like structures (Figure 2A). Immunocytochemical staining of individual EBs indicated that significant numbers of cells had differentiated into cells expressing definitive endoderm markers FoxA2 and Sox17. The content of differentiated cells per EB varied widely and ranged from ~25-80% of cells in individual EBs (Figure 2B). However, most of the Sox17+ and FoxA2+ cells contained in individual EBs co-expressed Hnf4α and Hnf1β (Figure 2B). These markers were found in the primitive gut tube, which gave rise to the pancreas and other endoderm organ primordia [21]. Furthermore, a significant fraction of FoxA2+ cells also expressed Pdx1, Nkx2.2, Isl1, or Nkx6.1 (Figure 2B). These factors are markers of committed pancreatic epithelium, and play key roles in endocrine cell differentiation [22]. In fact, a small number of cells in these structures were C-peptide- or glucagon-immunoreactive (Figure 2B), suggesting a capacity to differentiate into endocrine cells.
Figure 1.
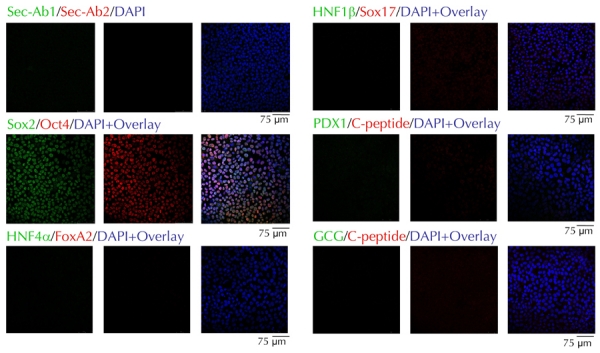
hES4 cells express Sox2 and Oct4, but not markers of definitive or pancreatic endoderm.
Figure 2. Differentiation of hES4 cells into pancreatic endoderm.
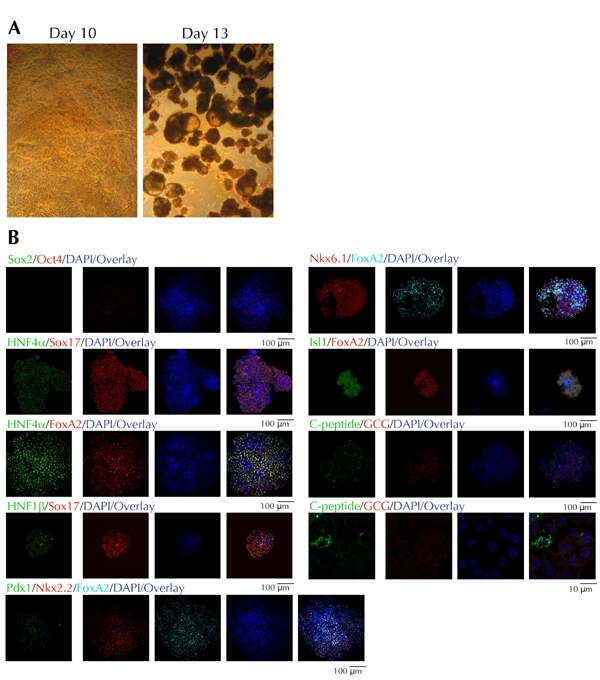
A: Optical images of a monolayer at day 10 of differentiation, immediately prior to tissue slicing, and at day 13, three days after mechanical slicing of the monolayers and growth on ultra-low attachment plates. B: Confocal microscopy images of individual EB structures stained with ES cell and differentiation markers. Each EB could only be stained with a single combination of antibodies. CGC: glucagon.
We next asked if this protocol could generate pancreatic endoderm from iPS cells reprogrammed from human keratinocytes [20]. For this purpose, we used the KiPS3F1 line generated by reprogramming human foreskin keratinocytes through retroviral transduction with Oct4, Sox2, and Klf4. These keratinocytes have been extensively characterized and shown to be fully reprogrammable [20]. Also, they appear indistinguishable from hES cells regarding colony morphology, growth properties, expression of pluripotency-associated transcription factors and surface markers, global expression profiling, as wells as in vitro and in vivo differentiation potential [20]. The data shown below were obtained with KiPS3F1 cells, but similar results were obtained with KiPS4F1 cells [20], which represent a pool of ~20 independent KiPS colonies generated with Oct4, Sox2, Klf4, and c-Myc.
As shown in Figure 3A, KiPS cells co-expressed Sox2 and Oct4, in addition to other markers, which are typically expressed by hES cells cultured under identical conditions, such as alkaline phosphatase, SSEA3, and SSEA4 ([20] and data not shown). Upon differentiation and mechanical processing, the monolayers and resulting EB structures were morphologically similar to those obtained with differentiated ES4 cells (Figure 3B). Nuclei of KiPS cells did not stain for Sox2 and Oct4 (Figure 4A). The resulting EBs contained significant, but variable portions of FoxA2+/Sox17+ cells, such as we have seen for EBs derived from ES4 cells (Figures 4B and 5). Most of the KiPS-derived EB cells displaying high nuclear content for FoxA2 or Sox17 co-expressed Hnf4α and Hnf1β, Pdx1, Nkx2.2, and Isl1 (Figure 5). Many of the hES and KiPS-derived EB cells that did not express these markers, contained nuclei that stained for Sox17, suggesting that they belonged to a less differentiated endoderm state (see Figure 2B, second row from top, and Figure 5, first and third rows from top). As in the case of ES4-derived EBs, a very small fraction of cells were C-peptide- and/or glucagon-immunoreactive (Figure 5).
Figure 3. Characterization of KiPS3F1 cells and differentiation into embryoid bodies.
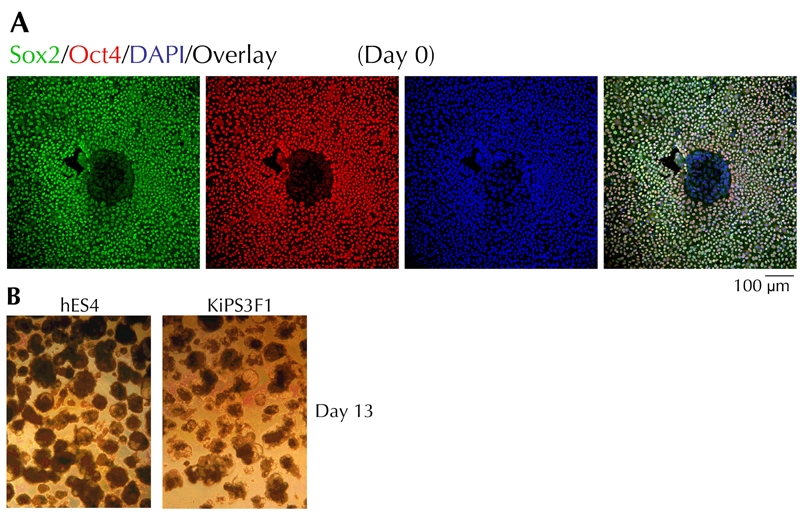
A: Confocal microscopy images of KiPS3F1 cells stained for Sox2 and Oct4 on day 0. B: Optical images of EBs of differentiated KiPS3F1 and hES4 cultures on day 13.
Figure 4. Characterization of embryoid bodies differentiated from KiPS3F1 and hES4 cells.
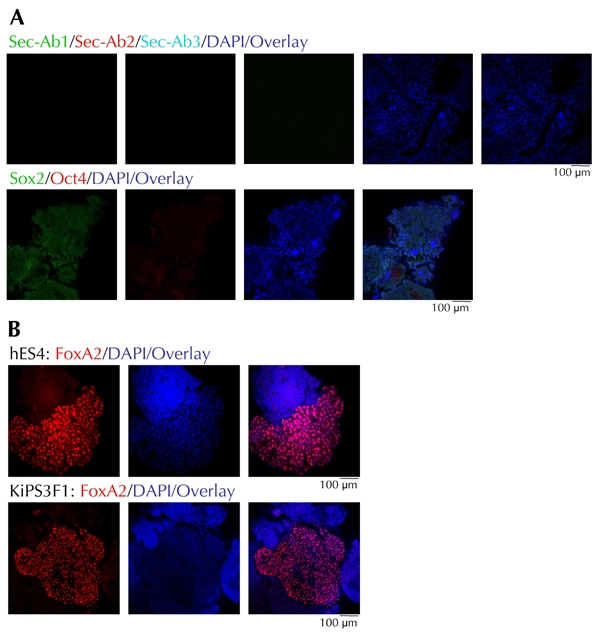
A: Confocal microscopy of individual EB structures stained with Sox2- and Oct4-specific Abs or secondary antibodies alone. B: Comparison of FoxA2 content in representative EBs from differentiated hES4 and KiPS3F1 cells.
Figure 5. Differentiation of KiPS3F1 cells into pancreatic endoderm.
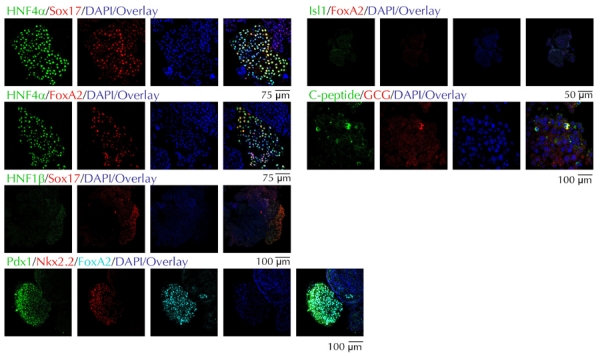
Expression of pancreatic endoderm markers in EBs from KiPS3F1 cells.
We determined whether there were any substantial differences in the ability of ES4 and KiPS cells to progressively differentiate into pancreatic endoderm in vitro. For this purpose, we measured changes in marker expression by flow cytometry and/or Taqman real-time PCR at the following different stages:
- day 1 (after culture in sodium butyrate, Wnt3a, and activin A)
- day 3 (after culture in activin A and 0.2% FBS)
- day 7 (after culture in FGF7 and 0.2% FBS)
- day 10 (after culture in KAAD, RA, and noggin)
- day 13 (EB-like structures cultured in DMEM supplemented with 1% B27).
As shown in Figure 6, ES4 and KiPS cells underwent remarkably similar changes in gene expression in response to these in vitro developmental cues. Culture in the presence of activin A and Wnt3a induced a marked upregulation of the mix1 homeobox-like 1 gene (Mixl1), a primitive streak marker, and T (brachyury) and Gsc, which are expressed in the mesendoderm (Figure 6A). This was associated with a slight upregulation of the endoderm-specific marker FoxA2 (Figure 6A). Removal of Wnt3a and addition of FBS led to further upregulation of FoxA2 and appearance of Hnf4α, a primitive gut tube marker, as well as Hex and Isl1, a pancreatic endoderm marker, in these Mixl1+/T+/Gsc+ cells.
Figure 6. Real-time PCR and flow cytometry.
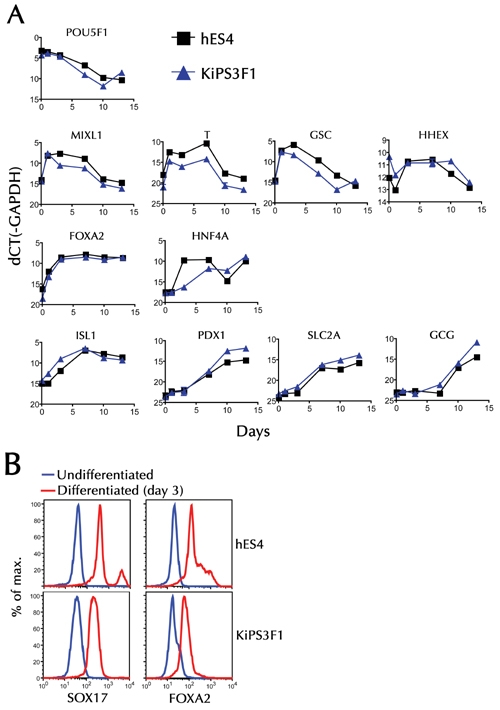
A: Changes in the levels of different mRNAs in undifferentiated, and progressively differentiated, hES and iPS cells. A series of plates were cultured, as described in the materials and methods section. These were used for RNA extraction at the end of each differentiation stage, immediately prior to change in culture conditions (days 1, 3, 7, 10, and 13). B: Flow cytometric analysis of Sox17 and FoxA2 expression on cells harvested on day 3.
To determine the extent of the culture conditions leading to the generation of definitive endoderm cell types, we compared undifferentiated ES4 and KiPS cells, and the corresponding day 3 differentiated cells for reactivity to FoxA2- and Sox17-specific antibodies by flow cytometry. As shown in Figure 6B, most day 3 cells, but not their undifferentiated precursors, were FoxA2+/Sox17+, consistent with efficient differentiation into definitive endoderm. From days 3-7 (withdrawal of activin A and exposure to FGF7), the cultures downregulated Gsc and upregulated Hnf4α, Isl1, Pdx1, and Slc2A (Glut2), while maintaining similar levels of Mixl1, T, and FoxA2. By day 10 (in response to RA, noggin, and inhibition of hedgehog signaling), the cells had downregulated Mixl1, T, and Gsc to the levels seen in undifferentiated cells. Also, they expressed maximal or near-maximal levels of FoxA2, Hnf4α, Isl1, Pdx1, and Slc2A, which are markers of committed pancreatic epithelium, and began to express Gcg. Whereas, we could not detect significant changes in insulin mRNA expression (not shown), presumably because the percentage of C-peptide-expressing cells was very low, as shown above (Figure 5). These data are consistent with a previously reported role for RA signaling in facilitating the conversion of mesendoderm into pancreatic endoderm [10]. Furthermore, they accord with the immunofluorescence microscopy analyses shown above, and demonstrate that ES4 and KiPS cells respond in a remarkably similar fashion to the in vitro developmental cues explored herein.
Several terminally-differentiated cell types other than fibroblasts, including mouse B lymphocytes [23], and liver and gastric cells [24], have been reprogrammed to iPS cells by transduction with Oct4, Sox2, Klf4, and c-Myc. We have recently shown that human keratinocytes, derived from the epidermis, are amenable to reprogramming into iPS cells by transduction with Oct4, Sox2, and Klf4, with or without c-Myc. In fact, we provided evidence that keratinocytes are more amenable to reprogramming than other cell types. This may be because they display a transcriptional profile significantly closer to hES cells than the profile seen in fibroblasts [20]. In our hands, keratinocytes typically yield iPS cell colonies within 10 days after retroviral transduction, as compared to more than 20 days for fibroblasts [17]. Already at this early stage of reprogramming, a significant fraction of the cells within these colonies expressed several hES cell markers, including alkaline phosphatase, Tra-1-81, SSEA4, Sox2, and Oct4, and lacked expression of keratinocyte markers, such as keratin 14. Furthermore, the overall reprogramming efficiency of keratinocytes approached 1%, which is ~100-fold of that reported for fibroblasts [20, 25].
It is still not clear whether iPS cells derived from all cell types can differentiate into pancreatic endoderm, or insulin-producing islet cells. The fact that iPS cells derived from fibroblasts can do so [26], should not be taken as proof that iPS cells derived from other cell types are equally amenable to differentiation into these structures. Likewise, it is not clear to what extent differentiation of iPS cells into pancreatic endoderm mimics the differentiation of hES cells into pancreatic endoderm. In the present study, we have shown for the first time that keratinocyte-derived iPS cells can be used to generate pancreatic endoderm in vitro. We found that the efficiency of pancreas endoderm formation is comparable to that seen with conventional hES cells (albeit lower than that reported for CyT203 hES cells) [13]. Furthermore, we have shown that both hES and iPS cells undergo remarkably similar changes in the expression of several different differentiation markers. We have not determined whether iPS-derived pancreatic endoderm can differentiate into mature, glucose-responsive β-cells in vivo. However, the presence of a few C-peptide and glucagon immunoreactive cells in the endoderm structures derived from both hES and iPS cells, suggests that the β-cell-differentiation potential of hES and KiPS cells is similar. These observations, coupled with the reported ability of pancreatic endoderm grafts to differentiate into islet cells in vivo [13], suggest that it may one day be possible to transplant diabetic patients with grafts derived from their own skin cells.
Disclosures (conflict of interests statement): The authors report no conflict of interests.
Acknowledgments
We thank Meritxell Carrió and Yvonne Richaud for assistance with cell culture, Cristina Pardo for assistance with immunocytochemistry, and Esther Melo and Mercé Marti for confocal microscopy. Pere Santamaria is a scientist of Alberta Innovates Health Solutions, and a JDRF Scholar. This work was supported by grants from the Ministerio de Educación y Ciencia, European Commission Marie-Curie Reintegration, Fondo de Investigaciones Sanitarias, Marató de TV3, the G. Harold and Leila Y. Mathers Charitable Foundation and Fundación Cellex (to A.R. and J.-C. I.B.), and a sabbatical AHFMR fellowship (to P.S.).
References
- 1.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC. et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 2.Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F. Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 3.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 4.Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, Wobus AM. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A. 2003;100:998–1003. doi: 10.1073/pnas.0237371100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ku HT, Zhang N, Kubo A, O'Connor R, Mao M, Keller G, Bromberg JS. Committing embryonic stem cells to early endocrine pancreas in vitro. Stem Cells. 2004;22:1205–1217. doi: 10.1634/stemcells.2004-0027. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki S, Yamato E, Miyazaki J. Regulated expression of pdx-1 promotes in vitro differentiation of insulin-producing cells from embryonic stem cells. Diabetes. 2004;53:1030–1037. doi: 10.2337/diabetes.53.4.1030. [DOI] [PubMed] [Google Scholar]
- 7.Segev H, Fishman B, Ziskind A, Shulman M, Itskovitz-Eldor J. Differentiation of human embryonic stem cells into insulin-producing clusters. Stem Cells. 2004;22:265–274. doi: 10.1634/stemcells.22-3-265. [DOI] [PubMed] [Google Scholar]
- 8.D'Amour K, Bang A, Eliazer S, Kelly O, Agulnick A, Smart N, Moorman M, Kroon E, Carpenter M, Baetge E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotech. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar A. Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 2007;25:1940–1953. doi: 10.1634/stemcells.2006-0761. [DOI] [PubMed] [Google Scholar]
- 10.Shim J, Kim S, Woo D, Kim S, Oh C, McKay R, Kim J. Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia. 2007;50:1228–1238. doi: 10.1007/s00125-007-0634-z. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Shi Y, Zhao D, Chen S, Yong J, Zhang J, Qing T, Sun X, Zhang P, Ding M, Li D, Deng H. In vitro derivation of functional insulin-producing cells from human embryonic stem cells. Cell Res. 2007;17:333–344. doi: 10.1038/cr.2007.28. [DOI] [PubMed] [Google Scholar]
- 12.Shiraki N, Yoshida T, Araki K, Umezawa A, Higuchi Y, Goto H, Kume K, Kume S. Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells. 2008;26:874–885. doi: 10.1634/stemcells.2007-0608. [DOI] [PubMed] [Google Scholar]
- 13.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J. et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;131:861–872. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 15.Maherali N, Sridharan R, Xie W, Utikal J, Eminli S, Arnold K, Stadtfeld M, Yachechko R, Tchieu J, Jaenisch R. Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell. 2007;1:55–70. doi: 10.1016/j.stem.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Park I, Zhao R, West J, Yabuuchi A, Huo H, Ince T, Lerou P, Lensch M, Daley G. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Yu J, Vodyanik M, Smuga-Otto K, Antosiewicz-Bourget J, Frane J, Tian S, Nie J, Jonsdottir G, Ruotti V, Stewart R, Slukvin I, Thomson J. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 19.Raya A, Rodriguez-Piza I, Aran B, Consiglio A, Barri P, Veiga A, Belmonte JC. Generation of cardiomyocytes from new human embryonic stem cell lines derived from poor-quality blastocysts. Cold Spring Harb Symp Quant Biol. 2008;73:127–135. doi: 10.1101/sqb.2008.73.038. [DOI] [PubMed] [Google Scholar]
- 20.Aasen T, Raya A, Barrero MJ, Garreta E, Consiglio A, Gonzalez F, Vassena R, Bilic J, Pekarik V, Tiscornia G, Edel M, Boue S, Belmonte JC. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat Biotechnol. 2008;26:1276–1284. doi: 10.1038/nbt.1503. [DOI] [PubMed] [Google Scholar]
- 21.Wells J, Melton D. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 22.Wilson M, Scheel D, German M. Gene expression cascades in pancreatic development. Mech Dev. 2003;120:65–80. doi: 10.1016/s0925-4773(02)00333-7. [DOI] [PubMed] [Google Scholar]
- 23.Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M, Creyghton MP, Steine EJ, Cassady JP, Foreman R. et al. Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell. 2008;133:250–264. doi: 10.1016/j.cell.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K, Chiba T, Yamanaka S. Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science. 2008;321:699–702. doi: 10.1126/science.1154884. [DOI] [PubMed] [Google Scholar]
- 25.Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601–31607. doi: 10.1074/jbc.M806597200. [DOI] [PubMed] [Google Scholar]
- 26.Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R, Leibel RL, Melton DA. Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci USA. 2009. 106:15768–15773. doi: 10.1073/pnas.0906894106. [DOI] [PMC free article] [PubMed] [Google Scholar]


