Abstract
Background. Patients with IgA nephropathy (IgAN) have an increased amount of abnormally O-glycosylated IgA1 in circulation, in glomerular deposits and produced by tissue cells in vitro. Although increased production of Th2 cytokines by peripheral blood lymphocytes and a functional abnormality of core 1 β1,3-galactosyltransferase (C1β3Gal-T) have been proposed as mechanisms underlying pathogenesis of IgAN, they are still obscure and are not connected.
Methods. To clarify the effect of T-cell cytokines, we analysed the mRNA levels of C1β3Gal-T and its molecular chaperone Cosmc, C1β3Gal-T activity and subsequent O-glycosylation of IgA1 in a human B-cell line stimulated with these cytokines. The surface IgA1-positive human B-cell line was cultured with recombinant human IFN-γ, IL-2, IL-4 or IL-5. The production and glycosylation of IgA1 were determined by sandwich ELISA and enzyme-linked lectin binding assay, respectively. The mRNA levels of C1β3Gal-T and Cosmc were quantitatively measured by real-time PCR. C1β3Gal-T activity was analysed using high-performance liquid chromatography.
Results. IgA1 production by IL-4-stimulated cells was significantly higher than controls or after IFN-γ or IL-5. The terminal glycosylation of secreted IgA1 was altered in response to IL-4. IL-4 stimulation significantly decreased the mRNA levels of both C1β3Gal-T and Cosmc and of C1β3Gal-T activity. IL-4 stimulation was clearly blocked by recombinant human IL-4 soluble receptor.
Conclusions. It appears that Th2 cytokine IL-4 may play a key role in controlling glycosylation of the IgA1 hinge region.
Keywords: IgA1 hinge region, T-cell cytokines, the surface IgA1-positive human B-cell line, Th2 response
Introduction
IgA nephropathy (IgAN) is the commonest form of glomerulonephritis in the world, and a significant proportion of patients progress to end-stage renal disease [1–5]. Human serum IgA consists of two subclasses, IgA1 and IgA2, structurally and functionally distinct. The diagnostic hallmark of the condition in IgAN is selective deposition of the IgA1 subclass, at least partly polymeric, in the glomerular mesangium. This subclass bias may arise from the higher ratio of IgA1 (85%) to IgA2 (15%) in serum [1–6]. IgA1 differs from IgA2, particularly at the hinge region; IgA1 contains an extended polyproline peptide bearing multiple serine and threonine residues and distinctive O-glycans. Core 1, Galβ1,3GalNAcα-serine/threonine, is the major constituent of the O-glycan structures normally present in the IgA1 hinge region. Recently, it was reported that O-linked Galβ1,3GalNAc content is significantly decreased in IgA1 purified from serum, glomerular mesangial deposits and tonsils of patients with IgAN compared to controls [6–11]. Therefore, the abnormal O-glycosylation of IgA1 in IgAN has been extensively investigated in recent years, and there is increasing evidence for its involvement in the pathogenesis of IgAN [12–18].
Core 1 β1,3-galactosyltransferase (C1β3Gal-T) transfers galactose (Gal) from UDP-Gal to an N-acetylgalactosamine (GalNAc) residue on glycoproteins, including IgA1 via a β1,3-linkage [19,20]. Although a functional abnormality of C1β3Gal-T has been proposed as a mechanism for altered O-glycosylation in IgAN, there is a paucity of information on the regulation of expression of this glycosyltransferase [6,21] or its molecular chaperone Cosmc, which is involved in the folding or stability of C1β3Gal-T [22]. Without Cosmc, translated C1β3Gal-T is lost, and there is deficiency of Gal residues on glycoprotein acceptors [22]. Mechanisms whereby C1β3Gal-T and/or Cosmc expression might be downregulated in B lymphocytes from IgAN patients remain unknown [23]. Although it is not known whether altered O-glycosylation of IgA1 is attributable to inherited defects or not, previous reports suggested that some stimuli, in a permissive genetic background, might cause low expression of the Cosmc gene and result in reduced C1β3Gal-T activity and subsequent aberrant glycosylation of IgA1 in IgAN [24–29].
IgAN is a glomerular disease that is likely Th2 dependent and is observed more commonly in developed nations [30]. An alteration in the balance of Th1 and Th2 T-cell subsets has been implicated as a mechanism to explain the pathogenesis of IgAN. Although it was reported that increased production of Th2 cytokines might result in the production of abnormally glycosylated IgA in mice, murine IgA has only N-glycosylated residues and lacks O-linked oligosaccharides [31–33]. Moreover, no investigation has attempted the analysis of IgA1 glycosylation using human B cells stimulated with T-cell cytokines. Interestingly, it was reported that O-glycosylation of IgA1 and IgD was differentially controlled during B-cell maturation by analysis of their lectin binding in patients with IgAN [25]. Compared with healthy controls, O-glycosylation in IgAN was incomplete in IgA1 but more complete in IgD. O-glycosylation of IgA1 in IgAN might be due to aberrant immunoregulation, whereas recent studies suggested that abnormal IgA1 glycosylation is an inherited rather than an acquired trait [28,29]. Therefore, in the present study, we focused on selective effects of T-cell cytokine stimulation upon C1β3Gal-T activity and subsequent glycosylation of IgA1 using IgA1-positive human B-cell line.
Materials and methods
Cell cultures and experimental protocols
The surface IgA1-positive human B lymphoma cell line, DAKIKI, was purchased from ATCC (Manassas, VA, USA) [34–36]. Cells were cultured in 75-cm2 flasks in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum (FCS), 1 mM sodium pyruvate, 2 mM glutamine, 100 U/mL penicillin and 100 mg/mL streptomycin and studied at passages 7–12 in RPMI 1640 medium containing 1% FCS at 37°C in a humidified atmosphere containing 5% CO2. To assess the effect of cytokine stimulation on cell proliferation and the production of IgA1, cells were cultured with 10 ng/mL of recombinant human IFN-γ, IL-2, IL-4 or IL-5 (R&D Systems, Minneapolis, MN, USA) at a density of 3 × 104/mL per well in 24-well culture plates for 5 days. For analysis of IgA glycosylation, cells were cultured with 10 ng/mL of each recombinant cytokine at a density of 3 × 104/mL in 100 mm culture dish for 6 days. Similarly, to assess the effect of cytokine stimulation on mRNA expression of C1β3Gal-T and Cosmc, cells were cultured in 6-well culture plates at a density of 1 × 105/mL, with or without added IFN-γ or IL-4 (10 ng/mL) for 12, 24 or 48 h. Recombinant human IL-4 soluble receptor (IL-4sR) (R&D Systems) (500 ng/mL) was added to parallel wells to assess the effect of IL-4. To measure C1β3Gal-T activity, cells were cultured in 225-cm2 flasks at a density of 3 × 105/mL with 10 ng/mL of IL-4 for 24 and 72 h. After incubation, the supernatants and cells were harvested for various assays. For analysis of IgA glycosylation, the supernatants were concentrated (Amicon® Ultra-4 50K, Millipore, Billerica, MA, USA). Cell viabilities, as assessed by trypan blue dye exclusion, were greater than 90% in all experiments. In each of more than two experiments, triplicate culture wells for each stimulation were established.
Measurement of IgA1 content
IgA1 content in the supernatant from each culture well was measured in duplicate using enzyme-linked immunosorbent assay (ELISA). All incubations were performed at room temperature except for capture antibody coating. Briefly, 96-well immunoplates (Thermo Fisher Scientific, Waltham, MA, USA) were coated with 5 µg/mL of F(ab′)2 fragment goat anti-human IgA antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, USA), in phosphate-buffered saline (PBS) at 4°C overnight. After three washes with PBS containing 0.05% Tween-20 (PBST), plates were blocked by adding PBS containing 1% bovine serum albumin (BSA) to the wells for 90 min. Next, 50 µL of supernatant sample or standard human IgA1 (CALBIOCHEM, La Jolla, CA, USA) was added to the reaction wells and then incubated for 90 min. After three washes, 0.75 µg/mL alkaline phosphatase conjugated goat anti-human IgA (Southern Biotechnology Associates, Birmingham, AL, USA) in 1% BSA/PBS was added to the reaction wells and then incubated for 90 min. Plates were washed three times and developed with a substrate solution of 1 mg/mL p-nitrophenyl phosphate disodium salt (SIGMA, St. Louis, MO, USA) in 0.1 M glycine buffer containing 1 mM MgCl2, 1 mM ZnCl2, pH 10.4. The optical density at 405 nm was determined in a microplate reader (Benchmark PlusTM Bio-Rad Laboratories, Hercules, CA, USA). IgA1 concentration in unknown duplicate samples was determined by interpolation of the respective optical density into the appropriate standard curve. IgG and IgM contents in the supernatant were measured using sandwich ELISA (IMMUNOtek®, ZeptoMetrix Corporation, Buffalo, NY, USA) according to the manufacturer’s instructions.
Enzyme-linked lectin binding assays
The O-glycosylation profile of IgA1 samples was measured by binding of the lectin, Helix aspersa (SIGMA), which is specific for terminal GalNAc [37]. Briefly, 2.5 µg/mL of F(ab′)2 fragment goat anti-human IgA antibody, which was the same antibody for IgA1 measurement, was utilized for this assay to capture IgA1. Plates were washed three times and blocked by adding 1% BSA/PBST to the wells for 3 h. The supernatants of each culture were diluted to a final concentration of 1 µg/mL IgA1 in 1% BSA/PBST, and 50 µL of each sample was added to the reaction wells and then incubated at 4°C overnight. The captured IgA1 was subsequently desialylated by treatment for 3 h at 37°C with 20 mU/mL neuraminidase from Vibrio cholerae (Roche, Penzberg, Germany) in 10 mM sodium acetate buffer, pH 5. After seven washes, 2 µg/mL of biotinylated H. aspersa lectin diluted in 1% BSA/PBST was added to the reaction wells, and they were then incubated for 3 h at 37°C. Plates were washed five times, and lectin binding was detected with avidin-horseradish peroxidase conjugate (ExtrAvidin®,SIGMA) diluted in 1% BSA/PBST, and the reaction was developed with the peroxidase chromogenic substrate o-phenylenediamine-H2O2 (SIGMA). The colour reaction was stopped with 2 N H2SO4, and the optical density in duplicate samples at 490 nm was determined in a microplate reader.
RNA extraction and real-time PCR
Total RNA was extracted from each culture sample using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Synthesis of cDNA by Moloney Murine Leukemia Virus (M-MLV) reverse transcriptase (Invitrogen) employed 1 µg RNA as a template and random primers. Resulting cDNA (1 µg) was amplified in real time, with a 25 µL reaction mixture containing SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), appropriate primer pairs and water in an ABI Prism 7500 Sequence Detection system (Applied Biosystems, Foster City, CA, USA). The samples were incubated at 95°C for 10 min to activate the Amplitaq Gold polymerase, followed by 40 cycles with 15 s denaturation at 95°C, 60 s annealing at 60°C and 60 s extension at 60°C. Quantification of specific mRNA in the samples was measured relative to the corresponding gene-specific standard curve. Real-time PCR reactions were carried out with 5-fold serial dilutions of cDNA as a template. The primer sets of C1β3Gal-T, Cosmc and human β-actin employed are in Table 1. An inverse linear correlation of the Ct value with the amount of template ensured reliable analysis of the mRNA level. The Ct values obtained were used to calculate the initial quantity of each specific cDNA by extrapolating from the standard curve derived for each primer set. All the samples were tested in triplicate with the reference gene human β-actin, used as a housekeeping gene to normalize data.
Table 1.
Sequences of primers used for real-time RT-PCR
| Gene | Primer sequence | Fragment size (bp) |
|---|---|---|
| C1β3Gal-T | 5′-AAG GTT GAC ACC CAG CCT AA-3′ (forward primer) | 226 |
| 5′-CTT TGA CGT GTT TGG CCT TT-3′ (reverse primer) | ||
| Cosmc | 5′-GCT CCT TTT TGA AGG GTG TG-3′(forward primer) | 242 |
| 5′-TAC TGC AGC CCA AAG ACT CA-3′ (reverse primer) | ||
| human β-actin | 5′-TCA CCC ACA CTG TGC CCA TCA TCG A-3′ (forward primer) | 295 |
| 5′-CAG CGG AAC CGC TCA TTG CCA ATG G -3′ (reverse primer) |
Analysis of C1β3Gal-T activity
Cells were harvested in 1 mL of ice-cold sample buffer containing 0.25 M sucrose, 10 mM Tris–HCl (pH 7.4), and a protease inhibitor cocktail (#P-8340; SIGMA). Suspensions containing 5 × 107 cells were sonicated three times for 10 s in an ice bath. The cell homogenates were centrifuged at 3,900 rpm for 10 min to remove cell debris, and the supernatants were centrifuged at 41,000 rpm for 1 h. Microsome fractions thus obtained in the pellet were resuspended in the sample buffer containing 0.25 M sucrose, 10 mM Tris–HCl (pH 7.4). All procedures were carried out at 4°C [38]. The protein concentration in each sample was determined by dye binding (BCA protein Assay Kit; PIERCE, Rockford, IL, USA) according to the manufacturer’s instructions. A commercially available compound, Galβ1,3GalNAcα-pNp (CALBIOCHEM), was used as a standard to estimate the β1,3-linkage structure. Cell homogenates were incubated in a 75-µL reaction mixture containing 0.25 mM GalNAcα-pNp (Tronto Research Chemicals, North York, Ontario, Canada), 50 mM MES buffer (pH 6.5), 20 mM MgCl2, 2 mM ATP, and 1 mM UDP-Gal (SIGMA) at 37°C for 2 h. After centrifugation (at 10,000 rpm for 10 min), the supernatants from the samples were subjected to high-performance liquid chromatography (HPLC) on an OSD-80Ts QA column (4.6 × 250 mm, Tosoh, Tokyo, Japan). The reaction products were eluted with 12% acetonitrile in water containing 0.1% trifluoroacetic acid at a flow rate of 1.0 mL/min at 40°C and monitored with an ultraviolet spectrophotometer (absorbance at 300 nm) in a Gilson dual-pump high-pressure mixing system with detector (Gilson Medical Electronics, Middleton, WI, USA). A unit of activity defined as the area under the curve was normalized by the protein concentration in each sample. Each HPLC peak was isolated and subjected to electrospray ionization-mass spectrometry (TSQ-Quantum, Thermo Electron Corporation, San Jose, CA, USA).
Statistical analysis
Values are shown as mean ± standard error (SE). Comparisons among groups and two groups were evaluated by ANOVA and Mann–Whitney’s U-test, respectively. Values of P < 0.05 were regarded as significant.
Results
Cell proliferation
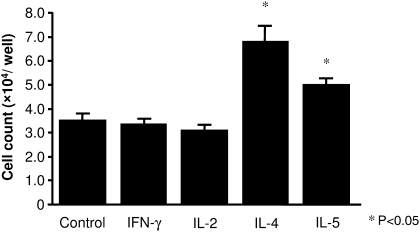
Incubation of DAKIKI cells in the presence of IL-5 (1.5-fold) or especially IL-4 (2.0-fold) evoked significant cell proliferation compared to cells maintained in medium (control) or in the presence of IFN-γ or IL-2 (P < 0.05) (Figure 1). There was a significant difference between the effect of IL-4 and that of IL-5 (P < 0.05).
Fig. 1.
Cell proliferation in response to cytokine stimulation. After incubation with IL-4 or IL-5, significantly more viable DAKIKI cells were recovered compared to cells incubated with either IFN-γ and IL-2 or to control cells in medium alone (P < 0.05). There was significant difference between IL-4 and IL-5 stimulation (P < 0.05). Neither IFN-γ nor IL-2 significantly affected cell proliferation compared to medium alone. Results are expressed as mean ± SE.
IgA1 production
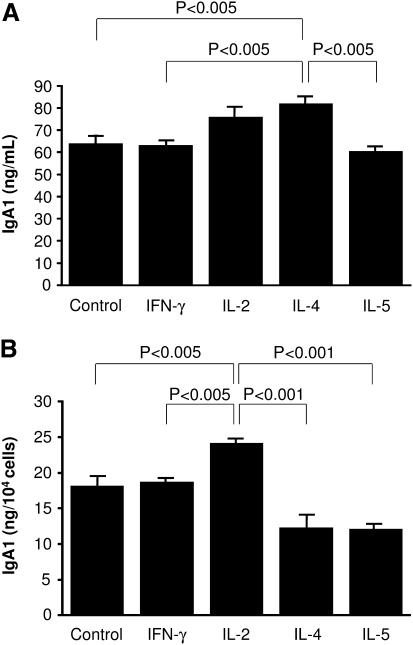
The concentration of IgA1 in supernatants from cells cultured with IL-4 was 28% higher than control cells in medium alone (P < 0.005) or cells cultured with IFN-γ or IL-5 (P < 0.005) (Figure 2A). Addition of IL-2 to the culture resulted in intermediate levels of IgA1 between medium control and IL-4 stimulation. However, on a per cell basis, IL-2 stimulation significantly increased IgA1 production by each cell (P < 0.005) (Figure 2B). Neither IFN-γ nor IL-5 altered the production of DAKIKI cells in culture, despite the capacity of IL-5 to increase cell number. IgG and IgM were not detected in the supernatant from the cell culture.
Fig. 2.
IgA1 production in response to cytokine stimulation. IgA1 concentration of supernatant was determined by sandwich ELISA. (A) IgA concentration of supernatant from cytokine stimulated cells. IgA1 content of supernatant from IL-4 stimulated cells was significantly higher than that of cells incubated with other cytokines, except for IL-2, and control (P < 0.005). (B) IgA1 production per cell stimulated by cytokine. Production of IgA1 from each cell stimulated by IL-2 was significantly higher than that of other cytokines or control (P < 0.005). Results are expressed as mean ± SE.
Effects of cytokine stimulation on IgA1 glycosylation
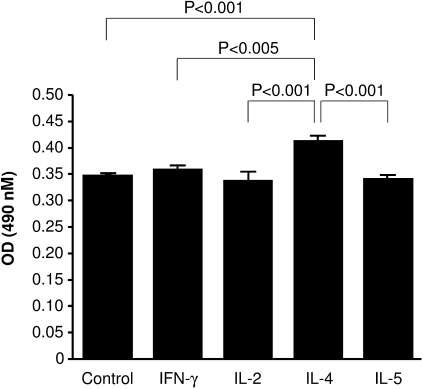
Specific lectin (H. aspersa) binding to IgA1 derived from cells stimulated by IL-4 was significantly (19%) higher than that from cells stimulated by the other cytokines or unstimulated cells (P < 0.005) (Figure 3). Because GalNAc occurs only in oligosaccharides of the hinge region of IgA1, the enhanced binding of the GalNAc-specific lectin H. aspersa is due to a deficiency of Gal in the O-linked side chains of the hinge region of IgA1. No significant increase in lectin binding was observed for IgA stimulated with the other cytokines relative to the unstimulated cultures, suggesting that no alteration of IgA1 glycosylation occurred. In summary, only IL-4 stimulation diminished the terminal Gal on IgA1.
Fig. 3.
Lectin binding to IgA1 derived from DAKIKI cells. Glycosylation of IgA1 was assessed by enzyme-linked lectin binding assay. Lectin binding to GalNAc of IgA1 was significantly higher after IL-4 stimulation than after stimulation by either other cytokines or medium control (P < 0.005). None of the other cytokines significantly increased binding of lectin relative to medium control. Results are expressed as mean ± SE.
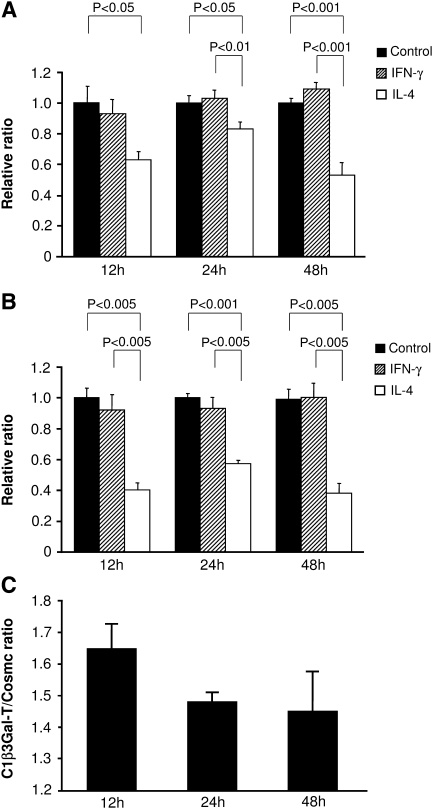
mRNA expression of C1β3Gal-T and Cosmc
The levels of mRNA encoding C1β3Gal-T and Cosmc in cells coincubated with IL-4 was significantly less than that in control after 12–48 h (P < 0.05) (Figure 4A and B). C1β3Gal-T/Cosmc ratio of mRNA expression stimulated by IL-4 at 12 h was higher than that at 24 or 48 h (Figure 4C). The level of C1β3Gal-T was decreased by 17–47% at each incubation time relative to control cells, whereas the level of Cosmc mRNA was reduced more powerfully (43–62%) after 12 or 24 h by stimulation of IL-4 (Figure 4A and B). Addition of IFN-γ had no significant effect on either C1β3Gal-T or Cosmc mRNA level. The effect of IL-4 on mRNA expression of C1β3Gal-T and Cosmc was significantly prevented by using IL-4sR at 24 h, 31% and 23%, respectively, and no significant difference was found in the results from the cells incubated with IL-4sR or unstimulated control.
Fig. 4.
C1β3Gal-T and Cosmc mRNA expression in response to cytokine stimulation. The level of mRNA encoding either C1β3Gal-T (A) or Cosmc (B) after addition of IL-4 was significantly less than that of control cells at each incubation time (P < 0.05 and P < 0.005, respectively). No significant difference was found between IFN-γ and control cultures at any incubation time. C1β3Gal-T/Cosmc ratio of mRNA expression with IL-4 stimulation was higher at 12 h than that of other incubation time (C). The effect of IL-4 on Cosmc mRNA levels was significantly greater than that on C1β3Gal-T at 12 and 24 h (P < 0.05). The efficiencies and the correlation index that were calculated from the slopes of the standard curves of C1β3Gal-T, Cosmc and β-actin were 0.984931–1.130224 and 0.98917–0.991649, respectively. Results are expressed as a relative ratio to control (A and B) or a ratio of C1β3Gal-T/Cosmc (C) of each incubation time, and mean ± SE.
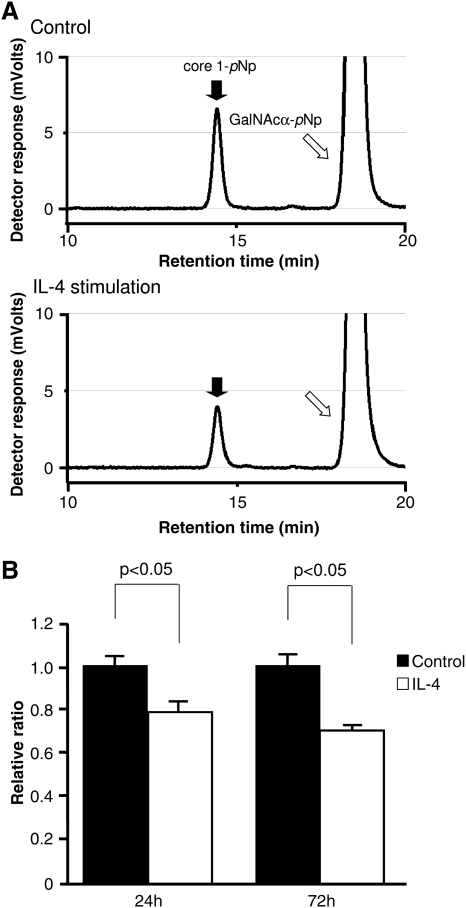
C1β3Gal-T activity
The retention time of the standard Galβ1,3GalNAcα-pNp (core 1-pNp) in HPLC system was 14.46 min (Figure 5A). Each reaction product was confirmed to be core 1-pNp by mass spectrometry (data not shown). Homogenate from cells incubated with IL-4 contained significantly less C1β3Gal-T activity compared to that of control cells at each incubation time (P < 0.05) (Figure 5B). Specifically, the enzymatic activity of C1β3Gal-T, relative to control, was decreased by 22% and 30% at 24 and 72 h, respectively. C1β3Gal-T activity in the presence of 10 mM UDP-Gal was not different from that in the presence of 1 mM UDP-Gal, indicating that the 1 mM level is at substrate excess (data not shown). No significant difference of C1β3Gal-T activity was observed between incubation times. Net enzymatic activity on endogenous human IgA1 is measured with the lectin binding assay in Figure 3, and the point of Figure 5 is to measure enzymatic activity objectively and quantitatively with a standard substrate. Comparison of Figure 3 with Figure 5 allows some evaluation as to the validity of the synthetic substrate representing IgA1 with a similar effect.
Fig. 5.
C1β3Gal-T activity in response to IL-4. (A) C1β3Gal-T activity was analysed by HPLC. Incorporation of the substrate GalNAcα-pNp (open arrow) into core 1-pNp (closed arrow) results in a shift in retention time to 14.46 min. The area under the peak at this time indicates galactosyltransferase activity. (B) C1β3Gal-T activity was significantly decreased by IL-4 stimulation compared to that of control at each incubation time, with longer incubation time resulting in a greater reduction relative to control. No significant difference of C1β3Gal-T activity was observed between incubation times. A unit of activity is defined as the area under the curve normalized to the protein concentration in each sample. Results are expressed as a relative ratio to control for each incubation time and mean ± SE.
Discussion
Lymphocyte functions, including cytokine profiles, have been investigated extensively in IgAN patients, and several abnormalities were identified [39]. It is noteworthy that T-cell cytokines known to be synthesized excessively in IgAN are closely related not only to immunoglobulin class switching and production but also to renal insufficiency [40–43]. Furthermore, it has been suggested that IgAN is a glomerular disease that is likely Th2 dependent [30].
Our results demonstrate that IL-4, one of the Th2 cytokines, alters the terminal glycosylation of IgA1. As anticipated, IL-4 stimulation significantly induced cell proliferation accompanied by increased IgA1 production in vitro [44,45]. The new findings here indicate that IL-4 also downregulates mRNA expression of both C1β3Gal-T and Cosmc and C1β3Gal-T enzymatic activity. Specific lectin binding revealed a significant deficiency of Gal in the O-glycans of IgA1 hinge region after IL-4 stimulation. This deficiency is the one implicated as a pathogenetic factor in IgAN [6–18]. No significant alteration of IgA1 glycosylation was observed by cytokine stimulation except for IL-4.
IL-4 regulates IgE and IgA synthesis, and increased production of IL-2 augments the IgA immune response in patients with IgAN [44,45]. Compared to healthy controls, blood mononuclear cells from patients with IgAN produce more IL-4 upon mitogen stimulation and express higher levels of mRNA encoding IL-4 and IL-5 [41,42]. Moreover, IgAN patients with severe renal dysfunction are more likely to hyperproduce Th2 cytokine and synthesize more IL-4 compared to patients with mild disease, although they have normal IL-4 responses during remission [41–43]. In an experimental model of IgAN induced by Sendai virus, Th2 predominant BALB/c mice developed more severe nephritis with acute renal insufficiency compared to the Th1 biased C3HeB strain of mice, which rarely developed renal insufficiency [32]. IL-4 is recognized for its many effects on lymphocytes and other leukocytes, but this is the first report implicating a role for IL-4 in the regulation of post-translational modifications of glycosylation in human B cell. IL-2 increased IgA1 secretion by each cell but had little or no effect on cell proliferation or terminal glycosylation. In contrast to IL-2, IL-5 induced significant cell proliferation but had little or no effect on IgA1 secretion or terminal glycosylation. Therefore, the rate of IgA1 synthesis itself and increased cell proliferation are not critical determinants of glycosyltransferase activity.
Core 1, the major constituent of O-glycans in IgA1 molecules, is synthesized by C1β3Gal-T [19]. Recently, the genes encoding C1β3Gal-T and its molecular chaperone Cosmc have been cloned [19,22]. The C1β3Gal-T gene lies on chromosome 7p14–p13, with a 1794-bp cDNA sequence encoding a protein of 363 amino acids [19], the Cosmc gene is on chromosome Xq23, with a 1471-bp cDNA sequence encoding a protein of 318 amino acids [22]. Cosmc is involved in the folding or stability of C1β3Gal-T [22]. A functional abnormality of the C1β3Gal-T responsible for the O-glycosylation of IgA1 has been proposed as a mechanism for altered O-glycosylation in IgAN [19,21]. Recently, it was reported that a mutation in Cosmc causes a loss of C1β3Gal-T activity [22,38]. Therefore, the aberrant IgA1 O-glycosylation in IgAN patients might be a consequence of reduced Cosmc expression in B lymphocytes of patients [23]. Although we have not yet clarified which molecule is more essential to C1β3Gal-T activity, mRNA expression of both C1β3Gal-T and Cosmc was reduced by IL-4 treatment in vitro collaterally with reduced C1β3Gal-T activity and subsequent glycosylation of IgA1.
Previous reports suggested that aberrantly glycosylated IgA1 molecules have an increased tendency both to self-aggregate and to form antigen–antibody complexes with IgG antibodies directed against IgA1 hinge epitope, favouring the generation of macromolecular aggregates of pIgA1 and IgA immune complexes [12,13,46]. Aberrantly, O-glycosylated IgA1 also has increased affinity for extracellular matrix components such as type IV collagen, fibronectin and laminin [15]. Therefore, the removal of sialic acid and Gal residues in IgA1 can promote mesangial deposition in IgAN by several mechanisms, likely synergistic [15]. In contrast to IgA1, IgD from patients with IgAN was more heavily O-galactosylated but less sialylated than IgA1, suggesting that the pattern of immunoglobulin O-glycosylation is differentially controlled at different stages of B-cell development [25]. Overall, altered regulation of glycosyltransferases at a particular stage of B-cell differentiation in patients with IgAN may have pathogenic significance for IgAN.
In summary, these findings suggest that aberrant immunoregulation manifest as enhanced IL-4 production in IgAN patients might contribute to the altered O-glycosylation of the IgA1 hinge region by down-regulation of C1β3Gal-T activity, regulated by not only C1β3Gal-T but also its molecular chaperone Cosmc. We have little information of relevance between increased IL-4 and altered IgA1 glycosylation in diseases other than IgAN. Although increased IL-4 might alter IgA1 glycosylation in other inflammatory diseases, several other factors also could be involved in the pathogenesis of IgAN. Further investigation is required to clarify the mechanisms whereby IL-4 downregulates both C1β3Gal-T and Cosmc mRNA levels and whether defects in Cosmc expression and/or in the direct expression of the C1β3Gal-T are involved in the defective IgA1 glycosylation in patients with IgAN. Resolution of these issues might provide a basis for developing better methods for treating IgAN.
Acknowledgments
We thank Drs. Hisashi Narimatsu and Kouichi Tachibana, Glycogene Function Team, Research Center for Glycoscience, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, for helpful discussions on analysis of enzymatic activity, and Hikari Taka and Terumi Shibata for helpful technical support. This work was supported in part by a grant from the study group on IgA nephropathy in Japan.
Conflict of interest statement. None declared.
References
- 1.Tomino Y. IgA nephropathy. From molecules to men. Contrib Nephrol. 1999;126:1–115. [PubMed] [Google Scholar]
- 2.Emancipator SN. IgA nephropathy and Henoch–Schönlein syndrome. In: Jennette JC, et al., editors. Heptinstall’s Pathology of the Kidney. 5th edn. Philadelphia: Lippincott-Raven; 1998. pp. 479–540. [Google Scholar]
- 3.Emancipator SN, Mestecky J, Lamm ME. IgA nephropathy and related diseases. In: Ogra PL, et al., editors. Mucosal Immunology. 2nd edn. New York: Academic Press; 1999. pp. 1365–1380. [Google Scholar]
- 4.Barratt J, Feehally J. IgA nephropathy. J Am Soc Nephrol. 2005;16:2088–2097. doi: 10.1681/ASN.2005020134. [DOI] [PubMed] [Google Scholar]
- 5.Julian BA, Novak J. IgA nephropathy: an update. Curr Opin Nephrol Hypertens. 2004;13:171–179. doi: 10.1097/00041552-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Mestecky J, Tomana M, Crowley-Nowick PA, et al. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contrib Nephrol. 1993;104:172–182. doi: 10.1159/000422410. [DOI] [PubMed] [Google Scholar]
- 7.Allen AC, Harper SJ, Feehally J. Galactosylation of N- and O-linked carbohydrate moieties of IgA1 and IgG in IgA nephropathy. Clin Exp Immunol. 1995;100:470–474. doi: 10.1111/j.1365-2249.1995.tb03724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiki Y, Tanaka A, Kokubo T, et al. Analyses of IgA1 hinge glycopeptides in IgA nephropathy by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Am Soc Nephrol. 1998;9:577–582. doi: 10.1681/ASN.V94577. [DOI] [PubMed] [Google Scholar]
- 9.Allen AC, Bailey EM, Brenchley PE, et al. Mesangial IgA1 in IgA nephropathy exhibits aberrant O-glycosylation: observations in three patients. Kidney Int. 2001;60:969–973. doi: 10.1046/j.1523-1755.2001.060003969.x. [DOI] [PubMed] [Google Scholar]
- 10.Hiki Y, Odani H, Takahashi M, et al. Mass spectrometry proves under-O-glycosylation of glomerular IgA1 in IgA nephropathy. Kidney Int. 2001;59:1077–1085. doi: 10.1046/j.1523-1755.2001.0590031077.x. [DOI] [PubMed] [Google Scholar]
- 11.Horie A, Hiki Y, Odani H, et al. IgA1 molecules produced by tonsillar lymphocytes are under-O-glycosylated in IgA nephropathy. Am J Kidney Dis. 2003;42:486–496. doi: 10.1016/s0272-6386(03)00743-1. [DOI] [PubMed] [Google Scholar]
- 12.Kokubo T, Hiki Y, Iwase H, et al. Evidence for involvement of IgA1 hinge glycopeptide in the IgA1-IgA1 interraction in IgA nephropathy. J Am Soc Nephrol. 1997;8:915–919. doi: 10.1681/ASN.V86915. [DOI] [PubMed] [Google Scholar]
- 13.Tomana M, Novak J, Julian BA, et al. Circulating immune complexes in IgA nephropathy consist of IgA1 with galactose-deficient hinge region and antiglycan antibodies. J Clin Invest. 1999;104:73–81. doi: 10.1172/JCI5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiki Y, Kokubo T, Iwase H, et al. Underglycosylation of IgA1 hinge plays a certain role for its glomerular deposition in IgA nephropathy. J Am Soc Nephrol. 1999;10:760–769. doi: 10.1681/ASN.V104760. [DOI] [PubMed] [Google Scholar]
- 15.Kokubo T, Hiki Y, Iwase H, et al. Protective role of IgA1 glycans against IgA1 self-aggregation and adhesion to extracellular matrix proteins. J Am Soc Nephrol. 1998;9:2048–2054. doi: 10.1681/ASN.V9112048. [DOI] [PubMed] [Google Scholar]
- 16.Amore A, Cirina P, Conti G, et al. Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol. 2001;12:1862–1871. doi: 10.1681/ASN.V1291862. [DOI] [PubMed] [Google Scholar]
- 17.Novak J, Tomana M, Matousovic K, et al. IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int. 2005;67:504–513. doi: 10.1111/j.1523-1755.2005.67107.x. [DOI] [PubMed] [Google Scholar]
- 18.Xu LX, Zhao MH. Aberrantly glycosylated serum IgA1 are closely associated with pathologic phenotypes of IgA nephropathy. Kidney Int. 2005;68:167–172. doi: 10.1111/j.1523-1755.2005.00390.x. [DOI] [PubMed] [Google Scholar]
- 19.Ju T, Brewer K, D’Souza A, et al. Cloning and expression of human core 1 β1,3-galactosyltransferase. J Biol Chem. 2002;277:178–186. doi: 10.1074/jbc.M109060200. [DOI] [PubMed] [Google Scholar]
- 20.Brockhausen I, Schutzbach J, Kuhns W. Glycoproteins and their relationship to human disease. Acta Anat (Basel) 1998;161:36–78. doi: 10.1159/000046450. [DOI] [PubMed] [Google Scholar]
- 21.Allen AC, Topham PS, Harper SJ, et al. Leucocyte β1, 3 galactosyltransferase activity in IgA nephropathy. Nephrol Dial Transplant. 1997;12:701–706. doi: 10.1093/ndt/12.4.701. [DOI] [PubMed] [Google Scholar]
- 22.Ju T, Cummings RD. A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc Natl Acad Sci USA. 2002;99:16613–16618. doi: 10.1073/pnas.262438199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin W, Zhou Q, Yang LC, et al. Peripheral B lymphocyte β1, 3-galactosyltransferase and chaperone expression in immunoglobulin A nephropathy. J Intern Med. 2005;258:467–477. doi: 10.1111/j.1365-2796.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- 24.Qin W, Zhong X, Fan JM, et al. External suppression causes the low expression of the Cosmc gene in IgA nephropathy. Nephrol Dial Transplant. 2008;23:1608–1614. doi: 10.1093/ndt/gfm781. [DOI] [PubMed] [Google Scholar]
- 25.Smith AC, de Wolff JF, Molyneux K, et al. O-glycosylation of serum IgD in IgA nephropathy. J Am Soc Nephrol. 2006;17:1192–1199. doi: 10.1681/ASN.2005101115. [DOI] [PubMed] [Google Scholar]
- 26.Malycha F, Eggermann T, Hristov M, et al. No evidence for a role of cosmc-chaperone mutations in European IgA nephropathy patients. Nephrol Dial Transplant. 2009;24:321–324. doi: 10.1093/ndt/gfn538. [DOI] [PubMed] [Google Scholar]
- 27.Buck KS, Smith AC, Molyneux K, et al. B-cell O-galactosyltransferase activity, and expression of O-glycosylation genes in bone marrow in IgA nephropathy. Kidney Int. 2008;73:1128–1136. doi: 10.1038/sj.ki.5002748. [DOI] [PubMed] [Google Scholar]
- 28.Gharavi AG, Moldoveanu Z, Wyatt RJ, et al. Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol. 2008;19:1008–1014. doi: 10.1681/ASN.2007091052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki H, Moldoveanu Z, Hall S, et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest. 2008;118:629–639. doi: 10.1172/JCI33189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurtado A, Johnson RJ. Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int. 2005;68:S62–S67. doi: 10.1111/j.1523-1755.2005.09711.x. [DOI] [PubMed] [Google Scholar]
- 31.Chintalacharuvu SR, Emancipator SN. The glycosylation of IgA produced by murine B cells is altered by Th2 cytokines. J Immunol. 1997;159:2327–2333. [PubMed] [Google Scholar]
- 32.Chintalacharuvu SR, Nagy NU, Sigmund N, et al. T cell cytokines determine the severity of experimental IgA nephropathy by regulating IgA glycosylation. Clin Exp Immunol. 2001;126:326–333. doi: 10.1046/j.1365-2249.2001.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckmann L, Morzycka-Wroblewska E, Smith JR, et al. Cytokine-induced differentiation of IgA B cells: studies using an IgA expressing B-cell lymphoma. Immunology. 1992;76:235–241. [PMC free article] [PubMed] [Google Scholar]
- 34.Xiang SD, Benson EM, Dunn IS. Tracking membrane and secretory immunoglobulin α heavy chain mRNA variation during B-cell differentiation by real-time quantitative polymerase chain reaction. Immunol Cell Biol. 2001;79:472–481. doi: 10.1046/j.1440-1711.2001.01033.x. [DOI] [PubMed] [Google Scholar]
- 35.Yu LM, Peng C, Starnes SM, et al. Two isoforms of human membrane-bound αIg resulting from alternative mRNA splicing in the membrane segment. J Immunol. 1990;145:3932–3936. [PubMed] [Google Scholar]
- 36.Weckert HA, Hughes JA, Benson EM, et al. Quantifiable analysis of human immunoglobulin heavy chain class-switch recombination to all isotypes. J Immunol Methods. 2000;233:141–158. doi: 10.1016/s0022-1759(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 37.Moore JS, Kulhavy R, Tomana M, et al. Reactivities of N-acetylgalactosamine-specific lectins with human IgA1 proteins. Mol Immunol. 2007;44:2598–2604. doi: 10.1016/j.molimm.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kudo T, Iwai T, Kubota T, et al. Molecular cloning and characterization of a novel UDP-Gal:GalNAcα peptide β1, 3-glactosyltransferase (C1Gal-T2), an enzyme synthesizing a core 1 structure of O-glycan. J Biol Chem. 2002;277:47724–47731. doi: 10.1074/jbc.M205839200. [DOI] [PubMed] [Google Scholar]
- 39.Emancipator SN. Immunoregulatory factors in the pathogenesis of IgA nephropathy. Kidney Int. 1990;38:1216–1229. doi: 10.1038/ki.1990.337. [DOI] [PubMed] [Google Scholar]
- 40.Snapper CM, Mond JJ. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993;14:15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- 41.Scivittaro V, Gesualdo L, Ranieri E, et al. Profiles of immunoregulatory cytokine production in vitro in patients with IgA nephropathy and their kindred. Clin Exp Immunol. 1994;96:311–316. doi: 10.1111/j.1365-2249.1994.tb06559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai KN, Leung JC, Li PK, et al. Cytokine production by peripheral blood mononuclear cells in IgA nephropathy. Clin Exp Immunol. 1991;85:240–245. doi: 10.1111/j.1365-2249.1991.tb05712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lai KN, Ho RT, Leung JC, et al. CD4-positive cells from patients with IgA nephropathy demonstrate increased mRNA of cytokines that induce the IgA switch and differentiation. J Pathol. 1994;174:13–22. doi: 10.1002/path.1711740104. [DOI] [PubMed] [Google Scholar]
- 44.Yano N, Endoh M, Miyazaki M, et al. Altered production of IgE and IgA induced by IL-4 in peripheral blood mononuclear cells from patients with IgA nephropathy. Clin Exp Immunol. 1992;88:295–300. doi: 10.1111/j.1365-2249.1992.tb03076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van den Wall Bake AW, Crowley-Nowick PA, Kulhavy R, et al. Cytokine-induced immunoglobulin production in primary IgA nephropathy. Am J Kidney Dis. 1992;20:611–617. doi: 10.1016/s0272-6386(12)70228-7. [DOI] [PubMed] [Google Scholar]
- 46.Tomino Y, Sakai H, Miura M, et al. Detection of polymeric IgA in glomeruli from patients with IgA nephropathy. Clin Exp Immunol. 1982;49:419–425. [PMC free article] [PubMed] [Google Scholar]